Abstract
EVI1 was first identified as a preferential integration site of ecotropic retroviruses in the MDS1/EVI1 genomic locus leading to myeloid tumors in susceptible mice. Later studies showed that retroviral integration in the MDS1/EVI1 locus results in the emergence of a non-malignant dominant hematopoietic stem cell clone in non-susceptible mice strains, in non-human primates, and in patients, suggesting that a gene encoded by the locus could affect the self-renewal potential of a cell. The locus encodes two genes. One of them, EVI1, has long been associated with myeloid leukemia. To understand whether EVI1 has a role in self-renewal control, we forcibly expressed EVI1 in the bone marrow progenitors of two mice strains that differ in their proliferation and self-renewal potential. By comparing the response of the hematopoietic cells to EVI1, we conclude that EVI1 has a role in prolonging the self-renewal potential of the cells and that this ability of EVI1 is limited and modulated by inherent characteristics of the cells.
Keywords: EVI1, hematopoietic stem cell (HSC), self-renewal, bone marrow
INTRODUCTION
The ecotropic virus integration site-1 (EVI1) gene plays important roles both in normal development and in oncogenesis. EVI1 is in general expressed at low level in adult mouse tissues, but has an essential function during embryogenesis as well as in cellular proliferation and differentiation [1]. The human EVI1 gene is located on chromosome band 3q26.2 and encodes a zinc finger nuclear protein with a molecular mass of about 145 kDa. The ten zinc finger motifs of EVI1 are grouped in a proximal domain containing 7 motifs and a distal domain of 3. EVI1 was first identified as a preferential integration site of ecotropic retroviruses in the MDS1/EVI1 genomic locus in susceptible mice leading to myeloid tumors [2]. This complex genomic locus spans over 600 kb and normally leads to the expression of at least two proteins. One of them, EVI1, is not identified in hematopoietic cells and is associated with myeloid human leukemia when inappropriately expressed. The second protein, called MDS1/EVI1, is expressed in several tissues and does not appear to be involved in leukemia. More recently it was reported that there is a significant long-term survival of hematopoietic clones after retroviral integration in the MDS1/EVI1 locus in non-susceptible mouse strains. However in these animals the retroviral integration does not induce leukemia but it rather leads to the expansion of a nonmalignant clone in long-term hematopoietic progenitors [3]. This effect of retroviral integration in the MDS1/EVI1 locus leading to the emergence of a dominant hematopoietic stem cell clone is not limited to the mouse. Two other groups analyzed hundreds of integration sites either in non-human primates seven years after transplantation of autologous CD34+ cells transduced with a retroviral vectors [4] or in two patients following a gene-therapy treatment a year earlier that had required infection of autologous bone marrow with a retrovirus [5]. In both studies the investigators observed a significant frequency of insertion into the MDS1/EVI1 region and the retrovirus was detected primarily in long-term myeloid cells, suggesting that the self-renewal and engraftment potential of CD34+ progenitor cells are enhanced by insertion mutagenesis at this specific locus across species. As with the mice, these investigators noted no evidence of in vivo malignant clones in the MDS1/EVI1 populations and the hematopoietic system of all animals and patients appeared normal without evidence of leukemia [4, 5].
At this time, there is no clear information on whether the integration of the retrovirus in the locus leads to inappropriate expression of EVI1 or MDS1/EVI1. Given the association of EVI1 with myeloid leukemia, the potential involvement of this gene in clonal but (as yet) benign expansion of hematopoietic stem cells would suggest that during embryogenesis this protein could function as a positive regulator of the hematopoietic stem cell (HSC) self-renewal. This finding would be of considerable interest not only for EVI1-leukemia but also to understand the pathways that regulate establishment and maintenance of self-renewal in stem cells.
Here we have evaluated whether EVI1 can indeed prolong or sustain self-renewal potential in hematopoietic stem cells of different murine strains and whether this ability is independent of intrinsic cellular functions or must work in concert with unknown cellular characteristics that are genetically encoded. To do so, we have taken advantage of the different properties of the HSCs of two murine strains, C57BL/J6 and DBA2. Very elegant studies by several investigators have shown that, even within the same species, not all HSCs are created equal and that the HSCs of these two strains of mice have different genetically encoded proliferation and self-renewal potential, with C57BL/6J HSCs undergoing about 30 replications before senescence whereas the DBA2 stem cells are shorter-lived and undergo only about 20 replications before senescence or terminal differentiation. These studies were based on the generation of chimeric mice by combining C57BL/6J and DBA2 embryos [6–8].
Our results indicate that while EVI1 prolongs the self-renewal potential of a HSC, this effect is modulated by inherent characteristics of the cell and by the cell’s intrinsic capability of undergo a number of divisions before lineage commitment.
MATERIAL AND METHODS
Retroviral vectors
The MSCVneo vector was purchased from Clontech (Mountain View, CA USA). The MSCV-EVI1 was generated by insertion of the EVI1 fragment into MSCVneo by using EcoRI and BglII sites.
Strains of mice
C57BL/6J and DBA2 mice were obtained from The Jackson Laboratories (Bar Harbor, ME). The mice were kept in the animal facilities of the University of Illinois at Chicago under specific pathogen-free conditions. All animal studies were performed in accordance with the guidelines of the Animal Care Committee of the University of Illinois at Chicago. Mice were sacrificed at 6/8 weeks of age five days after a subcutaneous injection of 5-Fluorouracil (5-FU) at a dose of 150 mg/kg in saline.
Enrichment of hematopoietic stem cells
Bone marrow cells were flushed from the femora and tibiae of C57BL/6J or DBA2 mice using a 21-gauge needle and syringe and placed into Hank’s balanced salt solution (HBSS) containing 2% fetal calf serum. Erythrocytes were eliminated by hypotonic lyses, and the remaining leukocytes were incubated with saturating amounts of biotin-conjugated monoclonal antibodies (mAbs) specific for murine CD5, CD11b/Mac-1, CD45R/B220, Gr-1/Ly-6G/C, 7-4 and Ter119. The labeled cells were incubated with micro-magnetic beads conjugated to a monoclonal anti-biotin monoclonal antibody (clone:Bio3-18E7.20) at a bead:cell ratio of approximately 4:1. Mature lymphoid and myeloid cells were removed by exposure to a magnetic field (MACS Miltenyi Biothech GmbH, Germany) and the flow-through contained a population of cells greatly enriched in lineage-negative (Lin-) progenitor and stem cells.
Retroviral infection of primary bone marrow cells
Phoenix retroviral packaging cells (ATCC) were maintained and transfected as previously described [9]. The viral supernatant was collected from the vector- or EVI1-transfected Phoenix cells, filtered and used for BM progenitors infection. The separated Lin- bone marrow (BM) cells were allowed to rest overnight in RPMI medium with 10% fetal bovine serum (FBS) and cytokines (GM-CSF, IL-3, IL-6 and SCF). The following day, the cells were counted and 5×105 cells were combined with 3 ml of retroviral supernatant and 3μl of polybrene (final concentration 4μg/ml) and centrifuged at 3000 rpm for 2–4 hours at 32°C. This procedure was repeated twice.
Colony-Formation Assay
The infected hematopoietic progenitors were selected in methylcellulose cultures supplemented with GM-CSF (10 μg/ml), IL3 (10 μg/ml), IL6 (10 μg/ml), SCF (100μg/ml), and G418 (1 mg/ml). After 7 days, the cells were re-plated in the same cytokine-cocktail without G418, and the colonies were counted 7 days later.
RESULTS
Higher percentage of Lin-negative progenitor cells in the bone marrow of DBA2 mice
To confirm previous reports that BM hematopoietic progenitor and stem cells are more abundant in DBA2 mice than in C57BL/6J mice [8], we isolated the Lin- BM cells from 15 C57BL/6J and 15 DBA2 six-weeks old mice as described in Materials and Methods. After negative separation by column and counting of the recovered cells, we confirmed that the number of progenitor/HSC cells recovered from total BM of DBA2 mice was higher than the number of Lin- cells recovered from the same number of C57BL/6J BM cells (Table 1).
TABLE 1.
Higher percentage of Lin-negative progenitor cells in the bone marrow of DBA2 mice
| C57BL/6J | DBA2 | |
|---|---|---|
| Number of mice | 15 | 15 |
| Total BM cells collected | 135,000,000 | 135,000,000 |
| Lin- cells recovered | 3,500,000 | 5,400,000 |
| Percentage of Lin- progenitors | 2.5% | 4% |
Intrinsic strain-specific properties determine the potential for cell self-renewal in murine HSC
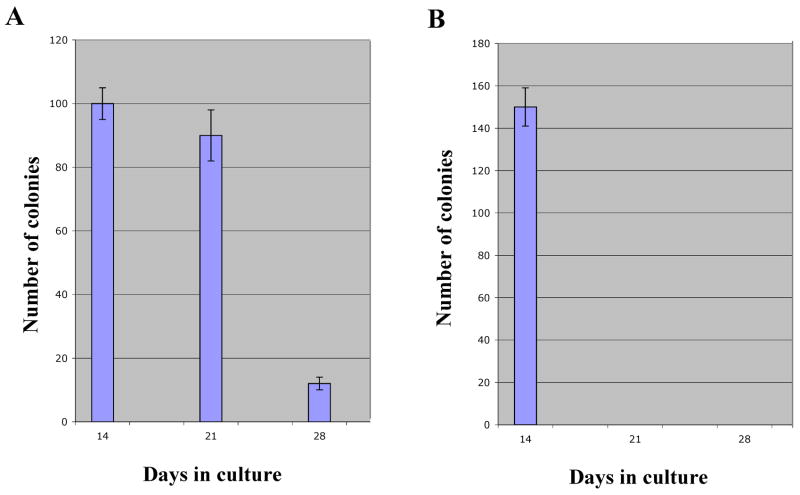
Self-renewal potential determines the ability of a progenitor cell to divide and generate daughter cells with self-renewal ability. These cells have the potential of generating in vitro colony-forming progenitor cells. This property is a hallmark of immortalized or transformed cell lines and is transiently observed in normal stem cells before they terminally differentiate or senesce. We first directly compared the self-renewal potential of C57BL/6J and DBA2 stem cells by determining the number of consecutive platings that generate visible colonies with HSCs of both strains. We found that the two types of HSCs significantly differ in their ability to generate colonies with time. As shown in Fig. 1, while visible C57BL/6J colonies are observed for about 3 platings before terminal differentiation, the DBA2 colonies virtually disappear after 2 platings, suggesting that DBA2 HSCs have a more limited potential for self-renewal.
Figure 1. Intrinsic strain-specific properties determine the potential for cell self-renewal in vitro.
In vitro cultured Lin- C57BL/6J BM cells generate visible colonies in 3 consecutive platings (panel A). Progenitor cells obtained from DBA2 mice lose their clonogenicity earlier (panel B). Note that DBA2 cells consistently generate a higher number of colonies.
EVI1 enhancement of clonogenicity and colony formation is strain-independent
We reported earlier that EVI1 significantly increases the ability to form hematopoietic colonies when expressed in C57BL/6J Lin- cells [10, 11]. To determine whether this property is strain-specific or depends exclusively on EVI1 itself, we stably expressed EVI1 in C57BL/6J and DBA2 Lin- cells by infection with a recombinant retrovirus as described in Materials and Methods. After seven days of culture in presence of G418 to eliminate the non infected cells, 20,000 cells for each BM sample were plated in presence of SCF, IL-3, IL-6 and GM-CSF to induce growth and myeloid differentiation. Seven days after G418 selection, visible colonies were observed and counted. As we previously showed [10, 11], the expression of EVI1 increases the clonogenic potential of C57BL/6J hematopoietic progenitor cells in vitro, resulting in a significantly higher number of visible colonies than for the control cells. A similarly proportional increase in colony number was also observed for the DBA2 cells suggesting that this property of EVI1 very likely does not depend on genes that have been reported to regulate the different self-renewal potential of HSCs in the two strains [12, 13].
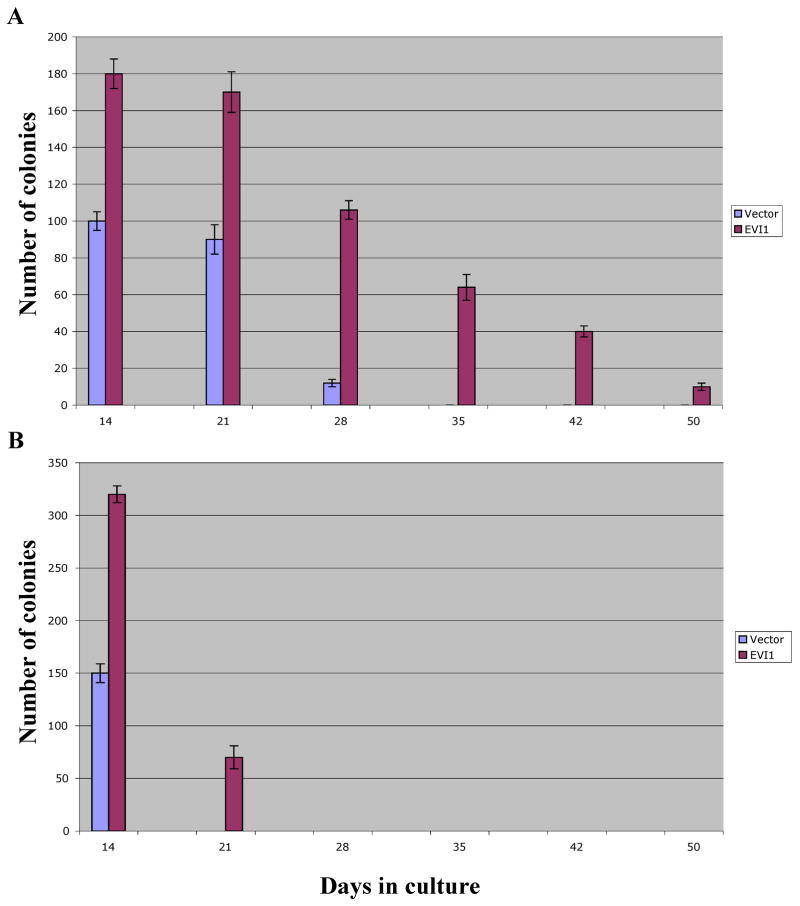
In vitro EVI1 doubles the self renewal potential of murine primary HSCs independently of the mouse strain
We have previously reported that in vitro EVI1 is unable to immortalize primary C57BL/6J HSCs but consistently prolongs their ability to double the number of replatings that yield visible colonies. This effect suggests that in vitro EVI1 enhances the self-renewal of these cells. To determine whether other HSCs with significantly different inherent self-renewal potential are similarly affected by EVI1, we compared C57BL/6J and DBA2 cells. Lin- cells were infected, selected, counted and sequentially plated as described. As reported by us and other investigators, C57BL/6J Lin- cells are capable of generating visible colonies in consecutive platings for about 30 days after their isolation from bone marrow. After this time, the cells deplete their self-renewal potential and terminally differentiate. We confirmed our previous data that when EVI1 is expressed in these cells by retroviral infection, the time until terminal differentiation is approximately doubled to about 50 days, suggesting that EVI1 significantly increases the self-renewal ability of the HSC. This is clearly shown in Fig. 3A. The results obtained using the DBA2 mice are shown in Fig. 3B. The control Lin- DBA2 cells infected by the empty retrovirus survived for only one plating in culture and after 14 days no colonies were observed any longer, very likely because of the cells reduced self-renewal potential compared to C57BL/6J cells. When EVI1 was expressed in DBA2 cells, there was a significant increase in replating potential and colonies were visible for over 20 days. This time extension corresponds roughly to a doubling of what noted for the control cells and is similar to that observed for the C57BL/6J cells, suggesting that this effect of EVI1 is not independent of those primary and fundamental mechanisms that are genetically encoded and that define a cell’s survival and differentiation in vitro.
Figure 3. In vitro EVI1 doubles the self-renewal potential of murine primary HSCs independently of the mouse strain.
EVI1 (red bars) doubles the number of consecutive platings that generate visible colonies for C57BL/6J cells (panel A) and DBA2 cells (panel B).
Morphologic comparison of EVI1-positive C57BL/6J and DBA2 cells
Fourteen days after infection and 7 days after the first plating, visible colonies were observed in EVI1-cells and control cells. To evaluate the cells’ morphology, the colonies were isolated and disaggregated, and cytospin preparations of C57BL/6J and DBA2 BM cells were made for Wright-Giemsa staining (Fig. 4). Clear morphology differences were seen at this time between the two strains. The DBA2 control cells were in a very advanced state of differentiation characterized by large cytoplasm and compact nuclei, the cell morphology was homogeneous, and the cells appeared to be overall synchronized in their differentiation progress (lower left panel). In contrast, the control C57BL/6J cells were not homogeneous in the extent of their differentiation with cells showing the extensive cytoplasm and compact nuclei characteristic of cells near their terminal monocytic differentiation (upper left panel, right side of the panel) mixed to more immature cells with less condensed larger nuclei and less cytoplasm (upper left panel, left side of the panel). This heterogeneous differentiation is likely due to the longer time that the cells can proliferate and differentiate in vitro allowing for the establishment of asynchronous progenitors. This difference between the DBA2 and C57BL/6J cells was reproduced when EVI1 was expressed (right panels, top and bottom). However in both strains a left shift was clearly observed with a larger number of immature cells visible. These results suggest that while EVI1 overall delays the terminal differentiation of BM precursors in vitro by enhancing the cells self-renewal, this effect appears to be strictly limited by intrinsic cellular properties that are genetically encoded and that can differ between intra-species strains.
Figure 4. Morphologic comparison of EVI1-positive C57BL/6J and DBA2 cells.
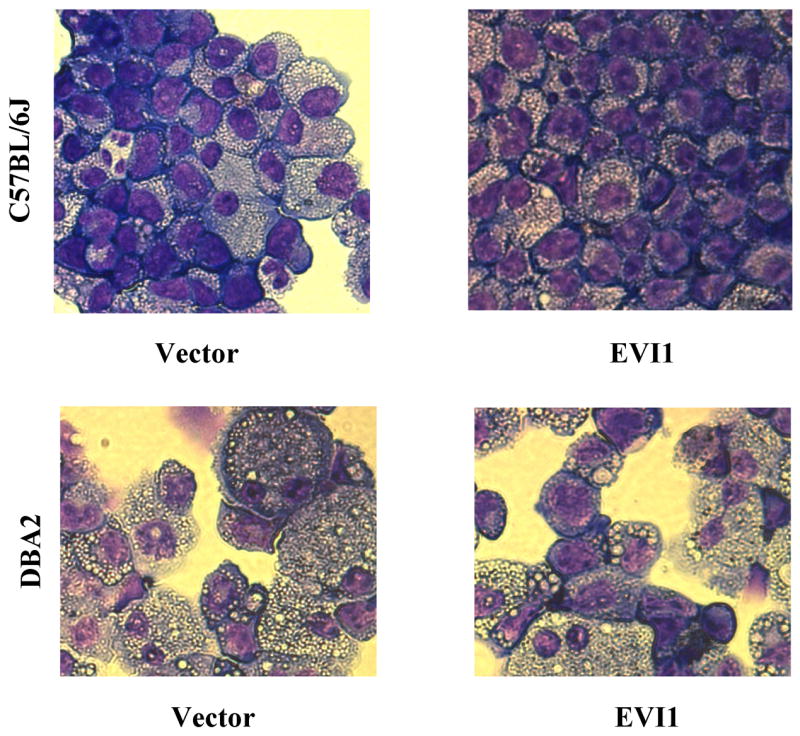
In contrast to C57BL/6J control cells (upper left panel), the DBA2 control cells (lower left panel) show an advanced state of differentiation characterized by large cytoplasm and compact nuclei. The expression of EVI1 in C57BL/6J cells (upper right panel) and DBA2 (lower right panel) cells delays terminal differentiation in vitro.
DISCUSSION
Mature blood cells have in general a very short life span. Their shortage is continuously replenished by newly differentiated cells that derive from hematopoietic stem cells. Among all normal cells, the HSCs are unique for their ability either to undergo self-renewal divisions or to generate progeny that differentiates into mature cells of different hematopoietic lineages. A constellation of intrinsic and extrinsic cellular mechanisms regulates the balance of self-renewal and differentiation in all stem cells and the genetic and molecular mechanisms responsible for the choice of a HSC between these two directions remain to be elucidated. It has recently been reported that retroviral integration in the MDS1/EVI1 locus leads to the emergence of a dominant hematopoietic clone with enhanced expansion and self-renewal potential [3–5]. The MDS1/EVI1 locus encodes 2 major proteins, MDS1/EVI1 and EVI1, but it is not yet known whether one of them is responsible for the emergence of the dominant hematopoietic clone. In this paper we have examined phenotypic changes after expression of EVI1 in normal HSCs obtained from two different murine strains to determine whether this protein has a role in the delicate equilibrium between self-renewal and differentiation of these cells and whether this potential role of EVI1 is dependent on intrinsic cell characteristics. We compared the ability of the BM progenitors obtained from C57BL/J6 and DBA2, two mice strains carrying stem cells with distinct characteristics of growth and self-renewal. We showed that the expression of EVI1 in the hematopoietic stem cells of these two different strains induces a proportionally similar increase in the ability of the HSCs to generate in vitro colonies in consecutive plating assays. These results would indicate that an increase or an induction of EVI1 expression after retroviral integration could result in the expansion of selected hematopoietic clones as reported. These data also suggest that the mechanisms/pathways utilized by EVI1 are not independent of genetically encoded cellular characteristics. These data are in agreement with those of investigators who suggested that EVI1 might have a crucial function in cellular proliferation and differentiation [1] and that might promote the cell proliferation [14].
While most of the published reports on EVI1 focus on the aggressive activity of this protein to induce human and murine leukemia when inappropriately expressed of EVI1, there is no evidence that by itself EVI1 can transform a cell, and one could propose a model in which EVI1 first leads to the expansion of a benign stem cell clone, which with time could be targeted by a second cooperating mutation to induce transformation. Although the molecular mechanisms controlling stem cell self-renewal are still unknown, several regulators have been identified. One of them is HOXB4, which, when transduced in HSCs leads to a 40-fold expansion in vitro in a period of 12 days and acts as a transcriptional regulator of genes that govern self-renewal [15]. EVI1 seems to act in a similar way, perhaps by up-regulating genes controlling HSC maintenance and proliferation such as GATA2 [16].
Figure 2. EVI1 enhancement of clonogenicity and colony formation is strain-independent.
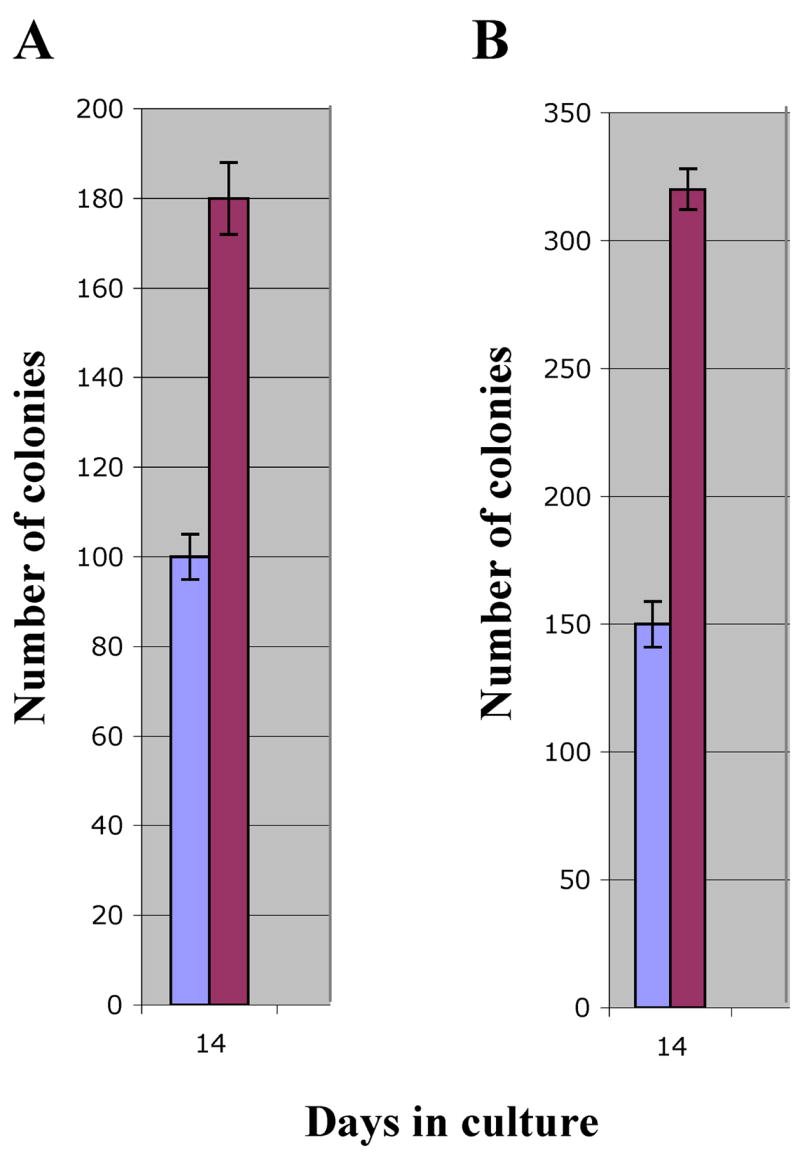
EVI1 equally affects the ability to form hematopoietic colonies when expressed in Lin- cells of two different mice strains. The ratio between the number of EVI1 colonies (red bars) and vector colonies (blue bars) for C57BL/6J cells (panel A) is approximately the same as for DBA2 cells (panel B).
Acknowledgments
This work was supported by NIH grants: R01 HL79580 and R01 HL082935 (GN). This paper is based on a presentation at the Seventh International Workshop on Molecular Aspects of Myeloid Stem Cell Development and Leukemia in Annapolis, Maryland, May 13-16, 2007, sponsored by The Leukemia & Lymphoma Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perkins AS, Mercer JA, Jenkins NA, Copeland NG. Patterns of Evi-1 expression in embryonic and adult tissues suggest that Evi-1 plays an important regulatory role in mouse development. Development. 1991;111:479. doi: 10.1242/dev.111.2.479. [DOI] [PubMed] [Google Scholar]
- 2.Mucenski ML, Taylor BA, Ihle JN, Hartley JW, Morse HC, 3rd, Jenkins NA, Copeland NG. Identification of a common ecotropic viral integration site, Evi-1, in the DNA of AKXD murine myeloid tumors. Mol Cell Biol. 1988;8:301. doi: 10.1128/mcb.8.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kustikova O, Fehse B, Modlich U, Yang M, Düllmann J, Kamino K, von Neuhoff N, Schlegelberger B, Li Z, Baum C. Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science. 2005;308:1171–74. doi: 10.1126/science.1105063. [DOI] [PubMed] [Google Scholar]
- 4.Calmels B, Ferguson C, Laukkanen M, Adler R, Faulhaber M, Kim HJ, Sellers S, Hematti P, Schmidt M, von Kalle C, Akagi K, Donahue RE, Dunbar CE. Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells. Blood. 2005;106:2530–3. doi: 10.1182/blood-2005-03-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, Glimm H, Kühlcke K, Schilz A, Kunkel H, Naundorf S, Brinkmann A, Deichmann A, Fischer M, Ball C, Pilz I, Dunbar C, Du Y, Jenkins NA, Copeland NG, Lüthi U, Hassan M, Thrasher AJ, Hoelzer D, von Kalle C, Seger R, Grez M. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–9. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 6.de Haan G, van Zant G. Genetic analysis of hemopoietic cell cycling in mice suggests its involvement in organismal life span. FASEB J. 1999;13:707–13. doi: 10.1096/fasebj.13.6.707. [DOI] [PubMed] [Google Scholar]
- 7.de Haan G, Szilvassy SJ, Meyerrose TE, Dontje B, Grimes B, van Zant G. Distinct functional properties of highly purified hematopoietic stem cells from mouse strains differing in stem cell numbers. Blood. 2000;96:1374–9. [PubMed] [Google Scholar]
- 8.de Haan G, Nijhof W, van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–50. [PubMed] [Google Scholar]
- 9.Laricchia-Robbio L, Fazzina R, Li D, Rinaldi CR, Kisaly K, Sinha KK, Chakraborty S, Nucifora G. Point mutations in two EVI1 Zn fingers abolish EVI1-GATA1 interaction and allow erythroid differentiation of murine bone marrow cells. Mol Cell Biol. 2006;26:7658–7666. doi: 10.1128/MCB.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonamici S, Li D, Chi Y, Zhao R, Wang X, Brace L, Ni H, Saunthararajah Y, Nucifora G. EVI1 induces myelodisplastic syndrome in mice. J Clin Investig. 2004;114:713–719. doi: 10.1172/JCI21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonamici S, Li D, Mikhail FM, Sassano A, Platanias LC, Colamonici O, Anastasi J, Nucifora G. EVI1 abrogates interferon-alpha response by selectively blocking PML induction. J Biol Chem. 2005;280:428–36. doi: 10.1074/jbc.M410836200. [DOI] [PubMed] [Google Scholar]
- 12.van Zant G, de Haan G. Genetic control of lifespan: studies from animal models. Expert Rev Mol Med. 1999:1–12. doi: 10.1017/S1462399499001441. [DOI] [PubMed] [Google Scholar]
- 13.de Haan G, Bystrykh LV, Weersing E, Dontje B, Geiger H, Ivanova N, Lemischka IR, Vellenga E, van Zant G. A genetic and genomic analysis identifies a cluster of genes associated with hematopoietic cell turnover. Blood. 2002;100:2056–62. doi: 10.1182/blood-2002-03-0808. [DOI] [PubMed] [Google Scholar]
- 14.Hoyt PR, Bartholomew C, Davis AJ, Yutzey K, Gamer LW, Potter SS, Ihle JN, Mucenski ML. The Evi1 proto-oncogene is required at midgestation for neural, heart, and paraxial mesenchyme development. Mech Dev. 1997;65:55–70. doi: 10.1016/s0925-4773(97)00057-9. [DOI] [PubMed] [Google Scholar]
- 15.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 16.Yuasa H, Oike Y, Iwama A, Nishikata I, Sugiyama D, Perkins A, Mucenski ML, Suda T, Morishita K. Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression. EMBO J. 2005;24:1976–1987. doi: 10.1038/sj.emboj.7600679. [DOI] [PMC free article] [PubMed] [Google Scholar]