Abstract
One concern with traditional smallpox vaccination is inadvertent spread of virus to atopic or immunocompromised contacts. To reduce this risk, we tested the ability of povidone iodine to inactivate infectious virus at the vaccination site beginning at 7 days after transcutaneous smallpox vaccination. This ointment rapidly inactivated virus on the skin without reducing neutralizing antibody titers or antiviral T cell responses. Moreover, there was no delay in healing/eschar separation following povidone iodine application. Together, this indicates that administration of an antiviral/antimicrobial cream can effectively block virus shedding after traditional smallpox vaccination and reduce the risks of autoinoculation or contact spread.
Keywords: Smallpox vaccination, Vaccinia, Povidone iodine ointment
1. Introduction
Although extinct in nature, smallpox has remained a public health concern due to the remote possibility that it could be used as a biological weapon [1–3]. In addition, a closely related orthopoxvirus, monkeypox, continues to cause a substantial number of outbreaks in the Democratic Republic of Congo [4] and recent studies have documented monkeypox outbreaks in other neighboring African countries such as Sudan [5]. The first imported monkeypox outbreak recorded in the Western Hemisphere occurred in the United States in 2003, resulting in >70 cases of monkeypox [6, 7] that ranged from asymptomatic infection in a proportion of previously vaccinated individuals [6, 7] to life-threatening infections in a small percentage of unvaccinated monkeypox patients [8, 9].
Smallpox vaccination is accomplished through cutaneous infection with live vaccinia virus, an orthopoxvirus that provides protective immunity against other members of the orthopoxvirus genus including smallpox, monkeypox, and cowpox [10]. General flu-like symptoms are the most common adverse event observed following smallpox vaccination, but other rare complications such as generalized vaccinia, autoinoculation, or ocular infection are known to occur. Smallpox vaccination can also be fatal, with an estimated 1 to 8 deaths occurring for every one million vaccinations administered during the modern era of universal smallpox vaccination [11], which ended in 1980 after eradication of naturally occurring smallpox. Most severe complications occur in the person who is directly vaccinated (e.g., vaccinia necrosum), but severe and sometimes lethal infections may occur due to contact spread from a recently vaccinated individual to atopic contacts, resulting in eczema vaccinatum (EV) [12]. Following mass vaccination of 3.2 million people in England and Wales in 1962, there were 185 cases of EV reported and 11 EV-associated deaths (~6% case mortality rate) [13]. There were 137 EV patients in which full clinical details were obtained and it was realized that at least 89 cases (65%) were due to contact spread from a close contact who had recently received smallpox vaccination. Likewise following vaccination of approximately 6.4 million people in New York in 1947, there were 45 cases of generalized vaccinia or EV and 28/45 (62%) of these cases occurred due to contact spread [14]. In an extensive epidemiological analysis of smallpox-vaccine associated deaths examined over a 9-year span of time, it was determined that 12/60 (20%) deaths attributed to primary smallpox vaccination in the US were due to EV [15]. All 12 EV-associated deaths in the US occurred in unvaccinated children who contracted their infections through contact spread from recently vaccinated individuals. Most recently, a 2-year-old Indiana boy contracted a severe case of EV from his father, a vaccinated soldier [16]. The child’s rash progressed to umbilicated lesions covering 50% of his keratinized skin. Despite sedation, intubation, mechanical ventilation, and treatment with vaccinia immune globulin (VIG), the child underwent hyperthermia and hemodynamic instability that required vasopressor support [16]. The child survived this life-threatening infection following hospitalization for 48 days and treatment with Cidofovir and the experimental drug, ST-246, in addition to VIG [16]. If virus shedding can be effectively reduced following smallpox vaccination, then spread to susceptible contacts and these ensuing events may be preventable.
In this study, we determined if one could efficiently reduce the risk of virus shedding and contact spread from the inoculation site following smallpox vaccination by applying an antiviral cream (povidone iodine ointment) as early as 7 days after vaccination. We also determined whether this simple safety precaution would interfere with the development of antiviral antibody or T cell responses or delay the time required for eschar separation. We believe that the approach described here will be useful in preventing autoinoculation, inadvertent ocular infection, and virus transmission to vulnerable contacts, which together will reduce at least one parameter of the morbidity and mortality associated with traditional smallpox vaccination.
2. Methods
2.1 Subjects
Subjects were screened for any contraindications for smallpox vaccination and provided informed written consent before signing research authorization forms complying with the US Health Insurance Portability Act and filling out a medical history questionnaire. Inoculations were performed using a bifurcated needle holding a drop of vaccine (Dryvax) that was pressed 15 times into the skin of the upper arm. The vaccination site was covered with a semi-permeable adhesive membrane over gauze until day 7 post-vaccination, after which subjects either continued to use the occlusive dressings or gauze plus tape. In this observational study, subjects were vaccinated due to occupational risk and were grouped based on occupation; health care workers (vaccinated as part of Oregon’s bioterrorism preparedness effort) represented the Control group and employees of Oregon Health & Sciences University (OHSU) or the Oregon National Primate Research Center (ONPRC) represented the Povidone Iodine Ointment (PIO) group (vaccinated due to work related to orthopoxvirus research). Other samples obtained for comparison were from primary vaccinees who were all OHSU/ONPRC employees.
In the PIO-treated groups of subjects, undiluted povidone iodine ointment (10% povidone-iodine; 1% available iodine, PDI®, Professional Disposables, Inc.) was applied directly to the vaccination site and re-applied at each bandage change (~3–5 days apart) from day 7 post-vaccination until 2–3 days after the eschar sloughed off. PIO was not administered prior to day 7 post-vaccination in order to allow the vaccination site to form a successful Jennerian pustule, the only formal standard used for determining vaccine efficacy. Each subject was given instructions on PIO administration and a vaccination diary to keep daily records of any reactions to the vaccination. Compliance with the study protocol was monitored at each study visit.
Blood samples (50 mL) were collected prior to vaccination and at 4–6 weeks after vaccination. Peripheral blood mononuclear cells (PBMC) were cryopreserved in aliquots and stored in liquid nitrogen. Plasma and serum samples were stored at −20°C or −80°C. For virus shedding analysis, n = 5 primary vaccinees and 17 revaccinated subjects (Controls, no PIO administration) and for the PIO groups, n = 6 primary vaccinees and 18 revaccinated subjects. For immunological analysis, n = 17 Control subjects and 18 PIO subjects (all represent revaccinations) and for eschar separation analysis, n = 22 Control subjects and 18 PIO subjects (all represent revaccinations). The Institutional Review Board of Oregon Health & Science University approved all clinical studies.
2.2 Vaccinia titrations and in vitro studies
To determine the antiviral effects of PIO in vitro, we used a vaccinia (strain WR)-infected BSC40 cell lysate prepared by 2 rounds of freeze/thaw cycles followed by sonication and centrifugation to remove cellular debris. Twenty µL aliquots of virus were added to 180 µL of PIO diluted in heat-inactivated human plasma (30 minutes at 56°C) and samples were incubated for the indicated periods of time prior to addition of 1.8 mL of medium immediately prior to plating onto multiple wells of a 6-well plaque assay plate as described below. For comparison, a 10% solution of Thermacine or Chlorhexidine were tested for antiviral activity against vaccinia by the plaque assay technique.
2.3 Vaccinia titrations before and after topical administration of PIO
Levels of infectious virus at the vaccination site were determined by swabbing the skin 8 times vertically and 8 times horizontally with a sterile cotton swab pre-wetted in sterile water. The swab was then rinsed in 1 ml of PBS containing 1% FBS. Plaque assays were performed on the same day that the swabbing occurred. Plaque assays were carried out by incubating 200 µL of serial ten-fold dilutions of each sample on Vero cells (70–100% confluent) in 6-well plates. After 1h at 37°C, the wells were overlaid with 3 mL 0.5% agarose in EMEM containing 2.5% FBS, and incubated for 4 days to allow plaque formation. Residual PIO was diluted to <0.5% final volume once the agar overlay was performed. This diluted the PIO to low levels at which antiviral activity is no longer observed (see Figure 1). Monolayers were fixed with 75% methanol/25% acetic acid, the agarose removed, and the plaques visualized by staining with 0.1% crystal violet in PBS containing 0.2% formaldehyde.
Figure 1. Effective vaccinia virus inactivation following exposure to Poviodine-iodine ointment.
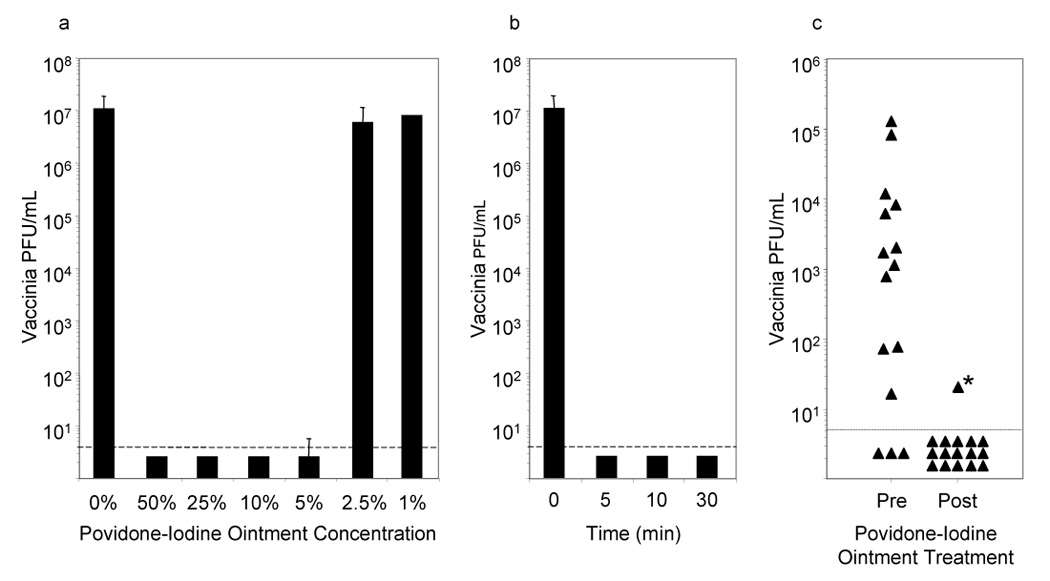
Vaccinia (107 PFU) was diluted in heat-inactivated human plasma and mixed with varying concentrations of Povidone-iodine ointment (PIO) for 60 minutes prior to determining infectious virus levels by plaque assay (1A). Shorter incubation times were tested (1B) using 10% PIO, which effectively inactivates vaccinia within 5 minutes. The efficiency of inactivating virus directly on the vaccination site were tested by taking swabs from the treatment group on day 7, before and 1–2 hours after application of PIO (1C). Of 15 subjects with pre-existing virus titers, only 1/15 had detectable virus after PIO administration, resulting in a significant decline in the potential to shed infectious virus (P < 0.001; binomial calculation).
*In this sample, the swabbing procedure unroofed the eschar, exposing infectious virus that may not have been in contact with PIO. Dashed lines indicate the limit of detection.
2.4 Vaccinia-specific T cell assays
Intracellular cytokine staining was performed as previously described [17]. Briefly, PBMC were cultured in 96-well round-bottomed plates at 37°C with 6% CO2, RPMI containing 20 mM HEPES, L-glutamine, antibiotics, and 5% heat inactivated FBS (Hyclone), with or without vaccinia virus [sucrose gradient-purified intracellular mature virus (IMV), vaccinia strain Western Reserve] at an optimized MOI of 0.1. After 12 h of culture, 20 µl of Brefeldin A (ICN) was added carefully without disturbing the cells, at a final concentration of 2 µg/mL for an additional 6 h. The cells were stained overnight at 4°C with antibodies specific for CD8β (clone 2ST8.5H7, Beckman Coulter) and CD4 (clone L200, PharMingen). Cells were fixed, permeabilized and stained intracellularly using antibodies to IFNγ (clone 4S.B3) and TNFα (clone Mab11), both from PharMingen. Samples were acquired on a FACS Calibur (Beckton Dickinson) using CELLQuest software (Beckton Dickinson), acquiring 1–2 million events per sample. Data was analyzed using CELLQuest software and a live cell gate was performed based on forward and side scatter characteristics. The number of IFNγ+TNFα+ T cells was quantitated after first gating on live CD4+CD8- or CD4−CD8+ cells and subtracting the number of IFNγ+TNFα+ events from uninfected cultures. Each assay contained PBMC from a positive control subject (~1 year post-smallpox vaccination), which scored 682±235 (s.d.) IFNγ+TNFα+ CD4+ T cells per 106 CD4+ T cells and 2096±523 (s.d.) IFNγ+TNFα+ CD8+T cells per 106 CD8+ T cells, respectively. One negative control sample consisting of PBMC from a vaccinia-naïve subject was included in each assay for quality control purposes (naïve = 4±7 IFNγ+TNFα+ CD4+ T cells per 106 CD4+ T cells and 10±19 (s.d.) IFNγ+TNFα+CD8+T cells per 106 CD8+ T cells, respectively).
2.5 ELISA and neutralizing assays
Vaccinia-specific ELISA assays were performed as previously described [17] using vaccinia whole virus lysate (inactivated by pretreatment with 3% H202 for 2 hours). An internal positive control standard was included on each plate to normalize ELISA values between plates and between assays performed on different days. Antibody titers were determined by log-log transformation of the linear portion of the curve, with 0.1 optical density (O.D.) units used as the endpoint and conversion performed on final values.
Neutralizing assays were performed by preparing serial 2-fold dilutions of heat-inactivated serum and incubating them with vaccinia (~100 pfu) for 2 h at 37°C prior to plating the mixtures on Vero cell monolayers and measuring plaque forming virus as described above. The NT50 was defined as the serum dilution required for 50% reduction of plaques. We used logarithmic transformation of the linear portion of the curve to calculate the titer and conversion was performed on the final values.
2.6 Statistical analysis
Each outcome measure was log (base-e) transformed prior to analysis to adjust for outliers and skewed (non-normal) distributions. Population characteristics were compared between the treated and untreated subjects using a two-sample t-test for continuous measures and a Fisher’s exact test for the categorical measures. Following smallpox vaccination, an exact binomial calculation was used to assess whether there were more subjects responding to PIO by loss of detectable infectious virus on the skin than would be expected under random conditions. Since the probability of response under random conditions was unknown, but suspected to be small (<<0.5), we conservatively compared whether the probability of the response was different from 0.5. The average baseline and 30-day antibody, T-cell, NT50 counts and the ratio of the 30-day to baseline counts for the PIO treated and control subjects were first compared using a two-sample t-test. ANCOVA models were fitted to adjust for subject characteristics (years post previous vaccination, current age, gender, and presence of allergies or asthma) and for the ratio outcomes, we additionally adjusted for baseline antibody, NT50, CD4 or CD8 levels, respectively. The time until eschar separation curves for the two groups were estimated using a Kaplan-Meier analysis, and the equivalence of the survival curves was tested using a log-rank test. P-values less than 0.05 are considered significant.
3. Results
3.1 Antiviral activity of povidone iodine ointment in vitro
A variety of disinfectants have been used to inactivate orthopoxviruses in the laboratory setting [18–21] but many of these are unsuitable for topical use due to their toxicity and/or inherent health risks [21]. In contrast, PIO is an effective iodine-based broad-spectrum antimicrobial cream that has been used extensively as a topical antiseptic. In our first experiments, we measured its specific antiviral activity against infectious vaccinia virus in vitro (Fig. 1). We used a high concentration of vaccinia (107 PFU/mL) because virus titers on skin lesions from monkeypox patients, smallpox patients, or smallpox vaccinees rarely reach this high level and we presumed that if we could inactivate a high titer of virus, then lower titers would also be efficiently inactivated. Protein and other contaminants can interfere with many types of antiseptics [20, 22–26] and orthopoxvirus lesions may release a substantial volume of virus-infected proteinacious exudate once they reach the pustular stages of development. For this reason, we tested the direct antiviral activity of PIO alone or after diluting it in naïve human plasma to mimic the exudate released by orthopoxvirus lesions. Virus titers were measured by plaque assay after exposure to different concentrations of PIO (Fig. 1a). Within 1 hour, we achieved a greater than one million-fold reduction in virus titer when vaccinia was incubated in plasma containing as little as 5% volume:volume concentration of PIO. At higher concentrations, vaccinia titers dropped below our limit of detection (<5 PFU/mL) but at a concentration of 5% PIO, infectious virus was detected in 1 of 3 experiments (6 PFU/mL compared to 107 PFU/mL in the untreated control). Lower concentrations of PIO (2.5% and 1%) were no longer effective at reducing virus titers in vitro but it is unlikely that this amount of dilution would occur on the skin surface. In terms of other clinically relevant topical agents, a solution containing 10% Chlorhexidine or 10% Thermacine reduced vaccinia titers in human plasma from 1.1 × 107 PFU/mL to 1.1 × 106 PFU/mL or 8.1 × 103 PFU/mL, respectively (data not shown). This indicated a 1-log10 to 3-log10 reduction in virus titer, but this is largely inefficient compared to the >6-log10 reduction in virus levels obtained with a similar concentration of PIO.
3.2 Kinetics of virus inactivation following exposure to povidone iodine ointment in vitro
The speed at which virus titers could be depleted was also determined (Fig. 1b). In these experiments, we observed a greater than one million-fold reduction of infectious virus in as little as 5 minutes using vaccinia-spiked plasma containing 10% PIO. This indicates that the inactivation of infectious virus is rapid under in vitro conditions that mimic the protein-rich exudate observed on the pustular lesions of orthopoxvirus-infected skin.
3.3 Vaccinia virus inactivation by povidone iodine ointment following smallpox vaccination
After determining that PIO worked the best for inactivating infectious orthopoxvirus in vitro, we tested its antiviral effects as a topic antiseptic on a cohort of subjects undergoing traditional smallpox vaccination. Revaccination against smallpox was performed on 35 subjects who had received prior smallpox vaccination greater than 17 years ago (Table 1). The most common adverse events recorded were itching at the vaccination site and swollen/tender lymph nodes. There were no serious adverse events reported. To prevent early virus shedding, subjects wore semipermeable Tegaderm dressings that were changed every 3–5 days. On day 7 post-vaccination, the vaccine takes were recorded and the vaccination site was swabbed for replication-competent virus. Levels of infectious virus are typically lower following revaccination compared to primary vaccination [27] and although 100% of subjects who received PIO demonstrated infectious virus at one or more time points (day 3, 5, or 7; data not shown), the titers ranged from below detection (n = 3) to as high as 1.3 × 105 PFU/mL at the time of PIO administration on day 7 post-vaccination. The mean titer of 7.9 × 103 PFU/mL is similar to a previous study which also noted a peak of approximately 103 PFU at day 10 after revaccination [27]. PIO was applied directly to the site followed by applying a fresh Tegaderm patch and the vaccination site was swabbed again for infectious virus within 1 to 2 hours after the initial application. After administration of PIO to the vaccination site, infectious virus dropped below the limits of detection (<5 PFU/mL) in 17/18 subjects. One subject with a pre-treatment titer of 8.0 × 104 PFU/mL had residual virus remaining at a titer of 20 PFU/mL (4,000-fold reduction in titer). However, after review of clinical notes it was realized that this was the only subject in which the eschar sloughed off during the second swabbing procedure and it is likely that the residual infectious virus had been sequestered under the eschar and not in direct contact with the PIO. Subjects undergoing primary smallpox vaccination exhibited higher virus shedding at day 7 post-infection (mean = 3.7 × 104 PFU/mL, n = 6) but again, PIO treatment eliminated infectious virus to levels below detection in all 6 subjects (data not shown). The loss of infectious virus observed during the second swabbing of the vaccination sites could be due, at least in part, to removing infectious virus during the first swabbing procedure. To determine if this played a role in the outcome of these experiments, we swabbed the vaccination site of another group of 5 primary vaccinees who did not apply PIO. The mean vaccinia titer at 7 days post-vaccination dropped slightly from 9 × 104 PFU/mL following the first swabbing to 4 × 104 PFU/mL following the second swabbing procedure performed 1–2 hours later. This indicates that physical removal of infectious virus played only a minor role in the loss of infectious virus observed following administration of PIO directly to the site of vaccination.
Table 1.
Study subject characteristics.
| Characteristic | Control (n = 17) | PIO (n = 18) |
|---|---|---|
| Age, average (range) | 51 (38–58) | 44 (33–59) |
| Number of vaccinations, average (range) | 1.6 (1–6) | 1.2 (1–2) |
| Years after last vaccination, average (range) | 43 (32–56) | 36 (17–50) |
| Male | 12% | 50% |
| Caucasian | 88% | 83% |
| Allergy/asthma | 24% | 33% |
Table 1 contains the summary statistics of revaccinated subjects divided by treatment group on the medical information available on each subject. The PIO treatment group was significantly younger and therefore, the time from the previous vaccination was shorter (P = 0.02, 0.03, respectively). The two populations had a similar number of previous smallpox vaccinations (P = 0.22) and did not differ significantly by race (P > 0.99). The PIO treated group had a balance of males and females, while the untreated group was predominately female (P = 0.03). The total population was 91% non-smokers with 29% reporting allergies or asthma and these parameters did not differ between the two treatment groups (P > 0.71).
3.4 Reduction in vaccinia virus shedding following administration of povidone iodine ointment
A number of studies have examined the frequency of detecting infectious virus on the outside of different bandages used to cover the inoculation site following smallpox vaccination [28–31]. As a more rigorous approach, we measured infectious virus on the inside of the Tegaderm patches/bandages used to cover the vaccination site from subjects who were treated with PIO. Of 55 gauze pads tested after PIO administration began (7, 10 or 15 days post-vaccination), 0/55 bandages contained detectable levels of infectious virus (limit of detection, 25 PFU/mL) even though they were in direct contact with the vaccination site and absorbed exudate/PIO from the pustular lesions during the period with the highest likelihood of virus shedding [31]. We also tested the gauze pads of 6 primary vaccinees who used PIO and found 0/15 bandages contained detectable virus when examined on 7, 10, or 15 days after vaccination. In contrast, we detected infectious virus on the inside of 13/15 (87%) gauze pads obtained at 7, 10, or 15 days after vaccination from 5 primary vaccinees who did not apply PIO (range, <5 PFU/mL to 1.4 × 105 PFU/mL). Together, this indicates that PIO is able to effectively inactivate infectious virus on the skin surface as well as inside protective bandages and is likely to significantly reduce inadvertent virus shedding and/or spread to susceptible contacts.
3.5 Effect of PIO treatment on vaccine-induced antiviral immunity
3.5.1 Virus-specific T cell responses
One concern with inactivating infectious virus at the site of inoculation is that this might result in decreased antigenic load and reduced antiviral immunity following smallpox vaccination. To examine this issue, we drew blood samples prior to revaccination and at 4–6 weeks post-vaccination and measured virus-specific T cell responses by intracellular cytokine staining (ICCS) analysis as previously described [7, 17] (Fig. 2). Following 18 hours of direct ex vivo stimulation with an optimized concentration of purified vaccinia, the virus-specific T cell responses were measured by staining the cells for CD4, CD8, IFNγ and TNFα. The number of virus-specific T cells was determined by quantitating the number of IFNγ+TNFα+ T cells in cultures stimulated with vaccinia after subtracting any non-specific cytokine-positive events from cultures incubated in medium alone. The subjects in the Control group (i.e., no PIO) and the PIO group had received smallpox vaccination in the distant past and the majority retained pre-existing CD4+ T cell memory that could be detected prior to revaccination (Fig. 2a). We found no significant difference in CD4+ T cell memory between these groups prior to vaccination (P = 0.064) and both groups demonstrated a sharp rise in virus-specific CD4+ T cell numbers within 4–6 weeks after revaccination, again with no significant difference between groups (P = 0.68). In contrast to CD4+ T cell memory, approximately half of individuals who received smallpox vaccination in the distant past had antiviral CD8+ T cell responses below the limits of detection (<1–10/106 T cells) within 20 years post-vaccination [17, 32]. Similar to these previous results, a proportion of subjects in each group (n = 6 Control and n = 4 PIO subjects, P = 0.45) had lost detectable CD8+ T cell memory following smallpox vaccination ≥17 years previously. Following revaccination, 16/18 (89%) of the Control group subjects and 17/17 (100%) of the PIO -treated subjects elicited a demonstrable boost in their vaccinia-specific CD8+ T cell memory with no significant difference in overall levels of T cell memory following revaccination (P = 0.56). Together, this indicates that topical application of PIO does not alter the induction of vaccinia-specific T cell responses following revaccination against smallpox.
Figure 2. Virus-specific T cell and antibody responses are unaltered by treatment with PIO.
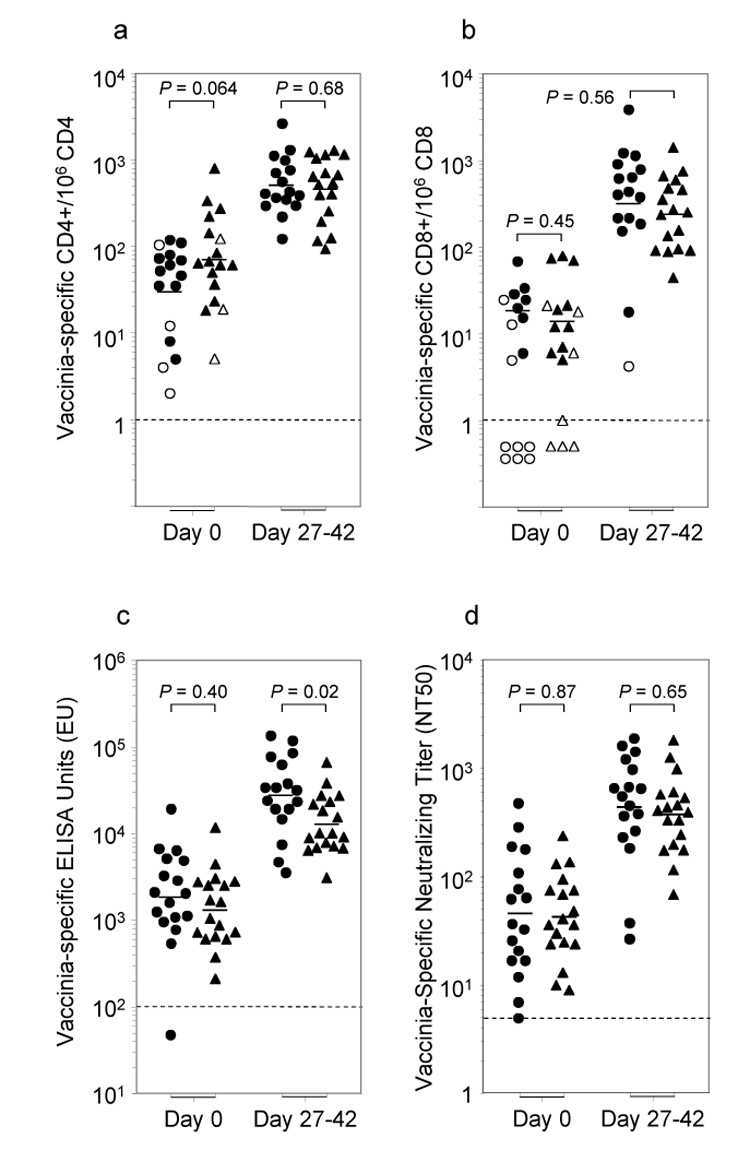
Samples were tested prior to vaccination (day 0) and 30 days (range 27–42 days) after traditional smallpox revaccination. Number of vaccinia-specific CD4+ and CD8+ T cells was measured by the number of IFNγ+TNFα+CD4+ per 106 CD4+ T cells (2A) or IFNγ+TNFα+CD8+ per 106 CD8+ T cells (2B). Open symbols in (2A) and (2B) represent scores that were <2-fold over the background levels observed after incubation with medium alone.
Vaccinia-specific antibody titers were determined by ELISA (2C), which measures total virus-specific IgG(γ) (ELISA Units) and by neutralizing antibody assays (2D), which measure the ability of antiviral serum antibodies to neutralize infectious virus particles.
●Control, ▲ PIO — Geometric mean, ---- Limit of detection.
3.5.2.Virus-specific antibody responses
Orthopoxvirus-specific serum antibody plays an important role in protection against this class of viruses [33–35] and has been shown to be both necessary and sufficient for protecting non-human primates against lethal monkeypox infection – a model similar to human smallpox infection [36]. To determine if PIO administration would alter humoral immunity following smallpox vaccination, we examined antiviral antibody responses by two independent approaches; a vaccinia-specific ELISA assay to measure total virus-specific antibody responses (Fig. 2c) and a vaccinia-specific neutralizing assay to measure biologically relevant antibody responses (Fig. 2d). The Control group had marginally higher pre-existing ELISA titers prior to vaccination that was not statistically significant (P = 0.40) and following revaccination this increased to approximately a 2-fold difference in geometric mean titers between groups (Fig. 2c). Although this was modestly significant (P = 0.02), it was no longer statistically significant after adjustment for baseline titers and other demographic characteristics between the two groups (P = 0.87; data not shown). Another benchmark for evaluating successful smallpox vaccination is the development of a 4-fold or higher increase in antibody levels [27]. In this regard, 15/17 (88%) Controls and 15/18 (83%) PIO-treated subjects exhibited a 4-fold or greater increase in antibody levels by ELISA, with no significant difference between groups (P > 0.99, Fisher’s exact test).
In the case of virus-specific neutralizing antibody titers, there was no significant difference between groups either before (P = 0.87) or after (P = 0.65) revaccination (Fig. 2d), or after adjustment for baseline titers and other group characteristics (data not shown). One study indicated that vaccinia-specific neutralizing titers of 1:32 or higher could be considered fully protective against smallpox [37] and based on this assumption, 16/17 (94%) Controls and 18/18 (100%) of PIO-treated subjects achieved successful smallpox vaccination. Together, this indicates that biologically relevant antibody responses are not inhibited by the administration of PIO.
3.6 Effect of PIO treatment on clinical morphology and kinetics of vaccination site healing
Individuals undergoing traditional smallpox vaccination are generally considered infectious until after detachment of the eschar from the vaccination site. In routine practice, the vaccination site is kept dry in order to aid in wound healing and eschar separation. Two potential caveats of our approach is that repeated application of PIO could result in a delay in healing and eschar separation or could lead to contact dermatitis. In our studies, there was no evidence of contact dermatitis at any POI-treated sites. Vaccination sites of the POI group displayed the typical morphologic progression from papule, to vesicle, to pustule and to eschar as seen in untreated sites (Fig. 3a). The median time until eschar separation for PIO-treated subjects was 20.0 days (95% CI: 18.3, 21.7) and 21.0 days (95% CI: 18.7, 23.3) for Control subjects. The median time until eschar separation did not differ statistically between these two groups (Fig. 3b, P = 0.43, log-rank test). The time until eschar separation is similar to the results of previous studies [31] and indicates that prolonged topical administration of PIO is able to inactivate infectious virus at the vaccination site without delaying wound healing time or eschar separation.
Figure 3. Normal lesion progression and eschar separation following administration of PIO.
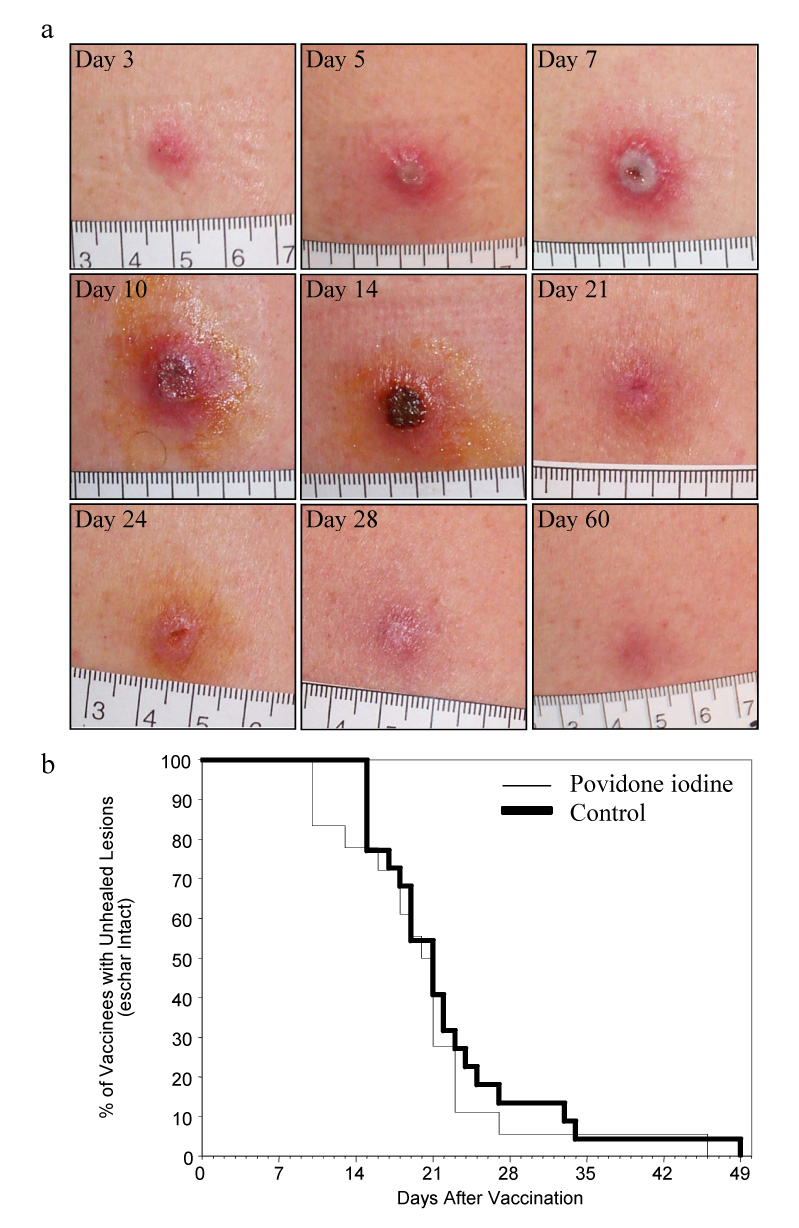
(3A) Photographs of a representative subject showing the cutaneous reaction following smallpox revaccination and treatment with povidone-iodine beginning at day 7. (3B) The application of povidone-iodine did not adversely affect healing times and no cases of contact dermatitis occurred.
4. Discussion
In these studies, we tested the efficacy of topical PIO administration as a means to reduce virus shedding from the skin following traditional smallpox vaccination. This topical agent was highly effective at reducing or eliminating infectious virus on the skin surface and on the bandages used to cover the vaccination site. Although we did not begin PIO application until day 7 post-vaccination, in future studies it will be important to determine if topical application could occur at an earlier time point, such as 3–4 days after inoculation. Importantly, these studies show that PIO administration did not interfere with the development of cellular or humoral immune responses nor did it impede the healing process and eschar separation following smallpox vaccination.
Despite proper instruction and care of the vaccination site, contact spread of vaccinia virus and ocular infections continue to be a problem among vaccinated military personnel [38]. This has led to secondary and even tertiary transfer of the virus in rare instances [39]. One unfortunate example is described in a case of inadvertent household spread from a recently vaccinated US soldier that resulted in the infection of his breastfeeding wife who then spread the virus to their infant daughter who developed a vaccinia-culture positive vesicle on her labial philtrum, as well as lesions on her cheek, and tongue [40]. To prevent further autoinoculation or ocular infection, goggles were placed on the infant and she was held in soft restraints while awake or not in her mother’s arms. Experiences like this could have been prevented if virus shedding was reduced or eliminated. Health care workers have used semipermeable dressings to reduce transmission, but this may not be financially feasible for mass vaccination programs such as that initiated by the US military. Povidone iodine ointment can be used by itself with semipermeable dressings or with simple gauze and tape to effectively and affordably reduce inadvertent spread of vaccinia to close contacts. The use of this antiviral cream may be most important during the pustular stages of the infection when dressings sometimes become inundated by the volume of exudate and leak infectious virus through the adhesive of the protective covering.
Interestingly, semipermeable dressings reduce, but do not necessarily eliminate infectious virus transmission. Recovery of vaccinia from the surface of semipermeable dressings is variable with 0% [31], 7% [30] or 18.2% [28] of samples testing positive for vaccinia on the outer surface. Direct comparisons between gauze and semipermeable dressings have given divergent results in which the more occlusive dressings were either more effective [31] or equivalent [29] to gauze in terms of reducing virus shedding. In our studies, we measured infectious virus on the inner surfaces of the dressings and found that PIO administration resulted in complete inactivation of vaccinia. If infectious virus is inactivated under the protective dressing, then it is even more unlikely that virus shedding could occur and we would recommend PIO administration in combination with any protective dressing to further minimize the potential for inadvertent contact spread following smallpox vaccination. Another potential use for topical antivirals may also be as a form of primary prophylaxis by administering ointment on exposed skin of medical personnel or predisposed individuals with atopic dermatitis if they inadvertently come into contact with a recently vaccinated individual. Although washing with soap and water is typically recommended, some detergents (data not shown) and commercial soaps [41] perform poorly at inactivating vaccinia virus. Because PIO administration is generally well tolerated, application of this antimicrobial ointment may be useful for treating other orthopoxvirus infections besides vaccinia. If smallpox were to be released during a bioterrorism event, PIO could be administered directly onto the skin lesions of the patients in order to reduce the risk of fomite spread in the hospital or clinic and/or during transport to designated quarantine sites. This may also be a useful approach to treating monkeypox patients during outbreaks that continue to occur in the Democratic Republic of Congo and possibly other neighboring countries [42, 43]. In this setting, PIO represents an affordable method for reducing virus transmission while at the same time helping to prevent secondary bacterial infections during the pustular stages of disease.
Povidone-iodine ointment does not appear to block virus replication under the skin (or eschar), but instead only inactivates virus that is released on the skin surface and comes into direct contact with it. This assumption is based mainly on one subject who failed to apply PIO at day 10 post-vaccination and instead removed the protective bandages and washed away the residual PIO with soap and water. We swabbed the vaccination site within 3 hours after realizing this oversight and recovered infectious virus (245 PFU/mL) – even though she had been negative for infectious virus (<10 PFU/mL) after administration of PIO 3 days earlier on day 7 post-vaccination. This suggests that as long as the PIO is applied at each dressing change, then infectious virus that is released on the skin surface is rapidly inactivated. However, if there is virus that is not in direct contact with the topical cream, then it will continue to replicate until the host immune system is able to clear the infection and the eschar separates. This likely explains why inactivation of surface vaccinia virus had no significant effect on the activation of cellular and humoral immune responses (Figure 2).
Remarkably, Edward Jenner and his colleagues were the first to describe virus inactivation at the inoculation site following smallpox vaccination. In his second paper on smallpox vaccination [44], Dr. Jenner realized that some of the “takes” were too robust and caused substantial alarm due to extensive inflammation. In one case (M.H., 12 years of age), he wrote, “The pustule, beginning to show a disposition to spread, was dressed with an ointment composed of hydrarg. nit.rub. and ung. cerae. The efflorescence itself was covered with a plaster of ung.hydr. fort.” This topical application continued daily for 10 days until, “The girl, after the tenth day, when, as has been observed, she became a little ill, showed not the least symptom of indisposition. She was afterwards exposed to the action of variolous matter, and completely resisted it.” In other words, he tested whether or not inactivation of surface virus resulted in the loss of protective antiviral immunity by directly inoculating the patient with smallpox and looking for resistance to infection. Smallpox inoculation (i.e., variolation) was the standard of care prior to development of smallpox vaccination. Jenner performed several similar trials and noted that the virus could be inactivated shortly after vaccination, “… the virus on the arm was destroyed soon after it had produced a perceptible sickening.” and yet the children were fully protected against cutaneous challenge with smallpox, “The appearance and progress of the infected arm was, in every respect, similar to that which we generally observe when variolous matter has been inserted into the skin of a person who has previously undergone either the cow-pox or the smallpox.”. Further details and examples were provided in Jenner’s third paper on smallpox vaccination [45] in addition to publishing letters that he received from other physicians who also inactivated virus at the inoculation site within a few days after vaccination using vinegar and water or vitriolic acid (Joseph H. Marshall, letter to Edward Jenner [45]) or by applying “…mercurial ointment to the inflamed part, which was repeated daily until the inflammation went off…” (M.J. Tieny, letter to Edward Jenner [45]). Based on these historical findings, our studies using povidone iodine ointment to inactivate vaccinia from the infected skin at the vaccination site are actually a simple modern extension of studies performed over 200 years ago. It is surprising that these findings have been largely forgotten, but still they provide further evidence that inactivation of surface virus does not interfere with the protective immunity afforded by smallpox vaccination – even in cases wherein subjects were directly challenged with smallpox itself.
Acknowledgements
We thank the volunteers for their generous participation in this research study and Dr. Paul Sehdev for administering the smallpox vaccine. This project has been funded with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services contract number HHSN266200400029 (to JH and MKS), RR000163 (to MKS), RR000334 (to NEC), and RR023424 (to NEC). ELS is a recipient of a Dermatology Foundation Physician Scientist Career Development Award.
Glossary
- POI
Povidone Iodine Ointment
- EV
eczema vaccinatum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson DA. The looming threat of bioterrorism. Science. 1999;283(5406):1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- 2.Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, et al. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. Jama. 1999;281(22):2127–2137. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 3.Smith GL, McFadden G. Smallpox: anything to declare? Nat Rev Immunol. 2002;2(7):521–527. doi: 10.1038/nri845. [DOI] [PubMed] [Google Scholar]
- 4.Rimoin AW, Kisalu N, Kebela-Ilunga B, Mukaba T, Wright LL, Formenty P, et al. Endemic human monkeypox, Democratic Republic of Congo, 2001–2004. Emerg Infect Dis. 2007;13(6):934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damon IK, Roth CE, Chowdhary V. Discovery of monkeypox in Sudan. N Engl J Med. 2006;355(9):962–963. doi: 10.1056/NEJMc060792. [DOI] [PubMed] [Google Scholar]
- 6.Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 7.Hammarlund E, Lewis MW, Carter SV, Amanna I, Hansen SG, Strelow LI, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11(9):1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 8.Sejvar JJ, Chowdary Y, Schomogyi M, Stevens J, Patel J, Karem K, et al. Human monkeypox infection: a family cluster in the midwestern United States. J Infect Dis. 2004;190(10):1833–1840. doi: 10.1086/425039. [DOI] [PubMed] [Google Scholar]
- 9.Lewis MW, Graham MB, Hammarlund E, Hanifin J, Slifka MK. Monkeypox without exanthem. N Engl J Med. 2007;356(20):2112–2114. doi: 10.1056/NEJMc062788. [DOI] [PubMed] [Google Scholar]
- 10.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization; The pathogenesis, immunology, and pathology of smallpox and vaccinia. 1988
- 11.Kretzschmar M, Wallinga J, Teunis P, Xing S, Mikolajczyk R. Frequency of Adverse Events after Vaccination with Different Vaccinia Strains. PLoS Med. 2006;3(8) doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neff JM, Lane JM, Fulginiti VA, Henderson DA. Contact vaccinia--transmission of vaccinia from smallpox vaccination. Jama. 2002;288(15):1901–1905. doi: 10.1001/jama.288.15.1901. [DOI] [PubMed] [Google Scholar]
- 13.Copeman PW, Wallace HJ. Eczema Vaccinatum. Br Med J. 1964;2(5414):906–908. doi: 10.1136/bmj.2.5414.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg M. Complications of vaccination against smallpox. Am J Dis Child. 1948;76:492–502. doi: 10.1001/archpedi.1948.02030030505002. [DOI] [PubMed] [Google Scholar]
- 15.Lane JM, Ruben FL, Abrutyn E, Millar JD. Deaths attributable to smallpox vaccination, 1959 to 1966, and 1968. Jama. 1970;212(3):441–444. [PubMed] [Google Scholar]
- 16.CDC. Household transmission of vaccinia virus from contact with a military smallpox vaccinee--Illinois and Indiana, 2007. MMWR Morb Mortal Wkly Rep. 2007;56(19):478–481. [PubMed] [Google Scholar]
- 17.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nature Medicine. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 18.Fenner F. Mouse-pox; infectious ectromelia of mice; a review. J Immunol. 1949;63(4):341–373. [PubMed] [Google Scholar]
- 19.Tanabe I, Hotta S. Effect of disinfectants on variola virus in cell culture. Appl Environ Microbiol. 1976;32(2):209–212. doi: 10.1128/aem.32.2.209-212.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrier A, Garin D, Crance JM. Rapid inactivation of vaccinia virus in suspension and dried on surfaces. J Hosp Infect. 2004;57(1):73–79. doi: 10.1016/j.jhin.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Butcher W, Ulaeto D. Contact inactivation of orthopoxviruses by household disinfectants. J Appl Microbiol. 2005;99(2):279–284. doi: 10.1111/j.1365-2672.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 22.Wallbank AM, Drulak M, Poffenroth L, Barnes C, Kay C, Lebtag I. Wescodyne: lack of activity against poliovirus in the presence of organic matter. Health Lab Sci. 1978;15(3):133–7. [PubMed] [Google Scholar]
- 23.Weber DJ, Barbee SL, Sobsey MD, Rutala WA. The effect of blood on the antiviral activity of sodium hypochlorite, a phenolic, and a quaternary ammonium compound. Infect Control Hosp Epidemiol. 1999;20(12):821–827. doi: 10.1086/501591. [DOI] [PubMed] [Google Scholar]
- 24.Hanson PJ, Gor D, Jeffries DJ, Collins JV. Chemical inactivation of HIV on surfaces. Bmj. 1989;298(6677):862–864. doi: 10.1136/bmj.298.6677.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maris P. Virucidal efficacy of eight disinfectants against pneumovirus, coronavirus and parvovirus. Ann Rech Vet. 1990;21(4):275–279. [PubMed] [Google Scholar]
- 26.Noda M, Matsuda S, Kobayashi M. Virucidal activity of disinfectants. Influence of the serum protein upon the virucidal activity of disinfectants. Kansenshogaku Zasshi. 2000;74(8):664–669. doi: 10.11150/kansenshogakuzasshi1970.74.664. [DOI] [PubMed] [Google Scholar]
- 27.Frey SE, Newman FK, Yan L, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. Jama. 2003;289(24):3295–3299. doi: 10.1001/jama.289.24.3295. [DOI] [PubMed] [Google Scholar]
- 28.Graham BS, Belshe RB, Clements ML, Dolin R, Corey L, Wright PF, et al. Vaccination of vaccinia-naive adults with human immunodeficiency virus type 1 gp160 recombinant vaccinia virus in a blinded, controlled, randomized clinical trial. The AIDS Vaccine Clinical Trials Network. J Infect Dis. 1992;166(2):244–252. doi: 10.1093/infdis/166.2.244. [DOI] [PubMed] [Google Scholar]
- 29.Waibe KH, Ager EP, Topolski RL, Walsh DS. Randomized trial comparing vaccinia on the external surfaces of 3 conventional bandages applied to smallpox vaccination sites in primary vaccinees. Clin Infect Dis. 2004;39(7):1004–1007. doi: 10.1086/423967. [DOI] [PubMed] [Google Scholar]
- 30.Hepburn MJ, Dooley DP, Murray CK, Hospenthal DR, Hill BL, Nauschuetz WN, et al. Frequency of vaccinia virus isolation on semipermeable versus nonocclusive dressings covering smallpox vaccination sites in hospital personnel. Am J Infect Control. 2004;32(3):126–130. doi: 10.1016/j.ajic.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Talbot TR, Peters J, Yan L, Wright PF, Edwards KM. Optimal bandaging of smallpox vaccination sites to decrease the potential for secondary vaccinia transmission without impairing lesion healing. Infect Control Hosp Epidemiol. 2006;27(11):1184–1192. doi: 10.1086/508827. [DOI] [PubMed] [Google Scholar]
- 32.Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J Virol. 2004;78(8):3811–3816. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couzi G, Kircher JP. Immunotherapie de la Variole. Bulletin de l'Institut d'hygiene du Maroc. 1941;1:59–68. [Google Scholar]
- 34.Kempe CH. Studies on smallpox and complications of smallpox vaccination. Pediatrics. 1960 August;25:176–189. [PubMed] [Google Scholar]
- 35.Kempe CH, Bowles C, Meiklejohn G, Berge TO, St. Vincent L, Babu BVS, et al. The use of vaccinia hyperimmune gammaglobulin in the prophylaxis of smallpox. Bull WHO. 1961;25:41–48. [PMC free article] [PubMed] [Google Scholar]
- 36.Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 37.Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21(2):214–218. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 38.Fillmore GL, Ward TP, Bower KS, Dudenhoefer EJ, Grabenstein JD, Berry GK, et al. Ocular complications in the Department of Defense Smallpox Vaccination Program. Ophthalmology. 2004;111(11):2086–2093. doi: 10.1016/j.ophtha.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Secondary and tertiary transfer of vaccinia virus among U.S. military personnel--United States and worldwide, 2002–2004. MMWR Morb Mortal Wkly Rep. 2004;53(5):103–105. [PubMed] [Google Scholar]
- 40.Garde V, Harper D, Fairchok MP. Tertiary contact vaccinia in a breastfeeding infant. Jama. 2004;291(6):725–727. doi: 10.1001/jama.291.6.725. [DOI] [PubMed] [Google Scholar]
- 41.Jonczy EA, Daly J, Kotwal GJ. A novel approach using an attenuated recombinant vaccinia virus to test the antipoxviral effects of handsoaps. Antiviral Res. 2000;45(2):149–153. doi: 10.1016/s0166-3542(00)00067-x. [DOI] [PubMed] [Google Scholar]
- 42.Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41(12):1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 43.Levine RS, Peterson AT, Yorita KL, Carroll D, Damon IK, Reynolds MG. Ecological niche and geographic distribution of human monkeypox in Africa. PLoS ONE. 2007;2:e176. doi: 10.1371/journal.pone.0000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenner E. An inquiry into the causes and effects of the variolae vaccinae. London: Sampson Low; 1798. [Google Scholar]
- 45.Jenner E. Further observations on the variolae vaccinae. London: Sampson Low; 1799. [Google Scholar]


