Abstract
Artificial neural networks (ANNs) have become increasingly sophisticated and are widely used for the extraction of patterns or meaning from complicated or imprecise datasets. At the same time, our knowledge of the biological systems that inspired these ANNs has also progressed and a range of model systems are emerging where there is detailed information not only on the architecture and components of the system but also on their ontogeny, plasticity and the adaptive characteristics of their interconnections. We describe here a biological neural network contained in the cephalopod statocysts; the statocysts are analogous to the vertebrae vestibular system and provide the animal with sensory information on its orientation and movements in space. The statocyst network comprises only a small number of cells, made up of just three classes of neurons but, in combination with the large efferent innervation from the brain, forms an ‘active’ sense organs that uses feedback and feed-forward mechanisms to alter and dynamically modulate the activity within cells and how the various components are interconnected. The neurons are fully accessible to physiological investigation and the system provides an excellent model for describing the mechanisms underlying the operation of a sophisticated neural network.
Keywords: statocyst, mollusc, balance
1. Introduction
Artificial neural networks (ANNs), inspired by the processing systems present in simple nervous systems, are now widely used for the extraction of patterns or meaning from complicated or imprecise datasets (e.g. Arbib 2003; Enquist & Ghirlanda 2005). Although modern ANNs have progressed considerably from the early basic feed-forward models to systems of significant sophistication, some with varying levels of feedback, modulation, adaptation, learning, etc. (e.g. Minsky & Papert 1969; Gurney 1997; Vogels et al. 2005), they rarely contain the full processing capabilities or adaptive power of real assemblies of nerve cells. Part of the problem in modelling such capabilities is that the detailed mechanisms underlying the operation of biological neural networks are not themselves fully identified or well understood, for there is a dearth of good biological model systems that possess a wide range of processing mechanisms but whose physiological processes and cellular interconnections can be fully investigated and characterized. One of the best biological model systems available is the vertebrate visual system, but even here the full range of cellular connections and interactions have not yet been characterized and hence cannot be developed into equivalent models (e.g. Van Hemmen et al. 2001; Wassle 2004).
In this report, we describe a real biological model system that comprises only three cell types but nevertheless demonstrates a wide range of complex and sophisticated cellular interactions, processing mechanisms and adaptive responses. This is the cephalopod vestibular system, a peripheral sense organ whose input can be specified and controlled, and which has a large feedback control system that can be monitored or mimicked.
Cephalopods have already supplied two model systems of fundamental importance to neuroscience and the development of neural networks. First, the squid giant axon preparation which, through the large size and accessibility of the axon to physiological and biochemical investigation, enabled the sub-cellular mechanisms underlying the nerve action potential to be elucidated and then described in precise mathematical formulation, e.g. Hodgkin & Huxley (1952). Second, the squid giant synapse, again through its large size and accessibility to physiological recording methods, has enabled the mechanism of neurotransmitter release at the synapse to be precisely described and modelled (reviewed by Llinas 1999).
Cephalopods, such as squid, cuttlefish and octopuses, are fast-moving predators that compete with fish and other marine vertebrates, and hence have developed motor and sensory systems of comparable performance (Packard 1972; Hanlon & Messenger 1996). Like all fast moving animals, cephalopods can sense the direction of gravity (linear accelerations) as well as the speed and direction of their turning movements (angular accelerations). The vestibular, or more correctly for a mollusc, the statocyst system used to detect these accelerations shows many parallels to vertebrate semicircular canal systems in both gross morphology and function (cephalopods: Budelmann 1977; vertebrate: Highstein et al. 2004). However, as a model for the investigation of the network properties underlying the operation of a complex but manageable neural system, the statocyst has clear advantages over analogous vertebrate systems. First, it is embedded in cartilage not bone and is hence easily accessible; second, precise physiological recordings can be obtained from all of the cellular elements comprising the peripheral network; and third, there is a very large and varied efferent feedback/feed-forward system whose influence on the operation of the system can be closely observed (Williamson & Chrachri 1994, 2004). These features are described below, as well as the likely operation of the neural network.
2. Statocyst and network structure
(a) Gross morphology
The gross morphologies of cephalopod statocysts have been described previously (e.g. Young 1960; Barber 1966; Budelmann et al. 1987a,b) but, in brief, consist of two endolymph-filled cavities lying within the cranial cartilage, just ventral and lateral to the brain. The left and right statocysts are mirror reversed with the precise shape of the cavity being species specific, presumably influencing the hydrodynamics of the system and hence the overall response characteristics (Maddock & Young 1984; Young 1989). Each statocyst contains two main areas of sensory epithelium, a macula/statolith area and a crista/cupula area. The macula system (figure 1a) consists of a plate of mechanosensory hair cells with an overlying statolith; the force exerted by the statolith mass upon the mechanosensory hair cells is dependent on the magnitude and direction of any applied linear accelerations, e.g. gravity. Octopuses have a single macula with a compact statolith, while squid and cuttlefish have three maculae carrying numerous small statoconia. Where three maculae are present, they are set in different planes thus enabling the acceleration(s) to be resolved into its directional components.
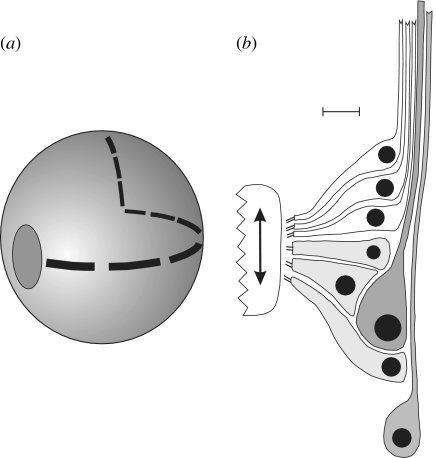
Figure 1.
(a) Diagram of the Octopus statocyst showing the ovoid plate of macula cells and the crista strip which runs around the inside of the statocyst sphere and is divided into nine segments. (b) Diagram of a greatly expanded transverse section through one of the crista segments showing the rows of primary sensory hair cells (white), the secondary sensory hair cells (light grey) and the afferent neurons (dark grey). The direction of travel of the overlying cupula is shown by the arrow. The efferent innervation is not shown. Scale bar in B=15 μm.
The crista/cupula system, the subject of this report, consists of a narrow strip of sensory epithelium that winds around the inside wall of the statocyst, such that it covers the three orthogonal planes (figure 1a). The strip comprises mechanosensory hair cells and afferent neurons, plus supporting cells, and is divided into segments: nine in octopuses and four in squids and cuttlefish. Each crista segment carries an overlying, sail-like cupula that is deflected during rotational movements of the animal by the flow of endolymph relative to the statocyst wall. Since the base of each cupula is in contact with the underlying mechanosensory hair cells, a cupula deflection may stimulate or inhibit these cells, depending on the direction of the cupula movement and the polarization of the hair cells.
(b) Crista morphology
A histological transverse section through the crista epithelium (figure 1b) shows that it is made up of three main cells types; these are primary sensory hair cells, secondary sensory hair cells and afferent neurons. The sensory hair cells are arranged in up to eight rows along the crista segment with each row containing only primary hair cells or secondary sensory hair cells. Primary sensory hair cells, which have a centripetally passing axon of their own, are common in invertebrates, while secondary hair cells, which have no axon but make synaptic contact with an afferent neuron, are usually found only in vertebrates (Budelmann et al. 1987a) and so this mixture of the two types in a single epithelium is unique to cephalopods. The introduction of secondary sensory hair cell here is most likely an evolutionary sophistication that permits greater flexibility in signal processing, through modulation and integration of the input, in the peripheral nervous system. All of these mechanosensory hair cells are morphologically and physiologically polarized such that they are excited by a cupula deflection in one specific direction and inhibited by a deflection in the opposite direction. For the cells so far examined, the primary and secondary sensory hair cells in a single segment have the opposite polarity (Budelmann 1977); thus, a cupula movement which excites the secondary sensory hair cells will also inhibit the primary sensory hair cells in the same segment and vice versa.
The afferent neurons within a segment fall into two populations: large afferent neurons, which lie mainly beneath the secondary hair cells within the crista strip, and small afferent neurons, which lie more ventrally (for the horizontally running segments)—mainly at the edge or just ventral to the crista strip. There is morphological evidence from Octopus indicating that the large secondary hair cells make synaptic contact with the large afferent neurons in a convergent manner with an estimated ratio of 4 : 1, whereas the smaller secondary hair cells make synaptic contact with the smaller afferent neurons in a divergent manner with an estimated ratio of 1 : 2 (Budelmann et al. 1987a). The axons from the afferent neurons and those from the primary hair cells project via the statocyst nerves to the central nervous system within the cranium.
3. Physiological connections of the crista/cupula network
(a) Peripheral connections and responses
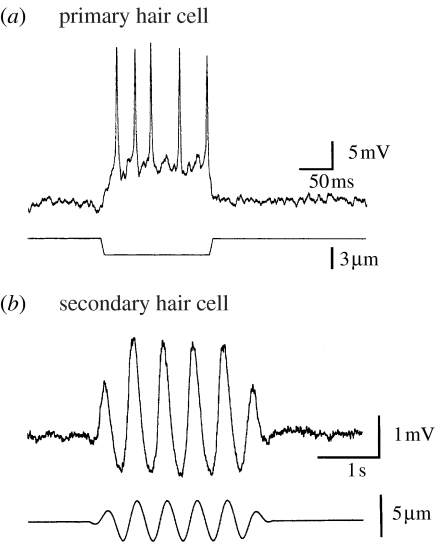
Unlike the analogous vertebrate semicircular canal system (e.g. Highstein et al. 2004; Eatock et al. 2005), all of the afferent cells in the cephalopod crista/cupula system have their somata within the peripheral sensory epithelium and hence it is possible to make sharp electrode intracellular recordings or whole-cell patch clamp recordings from all of the cellular components, including the afferent neurons. Such recordings from the mechanosensory hair cells (Williamson 1990; Chrachri & Williamson 1998) have shown that cupula deflections perpendicular to the segment direction (e.g. in an upward direction for horizontally oriented segments) result in a depolarization of the primary sensory hair cells and a train of action potentials in the cell axon (figure 2a). Similar recordings from secondary sensory cells show that they are polarized in the opposite direction and, as seen from the imposed sinusoidal displacement of the cupula (figure 2b), the response is asymmetric with much larger changes in membrane potential for displacements in the ventral direction than in the dorsal direction. This type of displacement/voltage response curve is also observed in recordings from vertebrate mechanosensory hair cells (e.g. Eatock et al. 2005). Depolarizing the secondary sensory hair cells in both vertebrate and cephalopod systems leads to an increased release of excitatory neurotransmitter, believed to be glutamate in both cases (Tu & Budelmann 1994; Highstein et al. 2004), and a subsequent excitation of the associated afferent neuron(s). For cephalopods, a range of neuromodulators have also been shown to influence the afferent cell activity (e.g. Tu & Budelmann 2000a,b). Note that in the vertebrate semicircular canal system, all of the sensory hair cells are polarized in one direction and hence stimuli applied in the opposite direction can only be registered by a decrease in any ongoing activity. Thus, in vertebrates, a steady resting discharge in the afferents is necessary, whereas this is not the case in cephalopods and this may have significant energy saving advantages.
Figure 2.
Responses of hair cells to cupula displacements. (a) Intracellular recording from a primary hair cell showing the depolarization and action potentials resulting from a downwards displacement of the cupula. The lower trace indicates the time course and amplitude of the cupula displacement. (b) Intracellular recording from a secondary hair cell showing the asymmetric voltage response to an imposed sinusoidal displacement of the cupula. Note the cell is depolarized by an upward displacement of the cupula. Lower trace indicates the time course and amplitude of the cupula displacement.
Unlike the primary sensory hair cells in the crista, the secondary sensory hair cells do not have afferent axons passing to the central nervous system; however, some secondary hair cells have long fine cellular processes extending from the cell's base along the direction of the crista segment (Williamson 1995; Chrachri & Williamson 1998). These processes are likely to be responsible for the spread of electrical coupling along the cells in a segment row beyond nearest neighbours, as described in §3c, but some appear much longer than their estimated space constant and hence are likely candidates for carrying regenerative action potentials. Small action potentials have been observed in intracellular recordings from the soma of some cephalopod secondary hair cells and whole cell patch clamp recordings from secondary hair cells have also detected an inward sodium current similar to that necessary for action potentials (Williamson 1995). It is therefore possible that these fine basal processes carry action potentials similar to the dendritic action potentials found in some vertebrate neurons (reviewed by Häusser et al. 2000), including retinal ganglion cells (Velte & Masland 1999).
Direct recordings of the afferent nerve activity from the crista during controlled imposed movements of the statocysts have shown that, like the vertebrate semicircular canals, the crista system acts mainly as a detector of angular velocity (Williamson & Budelmann 1985).
(b) The peripheral efferent system
The statocyst crista/cupula system receives a very large efferent innervation from the brain, with up to 75% of the axons in the statocyst nerve being efferent axons (Budelmann et al. 1987a,b) and the rest statocyst afferent fibres; this proportion of efferent fibres is much larger than, for example, the 8% found in some vertebrate vestibular nerves (Goldberg & Fernandez 1980). This statocyst efferent system forms a fine neural plexus beneath the crista ridge and innervates both the primary and the secondary mechanosensory hair cells as well as the afferent neurons (Budelmann et al. 1987b; Williamson & Chrachri 1994); a single hair cell can have up to 40 separate synaptic contacts from the efferent fibres (Budelmann et al. 1987b).
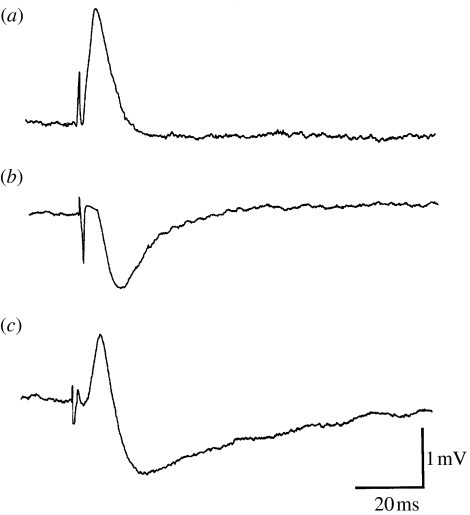
Activation of the efferent system has been found to enhance or depress the afferent input from the statocyst (Williamson 1985; Chrachri & Williamson 1998) and this is due to the direct depolarizing and/or hyperpolarizing effects on the primary and secondary hair cells, and the afferent neurons (Williamson 1989b; Chrachri & Williamson 1998). Thus, as shown in the intracellular recordings from secondary hair cells (figure 3), the efferent input to a cell can produce a depolarization, increasing its activity, a hyperpolarization, decreasing its activity, or even a mixture of the two. Here, the efferent fibres have been activated by direct electrical stimulation of the small crista nerve which contains some of the efferent fibres travelling from the brain to the crista. This is clear evidence for the existence of at least two populations of efferent fibres innervating the cephalopod statocyst, some excitatory and others inhibitory, and it is also apparent that individual cells receive multiple efferent contacts. An analogous excitatory and/or inhibitory efferent innervation is also present in the vertebrate semicircular canal system (Brichta & Goldberg 2000), but here it is much less extensive or influential. Note that because the cephalopod crista has primary and secondary sensory hair cells polarized in opposite directions, complex permutations of inhibiting or exciting the cells polarized in opposing directions are then possible.
Figure 3.
Intracellular recordings from three different secondary sensory hair cells showing the different efferent responses evoked by electrical stimulation of the crista nerve. (a) Depolarization of the cell, i.e. excitation. (b) Hyperpolarization of the cell, i.e. inhibition. (c) Mixed depolarization followed by a hyperpolarization.
The efferent inhibition is most likely achieved through activation of a cholinergic system (Auerbach & Budelmann 1986), whereas the excitation is through a catecholaminergic system (Budelmann & Bonn 1982; Williamson 1989c); however, a variety of other neurotransmitter and neuromodulator substances, including gamma aminobutyric acid and various peptides, have also been found to influence the statocysts' activity (Tu & Budelmann 1999, 2000a,b; Chrachri & Williamson 2004), and it may be that some or all of these are released from the efferent system, possibly as co-transmitters.
Recordings of the efferent fibre activity in semi-intact preparations of the statocysts and lower brain centres (Williamson 1986) have shown that, with the statocysts at rest, most efferent fibres cells display only low levels of spontaneous firing activity; however, during imposed sinusoidal movements of the statocysts, the efferent fibres fire bursts of activity and these are either synchronized in phase with the movement or in anti-phase with the movement. This evoked activity is presumably driven by the input from the statocysts themselves, through feedback and/or feed-forward pathways and/or from other mechanoreceptors.
(c) Extensive electrical coupling within the crista network
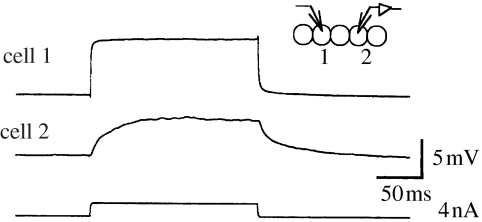
Simultaneous intracellular recordings from pairs of cells within the crista sensory epithelium (e.g. figure 4) have shown that groups of the cells are physiologically coupled through electrical synapses (Williamson 1989a; Chrachri & Williamson 1993, 1998). Thus, the secondary sensory hair cells along a row, within a single crista segment, are electrically coupled to their neighbours, with coupling ratios of up to 0.6. The electrical coupling ratio between cells is the ratio of the voltage change as seen in cell 2, divided by the voltage change imposed or observed in cell 1. This observed electrical coupling in the crista extends not only to an individual secondary hair cell's immediate neighbours, within a segment row, but also some distance along the row as has been confirmed by the persistence of the coupling even after the immediate neighbouring cells have been ablated (Williamson 1989a); this extended coupling probably arises because some of the secondary hair cells have fine processes extending along the crista row and these appear to make contact with multiple hair cells along the segment row (Chrachri & Williamson 1998).
Figure 4.
Electrical coupling between cells. Intracellular recordings from two secondary hair cells within a crista segment row showing the responses of both cells to the injection of a small, depolarizing current into cell 1. The synchronous depolarization in cell 2 indicates that these cells are electrically coupled.
Electrical coupling is also present between neighbouring primary sensory hair cells along a segment row, with coupling ratios here of up to 0.4 (Chrachri & Williamson 1993, 1998). These primary sensory cells produce action potentials when mechanically stimulated and such potentials are seen as sub-threshold depolarizations in neighbouring primary hair cells (Chrachri & Williamson 1998).
Although chemical synapses form the principal connections between the secondary sensory hair cells and the afferent neurons, low levels of electrical coupling have also been detected between these groups of cells (Chrachri & Williamson 1998). Coupling ratios of up to 0.18 have been detected between these cell types and, although this alone is unlikely to have a major influence on the activity of the afferent neuron, it has been found that the coupling effect can bring the afferent to fire action potentials if the membrane potential of the afferent neuron is just below threshold (Chrachri & Williamson 1998). Unlike the electrical coupling found between the other crista cell groups, the secondary hair cell to afferent neurons coupling is rectifying in that no electrical coupling was detected in the reverse direction between the afferent neurons and the secondary hair cells. A low level of electrical coupling was also found between neighbouring small afferent neurons, with coupling ratios of up to 0.3 (Chrachri & Williamson 1998), but as these cells do not lie in regular rows, as is the case with the hair cells, it is not clear how far along the segment this coupling extends.
Finally, no electrical coupling was detected between primary and secondary sensory hair cells across a crista segment, but such a finding would be surprising as these two types of hair cells are polarized in opposite directions and hence coupling here would act to diminish or cancel out any evoked responses.
(d) Modulation of electrical coupling
The strength of the electrical coupling between sensory hair cells has been found to vary, with coupling ratios of between 0.01 and 0.6 (e.g. Chrachri & Williamson 1998), but in addition, the coupling strength between individual pairs of cells can be modified by the application of a number of pharmacological agents, such as cAMP, forskolin and cGMP (Chrachri & Williamson 2001). Recent experiments have shown that bath application of the cholinergic agonist carbachol can reduce the electrical coupling between crista primary sensory hair cells (A. Chrachri & R. Williamson 2005, unpublished data) and a possible mechanism for this has been demonstrated in that acetylcholine has also been shown to modulate calcium entry into the hair cells and hence probably the intracellular calcium concentration (Chrachri & Williamson 2004); changes in intracellular calcium concentration have been shown to change cell coupling ratios in other systems (Chanson et al. 1999) and such a modulation of electrical coupling is also present in the vertebrate retina network (McMahon & Mattson 1996). Similarly, some of the pharmacologically active agents already reported to influence statocyst crista nerve activity, e.g. nitric oxide and the catecholamines, are also known to influence cell coupling in other systems (e.g. Rorig & Sutor 1996). These findings introduce the likelihood that the statocyst efferent innervation may act to modulate dynamically the strength of electrical coupling between groups of cells, as well as having a direct effect on cell membrane potentials.
(e) The central afferent and efferent projections
Nerve tracing studies have shown that the axons from the afferent neurons and from the primary sensory cells project through the statocyst crista nerves to a number of centres within the brain, with the most prominent projections being to the anterior and the posterior pedal lobes, as well as the ventral areas of the brachial and magnocellular lobes (Colmers 1982; Plän 1987). Similar tracer studies on the statocysts' efferent system have shown that the efferent axons originate from anterior palliovisceral lobes and the pedal lobes of the central brain complex. With the main afferent projections and efferent somata being co-localized, in the main, within the sub-oesophageal lobes of the brain (see fig. 4 of Williamson & Chrachri 2004), it is very likely that there are significant interconnections between these two statocyst systems and a number of appropriate inter-lobe connecting tracts have already been identified. Although the efferent fibres to the statocyst arise from at least two separate areas within the brain, there is no evidence, as yet, for functional divisions, e.g. the excitatory efferent fibres arising from one area and the inhibitory efferent fibres from another.
4. Network structure and operation
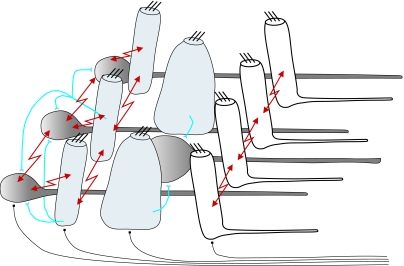
We have now identified the operation and many of the interconnections between the various cell types within the statocyst crista, e.g. the connection strengths and types, and can hence draw an outline network diagram (figure 5) and begin to speculate on how the network properties may influence the operation of the system.
Figure 5.
Outline network diagram of the crista epithelium showing the primary sensory hair cells (white), the secondary sensory hair cells (light grey), the small and large afferent neurons (dark grey) and the two types of synaptic connections: the electrical synapses (arrows) and the chemical synapses. Note the efferent nerve fibres, which innervate all of the cell components, coming from the crista nerve.
(a) The primary sensory hair cell network system
The primary sensory hair cell network system appears the simplest in the crista epithelium, with the sensory hair cells' input being driven by the mechanical movements of the overlying cupula to produce either a hyperpolarization or depolarization of the cells and hence a reduction or increase in any ongoing afferent activity; where spike firing is evoked, then, depending on the strength of coupling between neighbouring hair cells, the spiking activity in groups of neighbouring cells is likely to be in synchrony. Synchronous firing of receptor cells is also found in the vertebrate retina (e.g. Hu & Bloomfield 2003) and there it is said to preserve high-resolution spatial signals and compress information for efficient transmission across the limited capacity of the optic nerve. However, the primary hair cell axons in cephalopods are small and slow conducting, and hence the likely improvement in signal-to-noise ratio produced by the ensemble firing may be more important than the temporal signal. Synchronous firing of groups of cells from different areas may also be involved in a central ‘binding’ of related signals (O'Reilly & Busby 2001).
The direct effect of the efferent input on the hair cell membrane potentials could clearly act as a gain control, improving sensitivity during slow turning movements and reducing sensitivity during rapid movements, such as during jet-propelled escape response—where the entire sensory system is in danger of saturation. This type of usage could be incorporated into either a feedback or feed-forward system and the bursting activity recorded from the efferent nerves suggests a dynamic action of the efferent system.
A more specialized use of efferent fibres is found in the vertebrate auditory system, where activation can change the physical responses of the outer hair cells and hence the mechanical properties of the basilar membrane and its filter characteristics (e.g. Fettiplace 1999). Although there is no evidence that cephalopod sensory hair cells can actively change in length when stimulated by the efferent system, the possible change in coupling between the hair cells may result in significant changes in the apparent membrane capacitance and resistance of the cells, and hence the time constant of the responses produced by cupula stimulation. Thus, the frequency response or tuning characteristics of the receptor system could be increased or decreased by the impedance changes resulting from the changes in cell coupling.
Finally, the direct electrical coupling between the hair cells may also help remove uncorrelated noise from the cells' responses, as has been proposed for the vertebrate retinal cone cell network system (Smith 2002), and hence modulation of the cell coupling coefficients may further change response sensitivity.
(b) The secondary sensory hair cell network system
The membrane potentials of the secondary sensory hair cells are also modulated by the movements of the overlying cupula, responding to a cupula displacement with either a depolarization or a hyperpolarization depending on the direction of cupula movement and the direction of polarization of the hair cells. These hair cells do not have axons passing to the brain, and hence the effect of the membrane potential change will be to increase or decrease the rate of transmitter release from the synapses onto the afferent neurons and thereby change the rate of spiking discharge of these cells. However, some secondary hair cells have fine processes extending along the crista segment and it seems likely that these, as well as allowing the electrotonic spread of current, can also carry dendritic action potentials which may act to synchronize the hair cells' excitability along lengths of a segment by, for example, providing near-synchronous depolarizing inputs into groups of hair cells. Propagation of dendritic action potentials, particularly when involved in coincidence detection, has also been implicated in the promotion of synaptic plasticity (reviewed by Häusser et al. 2000) and it may be that these dendritic action potentials have a similar modulatory function here. Of course, such Hebbian modulation of hair cell and afferent neuron synapses is likely to occur anyway during development after hatching and during growth, for the statocysts continue to grow in both size and in cell numbers as the animal ages.
As with the primary hair cells, the electrical coupling between the hair cells could effect an averaging of the input signal, with a resulting improvement in signal-to-noise ratio and decrease in frequency sensitivity. Here again, the large efferent input onto the hair cells can act to increase or decrease the sensitivity, or possibly modulate the strength of coupling between neighbouring hair cells.
The convergence of the larger hair cell outputs onto the large afferent neurons may also improve detection sensitivity here by reducing the effect of synaptic noise on the spike generation, as has been postulated to occur in the vertebrate retinal network (e.g. Demb et al. 2004). The divergence of the signal from the smaller secondary hair cells onto the small afferent neurons may act to produce correlated, or even synchronous, firing in these afferents; this phenomenon has been observed in a number of sensory systems (Usrey & Reid 1999) and, although its functional significance is not fully understood, it may also lead to ensemble averaging in the higher processing centres. This correlated or synchronous firing of the afferent neurons may also be strengthened or weakened by modulation of the electrical coupling between cells via the efferent system.
(c) System complexity and flexibility
We have shown that there are only three neuronal cell types present in the statocyst crista epithelium (primary and secondary sensory hair cells and the afferent neurons) and that the system receives a very large efferent innervation from the brain which can excite and/or depress the activity in the individual cells, as well as possibly modulate the strength of electrical coupling between the cells. Despite the limited classes of neurons involved here, the resulting network complexity and plasticity appear to rival that seen in more extensive neural processing networks, such as the vertebrate retina, but it is not immediately clear why such a level of sophistication is required here. A possible reason for this may be that the mechanical input signal to the neural network, i.e. a sail-like cupula driven backwards and forwards by fluid movement, is not as simple as first appears. The statocyst cupulae are known to be rather soft and gelatinous (Williamson 1990) and therefore may flex or twist during turning movements that are not precisely perpendicular to the direction of the crista segment. Thus, nearly all movements are likely to cause a twisting or flexing of each of the four or nine cupulae within a statocyst and hence transmit a differential or uneven mechanical force to the sensory hair cells within the underlying crista segments; a specific head turn may then induce a pattern of differential excitation and inhibition across a single crista segment as well as across the multiple segments within both statocysts.
(d) Network considerations
We have argued here that the cephalopod statocyst system presents a model biological network of relatively simple architecture, fully accessible to physiological recording techniques, but which shows a range of dynamically modulated interconnections that transform a complex pattern of fluid flow within the statocyst cavity into a neural input signalling direction and magnitude of body movement. Clearly, this processing system cannot be modelled by a simple feed-forward network, for we know that it receives a large, complex and dynamically changing efferent input. At its simplest, the efferent activity could be interpreted as a recurrent signal, driven directly by the afferent input. However, this seems at odds with the magnitude and all encompassing nature of the efferent innervation and also, because the efferent axons are mainly small, the system may be too slow for such reactivity. A more likely scenario is that the multilayered efferent signal also contains an efference copy signal, based on the motor output, and representing the predicted, or re-afference, signal due to the animal's own intended movements. The effect of this could range from a simple reduction in input signal gain when a jet-propelled escape movement was imminent, or an increase in signal gain during fine hovering manoeuvres, to a finely tuned cancellation of the entire ‘expected’ input. Such efference copy systems have already been described in the mammalian vestibular system (Roy & Cullen 2004) and the fish electrosensory system (Bell 2001). The ability of the efferent system to modulate the level of electrical coupling between sets of cells would also enable the frequency response of the system to be adjusted and hence tuned, or matched, to the input.
A more speculative use of the efferent system would be to provide targeted co-activity along specific input pathways, thereby using Hebbian learning to increase or decrease the efficiency of specific neural pathways in an adaptive manner. Such a capability would enable a dynamic re-wiring of the network circuitry.
Finally, most neural network models consider that information processing results primarily from the properties of synapses and connectivity of individual neurons. However, we have shown here that single neurons can be non-spiking or carry dendritic spikes, both of which increase the likelihood of local signalling within a neuron, i.e. sub-sections of a neuron may be involved in separate, independent signalling pathways. In addition, we have also shown that groups of neurons may be dynamically connected through electrical coupling such that they act synchronously. Thus, the view of the single neuron as the basic element of the network must be revised to include the possibility of both sub-neuronal and supra-neuronal elements and these being dynamically interchangeable.
Acknowledgments
The experimental work incorporated above was supported by the Wellcome Trust and BBSRC. We would also like to thank Dr Sue Denham and Dr Guido Bugman for their helpful discussions on the operation of this network system.
Footnotes
One contribution of 15 to a Theme Issue ‘The use of artificial neural networks to study perception in animals’.
References
- Arbib M.A. 2nd edn. MIT Press; Cambridge, MA: 2003. Handbook of brain theory and neural networks. [Google Scholar]
- Auerbach B, Budelmann B.U. Evidence for acetylcholine as a neurotransmitter in the statocyst of Octopus vulgaris. Cell Tissue Res. 1986;243:429–436. doi:10.1007/BF00251060 [Google Scholar]
- Barber V.C. The fine structure of the statocysts of Octopus vulgaris. Z. Zellforsch. Mikroskop. Anat. 1966;70:91–107. doi:10.1007/BF00345067 [Google Scholar]
- Bell C.C. Memory-based expectations in electrosensory systems. Curr. Opin. Neurobiol. 2001;11:481–487. doi: 10.1016/s0959-4388(00)00238-5. doi:10.1016/S0959-4388(00)00238-5 [DOI] [PubMed] [Google Scholar]
- Brichta A.M, Goldberg J.M. Responses to efferent activation and excitatory response-intensity relations of turtle posterior-crista afferents. J. Neurophysiol. 2000;83:1224–1242. doi: 10.1152/jn.2000.83.3.1224. [DOI] [PubMed] [Google Scholar]
- Budelmann B.U. Structure and function of the angular acceleration receptor systems in the statocysts of cephalopods. Symp. Zool. Soc. Lond. 1977;38:309–324. [Google Scholar]
- Budelmann B.U, Bonn U. Histochemical evidence for catecholamines as neurotransmitters in the statocyst of Octopus vulgaris. Cell Tissue Res. 1982;227:475–483. doi: 10.1007/BF00204779. doi:10.1007/BF00204779 [DOI] [PubMed] [Google Scholar]
- Budelmann B.U, Sache M, Staudigl M. The angular acceleration receptor system of the statocyst of Octopus vulgaris: morphometry, ultrastructure, and neuronal and synaptic organization. Phil. Trans. R. Soc. B. 1987a;315:305–343. [Google Scholar]
- Budelmann B.U, Williamson R, Auerbach B. Structure and function of the angular acceleration receptor system of the statocyst of Octopus with special reference to its efferent innervation. In: Graham M.D, Kemmink J.L, editors. The Vestibular system: neurophysiologic and clinical research. Raven Press; New York, NY: 1987b. pp. 165–168. [Google Scholar]
- Chanson M, Mollard P, Meda P, Suter S, Jongsma H.J. Modulation of pancreatic acinar cell to cell coupling during ACh-evoked changes in cytosolic Ca2+ J. Biol. Chem. 1999;274:282–287. doi: 10.1074/jbc.274.1.282. doi:10.1074/jbc.274.1.282 [DOI] [PubMed] [Google Scholar]
- Chrachri A, Williamson R. Electrical coupling between primary hair cells in the statocyst of the squid, Alloteuthis subulata. Neurosci. Lett. 1993;161:227–231. doi: 10.1016/0304-3940(93)90300-a. doi:10.1016/0304-3940(93)90300-A [DOI] [PubMed] [Google Scholar]
- Chrachri A, Williamson R. Synaptic interactions between crista hair cells in the statocyst of the squid Alloteuthis subulata. J. Neurophysiol. 1998;80:656–666. doi: 10.1152/jn.1998.80.2.656. [DOI] [PubMed] [Google Scholar]
- Chrachri A, Williamson R. cAMP modulates electrical coupling between sensory hair cells in the squid statocyst. J. Physiol. Lond. 2001;536S:133–134. [Google Scholar]
- Chrachri A, Williamson R. Cholinergic modulation of L-type calcium current in isolated sensory hair cells of the statocyst of octopus, Eledone cirrhosa. Neurosci. Lett. 2004;360:90–94. doi: 10.1016/j.neulet.2004.01.050. doi:10.1016/j.neulet.2004.01.050 [DOI] [PubMed] [Google Scholar]
- Colmers W.F. The central afferent and efferent organization of the gravity receptor system of the statocyst of Octopus vulgaris. Neuroscience. 1982;7:461–476. doi: 10.1016/0306-4522(82)90280-9. doi:10.1016/0306-4522(82)90280-9 [DOI] [PubMed] [Google Scholar]
- Demb J.B, Sterling P, Freed M.A. How retinal ganglion cells prevent synaptic noise from reaching the spike output. J. Neurophysiol. 2004;92:2510–2519. doi: 10.1152/jn.00108.2004. doi:10.1152/jn.00108.2004 [DOI] [PubMed] [Google Scholar]
- Eatock, R. A., Fay, R. & Popper, A. (eds) 2005 Vertebrate hair cells Springer Handbook of Auditory Research, vol. 27. New York, NY: Springer.
- Enquist M, Ghirlanda S. Princeton University Press; Princeton, NJ: 2005. Neural networks and animal behavior. [Google Scholar]
- Fettiplace R. Mechanisms of hair cell tuning. Annu. Rev. Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. doi:10.1146/annurev.physiol.61.1.809 [DOI] [PubMed] [Google Scholar]
- Goldberg J.M, Fernandez C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J. Neurophysiol. 1980;43:986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Gurney K. UCL Press; London, UK: 1997. An introduction to neural networks. [Google Scholar]
- Hanlon R.T, Messenger J.B, editors. Cephalopod behaviour. Cambridge University Press; Cambridge, UK: 1996. [Google Scholar]
- Häusser M, Spruston N, Stuart G.J. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. doi:10.1126/science.290.5492.739 [DOI] [PubMed] [Google Scholar]
- Highstein, S. M., Fay, R. & Popper, A. N. (eds) 2004 The vestibular system Springer Handbook of Auditory Research, vol. 19. New York, NY: Springer.
- Hodgkin A.L, Huxley A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. Lond. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E.H, Bloomfield S.A. Gap junctional coupling underlies the short-latency spike synchrony of retinal ganglion cells. J. Neurosci. 2003;23:6768–6777. doi: 10.1523/JNEUROSCI.23-17-06768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R.R. Oxford University Press; New York, NY: 1999. The squid giant synapse: a model for chemical transmission. [Google Scholar]
- Maddock L, Young J.Z. Some dimensions of the angular-acceleration receptor systems of cephalopods. J. Mar. Biol. Assoc. UK. 1984;64:55–79. [Google Scholar]
- McMahon D.G, Mattson M.P. Horizontal cell electrical coupling in the giant danio: synaptic modulation by dopamine and synaptic maintenance by calcium. Brain Res. 1996;718:89–96. doi: 10.1016/0006-8993(96)00043-1. doi:10.1016/0006-8993(96)00043-1 [DOI] [PubMed] [Google Scholar]
- Minsky M, Papert S. MIT Press; Boston, MA: 1969. Perceptrons: an introduction to computational geometry. [Google Scholar]
- O'Reilly R.C, Busby R.S. Generalizable relational binding from coarse-coded distributed representations. In: Becker S, editor. Advances in neural information processing systems. MIT Press; Boston, MA: 2001. pp. 75–82. [Google Scholar]
- Packard A. Cephalopods and fish: the limits of convergence. Biol. Rev. 1972;46:241–307. [Google Scholar]
- Plän T. 1987 Functional neuroanatomy of sensory-motor lobes of the brain of Octopus vulgaris Ph.D. thesis, University of Regensburg.
- Rorig B, Sutor B. Regulation of gap junction coupling in the developing neocortex. Mol. Neurobiol. 1996;12:225–249. doi: 10.1007/BF02755590. [DOI] [PubMed] [Google Scholar]
- Roy J.E, Cullen K.E. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J. Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. doi:10.1523/JNEUROSCI.3988-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.G. Retina. In: Arbib M.A, editor. The handbook of brain theory and neural networks. MIT Press; Boston, MA: 2002. [Google Scholar]
- Tu Y.J, Budelmann B.U. The effect of l-glutamate on the afferent resting activity in the cephalopod statocyst. Brain Res. 1994;642:47–58. doi: 10.1016/0006-8993(94)90904-0. doi:10.1016/0006-8993(94)90904-0 [DOI] [PubMed] [Google Scholar]
- Tu Y.J, Budelmann B.U. Effects of l-arginine on the afferent resting activity in the cephalopod statocyst. Brain Res. 1999;845:35–49. doi: 10.1016/s0006-8993(99)01929-0. doi:10.1016/S0006-8993(99)01929-0 [DOI] [PubMed] [Google Scholar]
- Tu Y.J, Budelmann B.U. Inhibitory effect of cyclic guanosine 3′,5′-monophosphate (cGMP) on the afferent resting activity in the cephalopod statocyst. Brain Res. 2000a;880:65–69. doi: 10.1016/s0006-8993(00)02777-3. doi:10.1016/S0006-8993(00)02777-3 [DOI] [PubMed] [Google Scholar]
- Tu Y.J, Budelmann B.U. Effects of nitric oxide donors on the afferent resting activity in the cephalopod statocyst. Brain Res. 2000b;865:211–220. doi: 10.1016/s0006-8993(00)02222-8. doi:10.1016/S0006-8993(00)02222-8 [DOI] [PubMed] [Google Scholar]
- Usrey W.M, Reid R.C. Synchronous activity in the visual system. Annu. Rev. Physiol. 1999;61:435–456. doi: 10.1146/annurev.physiol.61.1.435. doi:10.1146/annurev.physiol.61.1.435 [DOI] [PubMed] [Google Scholar]
- Van Hemmen L.J, Cowan J.D, Domany E, editors. Models of neural networks: early vision and attention. vol. 4. Springer; New York, NY: 2001. [Google Scholar]
- Velte T.J, Masland R.H. Action potentials in the dendrites of retinal ganglion cells. J. Neurophysiol. 1999;81:1412–1417. doi: 10.1152/jn.1999.81.3.1412. [DOI] [PubMed] [Google Scholar]
- Vogels T.P, Rajan K, Abbott L.F. Neural network dynamics. Annu. Rev. Neurosci. 2005;28:357–376. doi: 10.1146/annurev.neuro.28.061604.135637. doi:10.1146/annurev.neuro.28.061604.135637 [DOI] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. doi:10.1038/nrn1497 [DOI] [PubMed] [Google Scholar]
- Williamson R. Efferent influences on the afferent activity from the Octopus angular acceleration receptor system. J. Exp. Biol. 1985;119:251–264. [Google Scholar]
- Williamson R. Efferent activity in the Octopus statocyst nerves. J. Comp. Physiol. A. 1986;158:125–132. doi:10.1007/BF00614526 [Google Scholar]
- Williamson R. Electrical coupling between secondary hair cells in the statocyst of the squid Alloteuthis subulata. Brain Res. 1989a;486:67–72. doi: 10.1016/0006-8993(89)91278-x. doi:10.1016/0006-8993(89)91278-X [DOI] [PubMed] [Google Scholar]
- Williamson R. Secondary hair cells and afferent neurones in the squid statocyst receive both inhibitory and excitatory efferent inputs. J. Comp. Physiol. A. 1989b;165:847–860. doi:10.1007/BF00610883 [Google Scholar]
- Williamson R. Electrophysiological evidence for cholinergic and catecholaminergic efferent transmitters in the statocyst of Octopus. Comp. Biochem. Physiol. C. 1989c;93:23–27. doi:10.1016/0742-8413(89)90004-2 [Google Scholar]
- Williamson R. The responses of primary and secondary sensory hair cells in the squid statocyst to mechanical stimulation. J. Comp. Physiol. A. 1990;167:655–664. [Google Scholar]
- Williamson R. Ionic currents in secondary sensory hair cells isolated from the statocysts of squid and cuttlefish. J. Comp. Physiol. A. 1995;177:261–271. doi:10.1007/BF00192416 [Google Scholar]
- Williamson R, Budelmann B.U. The responses of the Octopus angular acceleration receptor system to sinusoidal stimulation. J. Comp. Physiol. A. 1985;156:403–412. doi:10.1007/BF00610733 [Google Scholar]
- Williamson R, Chrachri A. The efferent system in cephalopod statocysts. Biomed. Res. 1994;15:51–56. [Google Scholar]
- Williamson R, Chrachri A. Cephalopod neural networks. Neurosignals. 2004;13:87–98. doi: 10.1159/000076160. doi:10.1159/000076160 [DOI] [PubMed] [Google Scholar]
- Young J.Z. The statocyst of Octopus vulgaris. Proc. R. Soc. B. 1960;152:3–29. doi: 10.1098/rspb.1960.0019. [DOI] [PubMed] [Google Scholar]
- Young J.Z. The angular-acceleration receptor system of diverse cephalopods. Phil. Trans. R. Soc. B. 1989;325:189–238. [Google Scholar]