Abstract
Background
Immunity to malaria develops naturally in endemic regions, but the protective immune mechanisms are poorly understood. Many vaccination strategies aim to induce T cells against diverse pre-erythrocytic antigens, but correlates of protection in the field have been limited. The objective of this study was to investigate cell-mediated immune correlates of protection in natural malaria. Memory T cells reactive against thrombospondin-related adhesive protein (TRAP) and circumsporozoite (CS) protein, major vaccine candidate antigens, were measured, as were frequencies of CD4+ CD25high T cells, which may suppress immunity, and CD56+ NK cells and γδ T cells, which may be effectors or may modulate immunity.
Methodology and Principal Findings
112 healthy volunteers living in rural Kenya were entered in the study. Memory T cells reactive against TRAP and CS were measured using a cultured IFNγ ELISPOT approach, whilst CD4+ CD25high T cells, CD56+ NK cells, and γδ T cells were measured by flow cytometry. We found that T cell responses against TRAP were established early in life (<5 years) in contrast to CS, and cultured ELISPOT memory T cell responses did not correlate with ex-vivo IFNγ ELISPOT effector responses. Data was examined for associations with risk of clinical malaria for a period of 300 days. Multivariate logistic analysis incorporating age and CS response showed that cultured memory T cell responses against TRAP were associated with a significantly reduced incidence of malaria (p = 0.028). This was not seen for CS responses. Higher numbers of CD4+ CD25high T cells, potentially regulatory T cells, were associated with a significantly increased risk of clinical malaria (p = 0.039).
Conclusions
These data demonstrate a role for central memory T cells in natural malarial immunity and support current vaccination strategies aimed at inducing durable protective T cell responses against the TRAP antigen. They also suggest that CD4+ CD25high T cells may negatively affect naturally acquired malarial immunity.
Introduction
Natural immunity that develops against P.falciparum malaria in endemic regions, providing protection from infection and/or disease, has yet to be clearly characterised. Acquisition of immunity against parasitaemia or disease is complicated. The stages of the parasite life cycle express different antigens against which the immune system responds with various effector mechanisms. Studying natural immunity not only improves our understanding of the pathogen-host interaction but may also provide clues for the design of malaria vaccines in terms of target antigens and optimal types of immunity. The role of immune responses that are not adaptive in nature, such as NK cells, γδ T cells should also be considered, as well as naturally-occurring CD4+CD25high regulatory T cells of unknown specificity. NK cells and γδ T cells may have direct effector functions or may modulate other immune responses [1], [2], whilst naturally-occurring CD4+CD25high FOXP3+ regulatory T cells may be suppress immunity [3], [4], [5]. Different individuals will randomly possess varying levels of all these cells. T cells against the liver stage of the malaria life cycle are one way in which sterile immunity may be effected, by eliminating infected hepatocytes or disrupting parasite development. This is considered the major mechanism by which immunisation with irradiated sporozoites elicits sterile protection in humans [6], as well as mediating the protective effect of various vaccines in animal models [7]. A number of studies in the field have implicated T cell responses to the pre-erythrocytic/liver stage antigen circumsporozoite (CS) protein as being associated with protection against malaria [8], [9], [10]. Thrombospondin-related adhesive protein (TRAP) is another pre-erythrocytic antigen that is a target for T cells and is one of the very few antigens that has generated protective immunity as a subunit vaccine in humans, and the only one in which such protection can be clearly attributed to vaccine-induced T cell responses [11]. Field studies in Africa have identified CD4+ and CD8+ T cell responses to TRAP induced by natural infection [12], and other studies suggest that TRAP is under diversifying selective pressure [13] further supporting a possible role in immune protection.
IFNγ-secreting T cells are a major effector mechanism for elimination of infected hepatocytes [14], together with cytotoxic T cells [15]. Immediate effector T cell responses can be measured in an ex-vivo 18 hour IFNγ ELISPOT assay, and are thought to comprise mainly effector-memory T cells which circulate shortly after antigenic priming or recall [16]. Resting memory T cells require antigenic re-stimulation and we have utilised an assay to measure such memory T cells [17], known as the “cultured IFNγ ELISPOT”, which reflects a potential IFNγ-secreting T cell capacity within PBMC. We have shown this assay to be capable of measuring a population of T cells distinct from effector responses [18], which appear to correlate more closely with protection from malaria following TRAP vaccination [19].
In this study we aimed to assess cell-mediated immunity as it relates to immune protection against malarial disease. We have previously examined ex-vivo T cell responses to TRAP in a cohort of subjects in a malaria-endemic region of coastal Kenya [20]. The study showed no relationship between ex-vivo IFNγ ELISPOT response against TRAP peptides, measured before a transmission season, and reduced malaria incidence in the following year. We now wished to investigated whether there was a relationship between resting memory T responses, as measured by cultured IFNγ ELISPOT against TRAP and CS, and subsequent development of clinical malaria, using stored cells from the same Kenyan cohort. We assessed how responses against these antigens, measured in different assays, relate to one another and to subject age. We also investigated potential correlations of the level of CD4+ CD25high T cells , NK cells, and γδ T cells with variable protection. We found that cultured responses to TRAP but not CS were significantly associated with reduced malaria, whilst increased numbers of CD4+ CD25high T cells were associated with increased malaria.
Methods
Subjects, Study Site and Follow up
Healthy volunteers between 1 month and 81 years of age were recruited from the Kenyan coastal district of Ngerenya. Informed consent (Consent form Text S2) was obtained from all subjects or their parent/guardian prior to the donation of 5 mls of venous blood into heparin anti-coagulant. Blood was taken in the first two weeks of September 1998, outside of the peak transmission periods. Ngerenya district is an area of moderate malaria transmission with an entomological inoculation rate of 10 infectious bites per person per year [21]. Ethical approval for this study (Ethical approval Text S1) was obtained from the Kenyan Medical Research Institute National Ethics Committee, as described previously [20]. Active follow up was performed for up to one year, with each participant visited weekly. Data for the entire cohort was analysed for 300 days after the early September start point. Those reporting subjective fever and/or having a documented temperature >37.5°C had finger prick blood smears prepared at Kilifi District Hospital, where parasite densities were counted. Clinical malaria was defined as a fever and/or temperature >37.5°C together with >2500 parasites/µl for those >1 year or <15 years of age, or any parasitaemia with fever and/or temperature >37.5°C in those under 1 year of age or >15 years, as determined in a previous study of this population [22]. A description of the subject population giving breakdown of ages represented and malaria incidence during the study are shown in Table 1. Due to limitations on sample, all tests could not be carried out on all volunteer samples (Table 2).
Table 1. Descriptive data on age groups tested and malaria incidence.
| Age range | Cultured TRAP | Cultured CS | FACS | No malaria | Malaria episode |
| 0–5 | 22* | 21 | 28 | 9 | 20 |
| 5–10 | 34 | 26 | 41 | 14 | 30 |
| 10–20 | 20 | 18 | 25 | 12 | 15 |
| >20 | 17 | 14 | 16 | 11 | 7 |
| Total (n) | 94 | 80 | 112 | 46 | 72 |
number of individuals in age group per test or according to malaria incidence
Table 2. Association between immune parameters and incidence of malaria.
| Survival (cont. variable): | Univariate | Multivariate | |||||
| Response | HR | 95% CI | p | HR | 95% CI | p | n |
| Cultured TRAP | 0.85 | 0.62–1.20 | 0.31 | 0.70 | 0.47–1.03 | 0.068 | 94 |
| Cultured CS | 1.07 | 0.76–1.51 | 0.69 | 1.33 | 0.88–2.032 | 0.176 | 80 |
| CD4+CD25high | 1.55 | 0.91–2.63 | 0.11 | 2.65 | 0.75–9.36 | 0.130 | 108 |
| CD56dim | 0.53 | 0.25–1.12 | 0.098 | 0.35 | 0.076–1.57 | 0.17 | 112 |
| CD56bright | 0.95 | 0.55–1.65 | 0.55 | 1.08 | 0.32–3.64 | 0.9 | 112 |
| γδ T cells | 1.30 | 0.42–4.06 | 0.65 | 2.5 | 0.55–11.34 | 0.24 | 64 |
| Logistic: | |||||||
| Response | OR | 95% CI | p | OR | 95% CI | p | n |
| Cultured TRAP | 0.68 | 0.41–1.12 | 0.13 | 0.50 | 0.27–0.93 | 0.028 | 94 |
| Cultured CS | 1.13 | 0.67–1.89 | 0.64 | 1.65 | 0.83–3.28 | 0.16 | 80 |
| CD4+CD25high | 2.12 | 0.92–4.91 | 0.079 | 2.52 | 1.05–6.08 | 0.039 | 108 |
| CD56dim | 0.27 | 0.076–1.01 | 0.076 | 0.33 | 0.084–1.29 | 0.11 | 112 |
| CD56bright | 0.80 | 0.33–1.94 | 0.63 | 0.70 | 0.28–1.78 | 0.46 | 112 |
| γδ T cells | 1.39 | 0.21–9.43 | 0.74 | 2.03 | 0.24–17.03 | 0.512 | 64 |
Univariate and Multivariate analysis with both Cox survival and logistic models were used to examine the data. HR hazards ratio, OR odds ratio, CI confidence interval, p<0.05 in bold
T cell responses
Peripheral blood mononuclear cells (PBMCs), separated from whole blood on a lymphoprep (Axis-Shield, UK) gradient, were washed and resuspended in RPMI 1640 medium (Sigma, UK) supplemented 1 in 100 with penicillin/streptomycin, L-glutamine (Invitrogen, UK) and 10% FCS (Biosera, UK). Cells were set up in 1 ml cultures, in 24-well plates, at 1×106/ml with peptides at 5–10 µg/ml, as optimized and described previously [19]. On days 3 and 7, 0.5 ml of culture medium was removed and replaced with medium containing 100 U/ml IL-2 (Chiron), resulting in a final concentration of 50 U/ml. On day 9 the cells were washed 3 times in medium and resuspended in 1 ml, and rested over night, before proceeding to a IFNγ ELISPOT assay. IFNγ ELISPOT kits were purchased from Mabtech (Sweden) and manufacturer's instructions followed, with modifications as previously described [19]. Antigenic stimuli used in vitro consisted of a pool of 57 peptides spanning TRAP and 48 peptides comprising CS (20mers overlapping by 10aa). Medium-only and PHA controls were used in all assays. Results are expressed as spot-forming cells (SFCs)/106 PBMCs (net antigen-stimulated spots less medium) on day 10 restimulation or ex-vivo.
Intracellular cytokine staining (ICS) and Flow Cytometry
Following the culture period, cell samples were examined for antigen-stimulated cytokine production by ICS, as previously optimized for mixed CD4/CD8 T cell responses [23], and recapitulating the cultured ELISPOT assay. Cells on day 10 were stimulated with peptides, or were unstimulated, for 20 hours, the last 18 hours of which were in the presence of 0.1 µg/ml Brefeldin A. Cells were washed, stained with surface antibodies (CD3, CD4, CD8) for 20 minutes before further washing and addition of Perm/Fix for 20 mins. After washing in perm/wash anti-IFNγ antibody was added for 20 minutes, and the cells were washed in PBS and examined using a FACScalibur. 100,000 cells were acquired. To examine other cell types in ex-vivo (uncultured) PBMC, cells were washed and stained for 20 mins with combinations of the following antibodies: CD56, CD3, CD4, CD25, γδ T cell receptor. For FOXP3 expression intracellular staining (ebiosciences, USA) as above was preceded by CD4, CD25 and CD127 staining. Appropriate isotype controls (mouse IgG2a-FITC, IgG1-PE, IgG1-APC, rat IgG2a-PE-Cy5) were used in each experiment (Figure S2). 50,000 cells were acquired on the FACScalibur. Data was analysed using Cellquest and FlowJo programs. All antibodies, Brefeldin A and ICS kit were purchased from BD (Oxford, UK).
Statistics
Multivariate analysis was carried out on data using both logistic and Cox survival models. Survival was additionally analysed using stratified Kaplan-Meier graphs and the log-rank test. Spearman's test was used for correlation. Stata 9 (StataCorp, Texas, USA) was used for all analysis. The cut-off value for statistical significance was p<0.05.
Results
Relating cultured IFNγ ELISPOT to age, and to ex-vivo IFNγ ELISPOT
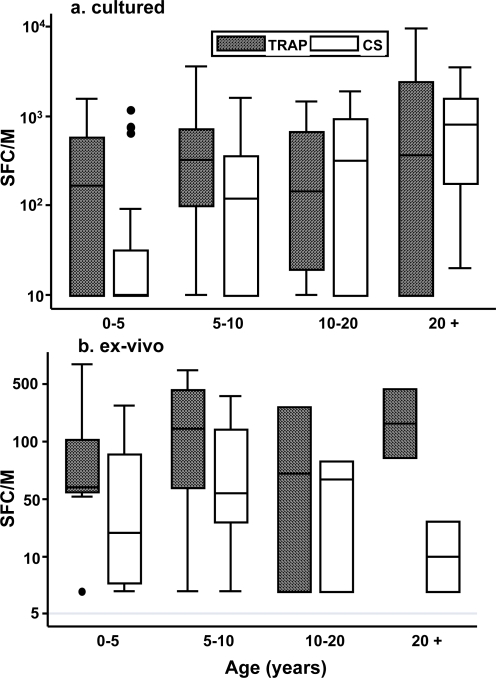
The cultured responses of individuals were stratified according to age into 4 groups: 0–5, 5–10, 10–20, and over 20. TRAP cultured ELISPOT responses were already high (mean 352 spot-forming cells (SFC)/M PBMC) in 0–5 year old children, and did rise further (517 SFC/M) in 5–10 year olds, but not significantly. In contrast, CS responses were lower in young (0–5 yr) children (137 SFC/M), and rose significantly with age (10–20 yr; 541 SFC/M)) (Fig. 1a). For the limited number of ex-vivo responses carried out, no significant rise was seen for TRAP or CS (Fig. 1b). Dot plots for these responses are shown in supporting information (Figure S1). Those individuals for whom sufficient PBMC were available were tested for cultured and ex-vivo responses against TRAP (n = 23) or CS (n = 14), and no correlation between the responses was observed for either TRAP (p = 0.61) or CS (p = 0.76) (data not shown). However, the ex-vivo TRAP and CS responses did correlate significantly (n = 23) (r = 0.57, p = 0.0041)(Fig. 2c), and there was a non-significant trend towards a positive correlation between cultured TRAP and CS responses (n = 70, r = 0.21, p = 0.08) (Fig. 2d).
Figure 1. Relationship between ELISPOT responses and age.
Cultured ELISPOT responses against (a) TRAP and (b) CS for individuals were stratified according to age into 4 groups: 0–5, 5–10, 10–20, and over 20. Median, 25th and 75th quartile, 5th and 95th quartile and outlying points are given by box and whisker plots.
Figure 2. Correlations between ELISPOT responses.
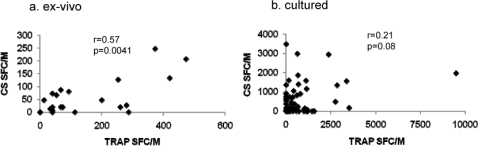
The relationship was examined between (a) TRAP and CS responses both ex-vivo, and (b) TRAP and CS responses both cultured.
Post Culture ICS and Surface Phenotyping
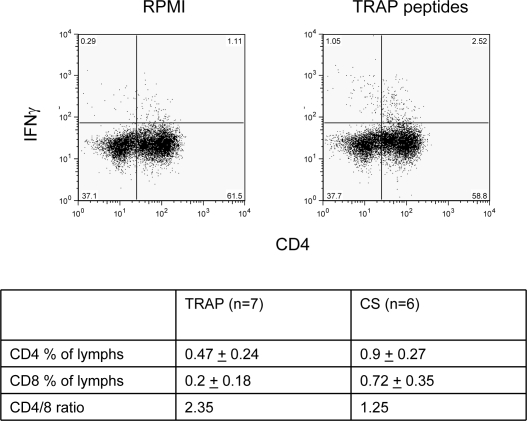
In order to determine the phenotype of T cells responding to antigens in the cultured ELISPOT assay, ICS was carried out on day 10 of the culture process. Cultured cells were stimulated with peptides, or unstimulated, and then stained for surface CD4 and CD8 and intracellular IFNγ. When unstimulated responses (RPMI only) were compared to peptide-stimulated, a significant increase was seen for both TRAP (p = 0.03) and CS (p = 0.004). For TRAP-responding T cells, 0.47% of lymphocytes that were IFNγ+ were CD4+ whilst 0.2% were CD8+, i.e. a 2.4 fold excess of CD4+ over CD8+ (n = 7)(Fig. 3). For CS the fold excess of CD4+ over CD8+ was 1.3.
Figure 3. Intracellular IFNγ Staining following culture.
ICS for IFNγ was carried out on cells by restimulation with peptides, following 10 days of culture, and co-staining with CD4 or CD8. The mean percentages (±standard deviation) of IFNγ+ cells possessing CD4 or CD8 (with medium controls subtracted) are shown together with the mean CD4:CD8 ratio.
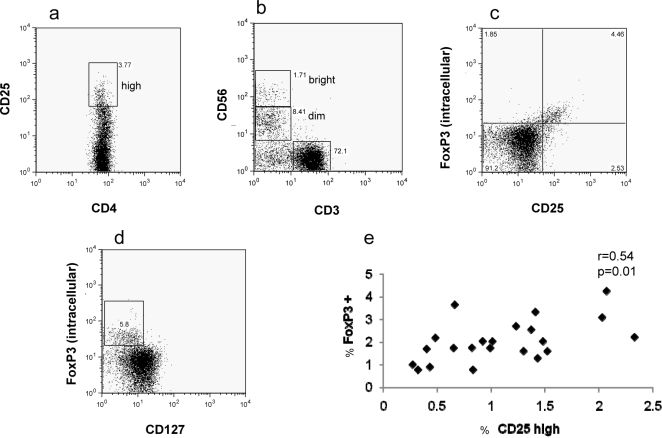
Unstimulated (ex-vivo) PBMC were examined for expression by lymphocytes of CD4/CD25, CD56 and γδ T cell receptor. Typical plots for CD4/CD25 and CD56 expression are shown (Fig. 4a,b), demonstrating CD4+ CD25high, and CD56dim/CD56bright status. The CD4+CD25high populations for a sub-group of the subjects were examined in more detail using intracellular staining for FOXP3 (Fig. 4c), where the majority of FOXP3+ T cells were CD25high, and for CD127 surface expression, where the majority were low/negative (Fig. 4d). Since a close correlation between CD25high and FOXP3+ was seen (Fig. 4e), CD25high was used for all further analysis.
Figure 4. Characterisation of regulatory T cells and NK cells.
Ex-vivo PBMC were stained for surface CD4, CD25 and CD127, and intracellularly for FoxP3. Typical dot plots show (a) the distribution of CD25 determining CD25high status, (b) the distribution of CD56 on CD3 negative (NK) cells determining CD56dim and CD56bright status, (c) that the majority of FoxP3+ cells are CD25high, and (d) that the FoxP3+ cells are predominantly CD127− [28]. (e) A correlation was demonstrated between CD25high and FoxP3 positivity.
Associations with malaria incidence
The initial univariate analyses of the relationship between immune response and malaria (Table 2), showed a tendancy for cultured TRAP response (p = 0.13) and CD56dim population (p = 0.076) to be associated with less malaria, and for CD4+ CD25high cells with increased malaria (p = 0.079). A similar pattern was seen for logistic and Cox survival analyses. When a multivariate model was applied, adjusting for age and including both cultured CS and TRAP responses, the association between TRAP responses and protection became stronger (p = 0.068 for Cox survival, p = 0.028 for logistic regression), whilst multivariate analysis showed a significant association between increased CD4+ CD25high population and increased malaria risk (p = 0.039).
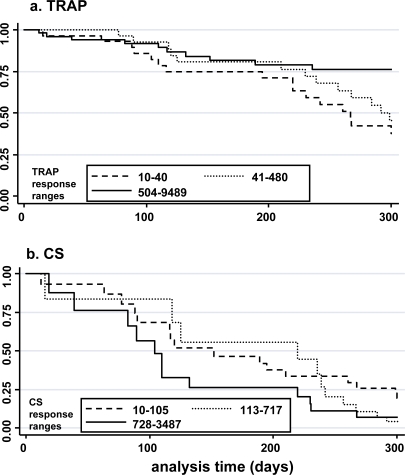
The adjusted survival functions of level of cultured ELISPOT response to TRAP or CS is shown for individuals stratified into three tertiles according to response, as Kaplan-Meier graphs (Fig. 5). When analysed by tertile, a similar pattern was seen to the previous analysis, with a significant association with reduced malaria observed for cultured ELISPOT response to TRAP (Fig. 5a)(p = 0.046), in contrast to CS (Fig. 5b)(p = 0.20).
Figure 5. Kaplan-Meier malaria-free survival plots.
Individuals were stratified into low, medium and high cultured ELISPOT responders (SFC/106) to (a) TRAP and (b) CS and probability of remaining free of clinical malaria is plotted over the 300 day monitoring period. Co-variants incorporated into the analysis are age and antigen response to the other antigen.
Multivariate analysis of the possession CD4+CD25high T cells showed a significant association with increased malaria incidence (p = 0.039). Since CD4+CD25high cells are known to have inhibitory effects on T cell responses, including proliferation, this population was compared to cultured ELISPOT responses by regression analysis. No negative or positive association between their frequency and cultured ELISPOT responses to TRAP (p = 0.9) or CS (p = 0.62) was observed (data not shown). No significant associations with survival were observed for the numbers of γδ T cells.
Discussion
The search for immune responses and parameters that may contribute to natural immunity and influence the incidence of infection and/or disease is important, despite the difficulties in obtaining blood samples from carefully monitored individuals, particularly of young age, in regions where malaria is endemic.
Our initial study on this cohort of individuals measured ex-vivo T cell responses against selected TRAP peptides and showed no association between responses and protection [20]. Previous data suggests that ex-vivo and cultured ELISPOT assays measure distinct populations of responder T cells [18], [19], possibly due to differing priming and homeostatic mechanisms. Further, it appeared that cultured responses may associate better with protection from malaria both in malaria-naïve individuals following TRAP vaccination in the UK [19] and in studies of responses to the CS protein in The Gambia [9]. This study therefore aimed to investigate whether naturally-acquired memory T cell responses to TRAP or CS, as measured by cultured ELISPOT, and other cell-mediated immune parameters, are associated with malaria incidence in the field.
Stratification of responses by age was carried out to determine whether immune response, as resistance to infection or disease, is simply acquired over time. TRAP cultured responses were established early, during the first 5 years of life, and did not significantly increase thereafter. In contrast, responses to CS developed more slowly with a significant rise over time. The ex-vivo responses showed a similar pattern of development of response over time, although the number of individuals examined >20 years were few. These data suggest that TRAP may be a more immunogenic antigen for T cell responses than CS since responses to it are generated more rapidly. Indeed, this is consistent with the weaker immunogenicity observed for CS compared to TRAP when DNA and viral vector vaccines were used in prime-boost regimens both in the UK [24], [25] and in the field [26], [27].
A comparison of antigen-specific responses showed that ex-vivo TRAP and CS responses correlated significantly, suggesting recent simultaneous exposure to these antigens in responding individuals. However, ex-vivo responses did not correlate with cultured responses to the same antigen, either for TRAP or CS, confirming our earlier findings [18]. There was a trend for cultured TRAP and CS responses to correlate, perhaps reflecting their differences in intrinsic immunogenicity but suggesting simultaneous exposure. Investigation of the phenotype of responding cells by ICS revealed a predominance of CD4+ IFNγ+ cells against TRAP, again similar to our previous findings [12], [23], compared to a more balanced CD4+/CD8+ composition for responses to CS.
In order to examine the relationship between immune parameters and malaria incidence, analysis models used survival as a continuous variable or logistically, and included age and all responses as co-variates in multivariate analysis (Table 2). Age is particularly important as resistance (“protective immunity”) to disease and infection develops over time, potentially mediated by numerous factors in addition to cell-mediated immunity. Throughout all the analyses the phenotypes consistently associated with subsequent malaria incidence were cultured TRAP response, and the CD4+CD25high population, but there was also weaker evidence of some association with the CD56dim population and reduced incidence. The cultured TRAP response was significantly associated with reduced malaria incidence. Depletion studies have demonstrated that the responding cells in cultured IFNγ ELISPOT possess the central memory T cell marker CCR7 prior to culture [17], [S Todryk unpublished]. Therefore, this suggests that the possession of such memory T cells against TRAP provides a protective advantage against malaria which would be generated by multiple contacts with antigen and remain at detectable levels once antigen levels have dropped, while the effector T cell numbers decline. These memory T cells become activated to proliferate and differentiate into effector cells upon re-exposure to antigen during a subsequent liver stage infection. Thus, a population of IFNγ-secreting T cells are provided that are capable of interfering with the liver stage of the life cycle, thus reducing or preventing merozoite release. The fact that the relationship between these cultured TRAP responses and malaria incidence is only moderately significant may not be surprising as many other factors are likely to contribute to protection in the field. These could include T cells reactive to other liver-stage antigens (although not CS, as shown in this study), antibody responses and innate mechanisms, and immunity to other stages of the life-cycle. Interestingly, the level of TRAP cultured ELISPOT response associated with protection here is in the range currently achievable by vectored vaccination regimes, at least in UK vaccinees, of around 400 SFC/M [19]. There was a difference between these findings and our recent studies (in conjunction with a vaccine trial [26]) in a neighbouring region of Kenya, where responses in non-vaccinated children were lower and not associated with protection. This may be due to reduced overall incidence in malaria in 2005 compared to 1998, as well as the locational difference, giving rise to less less T cell priming and not posing as testing a challenge as in 2005.
We found some weak evidence that increased CD56dim populations may be associated with reduced malaria incidence. CD56dim NK cells are known to be cytolytic [1] and so may have activity against host cells harbouring parasites and expressing parasite antigens or stress molecules.
Lastly, individuals with higher numbers of CD4+CD25high T cells were significantly associated with increased susceptibility to malaria. Cells of this phenotype will vary randomly within any human population dependant on a variety of extrinsic and intrinsic factors. The T cells are usually considered regulatory T cells when they express high levels of FOXP3. Such cells are known to inhibit proliferation of T cells in an antigen non-specific manner and may therefore inhibit parasite-specific T cells, as has been demonstrated in mouse [4] and human [5] malaria challenge models. In view of the many potential immune responses that could be affected by this CD4+CD25high cell population, further detailed analysis of this population in natural malarial immunity is warranted. We found no negative correlation between levels of these cells and levels of cultured ELISPOT reactivity against TRAP or CS suggesting an absence of inhibitory effects on these T cell responses, at least in vitro. The CD25high T cells could be affecting other T cell specificities, or other arms of the immune response.
Overall, these findings support a role of cell-mediated immunity in resistance to malaria infection, in particular T cell memory against TRAP. They also highlight the probability that both multiple adaptive and innate mechanisms together contribute to an individual's susceptibility to infection and/or disease. This complex relationship warrants further investigation in larger studies if we are to further dissect the mechanisms of natural protective immunity.
Supporting Information
Dot plots of individual responses against TRAP or CS measured by ex-vivo and cultured ELISPOT
(0.20 MB PPT)
Controls used in FACS analysis
(0.04 MB PPT)
Ethics Approval
(0.25 MB PDF)
Consent Form
(0.03 MB DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: We acknowledge funding support from the Wellcome Trust and the Medical Research council. KF was supported by a Wellcome Trust fellowship in Clinical Tropical Medicine. AVSH is a Wellcome Trust Principal Research Fellow. Funders had no role in any aspect of this project.
References
- 1.Roetynck S, Baratin M, Johansson S, Lemmers C, Vivier E, et al. Natural killer cells and malaria. Immunol Rev. 2006;214:251–63. doi: 10.1111/j.1600-065X.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T, Tachikawa S, Kanda Y, Kawamura T, Tomiyama-Miyaji C, et al. Malaria protection in beta 2-microglobulin-deficient mice lacking major histocompatibility complex class I antigens: essential role of innate immunity, including gammadelta T cells. Immunology. 2007;122:514–21. doi: 10.1111/j.1365-2567.2007.02661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 4.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 5.Walther M, Tongren JE, Andrews L, Korbel D, King E, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–96. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 7.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–62. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 8.Riley EM, Allen SJ, Bennett S, Thomas PJ, O'Donnell A, et al. Recognition of dominant T cell-stimulating epitopes from the circumsporozoite protein of Plasmodium falciparum and relationship to malaria morbidity in Gambian children. Trans R Soc Trop Med Hyg. 1990;84:648–57. doi: 10.1016/0035-9203(90)90133-y. [DOI] [PubMed] [Google Scholar]
- 9.Reece WH, Pinder M, Gothard PK, Milligan P, Bojang K, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–10. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 10.Luty AJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179:980–8. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 11.Hill AV. Pre-erythrocytic malaria vaccines: towards greater efficacy. Nat Rev Immunol. 2006;6:21–32. doi: 10.1038/nri1746. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan KL, Plebanski M, Akinwunmi P, Lee EA, Reece WH, et al. Broadly distributed T cell reactivity, with no immunodominant loci, to the pre-erythrocytic antigen thrombospondin-related adhesive protein of Plasmodium falciparum in West Africans. Eur J Immunol. 1999;29:1943–54. doi: 10.1002/(SICI)1521-4141(199906)29:06<1943::AID-IMMU1943>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Weedall GD, Preston BM, Thomas AW, Sutherland CJ, Conway DJ. Differential evidence of natural selection on two leading sporozoite stage malaria vaccine candidate antigens. Int J Parasitol. 2007;37:77–85. doi: 10.1016/j.ijpara.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, et al. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986;232:881–4. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 15.White KL, Snyder HL, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol. 1996;156:3374–81. [PubMed] [Google Scholar]
- 16.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 17.Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J Immunol. 2002;169:2210–4. doi: 10.4049/jimmunol.169.4.2210. [DOI] [PubMed] [Google Scholar]
- 18.Flanagan KL, Lee EA, Gravenor MB, Reece WH, Urban BC, et al. Unique T cell effector functions elicited by Plasmodium falciparum epitopes in malaria-exposed Africans tested by three T cell assays. J Immunol. 2001;167:4729–37. doi: 10.4049/jimmunol.167.8.4729. [DOI] [PubMed] [Google Scholar]
- 19.Keating SM, Bejon P, Berthoud T, Vuola JM, Todryk S, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175:5675–80. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 20.Flanagan KL, Mwangi T, Plebanski M, Odhiambo K, Ross A, et al. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am J Trop Med Hyg. 2003;68:421–30. [PubMed] [Google Scholar]
- 21.Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68:734–42. [PubMed] [Google Scholar]
- 22.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–9. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–55. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 24.Walther M, Thompson FM, Dunachie S, Keating S, Todryk S, et al. Safety, immunogenicity, and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus Ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infect Immun. 2006;74:2706–16. doi: 10.1128/IAI.74.5.2706-2716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci U S A. 2005;102:4836–41. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bejon P, Mwacharo J, Kai O, Mwangi T, Milligan P, et al. A Phase 2b Randomised Trial of the Candidate Malaria Vaccines FP9 ME-TRAP and MVA ME-TRAP among Children in Kenya. PLoS Clin Trials 2006. 2006;1:e29. doi: 10.1371/journal.pctr.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bejon P, Peshu N, Gilbert SC, Lowe BS, Molyneux CS, et al. Safety profile of the viral vectors of attenuated fowlpox strain FP9 and modified vaccinia virus Ankara recombinant for either of 2 preerythrocytic malaria antigens, ME-TRAP or the circumsporozoite protein, in children and adults in Kenya. Clin Infect Dis. 2006;42:1102–10. doi: 10.1086/501459. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dot plots of individual responses against TRAP or CS measured by ex-vivo and cultured ELISPOT
(0.20 MB PPT)
Controls used in FACS analysis
(0.04 MB PPT)
Ethics Approval
(0.25 MB PDF)
Consent Form
(0.03 MB DOC)