Abstract
The functions for neurosteroids during development and in response to nervous system injury are beginning to be identified. We focused on a mouse model in which we believed neurosteroid production would be altered, and which had a neurodegenerative phenotype. Niemann Pick Type-C (NP-C) is an autosomal recessive neurodegenerative disease caused by mutations in NPC1 (95%) or NPC2 (5%), resulting in lysosomal accumulation of unesterified cholesterol and glycolipids. The NIH mouse model of NP-C has a mutation in the NPC1 gene, and exhibits several pathological features of the most severe NP-C patients. How lysosomal storage and trafficking defects lead to neurodegeneration is unknown. We found that these mice had normal neurosteroidogenic enzyme activity during development, but lost this activity in the early neonatal period, prior to onset of neurological symptoms. Neurons that expressed P450scc, 3ß HSD, as well as those that expressed 3α HSD and 5α reductase were lost in adult NP-C brains, resulting in diminished concentrations of allopregnanolone. We treated NP-C mice with allopregnanolone and found that a single dose in the neonatal period resulted in a doubling of lifespan, substantial delay in onset of neurological symptoms, survival of cerebellar Purkinje and granule cell neurons, and reduction in cholesterol and ganglioside accumulation. The mechanism by which allopregnanolone elicited these effects is unknown. Our in vitro studies showed that Purkinje cell survival promoted by allopregnanolone was lost by treatment with bicuculline, suggesting GABAA receptors may play a role. We treated NP-C mice with a synthetic GABAA neurosteroid, ganaxolone (3α-hydroxy-3β-methyl-5α -pregnan-20-one). Ganaxolone treatment of NP-C mice produced beneficial neurological effects, but these effects were not as robust as those obtained using allopregnanolone. Thus, allopregnanolone may elicit its effects through GABAA receptors and through other mechanisms. Additional studies also suggest that allopregnanolone may elicit its effects through pregnane-X receptors (PXR). Our data suggest that mouse models of neurodegeneration may be beneficial in establishing both physiologic and pharmacologic actions of neurosteroids. These animal models further establish the wide range of functions of these compounds, which may ultimately be useful for treatment of human diseases.
Keywords: allopregnanolone, Niemann Pick Type C, GABAA receptor, pregnane-X-receptor
1. Introduction
Steroid hormones are essential for life. Glucocorticoids (cortisol, corticosterone) are necessary for carbohydrate metabolism and are synthesized and released in response to stress; mineralocorticoids (aldosterone) instruct the kidney to retain sodium; sex steroids (progesterone, testosterone, estradiol) are essential for reproduction. Neurosteroids are steroids synthesized de novo in the brain, or converted into neuroactive steroids in the brain from steroids derived from the circulation. In the 1980's, Etienne Baulieu coined the term “neurosteroid” to distinguish this class of steroids from glucocorticoids, mineralocorticoids, and sex steroids (Baulieu, et al., 1999,Compagnone and Mellon, 2000). This designation was ascribed to steroids that were synthesized de novo in the brain, as these steroids were identified in the rodent brain weeks after gonadectomy and adrenalectomy. At the same time, functions for neurosteroids, distinct from their function at nuclear receptors was being elucidated by several groups (Harrison and Simmonds, 1984,Majewska, et al., 1986). Over the past decade, the identification of the steroids found in the brains of many species has demonstrated a remarkable similarity. Functions associated with these neuroactive compounds has also been identified (reviewed in (Backstrom, et al., 2003,Barbaccia, 2004,Belelli, et al., 2006,Belelli and Lambert, 2005,Bernardi, et al., 2004,Brinton and Wang, 2006,Compagnone and Mellon, 2000,Finn, et al., 2004,Guarneri, et al., 2003,Lambert, et al., 2003,Mensah-Nyagan, et al., 2001,Morrow, et al., 2001,Reddy, 2002,Reddy, 2004,Rogawski and Reddy, 2002,Rupprecht and Holsboer, 1999,Schumacher, et al., 2004,Schumacher, et al., 2003,Stoffel-Wagner, 2003,Tsutsui and Mellon, 2006,Tsutsui, et al., 2004,Uzunova, et al., 2005,Vallee, et al., 2001). However, it is still unknown if these compounds are essential for life.
All steroids and neurosteroids are synthesized from cholesterol through the participation and concerted action of a series of steroidogenic enzymes (Miller and Tyrell, 1994,Miller, 1988). The presence or absence of particular steroidogenic enzymes dictates that pathway of steroidogenesis that will be taken by a particular steroidogenic organ or cell type.
In conjunction with the proposed effect of the neurosteroid allopregnanolone on GABAA receptors (Belelli and Lambert, 2005), there are several proposed roles for neurosteroids. Given exogenously, they are anxyiolytic, anticonvulsant compounds. The neurosteroid pregnenolone has been shown to enhance memory when given intrathecally (Flood, et al., 1992,Flood, et al., 1995,Mathis, et al., 1994,Mayo, et al., 1993,Robel, et al., 1995). In rodent models of alcohol intoxication, one mechanism through which alcohol elicits its effects is through increased synthesis of allopregnanolone in the brain (Brot, et al., 1997,Caldeira, et al., 2004,Finn, et al., 2004,Finn, et al., 2003,Follesa, et al., 2004,Grobin, et al., 2005,Janis, et al., 1998,VanDoren, et al., 2000). Finally, the neurosteroid allopregnanolone has been implicated in a severe form of premenstrual disorder, called premenstrual dysphoric disorder (Backstrom, et al., 2003,Bernardi, et al., 2004,Bicikova, et al., 1998,Bixo, et al., 1997,Epperson, et al., 2002,Friedman, et al., 1993,Girdler, et al., 2001,Monteleone, et al., 2000,Rapkin, et al., 1997,Schmidt, et al., 1994,Smith, et al., 1998). Most recently, changes in GABAA receptor subunit expression and sensitivity to the neurosteroid allopregnanolone has been implicated in changes in behavioral responses seen at puberty (Shen, et al., 2007).
In addition to behavioral effects of neurosteroids, neurosteroids have also been implicated in affecting neuronal function and differentiation (Brinton and Wang, 2006). These include neuroprotection against ischemia and stroke (Cutler, et al., 2005,Djebaili, et al., 2005,Hoffman, et al., 2003,Lapchak, 2004,Meffre, et al., 2007,Shear, et al., 2002,VanLandingham, et al., 2006), recovery of motor function after spinal cord injury (di Michele, et al., 2000,Fiore, et al., 2004,Labombarda, et al., 2006,Patte-Mensah, et al., 2004,Pomata, et al., 2000), regulation of myelination (Chavez-Delgado, et al., 2005,Gago, et al., 2001,Ghoumari, et al., 2005,Ghoumari, et al., 2003,Le Goascogne, et al., 2000,Schumacher, et al., 2000,Schumacher, et al., 2004,Schumacher, et al., 2003), proliferation of neuronal stem cells (Suzuki, et al., 2004,Wang, et al., 2005) , neurogenesis in the hippocampus (Keller, et al., 2004,Suzuki, et al., 2004,Wang, et al., 2005), and induction of analgesia (Pathirathna, et al., 2005,Todorovic, et al., 2004). Many of these actions of neurosteroids are discussed in detail in other papers in this issue.
We and others have taken several different approaches to understanding the role of neurosteroids in vivo. Mice in which several of the genes encoding several of the neurosteroidogenic enzymes have been ablated have been created. These include ablation of the P450scc (Hu, et al., 2002), P450c17 (Bair and Mellon, 2004), 5alpha reductase type I and 5alpha reductase type II (Mahendroo, et al., 2004,Mahendroo, et al., 2001,Mahendroo, et al., 1997). Among these knock out mice, only the P450c17 knock out mice are embryonic lethal at embryonic day 7. P450scc mice lack glucocorticoid production and need replacement at birth. Female mice lacking 5 alpha reductase type I and type II exhibited parturition and fecundity defects similar to those of animals without 5 alpha-reductase type 1 ; male mice are phenotypically relatively normal, and the data from the knockout mice indicate T appears to be the only androgen required for differentiation of the male urogenital tract in mice and the synthesis of DHT serves largely as a signal amplification mechanism. Thus, global ablation of these genes does not provide insight into the roles of the neurosteroids they are involved in synthesizing, in the nervous system. Alternatively, these results may suggest that the neurosteroids do not play obligate and unique roles in the nervous system.
To identify regions and cells of the nervous system that express the neurosteroidogenic enzymes, promoter-reporter constructs can be prepared, using the promoters for the genes encoding steroidogenic enzymes. Recent studies using a P450scc-cre reporter (Wu, et al., 2007) have shown that the P450scc promoter is expressed in the cortex, hippocampus, thalamus, hypothalamus (dorsomedial ventromedial hypothalamus and arcuate nucleus). We prepared P450c17-GFP transgenic mice, using a 1.5 kb promoter region of the rat P450c17 gene. In the brain, we found GFP expression in axonal tracts projecting rostrally from the midbrain and consolidating in the sub-cortical plate of the embryonic cortex and observed neuronal projections traversing along the dorsal ventral axis connecting the spinal cord and the brainstem extending basally towards the medulla and in condensed ganglia of the dorsal root. Hence these results are promising and may define neurosteroidogenic neurons and glia throughout development, and under different types of regulation.
Another strategy for understanding neurosteroid function in vivo is to identify existing mouse lines that may have altered neurosteroid production. We have used this approach, and have identified a mouse line for a childhood neurodegenerative disease, Niemann Pick Type C. We have used this mouse as a model for altered neurosteroidogenesis (Griffin, et al., 2004).
2. Niemann Pick Type C Disease
Niemann-Pick type C disease is a fatal autosomal recessive, childhood-onset, neurodegenerative disorder. This lysosomal lipid storage disorder is characterized by a defect in intracellular cholesterol trafficking, resulting in lysosomal accumulation of unesterified cholesterol (reviewed in Patterson, et al., 2001). The accumulation of cholesterol causes hepatomegaly with foamy macrophage infiltration, and chronic neurologic deterioration associated with accumulation of sphingomyelin and other glycolipids in neuronal tissues, leading to seizures, supranuclear ophthalmoplegia and progressive loss of motor and intellectual function in the second decade of life (Fink, et al., 1989,Norman, et al., 1967). NP-C has been linked to two genetic loci, NPC1 (major locus) and NPC2 (Millat, et al., 2001,Naureckiene, et al., 2000,Patterson, et al., 2001,Pentchev, et al., 1995,Vanier, et al., 1996). The human NPC1 gene encodes a protein of 1,278 amino acids (Carstea, et al., 1997) that shares homology with other proteins that regulate cholesterol homeostasis, including 3-hydroxy-3-methylglutaryl-CoA (HMG CoA) reductase, sterol regulatory element binding protein cleavage-activating protein (SCAP), with Patched, the receptor for sonic hedgehog (Loftus, et al., 1997), and the RND family of prokaryotic permeases, suggesting NPC1 may function as a transmembrane efflux pump (Davies, et al., 2000). More than 95% cases of NP-C are caused by mutations in NPC1 (Bauer, et al., 2002,Carstea, et al., 1997). NPC2, first identified as human epididymal protein 1 (HE1), is a widely expressed 151-amino acid lysosomal glycoprotein that binds cholesterol. About 5% of NP-C is caused by mutations in NPC2. NP-C patients from both complementation groups have similar clinical and biochemical phenotypes, suggesting that NPC1 and NPC2 may interact or function sequentially in a common metabolic pathway.
There are few data concerning the epidemiology of NP-C. The disease is pan-ethnic, and two genetic isolates have been described in French Arcadians in Nova Scotia, previously called NP-D (Crocker, 1961) and Spanish-Americans in southern Colorado (Wenger, et al., 1977). The prevalence of NP-C in the general population has been estimated at 1/150,000 live births (Patterson, et al., 2001). This estimate may be low, as about 50% of NP-C cases may present with neonatal liver disease (Kelly, et al., 1993,Vanier, et al., 1988). Thus, the true prevalence of NP-C is likely to be greater than 1/150.000.
Much of the work on NPC1 protein, neuronal histology, and cholesterol utilization has come from the mouse model of NP-C (Morris, et al., 1982), a strain of BALB/c mice with a retroposon insertion in NPC1 (Loftus, et al., 1997,Morris, et al., 1982). These mice have defects in cholesterol metabolism morphologically and biochemically similar to human NP-C, and show most of the same neurological phenotypes as human beings with NP-C, although the neurological demise is much more rapid in the mouse than in human beings. Nevertheless, both the murine model and patients with NP-C show similar widespread histopathological abnormalities in the central and peripheral nervous systems, including cerebellar degeneration (Gilbert, et al., 1981,Morris, et al., 1982) Purkinje cell degeneration, irregular dendritic trees, decreased numbers of dendritic spines (Higashi, et al., 1993) and progressive dysmyelination of the CNS (Higashi, et al., 1995,Weintraub, et al., 1987,Weintraub, et al., 1985,Xie, et al., 2000), suggesting progressively defective utilization of cholesterol. The mechanisms of neuronal dysfunction and degeneration are not fully understood. Cholesterol content does not appear to be elevated in cortical neurons, even though these cells exhibit neuronal storage abnormalities (Spence and Callahan, 1989,Vanier, et al., 1991), and the rate of sterol synthesis and loss is lower in NP-C mice (Xie, et al., 2000). Human NP-C brains have cortical neurons with distended cytoplasm, ballooned neurons (Anzil, et al., 1973,Braak, et al., 1983,Norman, et al., 1967), and neurofibrillary tangles (Suzuki, et al., 1995). Cholesterol and sphingomyelin are decreased in white matter due to demyelination (Braak, et al., 1983,Xie, et al., 2000). In addition to accumulating cholesterol, cells from NP-C mice also accumulate gangliosides and glycosphingolipids (Zervas, et al., 2001).
3. Neurosteroids and NP-C
In addition to abnormal cholesterol trafficking in NP-C neurons, NP-C mice also show abnormalities in testicular steroidogenesis (Roff, et al., 1993) and ovarian steroidogenesis (Gevry, et al., 2004). Since we believe that neurosteroids are necessary for neuronal and glial function, we hypothesized that alterations in sequestration of intracellular cholesterol would result in altered neurosteroidogenesis, which we hypothesize would subsequently alter neuronal and glial function.
As a first step in determining if altered neurosteroidogenesis could contribute to the neuropathology seen in NP-C, we analyzed brains of adult NP-C mice for the endogenous concentrations of some neurosteroids. We determined that the concentration of pregnenolone, DHEA and allopregnanolone were all significantly less in brains of NP-C mice than they were in brains of age-matched wild type mice (Griffin, et al., 2004). The concentrations of other neuroactive compounds such as tetrahydrodeoxycorticosterone or androstenediol were not assessed. Thus, NP-C mice have diminished neurosteroid concentrations, which could result from several different mechanisms.
We determined whether neurons and glia that express the steroidogenic enzymes required for allopregnanolone production are also diminished in brains of NP-C mice. Using immunohistochemistry, we found that adult NP-C brains had significantly diminished expression of P450scc, 3ßHSD, 5α reductase and 3α HSD. This reduction in expression of these neurosteroidogenic enzymes was seen in the cortex and in the cerebellum. In the cerebellum, Purkinje neurons that express these enzymes are lost in the adult NP-C mouse. In the cortex, it is unknown which particular neurons or glia express these enzymes. Thus it is unknown if those cells are also lost in NP-C mouse brains, or if there is a reduction in expression of neurosteriodogenic enzymes.
Analysis of neurosteroidogenic enzyme activity throughout the life of the NP-C mouse showed that neurosteroidogenesis (allopregnanolone production) is normal at least at the end of gestation. However, at birth, we found that NP-C mouse brains had a significant reduction in 3α HSD activity, and hence could not convert dihydroprogesterone to allopregnanolone. While not explicitly tested as substrates in these studies, it is also likely that NP-C mouse brains cannot convert corticosterone to tetrahydrodeoxycorticosterone, or testosterone to androstenediol, other neuroactive compounds. These conversion use the same enzymes as those used to convert progesterone to allopregnanolone (i.e. 5α reductase and 3α hydroxysteroid dehydrogenase).
The diminution in enzyme activity was seen in the cortex, midbrain and hindbrain, indicating that there was not region-specific reduction in enzymatic activity. Several weeks later, we also demonstrated a significant reduction in 5α reductase activity (conversion of progesterone to dihydroprogesterone) in all brain regions. This reduction in 5α reductase and 3α HSD activities preceded, by several weeks, onset of behavioral symptoms of ataxia, tremor, and weight loss.
4, Treatment of NP-C mice with allopregnanolone
If the loss of allopregnanolone production contributed to the neuropathology of NP-C, we reasoned that appropriately timed treatment of NP-C mice with allopregnanolone should reduce the symptoms and pathology seen in untreated NP-C mice. We tried several approaches to treatment with allopregnanolone – providing the neurosteroid in drinking water, as a timed-release pellet, and as an injection, and each treatment had some efficacy (Griffin, et al., 2004). Efficacy was assessed by survival of mice, by time of onset of neurological symptoms, as well as by weekly assessment of locomotor function and coordination. All these markers of effective treatment changed in parallel with effective treatment, i.e. effective treatment delayed weight loss and onset of ataxia and tremor, prolonged locomotor function, and increased survival.
We reasoned that since there was a reduction in neurosteriodogenic enzyme activity in the early neonatal period, that lack of allopregnanolone at that time might be crucial for appropriate brain development. Hence, we treated mice during the first two weeks of life (Griffin, et al., 2004). Our results indicated that a single injection of allopregnanolone beginning at weaning (postnatal day 23) or earlier (to postnatal day 7) was effective, and that efficacy depended upon the day at which treatment was given. Hence, treatment at postnatal day 7 was the most effective time to treat NP-C mice, and resulted in a doubling of lifespan, a 5-week delay in loss of locomotor function, and a 3-4 week delay in onset of symptoms.
Additional studies treating mice at postnatal day 0 or day 3 indicated that treatment at these times was less effective than treatment at day 7 (unpublished results), again suggesting a time-dependency to treatment.
Our studies have now been repeated by other laboratories, which have demonstrated similar effects of allopregnanolone treatment on their colonies of NP-C mice (Ahmad, et al., 2005,Langmade, et al., 2006).
5. Assessment of biochemical markers of NP-C disease
Allopregnanolone treatment significantly increased survival and locomotor function in NP-C mice. We assessed whether there were changes in neuronal survival in the mice. As discussed previously, untreated NP-C mice have substantial loss of cerebellar Purkinje neurons at the end of life (∼60 days). Analysis of brains of NP-C mice treated with allopregnanolone at postnatal day 7 indicated that those mice had substantial survival of cerebellar Purkinje neurons, which was seen in all lobes of the cerebellum (Griffin, et al., 2004,Langmade, et al., 2006). Concomitant with increased Purkinje neuronal survival, we also found that allopregnanolone-treated NP-C mice had significant reduction in both cortical and cerebellar ganglioside concentrations, and reduction in accumulation of cholesterol in the brain. Thus, many hallmarks of NP-C disease progression are ameliorated by a single injection of allopregnanolone at postnatal day 7.
6. Mechanism of allopregnanolone action: GABAA receptor
Studies by other laboratories have demonstrated that one mechanism by which allopregnanolone functions is by augmentation of GABAA receptor channel opening, through alteration of the kinetics of entry to and exit from desensitized states of the receptor (Zhu and Vicini, 1997,Zhu, et al., 1996). We used another GABA-ergic neurosteroid, ganaxolone, as a treatment in NP-C mice. Ganaxolone is a C3-β–methyl derivative of allopregnanolone, and was developed as an orally effective neurosteroid, presumably because of the inhibition of C3-hydroxyl oxidation due to the presence of the methyl group (Beekman, et al., 1998,Carter, et al., 1997,Gasior, et al., 2000,Gee, 1996). Pharmacokinetic and in vivo studies have shown that ganaxolone has greater efficacy than allopregnanolone at GABAA receptors (Beekman, et al., 1998,Carter, et al., 1997,Gasior, et al., 2000,Gee, 1996,Kerrigan, et al., 2000,Laxer, et al., 2000,Monaghan, et al., 1997,Reddy and Rogawski, 2000,Reddy and Rogawski, 2000,Robichaud and Debonnel, 2005,Ungard, et al., 2000). We treated mice at postnatal day 7, using the same dose of ganaxolone as we used for allopregnanolone (25 mg/kg). We assessed onset of symptoms, locomotor function and coordination, and survival of mice. These data are shown in figure 1. Ganaxolone-treated mice resulted in a significant increase in longevity (average of 93 days vs. 68 days in untreated NP-C mice). However, this increased longevity was not as great as that seen with allopregnanolone treatment (allopregnanolone, 124 days vs. ganaxolone 93 days, figure 1). In addition to increasing longevity, ganaxolone treatment resulted in a significant delay in tremor, ataxia and weight loss in treated NP-C mice (figure 2 A). When we compared these beneficial effects of ganaxolone with the results obtained from allopregnanolone treatment, ganaxolone treatment was not as effective.
Figure 1.
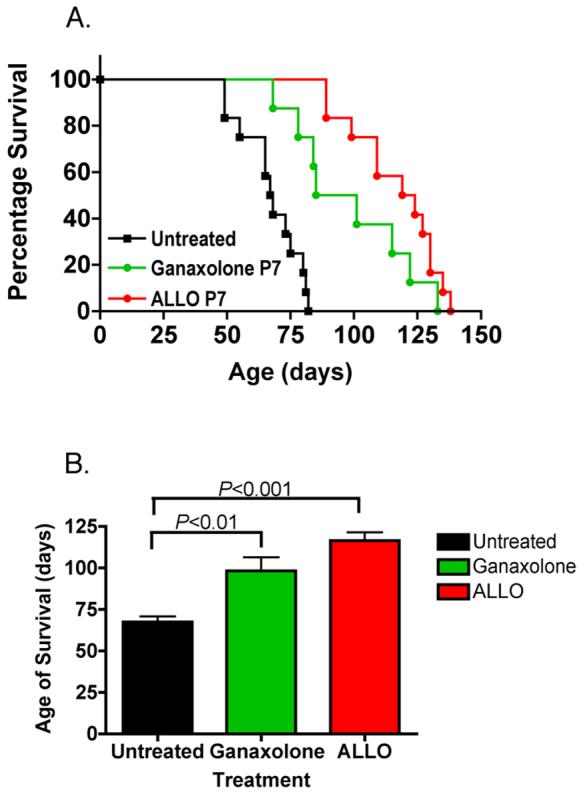
Effect of a single dose of allopregnanolone or ganaxolone on survival of NP-C mice. Allopregnanolone (Allo) or ganaxolone (25 mg/kg in 20% §-cyclodextrin) or nothing (untreated, 20% §-cyclodextrin only) was administered subcutaneously in a single injection at postnatal day 7. A. Survival curves for treatments. B. Average survival time. Data are means ± S.D. N=12 for untreated and allopregnanolone-treated mice; n=8 for ganaxolone-treated mice.
Figure 2.
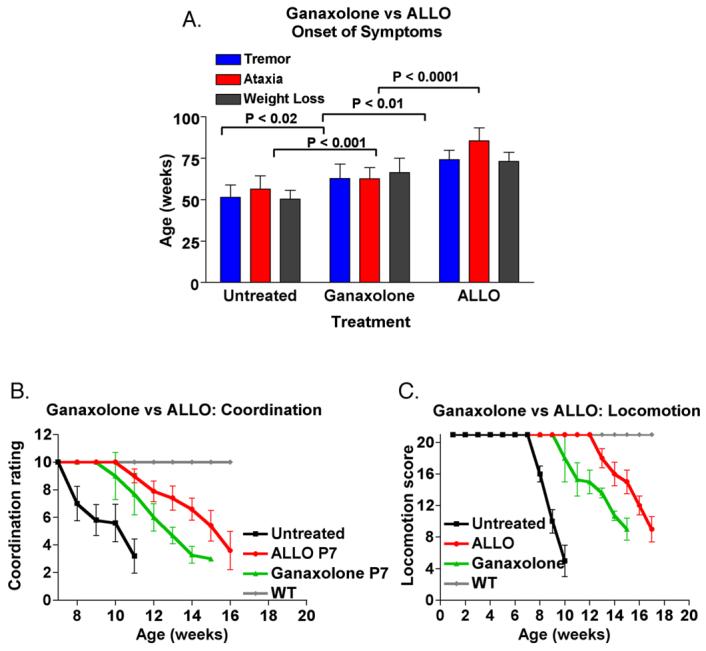
Effect of a single dose of allopregnanolone (allo) or ganaxolone on onset of symptoms of NP-C mice. A. Onset of symptoms: Animals were assessed weekly for weight, tremor, ataxia, and week of onset was noted. B. Motor coordination and C. Locomotor function: mice were assessed weekly for locomotor function and coordination. These assays used locomotor tests established for assessing spinal cord injuries and recovery (Basso, et al., 1995). The rater was blinded to treatment. Data are means ± S.D. N=12 for untreated and allopregnanolone-treated mice; n=8 for ganaxolone-treated mice.
We also assessed locomotor function and coordination in ganaxolone-treated NP-C mice. Ganaxolone treatment resulted in a significant delay in loss of locomotor function; furthermore, the loss of locomotor function was much more gradual in ganaxolone-treated mice than it was in untreated mice (Figure 2 B and C). When compared with allopregnanolone-treated mice, ganaxolone-treated mice had an earlier loss of locomotor function. Delays in loss of coordination were similar between allopregnanolone- and ganaxolone-treated mice. Both groups of treated mice had normal coordination for more than 4 weeks after untreated NP-C mice showed loss of coordination. The loss of coordination was also more gradual in both ganaxolone- and allopregnanolone-treated mice than it was in untreated NP-C mice. Taken together, these results indicate that ganaxolone, a synthetic neurosteroid that is being developed clinically for treatment of pediatric seizure disorders, is an effective treatment in NP-C mice. However, the differences we found in the effects of ganaxolone and allopregnanolone treatment suggest that the mechanism(s) by which allopregnanolone elicits its effects is not completely identical to that of ganaxolone. Since ganaxolone is thought to elicit its effects solely through GABAA-mediated mechanisms, our data suggest that in addition to affecting GABAA receptors, allopregnanolone likely has other mechanisms of action in vivo that are not mimicked in entirety by ganaxolone. Treatment of NP-C mice with other ligands of the GABAA receptor, such as benzodiazepines, has not been tested.
Another reason that ganaxolone may not be as effective as allopregnanolone maybe due to ganaxolone's inability to be metabolized to other neuroactive compounds, as allopregnanolone can. Allopregnanolone can be converted to dihydroprogesterone (5α-pregnan-3, 20-dione) by the enzyme 3 alpha hydroxysteroid dehydrogenase. This is a reversible enzymatic reaction, although the reduction (production of allopregnanolone) is favored enzymatically. Dihydroprogesterone, unlike allopregnanolone, is active at nuclear progesterone receptors. Because of the 3ß-methyl group on ganaxolone, it cannot be converted to a similar compound. Hence, some of the additional benefits of allopregnanolone may be due to its metabolism to other neuroactive compounds.
The data also suggest that the neurodegeneration that occurs in NP-C mice is a result of multiple different pathologies that result from lysosomal accumulation of cholesterol and other compounds, like gangliosides, that traffic via the same intracellular pathway. Hence, targeting several of these pathways would likely be more beneficial than targeting a single pathway. For example, NP-C mice fail to use lipoprotein-derived cholesterol for synthesis of 25- and 27-hydroxycholesterol (Frolov, et al., 2003,Zhang, et al., 2004). These compounds are ligands for the liver X receptor (LXR) that promote cellular cholesterol efflux and catabolism. Treatment of NP-C mice with both allopregnanolone and a synthetic LXR ligand resulted in even better neurological outcome than treatment with either compound alone (Langmade, et al., 2006). These data indicate that directing treatments toward multiple different mechanisms, pathways, and targets provides synergistic benefits. The data also indicate that these different ligands work through different mechanisms, since the result of their combined treatments are synergistic. These data may suggest that treatment of NP-C mice with allopregnanolone plus ganaxolone may result in a different, perhaps more beneficial outcome, than treatment with either compound alone.
7. Allopregnanolone is a ligand for the nuclear pregnane-X receptor (PXR)
How can a single injection of a neurosteroid in the early postnatal period, result in long-term effects on neuronal survival and intracellular cholesterol and ganglioside accumulation more than 10 weeks later? Furthermore, how can a GABAA receptor-mediated action elicit these results? The concentration of allopregnanolone used in our studies (25 mg/ml) (Griffin, et al., 2004) was a maximally effective dose (Figure 3, onset of symptoms). At this dose of treatment, we determined that the maximum concentration of allopregnanolone achieved in the brain is ∼25 μM (∼8 μg/g tissue). Allopregnanolone has a very short half life in the plasma of wild type and NP-C mice (t1/2 ∼ 30 minutes) as well as in human beings (t1/2 ∼ 45 minutes) (Timby, et al., 2006). In addition, allopregnanolone has a short half-life in the brain, its concentrations are maximal within 10 minutes, and has a mean retention time of less than one hour. These data suggest that while allopregnanolone may preferentially accumulate in the brain, it is unlikely that allopregnanolone given at postnatal day 7, remains in high enough concentration, to continue to elicit GABAA-mediated effects weeks later. Studies in human beings indicate that short-term doses of allopregnanolone elicit neurological effects within minutes. Thus, the question about additional mechanisms of allopregnanolone action persist.
Figure 3.
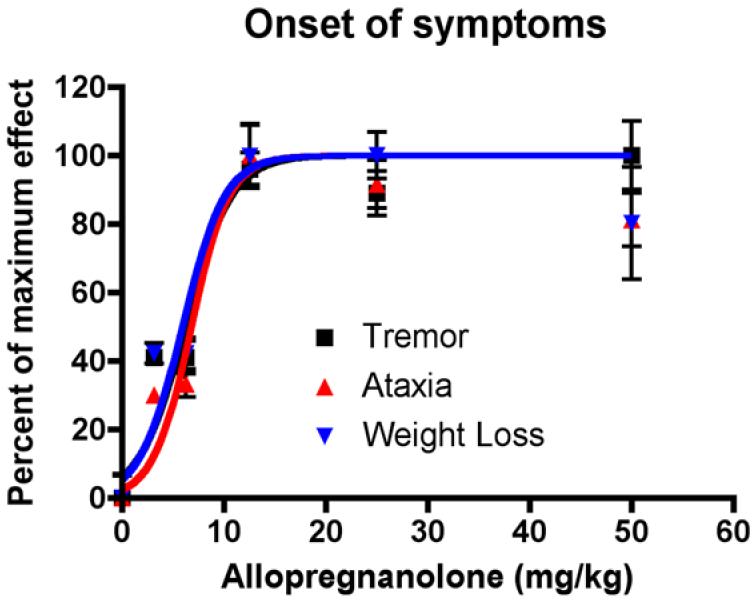
Dose-responsiveness of allopregnanolone treatment. NP-C mice were treated with a single dose of allopregnanolone (0 – 50 mg/kg) at postnatal day 7. Onset of symptoms (tremor, ataxia and weight loss) was assessed weekly. Data are means ± S.D. N=12 mice for each dose.
Studies by the Ory laboratory provide evidence of these additional mechanisms (Langmade, et al., 2006). The Ory laboratory used a synthetic enantiomer of allopregnanolone to shed light on additional mechanisms of action. They treated NP-C mice at postnatal day 7 with either allopregnanolone or the enantiomer of allopregnanolone, called ent-allopregnanolone (Covey, et al., 2000). Remarkably, they showed that treatment of NP-C mice with each of these compounds yielded indistinguishable results! Animals treated with either allopregnanolone or ent-allopregnanolone had identical survival profiles and locomotor profiles. Since ent-allopregnanolone has ∼ 1/300th the action of the naturally occurring compound at GABAA receptors (Alakoskela, et al., 2007,Covey, et al., 2000) the results suggest that the beneficial effects of allopregnanolone in NP-C mice are not mediated by GABAA receptors.
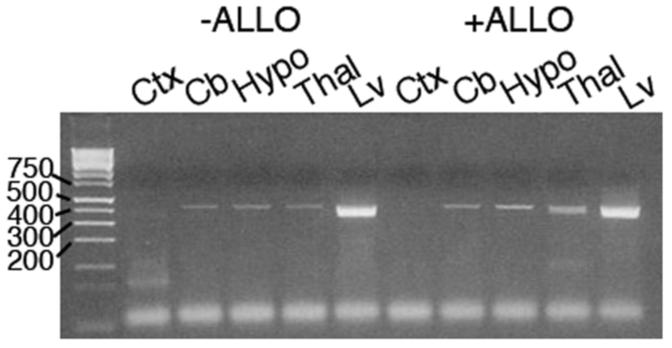
In addition to working through GABAA receptors, allopregnanolone is a ligand for the promiscuous pregnane-X-receptor (Kliewer, et al., 2002,Lamba, et al., 2004,Moore, et al., 2000,Watkins, et al., 2001). This receptor is found mainly in the liver; however, we have found that PXR is also expressed in the mouse brain (Figure 4), albeit at much lower concentrations than in the liver. Both allopregnanolone and ent-allopregnanolone cause increased expression of cyp3A13 mRNA, a PXR target gene. However, this increased gene expression requires 10-50 μM concentrations of the steroids, in contrast to the 10-100 nM concentration of allopregnanolone needed to augment GABAA-ergic function. In mice treated with either allopregnanolone or ent-allopregnanolone, there is increased brain expression of cyp3A13 within 24 hours of treatment, which persists 28 days later. Thus, induction of PXR may be an additional mechanism through which allopregnanolone elicits its effects, and results in beneficial treatment of NP-C. It is unknown if other PXR ligands would thus be equally (or even more) effective in treatment of NP-C. Similarly, if PXR is the mechanism through which both allopregnanolone and ent-allopregnanolone mediate their effects in vivo in NP-C mice, ablation of brain expression of PXR in NP-C mice should result in ineffective treatment of NP-C mice. Studies studying additional PXR and GABAA receptor ligands are currently ongoing.
Figure 4.
RT-PCR analysis of pregnane X receptor mRNA expression in the brain. Brains from postnatal day 7 mice were treated with nothing (−ALLO) or with 25 mg/kg allopregnanolone, subcutaneously (+ALLO), and brains were removed 24 hours later. Brains were separated into various regions, RNA was isolated and cDNA was prepared and amplified with primers specific for mPXR . Amplification was for 35 cycles, and PCR products of 418 bp were separated on 2% agarose gels. Ctx, cortex; Cb, cerebellum; Hypo, hypothalamus; Thal, thalamus; Lv, liver.
8. Conclusions
The results from these studies have demonstrated that a lack of allopregnanolone synthesis in the early neonatal period may contribute to the neuropathology seen in NP-C mice. The treatment studies suggest that allopregnanolone may function in the early postnatal period in the brain of mice, and that allopregnanolone's actions in NP-C mice may be time-specific. These actions may be related to specific cellular development, proliferation, and migration. The action of allopregnanolone on reducing cellular accumulation of gangliosides and cholesterol may likewise contribute to the beneficial effects of allopregnanolone treatment. The mechanism(s) through which allopregnanolone functions in NP-C is unknown. Our studies using the synthetic GABAA receptor ligand ganaxolone, suggests that this receptor may indeed play a role in the beneficial actions of allopregnanolone. Indeed, we have shown that allopregnanolone can mediate beneficial actions in cultured Purkinje neurons which are mediated through GABAA-receptors. However, the compelling studies using a GABAA-inactive ent-allopregnanolone suggest that GABAA-receptors may not be the only receptor involved in the beneficial in vivo mechanisms. These results also suggest that the pregnane-X-receptor may mediate some of the effects of allopregnanolone. Hence, studies using additional known GABAA agonists, such as benzodiazepines, or of other neurosteroids, such as tetrahydrodeoxycorticosterone and corticosterone-derived neuroactive steroids, in addition to studies using known ligands of the pregnane-X or the progesterone receptors may provide insight into the role of these receptors, and roles of neurosteroids, in successful treatment of neurodegeneration in NP-C. Additional in vivo and in vitro studies are needed to clarify the exact mechanism of action of allopregnanolone.
Our studies have demonstrated that NP-C represents a prototype of disordered neurosteroidogenesis. Other neurodegenerative diseases may also involved similar reductions in neurosteroid synthesis, either as primary effects, or due to specific loss of neurons that express neurosteroidogenic enzymes. Since many neurodegenerative diseases share common neuropathology, it may be likely that these diseases, like NP-C, may benefit from allopregnanolone treatment. Ultimately, understanding the mechanism of allopregnanolone action will clarify our understanding of the cellular processes that result in neurodegeneration.
Acknowledgements
This work was supported by grants NIH NS 049462 NSF 0090995, March of Dimes FY06-340/FY079-554, grants from The National Niemann Pick Disease Foundation, The Ara Parseghian Medical Research Foundation and The Lysosomal Storage Disease Research Consortium to SHM, and a fellowship from NIH Training Grant DK07161 to MDS. We thank Dr. Kelvin Gee, University of California, Irvine, for the ganaxolone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad I, Lope-Piedrafita S, Bi X, Hicks C, Yao Y, Yu C, Chaitkin E, Howison CM, Weberg L, Trouard TP, Erickson RP. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J Neurosci Res. 2005;82:811–821. doi: 10.1002/jnr.20685. [DOI] [PubMed] [Google Scholar]
- Alakoskela JM, Covey DF, Kinnunen PK. Lack of enantiomeric specificity in the effects of anesthetic steroids on lipid bilayers. Biochim Biophys Acta. 2007;1768:131–145. doi: 10.1016/j.bbamem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Anzil AP, Blinzinger K, Mehraein P, Dozic S. Niemann-Pick disease type C: case report with ultrastructural findings. Neuropadiatrie. 1973;4:207–225. doi: 10.1055/s-0028-1091741. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Andersson A, Andree L, Birzniece V, Bixo M, Bjorn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Stromberg J, Sundstrom-Poromaa I, Turkmen S, Wahlstrom G, Wang M, Wihlback AC, Zhu D, Zingmark E. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Bair SR, Mellon SH. Deletion of the mouse P450c17 gene causes early embryonic lethality. Mol Cell Biol. 2004;24:5383–5390. doi: 10.1128/MCB.24.12.5383-5390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML. Neurosteroidogenesis: relevance to neurosteroid actions in brain and modulation by psychotropic drugs. Crit Rev Neurobiol. 2004;16:67–74. doi: 10.1615/critrevneurobiol.v16.i12.70. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bauer P, Knoblich R, Bauer C, Finckh U, Hufen A, Kropp J, Braun S, Kustermann-Kuhn B, Schmidt D, Harzer K, Rolfs A. NPC1: Complete genomic sequence, mutation analysis, and characterization of haplotypes. Hum Mutat. 2002;19:30–38. doi: 10.1002/humu.10016. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P, Schumacher M, editors. Neurosteroids: A New Regulatory Function in the Nervous System. Human Press, Inc.; Totowa, NJ: 1999. [Google Scholar]
- Beekman M, Ungard JT, Gasior M, Carter RB, Dijkstra D, Goldberg SR, Witkin JM. Reversal of behavioral effects of pentylenetetrazol by the neuroactive steroid ganaxolone. J Pharmacol Exp Ther. 1998;284:868–877. [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Pluchino N, Begliuomini S, Lenzi E, Palumbo M, Luisi M, Genazzani AR. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol. 2004;19:344–353. doi: 10.1080/09513590400018223. [DOI] [PubMed] [Google Scholar]
- Bicikova M, Dibbelt L, Hill M, Hampl R, Starka L. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30:227–230. doi: 10.1055/s-2007-978871. [DOI] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Goebel HH. Isocortical pathology in type C Niemann-Pick disease. A combined Golgi-pigmentoarchitectonic study. J Neuropathol Exp Neurol. 1983;42:671–687. doi: 10.1097/00005072-198311000-00007. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer's disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3:185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li PK, Akwa Y, Savage DD, Engen JR, Valenzuela CF. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem. 2004;90:1530–1539. doi: 10.1111/j.1471-4159.2004.02686.x. [DOI] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Tagle DA, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Chavez-Delgado ME, Gomez-Pinedo U, Feria-Velasco A, Huerta-Viera M, Castaneda SC, Toral FA, Parducz A, Anda SL, Mora-Galindo J, Garcia-Estrada J. Ultrastructural analysis of guided nerve regeneration using progesterone- and pregnenolone-loaded chitosan prostheses. J Biomed Mater Res B Appl Biomater. 2005;74:589–600. doi: 10.1002/jbm.b.30243. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 2000;21:1–58. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS. Enantioselectivity of pregnanolone-induced gamma-aminobutyric acid(A) receptor modulation and anesthesia. J Pharmacol Exp Ther. 2000;293:1009–1016. [PubMed] [Google Scholar]
- Crocker AC. The cerebral defect in Tay-Sachs disease and Niemann-Pick disease. J Neurochem. 1961;7:69. doi: 10.1111/j.1471-4159.1961.tb13499.x. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Pettus EH, Hoffman SW, Stein DG. Tapered progesterone withdrawal enhances behavioral and molecular recovery after traumatic brain injury. Exp Neurol. 2005;195:423–429. doi: 10.1016/j.expneurol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290:2295–2298. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- di Michele F, Lekieffre D, Pasini A, Bernardi G, Benavides J, Romeo E. Increased neurosteroids synthesis after brain and spinal cord injury in rats. Neurosci Lett. 2000;284:65–68. doi: 10.1016/s0304-3940(00)00965-4. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Fink JK, Filling-Katz MR, Sokol J, Cogan DG, Pikus A, Sonies B, Soong B, Pentchev PG, Comly ME, Brady RO, et al. Clinical spectrum of Niemann-Pick disease type C. Neurology. 1989;39:1040–1049. doi: 10.1212/wnl.39.8.1040. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Long S, Tanchuck M, Phillips TJ. Neurosteroid consumption has anxiolytic effects in mice. Pharmacol Biochem Behav. 2003;76:451–462. doi: 10.1016/j.pbb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75:391–400. doi: 10.1002/jnr.10821. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci U S A. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABA(A) receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500:413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Friedman L, Gibbs TT, Farb DH. Gamma-aminobutyric acidA receptor regulation: chronic treatment with pregnanolone uncouples allosteric interactions between steroid and benzodiazepine recognition sites. Mol Pharmacol. 1993;44:191–197. [PubMed] [Google Scholar]
- Frolov A, Zielinski SE, Crowley JR, Dudley-Rucker N, Schaffer JE, Ory DS. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J Biol Chem. 2003;278:25517–25525. doi: 10.1074/jbc.M302588200. [DOI] [PubMed] [Google Scholar]
- Gago N, Akwa Y, Sananes N, Guennoun R, Baulieu EE, El-Etr M, Schumacher M. Progesterone and the oligodendroglial lineage: stage-dependent biosynthesis and metabolism. Glia. 2001;36:295–308. doi: 10.1002/glia.1117. [DOI] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–1196. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Gee KW. Epalons as anticonvulsants: actions mediated by the GABAA receptor complex. Proc West Pharmacol Soc. 1996;39:55. [PubMed] [Google Scholar]
- Gevry NY, Lopes FL, Ledoux S, Murphy BD. Aberrant intracellular cholesterol transport disrupts pituitary and ovarian function. Mol Endocrinol. 2004;18:1778–1786. doi: 10.1210/me.2003-0323. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135:47–58. doi: 10.1016/j.neuroscience.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O'Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Gilbert EF, Callahan J, Viseskul C, Opitz JM. Niemann-Pick disease type C. Pathological, histochemical, ultrastructural and biochemical studies. Eur J Pediatr. 1981;136:263–274. doi: 10.1007/BF00442993. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Grobin AC, VanDoren MJ, Porrino LJ, Morrow AL. Cortical 3 alpha-hydroxy-5 alpha-pregnan-20-one levels after acute administration of Delta 9-tetrahydrocannabinol, cocaine and morphine. Psychopharmacology (Berl) 2005;179:544–550. doi: 10.1007/s00213-004-2084-3. [DOI] [PubMed] [Google Scholar]
- Guarneri P, Cascio C, Russo D, D'Agostino S, Drago G, Galizzi G, De Leo G, Piccoli F, Guarneri M, Guarneri R. Neurosteroids in the retina: neurodegenerative and neuroprotective agents in retinal degeneration. Ann N Y Acad Sci. 2003;1007:117–128. doi: 10.1196/annals.1286.012. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of GABA receptor complex by a steroid anesthetic. Brain Res. 1984;323:284–293. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Murayama S, Pentchev PG, Suzuki K. Cerebellar degeneration in the Niemann-Pick type C mouse. Acta Neuropathol (Berl) 1993;85:175–184. doi: 10.1007/BF00227765. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Murayama S, Pentchev PG, Suzuki K. Peripheral nerve pathology in Niemann-Pick type C mouse. Acta Neuropathol (Berl) 1995;90:158–163. doi: 10.1007/BF00294315. [DOI] [PubMed] [Google Scholar]
- Hoffman SW, Virmani S, Simkins RM, Stein DG. The delayed administration of dehydroepiandrosterone sulfate improves recovery of function after traumatic brain injury in rats. J Neurotrauma. 2003;20:859–870. doi: 10.1089/089771503322385791. [DOI] [PubMed] [Google Scholar]
- Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol. 2002;16:1943–1950. doi: 10.1210/me.2002-0055. [DOI] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Keller EA, Zamparini A, Borodinsky LN, Gravielle MC, Fiszman ML. Role of allopregnanolone on cerebellar granule cells neurogenesis. Brain Res Dev Brain Res. 2004;153:13–17. doi: 10.1016/j.devbrainres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Kelly DA, Portmann B, Mowat AP, Sherlock S, Lake BD. Niemann-Pick disease type C: diagnosis and outcome in children, with particular reference to liver disease. J Pediatr. 1993;123:242–247. doi: 10.1016/s0022-3476(05)81695-6. [DOI] [PubMed] [Google Scholar]
- Kerrigan JF, Shields WD, Nelson TY, Bluestone DL, Dodson WE, Bourgeois BF, Pellock JM, Morton LD, Monaghan EP. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res. 2000;42:133–139. doi: 10.1016/s0920-1211(00)00170-4. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Pianos A, Liere P, Eychenne B, Gonzalez S, Cambourg A, De Nicola AF, Schumacher M, Guennoun R. Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology. 2006;147:1847–1859. doi: 10.1210/en.2005-0955. [DOI] [PubMed] [Google Scholar]
- Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol. 2004;199:251–265. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABA(A) receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. Pregnane X receptor (PXR) activation: A mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci U S A. 2006;103:13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA. The neuroactive steroid 3-alpha-ol-5-beta-pregnan-20-one hemisuccinate, a selective NMDA receptor antagonist improves behavioral performance following spinal cord ischemia. Brain Res. 2004;997:152–158. doi: 10.1016/j.brainres.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP. Assessment of ganaxolone's anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia. 2000;41:1187–1194. doi: 10.1111/j.1528-1157.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Le Goascogne C, Eychenne B, Tonon MC, Lachapelle F, Baumann N, Robel P. Neurosteroid progesterone is up-regulated in the brain of jimpy and shiverer mice. Glia. 2000;29:14–24. doi: 10.1002/(sici)1098-1136(20000101)29:1<14::aid-glia2>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- Mahendroo M, Wilson JD, Richardson JA, Auchus RJ. Steroid 5alpha-reductase 1 promotes 5alpha-androstane-3alpha,17beta-diol synthesis in immature mouse testes by two pathways. Mol Cell Endocrinol. 2004;222:113–120. doi: 10.1016/j.mce.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Hess DL, Russell DW. Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes. Endocrinology. 2001;142:4652–4662. doi: 10.1210/endo.142.11.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Landrum DP, Russell DW. Fetal death in mice lacking 5alpha-reductase type 1 caused by estrogen excess. Mol Endocrinol. 1997;11:917–927. doi: 10.1210/mend.11.7.9933. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mathis C, Paul SM, Crawley JN. The neurosteroid pregnenolone sulfate blocks NMDA antagonist-induced deficits in a passive avoidance memory task. Psychopharmacology (Berl) 1994;116:201–206. doi: 10.1007/BF02245063. [DOI] [PubMed] [Google Scholar]
- Mayo W, Dellu F, Robel P, Cherkaoui J, Le Moal M, Baulieu EE, Simon H. Infusion of neurosteroids into the nucleus basalis magnocellularis affects cognitive processes in the rat. Brain Res. 1993;607:324–328. doi: 10.1016/0006-8993(93)91524-v. [DOI] [PubMed] [Google Scholar]
- Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, Stein DG, Guennoun R. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148:2505–2517. doi: 10.1210/en.2006-1678. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Anatomical and biochemical evidence for the synthesis of unconjugated and sulfated neurosteroids in amphibians. Brain Res Brain Res Rev. 2001;37:13–24. doi: 10.1016/s0165-0173(01)00110-2. [DOI] [PubMed] [Google Scholar]
- Millat G, Chikh K, Naureckiene S, Sleat DE, Fensom AH, Higaki K, Elleder M, Lobel P, Vanier MT. Niemann-Pick disease type C: spectrum of HE1 mutations and genotype/phenotype correlations in the NPC2 group. Am J Hum Genet. 2001;69:1013–1021. doi: 10.1086/324068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W, Tyrell J. The Adrenal Cortex. In: Felig P, Baxter J, Frohman L, editors. Endocrinology & Metabolism. McGraw Hill; New York: 1994. pp. 555–711. [Google Scholar]
- Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia. 1997;38:1026–1031. doi: 10.1111/j.1528-1157.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Morris MD, Bhuvaneswaran C, Shio H, Fowler S. Lysosome lipid storage disorder in NCTR-BALB/c mice. I. Description of the disease and genetics. Am J Pathol. 1982;108:140–149. [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Fleming R, Penland S. Ethanol and neurosteroid interactions in the brain. Int Rev Neurobiol. 2001;46:349–377. doi: 10.1016/s0074-7742(01)46068-5. [DOI] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Norman RM, Forrester RM, Tingey AH. The juvenile form of Niemann-Pick disease. Arch Dis Child. 1967;42:91–96. doi: 10.1136/adc.42.221.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirathna S, Brimelow BC, Jagodic MM, Krishnan K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5alpha-reduced neuroactive steroids. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Patte-Mensah C, Penning TM, Mensah-Nyagan AG. Anatomical and cellular localization of neuroactive 5 alpha/3 alpha-reduced steroid-synthesizing enzymes in the spinal cord. J Comp Neurol. 2004;477:286–299. doi: 10.1002/cne.20251. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Vanier MT, Suzuki K, Morris JA, Carstea E, Neufeld EB, Blanchette-Mackie JE, Pentchev PG. Niemann-Pick Disease Type C: A Lipid Trafficking Disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. III. McGraw-Hill; New York: 2001. pp. 3611–3633. [Google Scholar]
- Pentchev PG, Vanier MT, Suzuki K, Patterson MC. Niemann-Pick Disease Type C: A cellular cholesterol lipidosis. In: Scriver CR, Beaudet AL, Sly WS, Valle DD, editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw Hill; New York: 1995. pp. 2625–2639. [Google Scholar]
- Pomata PE, Colman-Lerner AA, Baranao JL, Fiszman ML. In vivo evidences of early neurosteroid synthesis in the developing rat central nervous system and placenta. Brain Res Dev Brain Res. 2000;120:83–86. doi: 10.1016/s0165-3806(99)00181-9. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The clinical potentials of endogenous neurosteroids. Drugs Today (Barc) 2002;38:465–485. doi: 10.1358/dot.2002.38.7.820115. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy Res. 2004;62:99–118. doi: 10.1016/j.eplepsyres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000;294:909–915. [PubMed] [Google Scholar]
- Robel P, Young J, Corpechot C, Mayo W, Perche F, Haug M, Simon H, Baulieu EE. Biosynthesis and assay of neurosteroids in rats and mice: functional correlates. J Steroid Biochem Mol Biol. 1995;53:355–360. doi: 10.1016/0960-0760(95)00074-a. [DOI] [PubMed] [Google Scholar]
- Robichaud M, Debonnel G. Allopregnanolone and ganaxolone increase the firing activity of dorsal raphe nucleus serotonergic neurons in female rats. Int J Neuropsychopharmacol. 2005:1–10. doi: 10.1017/S146114570500595X. [DOI] [PubMed] [Google Scholar]
- Roff CF, Strauss J.F.r., Goldin E, Jaffe H, Patterson MC, Agritellis GC, Hibbs AM, Garfield M, Brady RO, Pentchev PG. The murine Niemann-Pick type C lesion affects testosterone production. Endocrinology. 1993;133:2913–2923. doi: 10.1210/endo.133.6.8243319. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Reddy DS. Neurosteroids and infantile spasms: the deoxycorticosterone hypothesis. Int Rev Neurobiol. 2002;49:199–219. doi: 10.1016/s0074-7742(02)49014-9. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuropsychopharmacological properties of neuroactive steroids. Steroids. 1999;64:83–91. doi: 10.1016/s0039-128x(98)00101-9. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH, Jr., Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Akwa Y, Guennoun R, Robert F, Labombarda F, Desarnaud F, Robel P, De Nicola AF, Baulieu EE. Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J Neurocytol. 2000;29:307–326. doi: 10.1023/a:1007152904926. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res. 2004;14(Suppl A):S18–33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABA(A) receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu F-C, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Spence MW, Callahan JW. Sphingomyelin-cholesterol lipidoses: the Niemann-Pick group of diseases. In: Scriver CR, Beaudet AL, Sly VS, Valle D, editors. The Metabolic Basis of Inherited Disease. McGraw Hill; New York: 1989. pp. 1655–1676. [Google Scholar]
- Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Parker CC, Pentchev PG, Katz D, Ghetti B, D'Agostino AN, Carstea ED. Neurofibrillary tangles in Niemann-Pick disease type C. Acta Neuropathol (Berl) 1995;89:227–238. doi: 10.1007/BF00309338. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci U S A. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timby E, Balgard M, Nyberg S, Spigset O, Andersson A, Porankiewicz-Asplund J, Purdy RH, Zhu D, Backstrom T, Poromaa IS. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology (Berl) 2006;186:414–424. doi: 10.1007/s00213-005-0148-7. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, Nilsson KR, Zorumski CF, Covey DF, Jevtovic-Todorovic V. 5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Mellon SH. Neurosteorids in the Brain Neuron: Biosynthesis, Action and Medicinal IMpact on Neurodegenerative Disease. Central Nervous System Agents in Medicinal Chemistry. 2006;6:73–82. [Google Scholar]
- Tsutsui K, Sakamoto H, Shikimi H, Ukena K. Organizing actions of neurosteroids in the Purkinje neuron. Neurosci Res. 2004;49:273–279. doi: 10.1016/j.neures.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ungard JT, Beekman M, Gasior M, Carter RB, Dijkstra D, Witkin JM. Modification of behavioral effects of drugs in mice by neuroactive steroids. Psychopharmacology (Berl) 2000;148:336–343. doi: 10.1007/s002130050060. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2005:1–11. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Koob GF, Le Moal M. Neurosteroids in learning and memory processes. Int Rev Neurobiol. 2001;46:273–320. doi: 10.1016/s0074-7742(01)46066-1. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier MT, Duthel S, Rodriguez-Lafrasse C, Pentchev P, Carstea ED. Genetic heterogeneity in Niemann-Pick C disease: a study using somatic cell hybridization and linkage analysis. Am J Hum Genet. 1996;58:118–125. [PMC free article] [PubMed] [Google Scholar]
- Vanier MT, Pentchev P, Rodriguez-Lafrasse C, Rousson R. Niemann-Pick disease type C: an update. J Inherit Metab Dis. 1991;14:580–595. doi: 10.1007/BF01797928. [DOI] [PubMed] [Google Scholar]
- Vanier MT, Wenger DA, Comly ME, Rousson R, Brady RO, Pentchev PG. Niemann-Pick disease group C: clinical variability and diagnosis based on defective cholesterol esterification. A collaborative study on 70 patients. Clin Genet. 1988;33:331–348. doi: 10.1111/j.1399-0004.1988.tb03460.x. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, Hammes SR, Jamnongjit M, Stein DG. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–2333. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Abramovici A, Sandbank U, Booth AD, Pentchev PG, Sela B. Dysmyelination in NCTR-Balb/C mouse mutant with a lysosomal storage disorder. Morphological survey. Acta Neuropathol (Berl) 1987;74:374–381. doi: 10.1007/BF00687215. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Abramovici A, Sandbank U, Pentchev PG, Brady RO, Sekine M, Suzuki A, Sela B. Neurological mutation characterized by dysmyelination in NCTR-Balb/C mouse with lysosomal lipid storage disease. J Neurochem. 1985;45:665–672. doi: 10.1111/j.1471-4159.1985.tb04044.x. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Barth G, Githens JH. Nine cases of sphingomyelin lipidosis, a new variant in Spanish-American Children. Juvenile variant of Niemann-Pick Disease with foamy and sea-blue histiocytes. Am J Dis Child. 1977;131:955–961. doi: 10.1001/archpedi.1977.02120220021002. [DOI] [PubMed] [Google Scholar]
- Wu HS, Lin HT, Wang CK, Chiang YF, Chu HP, Hu MC. Human CYP11A1 promoter drives Cre recombinase expression in the brain in addition to adrenals and gonads. Genesis. 2007;45:59–65. doi: 10.1002/dvg.20266. [DOI] [PubMed] [Google Scholar]
- Xie C, Burns DK, Turley SD, Dietschy JM. Cholesterol is sequestered in the brains of mice with Niemann-Pick type C disease but turnover is increased. J Neuropathol Exp Neurol. 2000;59:1106–1117. doi: 10.1093/jnen/59.12.1106. [DOI] [PubMed] [Google Scholar]
- Zervas M, Dobrenis K, Walkley SU. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J Neuropathol Exp Neurol. 2001;60:49–64. doi: 10.1093/jnen/60.1.49. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dudley-Rucker N, Crowley JR, Lopez-Perez E, Issandou M, Schaffer JE, Ory DS. The steroidal analog GW707 activates the SREBP pathway through disruption of intracellular cholesterol trafficking. J Lipid Res. 2004;45:223–231. doi: 10.1194/jlr.M300409-JLR200. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Wang JF, Krueger KE, Vicini S. Delta subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]