Abstract
Background
The aim of this paper is to determine the relationships between aortic wall calcification (AWC) including ascending and descending thoracic aortic calcification and gender, race/ethnicity, age, and traditional risk factors. Allison et al and Post et al previously described the relationship of noted risk factors and AWC as detected by computed tomography (CT) in smaller cohorts. We performed a cross-sectional study to determine which of these variables are independently associated with thoracic calcium.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) study population included a population based sample of four ethnic groups (12% Chinese, 38% White, 22% Hispanic and 28% black) of 6814 women and men ages 45–84 years old. CT scans were performed for all participants. We quantified AWC, which ranged from the lower edge of the pulmonary artery bifurcation to the cardiac apex. Multivariable logistic regression was used to evaluate relationships between AWC and measured cardiovascular risk factors.
Results
Overall prevalence of AWC was 28.0%. In the ethnic groups, prevalence of AWC was 32.4% Chinese, 32.4% White, 24.9% Hispanic and 22.4% Black. All age categories of females had a higher prevalence of thoracic calcification than males (total age prevalence: 29.1% and 26.8%, respectively). AWC were most strongly associated with hypertension and current smoking. In addition, diabetes, hypercholesterolemia, high LDL, low HDL, family history of heart attack and high CRP were all associated with increased AWC. Overall p-value for difference between genders for prevalence of AWC = 0.037. Overall p-value for difference between race for prevalence of AWC <0.001. The only significant gender differences distributed by race were for Chinese (p=0.035) and Hispanic (p=0.042) participants.
Conclusions
Risk factors for aortic calcification were similar to cardiovascular risk factors in a large population based cohort. Suprisingly, AWC was similar for the Chinese and white populations despite the fact that MESA demonstrated that coronary caclium was more prevalent in the white population. Further studies are needed to investigate whether aortic calcification is a risk factor for coronary disease, independent of coronary calcification.
Introduction
Cardiovascular disease is the leading cause of death in the United States1. Low density lipoproprotein (LDL) cholesterol is a cardiovascular risk factor for the development of coronary atherosclerosis.2 Calcifications are part of the development of atherosclerosis; they occur exclusively in atherosclerotic arteries and are absent in the normal vessel wall.3–5 Studies have demonstrated calcification in both coronary and aortic arteries to be a specific marker of underlying atherosclerosis in the respective vascular beds.6 Several preliminary studies have demonstrated that similar atherosclerotic risk factors contribute to the formation or presence of aortic wall calcification (AWC). AWC is common in the elderly and its presence is associated with increased risk of cardiovascular events3,4,5.
The aim of this paper is to determine the relationships between AWC, including ascending and descending thoracic aortic calcification (ATAC and DTAC, respectively), with traditional CV risk factors. Cardiac computed tomography (CT) is a well-established tool for the detection of coronary artery calcium (CAC). One previous study has shown that the presence of DTAC was associated with a higher prevalence of coronary artery disease by angiography6. No previous study has examined the relationship of known risk factors and thoracic aortic calcium as detected by computed tomography (CT) in a large population based participant cohort. We performed a cross-sectional study to determine which of these variables, at baseline, are independently associated with thoracic aortic calcium.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) was initiated in July 2000 to investigate the prevalence, correlates and progression of subclinical cardiovascular disease in individuals without known cardiovascular disease7. This prospective cohort study includes 6814 women and men ages 45–84 years old recruited from six U.S. communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN). There are 38% White (N=2624), 28% Black (N=1895), 22% Hispanic (N=1492), and 12% Chinese (N=803) individuals.
Medical history, anthropometric measurements, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002). Information about age, gender, ethnicity, and medical history were obtained by questionnaires. Information regarding physical activity was collected at the baseline examination with a combination of self-administered and interviewer-administered questionnaires. Physical activity was measured by self-reported leisure, conditioning, occupational and household activities, and quantitated by hours/day of activity. Current smoking was defined as having smoked a cigarette in the last 30 days. Alcohol use was defined as never, former, or current. Diabetes was defined as a fasting glucose ≥ 126 mg/dl or on hypoglycemic medication. Use of antihypertensive and other medications were based on clinic staff entry of prescribed medications.
Resting blood pressure was measured three times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, Florida) and the average of the 2nd and 3rd readings was recorded. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of medication prescribed for hypertension. Body mass index was calculated from the equation weight (kg)/height (m2).
Total and HDL cholesterol were measured from blood samples obtained after a 12-hour fast. LDL cholesterol was calculated with the Friedewald equation(11). CRP was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Analytical intra-assay CVs ranged from 2.3 – 4.4% and inter-assay CVs ranged from 2.1 – 5.7%.
All participants underwent two CT scans at the same time for evaluation of CAC, after signing informed consent. An ancillary study, supported by the National Institutes of Health, was performed to measure aortic and valvular calcification on the scans obtained for the MESA study. This study was approved by the Institutional Review Board of our institution. Three sites used an Imatron C-150XL CT scanner (GE-Imatron, San Francisco, CA), and three sites used a multidetector CT scanner (four slice). The method has been reported previously8. Image slices were obtained with the participant supine, with no couch angulation. A minimum of 35 contiguous images with a 2.5- or 3-mm slice thickness was obtained, starting above the left main coronary artery to the bottom of both ventricles. Each scan was obtained in a single breath hold. Section thickness of 3 mm, field of view of 35 cm, and matrix of 512 × 512 were used to reconstruct raw image data. The nominal section thickness was 3.0 mm for electron beam CT and 2.5 mm for four-detector row CT. Spatial resolution can be described by the smallest volume element, or voxel, for the protocol for each system: 1.15 mm3 for four-detector row CT (0.68 × 0.68 × 2.50 mm) and 1.38 mm3 for electron beam CT (0.68 × 0.68 × 3.00 mm). Ascending and descending thoracic aortic calcification (ATAC and DTAC, respectively) ranged from the lower edge of the pulmonary artery bifurcation to the cardiac apex (imaged on every study of coronary calcium) were quantified by using the same lesion definition for coronary calcification. AWC included both ATAC and DTAC on the portion of the aorta imaged by cardiac CT. Any calcified focus seen extending into the aortic root wall was excluded from the aortic wall calcium. The absence of AWC, ATAC and DTAC was assigned a score of zero.
Statistical Methods
Distributions of demographics, cardiovascular risk factors and the various calcium scores were compared across ethnic groups. Differences in characteristics were compared using ANOVA for continuous variables and χ2 tests for categorical variables. AWC including ATAC and DTAC was dichotomized as present (Agatston score > 0) or absent (=0). Because the prevalence of calcification is greater than 10% in our cohort, odds ratios (ORs) overestimate the relative risk (RR). Therefore, RR estimates are presented from the regression model y=exp(XTβ). The exponentiated parameters β are interpreted as relative risks. We assumed Gaussian error and used robust standard error estimates. Using this method we assessed the relationship between each risk factor and the presence of calcium, adjusting for all other risk factors in the model. The following covariates were used in a backward stepwise regression for multivariable adjustment: age, gender, body mass index, HDL, LDL, lipid lowering medication, smoking, hypertension, diabetes mellitus and education level. Among those with detectable AWC including ATAC and DTAC the relationship between risk factors and the quantity of calcification [(ln)Agatston score] by ethnicity was assessed with multivariable linear regression, controlled for all other risk factors in the model. The relationship was expressed as a percent difference in calcification for a given increment in the risk factor. The ‘Relative Difference’ is the anti-log of the regression coefficient using log-transformed calcium score as the dependent variable in each multiple linear regression analysis. Statistical analyses were performed with SPSS 13.0.1 software for Windows (SPSS Inc, Chicago, Ill) and STATA 8.0 for Windows (Stata Co, College Station, Tx).
Results
The study population (6814 individuals, 49% men and mean age: 63±10 years) was assessed as to the distribution and frequency of ATAC and DTAC. Overall 4904 (72%) did not demonstrate any detectable DTAC and ATAC. A total of 1675 (25%) had only DTAC, 56 (1%) had isolated ATAC, and 178 (3%) participants were found to have both DTAC and ATAC, respectively. One participant had uninterpretable data for DTAC.
Age had a stronger association with DTAC (RR 1.16 per year) than ATAC (RR 1.11 per year, P<0.05), and men had a lower prevalence of DTAC than women (RR 0.68, 95% CI 0.58–0.80, p<0.001). Figure 1 shows the prevalence of TAC variables by gender and increasing age-groups. Female participants had a higher prevalence of thoracic calcification than males, with the sole exception of ascending calcification in persons aged 55 years or more. With increasing age, the prevalence of thoracic calcifications abruptly increases in both genders.
Figure 1.
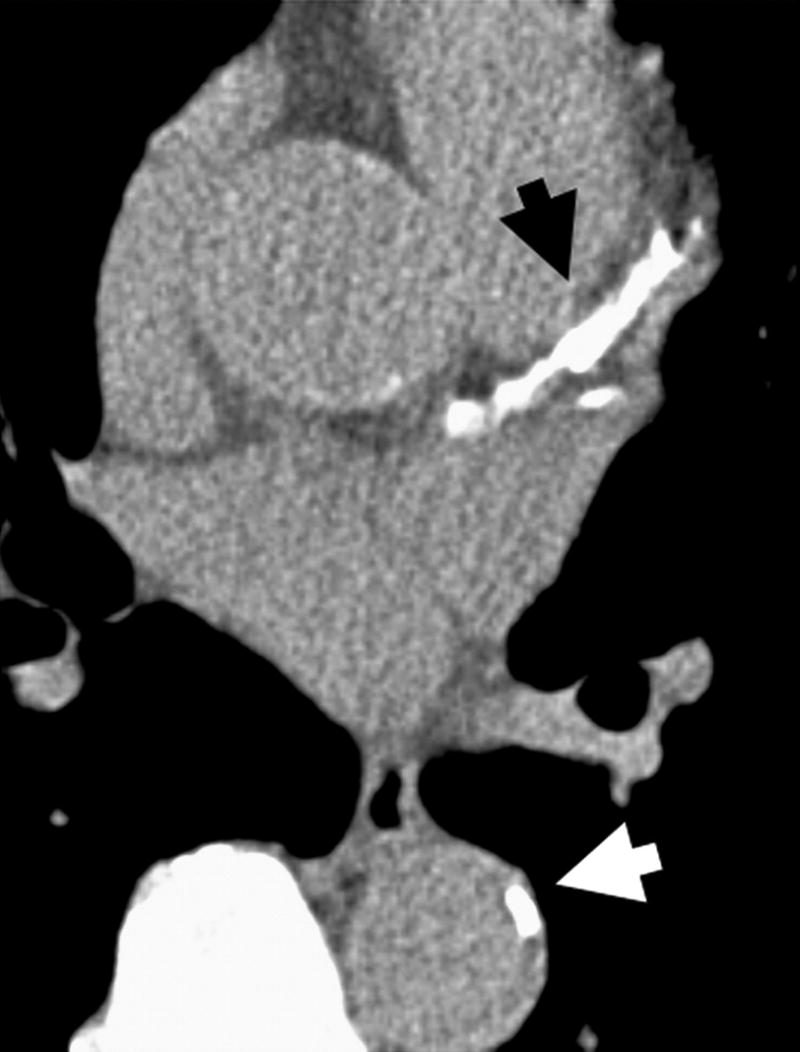
Table 1 demonstrates the baseline characteristics of the study population according to TAC variables. The ethnic makeup of the MESA cohort was: White 2623(38%), Chinese 803(12%), Black 1895(28%) and Hispanic 1492(22%). The prevalence of ATAC was highest among Black participants (4.5%, significantly greater than other ethnic subgroups, p<0.05). Conversely, white and Chinese participants had the highest prevalence of DTAC (32%, significantly greater than Black [21.4%] and Hispanics [24.3%], p<0.01). Smoking history was more significantly predictive of ATAC than DTAC. Former or current smokers had a 5% prevalence of ATAC, compared with a 2.2% prevalence in never smokers (p<0.01).
Table 1.
Characteristics of the MESA population.
| Characteristic | ATAC=0 | ATAC > 0 | DTAC=0 | DTAC > 0 | AWC=0 | AWC > 0 |
|---|---|---|---|---|---|---|
| N | 6578 | 235 | 4960 | 1853 | 4905 | 1907 |
| Age (years +/− S.D.) | 62 +/− 10 | 71+/− 8 ‡ | 59 +/− 9 | 71 +/− 8 ‡ | 59 +/− 9 | 71 +/− 8 ‡ |
| Female | 3492 (53%) | 108 (46%)† | 2579 (52%) | 1022 (55%)† | 2554 (52%) | 1046 (55%)† |
| Race | † | ‡ | ‡ | |||
| White (n=2623) | 2543 | 80 (3.0%) | 1788 | 835 (31.8%) | 1772 | 850 (32.4%) |
| Chinese (n=803) | 786 | 17 (2.1%) | 544 | 259 (32.3%) | 543 | 260 (32.3%) |
| African-American (n=1895) | 1812 | 83 (4.5%) | 1498 | 397 (21.4%) | 1470 | 425 (22.9%) |
| Hispanic (n=1492) | 1437 | 55 (3.7%) | 1130 | 362 (24.3%) | 1120 | 372 (24.9%) |
| Weight (lbs +/− S.D) | 174 +/− 38 | 169 +/− 37 | 176 +/− 39 | 166 +/− 36 ‡ | 176 +/− 39 | 166 +/− 36 ‡ |
| Height (cm) | 166 +/− 10 | 165 +/− 10 | 167 +/− 10 | 165 +/− 10 ‡ | 167 +/− 10 | 165 +/− 10 ‡ |
| BMI | 28 +/− 6 | 28 +/− 5 | 29 +/− 6 | 28 +/− 5 ‡ | 29 +/− 6 | 28 +/− 5 ‡ |
| Physical hours per day | 13 +/− 6 | 11 +/− 6 ‡ | 13 +/− 6 | 11 +/− 5 ‡ | 13 +/− 6 | 11 +/− 5 ‡ |
| Family history of heart attack | 2635 (43%) | 99 (46%) | 1918 (41%) | 815 (48%)‡ | 1896 (41%) | 837 (48%)‡ |
| Diabetes | 922 (14%) | 49 (21%)‡ | 620 (13%) | 351 (19%)‡ | 610 (13%) | 361 (19%)‡ |
| Hypertension | 2894 (44%) | 164 (70%)‡ | 1856 (37%) | 1201 (65%)‡ | 1823 (37%) | 1234 (65%)‡ |
| Systolic BP | 126 +/− 21 | 140 +/− 23 ‡ | 123 +/− 20 | 136 +/− 23 ‡ | 123 +/− 20 | 136 +/− 23 ‡ |
| Diastolic BP | 72 +/− 10 | 72 +/− 11 | 72 +/− 10 | 72 +/− 11 | 72 +/− 10 | 72 +/− 10 |
| Antihypertensive meds | 2400 (37%) | 136 (58%)‡ | 1568 (32%) | 967 (53%)‡ | 1541 (31%) | 994 (52%)‡ |
| Smoking status | ‡ | ‡ | ‡ | |||
| Never | 3342 (51%) | 75 | 2516 (51%) | 902 | 2502 (51%) | 915 |
| Former | 2372 (36%) | 115 | 1755 (36%) | 731 | 1730 (35%) | 756 |
| Current | 843 (13%) | 44 | 671 (14%) | 216 | 655 (13%) | 232 |
| Smoking (pack-yrs)* | 0.0 [0.0, 14.5] | 14.3 [0.0, 37.8] ‡ | 0.0 [0.0, 12.8] | 0.0 [0.0, 23.0] | 0.0 [0.0, 12.5] | 0.3 [0.0, 23.3] ‡ |
| Alcohol | ‡ | ‡ | ||||
| Never | 1340 (21%) | 50 | 942 (19%) | 448 | 935 (19%) | 455 |
| Former | 1563 (24%) | 61 | 1151 (23%) | 473 | 1139 (23%) | 485 |
| Current | 3627 (56%) | 121 | 2829 (58%) | 919 | 2793 (57%) | 954 |
| Total cholesterol | 194 +/− 36 | 198 +/− 41 | 194 +/− 35 | 195 +/− 36 | 194 +/− 35 | 195 +/− 37 |
| LDL | 117 +/− 31 | 122 +/− 36 † | 117 +/− 32 | 117 +/− 31 | 117 +/− 31 | 117 +/− 32 |
| HDL | 51 +/− 15 | 50 +/− 14 | 51 +/− 15 | 51 +/− 15 | 51 +/− 15 | 51 +/− 15 |
| Triglycerides* | 111 [77, 161] | 119 [86, 162] | 109 [76, 159] | 117 [83, 164] ‡ | 109 [76, 159] | 117 [83, 165] ‡ |
| Lipid lowering meds | 1041 (16%) | 59 (25%)‡ | 652 (13%) | 448 (24%)‡ | 644 (13%) | 456 (24%)‡ |
| CRP* | 1.91 [0.83, 4.25] | 2.40 [1.03, 4.51] † | 1.87 [0.81, 4.27] | 1.99 [0.92, 4.23] | 1.85 [0.80, 4.26] | 2.04 [0.92, 4.25] ‡ |
median[IQR]
p<0.05
p<0.01
Multivariable prevalence ratio estimates for factors associated with the presence of ATAC, DTAC, and AWC are shown in table 2. Males were less likely to have DTAC than females (0.68, 95% CI 0.58–0.80). Current smoking, in particular, most influenced the prevalence of ATAC (RR 4.35, 95% CI 2.83–6.70). Hypertension, hypercholesterolemia and current smoking increased all TAC variables. In addition, high LDL and low HDL, and taking lipid lowering meds independently increased the prevalence of all TAC variables while diabetes, and family history of heart attack increased DTAC alone (Tables 2–3). C-Reactive protein was weakly associated with aortic calcification (RR=1.07, 95% CI 1.00,1.14).
Table 2.
Multivariable relative risk estimates for factors associated with the presence of ATAC, DTAC, and AWC stratified by gender.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| ATAC | DTAC | AWC | ATAC | DTAC | AWC | |
| Variables* | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| Age (per year) | 1.12 (1.09, 1.15) | 1.17 (1.15, 1.18) | 1.16 (1.15, 1.18) | 1.11 (1.09, 1.14) | 1.15 (1.13, 1.16) | 1.15 (1.13, 1.16) |
| Race | ||||||
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Chinese | 1.01 (0.42, 2.43) | 1.37 (0.97, 1.93) | 1.34 (0.95, 1.90) | 0.66 (0.32, 1.39) | 0.97 (0.68, 1.38) | 0.93 (0.66, 1.32) |
| African-American | 1.60 (0.97, 2.65) | 0.37 (0.29, 0.48) | 0.39 (0.30, 0.50) | 1.26 (0.80, 1.98) | 0.41 (0.31, 0.54) | 0.48 (0.37, 0.62) |
| Hispanic | 2.03 (1.18, 3.49) | 0.68 (0.52, 0.89) | 0.69 (0.53, 0.90) | 1.02 (0.61, 1.71) | 0.64 (0.48, 0.84) | 0.65 (0.49, 0.85) |
| BMI | 0.98 (0.97, 1.00) | 0.98 (0.97, 1.00) | 0.97 (0.95, 1.00) | 0.97 (0.95, 1.00) | ||
| Family history of heart attack | 1.20 (0.99, 1.46) | 1.16 (0.96, 1.41) | 1.19 (0.96, 1.46) | 1.21 (0.99, 1.49) | ||
| Diabetes | 1.44 (1.09, 1.91) | 1.43 (1.08, 1.88) | 1.35 (1.03, 1.76) | 1.30 (1.00, 1.70) | ||
| Hypertension | 1.68 (1.07, 2.66) | 2.26 (1.84, 2.77) | 2.31 (1.89, 2.84) | 1.86 (1.23, 1.80) | 2.04 (1.65, 2.52) | 2.00 (1.62, 2.47) |
| Smoking status | ||||||
| Never | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Former | 1.81 (1.14, 2.87) | 0.99 (0.80, 1.24) | 1.02 (0.82, 1.27) | 2.67 (1.65, 4.32) | 1.48 (1.19, 1.84) | 1.53 (1.23, 1.90) |
| Current | 3.83 (2.10, 6.98) | 2.35 (1.70, 3.24) | 2.49 (1.81, 3.43) | 5.05 (2.73, 9.36) | 2.22 (1.59, 3.10) | 2.52 (1.81, 3.49) |
| LDL (per SD) | 1.21 (1.00, 1.48) | 1.21 (1.10, 1.33) | 1.22 (1.11, 1.35) | 1.37 (1.13, 1.66) | 1.11 (1.00, 1.23) | 1.12 (1.01, 1.25) |
| HDL (per SD) | 0.80 (0.64, 1.00) | 0.82 (0.74, 0.91) | 0.81 (0.73, 0.90) | 1.03 (0.81, 1.29) | 0.90 (0.79, 1.03) | 0.89 (0.79, 1.02) |
| Lipid lowering meds | 1.31 (0.82, 2.11) | 1.47 (1.16, 1.88) | 1.49 (1.17, 1.89) | 1.50 (0.95, 2.36) | 1.50 (1.17, 1.94) | 1.44 (1.12, 1.85) |
| CRP† | 1.03 (0.94, 1.13) | 1.03 (0.94, 1.13) | 1.15 (1.04, 1.27) | 1.16 (1.05, 1.28) | ||
Hypertension and current smoking have the the strongest independent relationships to TAC in both men and women.
variables selected from a backward stepwise regression where the following variables were allowed to enter the model: age, gender, race, BMI, physical activity, family history of heart attack, DM, HTN, smoking, alcohol, LDL, HDL, triglycerides, lipid lowering meds, and CRP.
natural logarithms used to transform the variable
Table 3.
Multivariable estimates of relative difference in calcification associated with each unit difference in risk factors among people with detectable calcium (i.e. calcium score > 0) stratified by gender
| Women | Men | |||||
|---|---|---|---|---|---|---|
| ATAC | DTAC | AWC | ATAC | DTAC | AWC | |
| Variables* | RD (95% CI) | RD (95% CI) | RD (95% CI) | RD (95% CI) | RD (95% CI) | RD (95% CI) |
| Age (per year) | 1.04 (1.01, 1.08) | 1.09 (1.07, 1.10) | 1.09 (1.08, 1.10) | 1.05 (1.00, 1.09) | 1.07 (1.06, 1.09) | 1.08 (1.06, 1.09) |
| Race | ||||||
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Chinese | 1.04 (0.76, 1.42) | 1.07 (0.78, 1.46) | 1.06 (0.74, 1.50) | 1.07 (0.75, 1.53) | ||
| African-American | 0.55 (0.43, 0.71) | 0.56 (0.44, 0.73) | 0.53 (0.39, 0.71) | 0.48 (0.36, 0.65) | ||
| Hispanic | 0.76 (0.58, 0.99) | 0.76 (0.58, 1.00) | 0.88 (0.64, 1.20) | 0.83 (0.61, 1.14) | ||
| Diabetes | 1.19 (0.92, 1.55) | 1.16 (0.89, 1.51) | 1.31 (0.98, 1.74) | 1.34 (1.00, 1.78) | ||
| Hypertension | 1.38 (1.12, 1.71) | 1.37 (1.11, 1.70) | 1.42 (1.12, 1.80) | 1.42 (1.12, 1.80) | ||
| Smoking status | ||||||
| Never | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Former | 1.34 (0.63, 2.87) | 1.67 (1.32, 2.10) | 1.60 (1.27, 2.01) | 1.39 (0.59, 3.31) | 1.20 (0.94, 1.54) | 1.22 (0.95, 1.57) |
| Current | 2.52 (0.91, 6.99) | 2.54 (1.81, 3.56) | 2.45 (1.75, 3.42) | 2.82 (0.97, 8.20) | 2.11 (1.44, 3.10) | 2.21 (1.52, 3.22) |
| Alcohol | ||||||
| Never | 1.00 (ref) | 1.00 (ref) | ||||
| Former | 0.87 (0.35, 2.14) | 1.90 (0.59, 6.12) | ||||
| Current | 0.72 (0.31, 1.68) | 4.13 (1.37, 12.44) | ||||
| Lipid lowering meds | 1.37 (1.09, 1.71) | 1.38 (1.10, 1.73) | 1.06 (0.81, 1.38) | 1.18 (0.90, 1.55) | ||
African Americans have the lowest prevalence of aortic atherosclerosis, while current smoking status is most strongly related. There is a strong relationship to current alcohol use in men, not seen in women.
RD = the anti-log of the regression coefficient using log-transformed calcium score as the dependent variable in each multiple linear regression analysis. The relative difference in Agatston score per increment in risk factor from a linear regression with Ln(Agaston) as the dependent variable, adjusted for all risk factors. An RD of 1.50 represents a 50% increase.
Variables were selected by backward stepwise regression for each outcome separately.
Ethnic differences were also observed. Overall Black and Hispanics were less likely to have any AWC compared to non-Hispanics whites. After multivariable analysis, blacks were much less likely to have descending thoracic calcification than whites (RR = 0.40, 95% confidence intervals 0.33–0.48). No such difference was seen for Chinese.
Discussion
Atherosclerosis has been demonstrated to coexist with osteoporosis, suggesting to some that these processes are inter-related.9,10,11,12,13 Arterial calcification has osteoblastic-like mechanisms, many bone-proteins expressed in arterial calcification of the coronary arteries and aorta.14,15,16,17,18,19.
Ascending Aortic Calcification
The distribution of calcification varies by segments of the aorta. Thoracic aorta is anatomically divided into three segments; ascending aorta, arch and descending aorta. A population based transesophageal echocardiography (TEE) study on thoracic atherosclerosis reported that the prevalence of atheroma in the descending thoracic aorta is the highest, followed by the aortic arch, with lowest prevalence in the ascending aorta20. In an autopsy study21, ascending aortic atherosclerosis was also found to have a significantly lower prevalence than other segments. Previous reports by CT demonstrated that the aortic arch had the highest prevalence of calcification22,23. In this study, the prevalence of ATAC was one eighth of DTAC (Figure 2). A possible explanation is related to the sheer forces on the wall. The ascending aorta has a very large diameter, high blood velocity and no branch vessels. While high velocity can cause shear stress, most raised lesions occur at sites where shear stresses are low but rapidly fluctuating, such as branch vessels or abrupt changes in vessel diameter.24.
Figure 2.
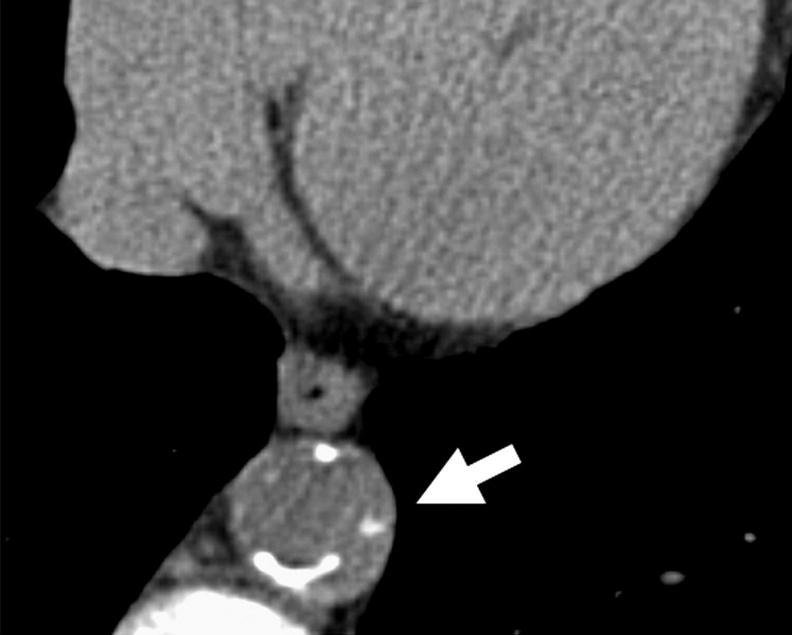
Left Panel – Patient with severe coronary calcium (black arrow) and mild thoracic aortic calcification (white arrow). Right panel – patient with severe thoracic calcification (white arrow) in descending aorta.
Smoking influenced ATAC more than DTAC. Nicotine, the component most characteristic of smoking, influences cardiac function by increasing systolic and diastolic pressure, heart rate, force of myocardial contraction, myocardial oxygen consumption, and myocardial excitability through the release of endogenous epinephrine25. It is possible that the ascending wall is more directly influenced by these variables.
Cardiovascular Risk Factors and Thoracic Calcification
The associations to cardiovascular risk factors were similar between ATAC and DTAC in this study, with positive associations with hypertension, tobacco use, LDL cholesterol, lipid lowering therapy and inversely with HDL cholesterol (table 2). Arai et al26 have shown in a study of participants modified with HMG-CoA reductase that progression of the abdominal aortic wall volume is inhibited by aggressively lower LDL-C below 125 mg/dl during the follow-up period. These results have suggested that serum cholesterol, especially LDL-C, strongly affect the progression or development of aortic atherosclerosis. In a recently published study, the measures of extra-coronary calcification were found to sufficiently reproducible to allow serial investigations27.
Allison et al compared calcifications of the coronary and extra-coronary arteries (carotid artery, aorta and iliac artery) in 650 asymptomatic subjects by whole-body electron beam tomography (EBT)28. The calcifications the vascular beds had similar associations to those found in this study. In the thoracic aorta, the prevalence of calcification increased more than twofold between the sixth and seven decades of life. The relationship of aging with increased likelihood of presence of calcification in aortic wall is consistent with similar relationships observed in other vascular beds.28
Extensive evidence exist that men are more likely to have calcification in the coronary arteries,29 however whether similar difference exists in other vascular beds is not well established. In our study, women had more prevalent DTAC even after adjusting for traditional atherosclerotic risk factors, except in the ascending aorta in women aged >55 years. Our data extends the findings of a previous report from the Reykjavik study that demonstrated that the prevalence of calcification in the abdominal aorta was more prevalent in women, independently associated with atherosclerotic risk factors, and a potential marker for both coronary and peripheral arterial disease. In a study of symptomatic men and women, Yamamoto et al30 also noted a higher prevalence of DTAC in women compared to men.
This study is among the first that demonstrates that the AWC distribution and associated risk factors parallel coronary atherosclerosis. Risk factors for TAC are largely similar coronary risk factors.31 Whether TAC has prognostic implications incremental or independent to CAC is actively being investigated in the Multi-Ethnic Study of Atherosclerosis.
This study has several limitations. This study only examined the aorta in the available range on calcium scanning (excluding the aortic arch and the infra-renal abdominal aorta, two places with noted higher prevalence of calcification).22,23 However the relative ease in identifying AWC during a standard CAC scan, without requiring additional scanning, is a potential advantage as it can be a good estimate of the presence and extent of overall calcific atheroma burden6,32. Aortic calcification was influenced by almost all of the traditional risk factors, suggesting this may just be another manifestation of cardiovascular atherosclerosis. ATAC has a much smaller sample size, and some variables which are statistically significant for DTAC may not reach significance for ATAC, despite a similar relationship. Thus, the findings from this study may be more pertinent for either DTAC or total AWC, which is primarily driven by the presence of DTAC. Further studies will be performed to better classify inflammatory pathways and bone metabolism mechanisms of aortic calcifications.
Acknowledgments
This research was supported by R01-HL-63963-01A1 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 3.Witteman JC, Kannel WB, Wolf PA, Grobbee DE, Hofman A, D’Agostino RB, Cobb JC. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 4.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 5.Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Witteman JC, Breteler MM. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003 Oct;34:2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 6.Takasu J, Mao S, Budoff MJ. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Acad Radiol. 2003;10:631–637. doi: 10.1016/s1076-6332(03)80081-8. [DOI] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi Y, Akashita M, De Souza AC, Nakamura T, Orimo H. Age-related loss of bone mass and aortic/aortic valve calcification: reevaluation of recommended dietary allowance of calcium in the elderly. Ann N Y Acad Sci. 1993;676:297–307. doi: 10.1111/j.1749-6632.1993.tb38743.x. [DOI] [PubMed] [Google Scholar]
- 10.Boukhris R, Becker KL. Calcification of the aorta and osteoporosis. JAMA. 1972;219:1307–1311. [PubMed] [Google Scholar]
- 11.Banks LM, Lees B, Macsweeney JE, Stevenson JC. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: links between osteoporosis and cardiovascular disease? Eur J Clin Invest. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 12.Laroche M, Pouilles JM, Ribot C, Bendayan P, Bernard J, Boccalon H, Mazieres B. Comparison of the bone mineral content of the lower limbs in men with ischaemic atherosclerotic disease. Clin Rheumatol. 1994;13:611–614. doi: 10.1007/BF02243003. [DOI] [PubMed] [Google Scholar]
- 13.Broulik PD, Kapitola J. Interrelations between body weight, cigarette smoking and spine mineral density in osteoporotic Czech women. Endocr Regul. 1993;27:57–60. [PubMed] [Google Scholar]
- 14.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993;92:2814–20. doi: 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirota S, Imakita M, Kohri K, Ito A, Morii E, Adachi S, Kim HM, Kitamura Y, Yutani C, Nomura S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am J Pathol. 1993;143:1003–1008. [PMC free article] [PubMed] [Google Scholar]
- 18.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 20.Agmon Y, Khandheria BK, Meissner I, Schwartz GL, Petterson TM, O’Fallon WM, Gentile F, Whisnant JP, Wiebers DO, Seward JB. Independent association of high blood pressure and aortic atherosclerosis: A population-based study. Circulation. 2000;102:2087–2093. doi: 10.1161/01.cir.102.17.2087. [DOI] [PubMed] [Google Scholar]
- 21.Bjurulf P. Atherosclerosis and body-build with special reference to size and number of subcutaneous fat cells. Acta Med Scand Suppl. 1959;349:1–99. [PubMed] [Google Scholar]
- 22.Takasu J, Takanashi K, Naito S, Onishi M, Miyazaki A, Aoyagi Y, Morooka N, Masuda Y, Inagaki Y. Evaluation of morphological changes of the atherosclerotic aorta by enhanced computed tomography. Atherosclerosis. 1992 Dec;97(2–3):107–21. doi: 10.1016/0021-9150(92)90124-y. [DOI] [PubMed] [Google Scholar]
- 23.Itani Y, Watanabe S, Masuda Y. Aortic calcification detected in a mass chest screening program using a mobile helical computed tomography unit. Relationship to risk factors and coronary artery disease. Circ J. 2004;68(6):538–41. doi: 10.1253/circj.68.538. [DOI] [PubMed] [Google Scholar]
- 24.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 25.Renaud S, Blache D, Dumont E, Thevenon C, Wissendanger T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther. 1984 Sep;36(3):389–95. doi: 10.1038/clpt.1984.193. [DOI] [PubMed] [Google Scholar]
- 26.Arai Y, Hirose N, Yamamura K, Kimura M, Murayama A, Fujii I, Tsushima M. Long-term effect of lipid-lowering therapy on atherosclerosis of abdominal aorta in patients with hypercholesterolemia: noninvasive evaluation by a new image analysis program. Angiology. 2002;53:57–68. doi: 10.1177/000331970205300108. [DOI] [PubMed] [Google Scholar]
- 27.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–72. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 28.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JAC, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of Coronary Artery Disease by Cardiac Computed Tomography, A Scientific Statement From the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114(16):1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto H, Shavelle DM, Takasu J, Lu B, Mao SS, Fisher H, Budoff MJ. Valvular and Thoracic Aortic Calcium as a Marker of the Extent and Severity of Angiographic Coronary Artery Disease. Am Heart J. 2003;146:153–9. doi: 10.1016/S0002-8703(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 31.Bild DE, Detrano R, Peterson D, et al. Ethnic Differences in Coronary Calcification The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 32.Wong ND, Sciammarella M, Arad Y, Miranda-Peats R, Polk D, Hachamovich R, Friedman J, Hayes S, Daniell A, Berman DS. Relation of thoracic aortic and aortic valve calcium to coronary artery calcium and risk assessment. Am J Cardiol. 2003;92:951–5. doi: 10.1016/s0002-9149(03)00976-7. [DOI] [PubMed] [Google Scholar]


