Abstract
Objective
IL-17 producing helper T cells have been proposed to represent a separate lineage of CD4+ cells, designated Th17 cells, which are regulated by the transcription factor RORγt. However, despite advances in understanding murine Th17 differentiation, a systematic assessment of factors that promote the differentiation of naïve human T cells to Th17 cells has not been reported. This present study was undertaken to assess the effects of cytokines known to promote murine Th17 cells on naïve human CD4+ T cells.
Methods
Human naïve and memory CD4+ T cells isolated from peripheral blood were activated and cultured with various cytokines. Cytokine production was measured by ELISA and flow cytometry. mRNA was measured by quantitative PCR.
Results
In response to CD3/CD28 stimulation alone, human memory T cells rapidly produced IL-17, whereas naïve T cells expressed low levels. TGF-β1 and IL-6 upregulated RORγt expression but did not induce Th17 differentiation of naïve CD4+ T cells. However, IL-23 upregulated its own receptor and was an important inducer of IL-17 and IL-22.
Conclusion
The present data demonstrate the differential regulation of IL-17 and RORγt expression in human CD4+ T cells compared to murine cells. Optimal conditions for the development of IL-17-producing T cells from murine naïve precursors are ineffective in human T cells. Conversely, IL-23 promoted generation of human Th17 cells but was also a very potent inducer of other proinflammatory cytokines. These findings may have important implications in the pathogenesis of human autoimmunity compared to mouse models.
INTRODUCTION
Classically, naïve CD4+ have been thought to differentiate into two possible helper lineages, T helper (Th)1 or Th2 cells. Th1 cells produce the signature cytokine, IFN-γ, a critical factor that promotes cellular immunity. Interleukin-12 acting via the transcription factor STAT4, in concert with T-BET is critical for Th1 differentiation. In contrast, Th2 development is initiated by IL-4 signaling with the participation of the transcription factors STAT6 and GATA3. The hallmark cytokine secreted by Th2 cells is IL-4, which is crucial for host defense against helminths and the pathogenesis of asthma and allergy. Th1 and Th2 lineage decision appears to be made at a very early stage of T helper differentiation with respective Th1/Th2 cytokines enforcing their own expression and inhibiting alternative commitment. This occurs by regulation of receptor levels, expression of transcription factors and epigenetic changes (1–3). Another important aspect of Th1/Th2 counterregulation is interchromasomal interactions between Th1- or Th2-specific cytokine genes (4). As a result of these mechanisms, Th1 and Th2 cells develop into mature effectors with stable phenotypes and important roles in host defense, as documented in a number of murine models.
While the simple dichotomous model of helper T cell differentiation fits well with many infection models, fitting autoimmune disease into such models has been problematic. CD4+ T cells can also differentiate to become regulatory T cells (Treg cells) and it is clear that dysregulation of this subset has major consequences with respect to the pathogenesis of autoimmunity (5). An additional complication relates to the discovery of another cytokine, IL-23. IL-23 is a dimer that shares a subunit with IL-12, IL-12p40 and both utilize a receptor subunit designated IL-12Rβ1 (6). The complex biology of IL-12 and IL-23 is relevant to the pathogenesis of autoimmunity in that gene targeting of IL-12p40 attenuates the development of disease in many models of autoimmunity. Similarly, anti-p40 antibody is efficacious in the treatment of Crohn’s disease (7). Although these effects were initially misattributed to interference with IL-12 actions, using specific deletion of IL-23 (p19−/− mice), it is now recognized that IL-23 and not IL-12 is the culprit, at least in many of the animal models (8–13).
One way that IL-23 is thought to promote autoimmune disease is through the regulation of IL-17A and IL-17F. IL-17A is a proinflammatory cytokine initially identified in mouse cytotoxic T cells more than 10 years ago. This family now includes 6 family members: IL-17 (IL-17A), IL-17B, IL-17C, IL-17D, IL-17E and IL-17F (14-17) that share 16–50% amino acid identity and have different tissue expression patterns. IL-17 acts on epithelial cells, endothelial cells, fibroblasts, synoviocytes and myeloid cells to induce secretion of a variety of mediators including IL-8, CXC ligand (CXCL) 1, CXCL6, IL-6, granulocyte macrophage colony-stimulating factor, granulocyte colony-stimulating factor, TNF-α and IL-1β. IL-17 family cytokines thus induce cellular infiltration and production of inflammatory cytokines. Dysregulated production of IL-17 is associated with human autoimmune diseases including multiple sclerosis, inflammatory bowel disease, and psoriasis (13, 18–20). Importantly, studies in a murine experimental arthritis model showed that IL-17 was involved in both the initiation and progression of the disease. Furthermore, elevated levels of IL-17 were detected in synovial fluid from patients with rheumatoid arthritis, and osteoclast formation was inhibited by anti-IL-17 antibody, suggesting an effect on bone resorption (21–25).
IL-17A was originally reported to be produced by activated CD4+ and CD8+ T cells. More recently, it has been proposed that IL-17-producing CD4+ T cells represent a distinct lineage (Th17), a lineage that does not produce IL-4 or IFN-γ (26–28). In fact, these products of Th1 and Th2 cells antagonize the differentiation of Th17 cells. Additionally, cytokines that promote Th17 differentiation are distinct from those that promote Th1 and Th2 differentiation. The current model is that whereas TGF-β1 promotes Treg cell differentiation, the combination of TGF-β1 and IL-6 promotes Th17 lineage commitment (29–31). This subset is expanded and maintained by IL-23. Both IL-6 and IL-23 activate the transcription factor Stat3, which directly binds to the IL-17 promoter to regulate IL-17 expression (32). Conversely, SOCS3 negatively regulates Th17 differentiation by inhibiting Stat3 phosphorylation (32, 33). In addition to Stat3, RORγt is an important transcription factor for initiation of Th17 differentiation (34).
The current models of Th17 differentiation are all derived from studies using murine cells. Given the pathogenic relevance of IL-17 family members, it is obviously important to understand how this family of cytokines is controlled in human T cells and to define the conditions under which human naïve CD4+ T cells might become Th17 cells. Previously, it has been reported that T cell receptor (TCR) crosslinking leads to IL-17 production (35). It has also been reported that IL-23 induces IL-17 expression (26). However, these studies did not separate effects on memory versus naïve cells. The ability of naïve human CD4+ T cells to differentiate into IL-17 producers has not been examined nor has a comprehensive assessment been undertaken to define the relative importance of various conditions in promoting the differentiation of this putative human lineage. Additionally, the extent to which human T cells subsets fully commit to, or extinguish IL-17 production has not been addressed. This is important not only with respect to the pathogenesis of human autoimmune disease but also because human T helper cell differentiation appears to exhibit much more plasticity than murine Th differentiation (36).
Here we show that human memory T cells upregulate RORγt and IL-17 in response to TCR occupancy. Importantly, in sharp contrast to murine naïve CD4+ T cells in which Th17 lineage commitment is initiated by TGF-β1 and IL-6, human Th17 differentiation does not occur in response to this cytokine combination, even though these cytokines promote RORγt expression. Rather, IL-23 upregulates its own receptor on T cells, increases RORγt and IL-17 expression. IL-23 also induces other proinflammatory cytokines including IL-22. These findings may have important implications in the pathogenesis of human autoimmunity compared to mouse models.
MATERIALS AND METHODS
Cell cultures
PBMC were isolated from peripheral blood of healthy donors (Department of Transfusion Medicine, the National Institutes of Health) by Ficoll-Paque PLUS (Amersham Pharmacia Biotech, Uppsala, Sweden). Naïve or memory CD4+ T cells were further purified using human naïve or memory CD4+ T cell isolation kit (Miltenyi Biotech, Bergisch-Gladbach, Germany). The purity of CD45RA+RO− cells was approximately 90%. Naïve CD4+ T cells were activated by plate-bound anti-CD3 and soluble anti-CD28 (BD Pharmingen, San Diego) and cultured under neutral (Th0), Th1 (IL-12, anti-IL-4), Th2 (IL-4, anti-IL-12, anti-IFN-γ) or Th17 (TGF-β1+ IL-6, anti-IFN-γ and anti-IL-4) polarizing conditions or stimulated with IL-23 (10 ng/ml, from R&D Systems, Minneapolis, MN). For 14 days of culture, on day 7, cells were restimulated with plate-bound anti-CD3 and anti-CD28 and continuously cultured until day 14 under Th0, Th1, Th2 or Th17 polarizing conditions.
Cytokine detection
Quantitation of cytokine production in cell culture supernatants was determined by ELISA (R & D Systems) according to the manufacturer’s instructions. Cytokine-producing cells were determined by intracellular staining using PE-anti-IFN-γ (BD Pharmingen), Alexa Fluor® 647 anti-human IL-17 (eBioscience, San Diego, CA) and Alexa Fluor® 488 anti-human FOXP3 (BioLegend, San Diego, CA). Briefly, cells were stimulated with PMA and ionomycin for four hours, and Golgiplug (BD Biosciences) was added after two hours. Cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% saponin, stained with fluorescent antibodies and analyzed on a FACS Calibur flow cytometer (BD Biosciences). CellQuest software (BD Biosciences) was used for data acquisition and Flow Jo software (Tree Star Inc, Ashland, OR) was used for analysis.
Real-Time quantitative PCR (Taqman) detection
Total RNA was isolated by RNeasy kit (Qiagen, Valencia, CA). cDNA was synthesized by TaqMan Reverse Transcription kit (Applied Biosystems, Foster City, CA) using random hexamers as primers according to the manufacture’s instruction. HPRT was used as an endogenous control. TaqMan primers and probes for human IL-17A (IL-17), IL-17B, IL-17C, IL-17D, IL-17E, IL-17F, IL-22, IFNγ, IL-4, T-bet, Gata3, IL-23R, RORγ and HPRT were purchased from Applied Biosystems, and samples were analyzed using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA).
Statistical analyses
Statistical analyses were performed using Small Stata 9.2 (Statacorp, College Station, TX). Geometric means were computed separately for each stimulation. The log difference (geometric mean) in IL-17 or IL-22 secretion between the two stimulations for each donor was used to conduct a paired t-test and compute a 95% confidence interval.
RESULTS
Human memory helper T cells produce high levels of IL-17
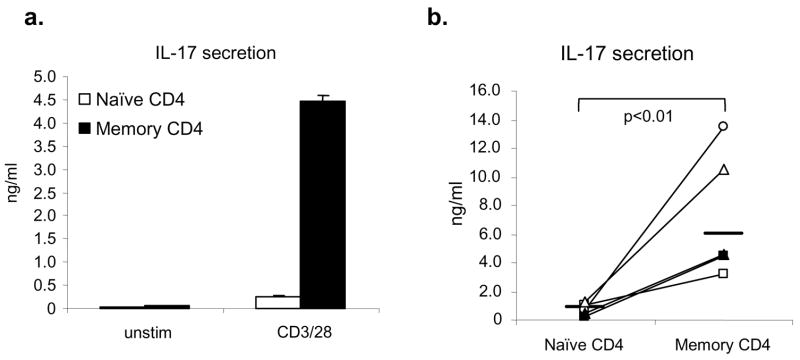
We first assessed whether IL-17 production is detected in normal human donors. To this end, we fractionated CD4+ cells into memory (CD45RO+) and naïve cells (CD45RA+). As shown in Figure 1a, freshly isolated memory CD4+ T cells secreted large quantities of IL-17 in response to anti-CD3 and anti-CD28. While there was considerable individual variation in the propensity to produce IL-17, memory CD4+ T cells made significantly more IL-17 than naïve CD4+ T cells (Fig 1b), although modest levels of IL-17 were clearly produced by naïve CD4+ T cells (approximately 15–20% of the levels produced by memory cells). In this setting, plate-bound antibodies and antibody-coated microbeads gave similar results (data not shown).
Figure 1. Human memory helper T cells produce significantly higher levels of IL-17.
(a,b). Isolated human naïve and memory CD4+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 for 24 hours. IL-17 production was detected by ELISA immunoassay kits. A representative result is shown in panel a and data from five healthy donors are shown in panel b, where the horizontal line indicates geometric mean. Statistical significance was calculated based on geometric mean using paired t-test.
Optimal conditions for murine Th17 cells do not generate human Th17 cells
Since IL-17-producing T cells were readily detectable in human memory CD4+ T cells, we next asked what conditions might favor the generation of such cells from naïve CD4+ T cells. Recent studies have reported that TGF-β1 and IL-6, combined with blockade of IL-4 and IFN-γ, quickly generates large numbers of IL-17-producing T cells from isolated naïve murine CD4+ T cells within a few days (supplemental figure 1) (29–31, 37). Therefore, we cultured naïve human CD4+ T cells under these conditions (denoted “Th17” conditions) to assess whether this regimen would generate human IL-17-producing T cells. Surprisingly, the combination of TGF-β1 and IL-6 with anti-IL-4 and anti-IFN-γ was no better than anti-CD3 and anti- CD28 alone in terms of IL-17 production (p =0.67, n=5) (Fig 2a). This was the case regardless of the concentration of anti-CD3 used, with or without anti-CD28 (supplementary figure 2). Kinetic analysis of IL-17 production during two-week culture further supported this contention (Fig 2b). The effect of TGF-β1 was evidenced by the upregulation of FOXP3, although surprisingly, the combination of TGF-β1 and IL-6 enhanced FOXP3 expression rather than inhibiting expression as is the case in murine cells (31) (Fig 2c). Some protocols for in vitro generation of murine Th17 cells employ mononuclear cells along with soluble anti-CD3/CD28 (29) and exogenous TGF-β1 and IL-6. However, this also failed to induce human IL-17-producing cells (data not shown); in fact activated monocyte-derived dendritic cells enhanced IFN-γ production and inhibited Th17 differentiation (data not shown).
Figure 2. Optimal conditions for murine Th17 differentiation do not enhance Th17 differentiation of human naïve CD4+ T cells.
a. Naïve CD4+ T cells from normal donors (n = 5) were activated with plate-bound anti-CD3 and anti-CD28 and cultured for two days with IL-2 (Th0) or optimal polarizing conditions for murine Th17 differentiation (TGF-β1+IL-6+anti-IFN-γ+anti-IL-4). IL-17 secretion in cell culture supernatants was detected by ELISA. Statistical significance was calculated using the paired Student’s t-test. b. Naïve CD4+ T cells were activated and cultured under Th0 or Th17 conditions for 7 days. At day 7, cells were activated with anti-CD3 and -CD28 and cultured under Th0 or Th17 conditions for another 7 days. IL-17 production in cell culture supernatants was measured by ELISA at various timepoints. c. Quantitation of IL-17- and FOXP3-producing cells after stimulation of naïve CD4+ T cells under Th0 or “Th17” conditions, as determined by intracellular cytokine staining and flow cytometry. d. Quantitation of IL-17- and IFN-γ-producing cells at 24 h and 7 days post-stimulation was determined by intracellular cytokine staining and flow cytometry.
In murine CD4+ T cells, the combination of TGF-β1 and IL-6 with anti-IL-4 and anti-IFN-γ not only efficiently generates IL-17-producing T cells, but also extinguishes IFN-γ production. This is the basis of the idea that Th17 cells are a distinct functional lineage of mouse CD4+ effector cells (30, 31). Culture of human naïve CD4+ T cells with or without exogenous TGF β1 and IL-6 generated similar numbers of IL-17 producing cells, and notably half of the IL-17-producing T cells produced both IFN-γ and IL-17 (Fig 2d). We therefore next polarized naïve human CD4+ T cells under Th1 (IL-12 and anti-IL-4) and Th2 (IL-4 and anti-IL-12 and anti-IFN-γ) polarizing conditions and assessed their ability to produce IL-17. Surprisingly though, IL-17 production was not extinguished under these conditions (supplemental figure 3). Despite the absence of IL-17 mRNA in cells polarized under Th2 conditions for 7 days, restimulation via TCR occupancy permitted considerable IL-17 production, indicating the plasticity of human CD4+ T cells with respect to IL-17 production (Fig 3a). IFN-γ and IL-17 dual producers were also observed in freshly isolated human memory CD4+ T cells activated with anti-CD3/CD28, indicating the incomplete differentiation of human Th17 cells was not simply an in vitro phenomenon (Fig 3b).
Figure 3. Plasticity of human IL-17 producing T cells.
a. Isolated naïve CD4+ T cells were cultured under Th0, Th1, Th2 and Th17 conditions for 7 days, and IL-17 protein production was measured by ELISA directly or after restimulated with anti-CD3 and anti-CD28 for an additional 24 h. b. Isolated memory CD4+ T cells from two different donors were activated by plate-bound anti-CD3 and anti-CD28 for indicated time. IFN-γ and IL-17 producing cells were analyzed by intracellular cytokine staining using flow cytometry.
IL-23 upregulates its receptor and enhances IL-17 production by human CD4+ T cells
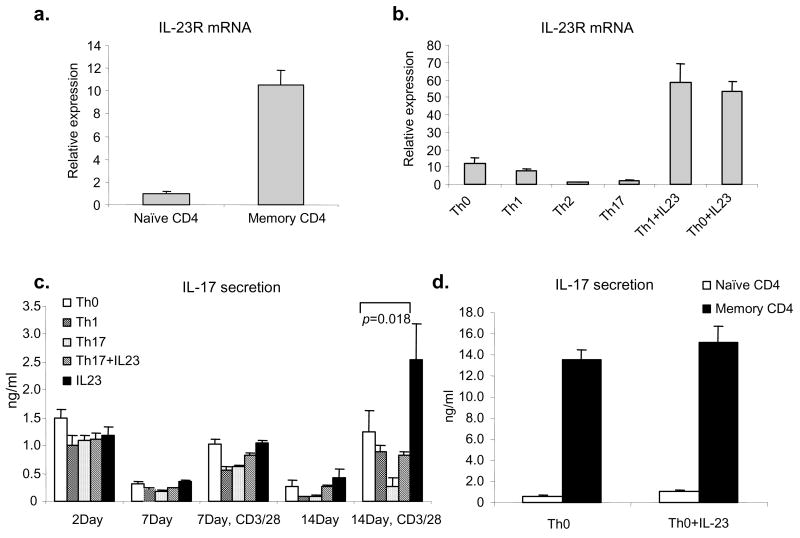
IL-23 is a heterodimeric cytokine that shares a ligand subunit (p40) and a receptor subunit (IL-12Rβ1) with IL-12 (38). IL-23 has been reported to be essential for the development of autoimmunity in mouse models and current data suggest that this is due to production of IL-17 (6, 8–10, 26, 39). We found that naïve CD4+ T cells expressed low levels of IL-23R mRNA compared to memory CD4+ T cells (Fig 4a). Under Th0, Th1, and Th2 the expression of this receptor remained low; however, IL-23R expression was strongly upregulated in CD3/CD28 activated T cells cultured with IL-23 (Fig 4b). Furthermore, IL-23R was inhibited by “Th17” conditions.
Figure 4. IL-23 upregulates its receptor and enhances IL-17 production in human CD4+ T cells.
(a). IL-23R mRNA expression in isolated naïve or memory CD4+ T cells was analyzed by real-time quantitative PCR. (b). Naïve CD4+ T cells were activated with plate-bound anti-CD3 and anti-CD28 and cultured under Th0, Th1, Th2, Th17 polarizing conditions or with IL-23 (10 ng/ml), anti-IFN-γ (10 μg/ml) and anti-IL-4 (10 μg/ml) for 2 days. IL-23R mRNA expression was detected by real time quantitative PCR. (c). Naïve CD4+ T cells were cultured under Th0 or Th17 conditions in the presence or absence of IL-23 and anti-IFN-γ and anti-IL-4 or Th1 conditions for 14 days. At day 14, cells were restimulated with anti-CD3/CD28 for 24 hours. IL-17 in cell culture supernatants was detected by ELISA. The figure depicts the mean of data from three separate donors analyzed in duplicate. Statistical significance was determined using the paired Student’s t-test, n=6. (d). Naïve or memory CD4+ T cells were activated by plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-23 for 24h. IL-17 was measured by ELISA.
In mice, IL-23 was initially thought to promote Th17 polarization, but was later viewed as a regulator of Th17 expansion/maintenance (29). We therefore next studied the effect of inclusion of IL-23 in cultures on the polarization of naïve human precursors compared to other conditions. As shown in figure 4c, anti-CD3/anti-CD28 induced IL-17 and IL-23 alone had little additional effect at early time points. Addition of IL-23 in “Th17” polarizing conditions was also ineffectual. However, after two weeks culture, cells cultured with IL-23 and restimulated with anti- CD3/CD28 produced significantly higher amounts of IL-17 compared to cells cultured under other conditions. Cells cultured under Th1 and Th17 conditions had poor IL-17 production and addition of IL-23 did not rescue cells cultured under Th17 conditions. IL-23 had relatively little effect on memory (CD45RO+CD4+) T cells to produce IL-17 (Fig 4d); this contrasts greatly with its effect on IL-22 (see below). Again, IFN-γ and IL-17 dual producers were observed under all these conditions (data not shown).
Regulation of RORγt expression in human T cells
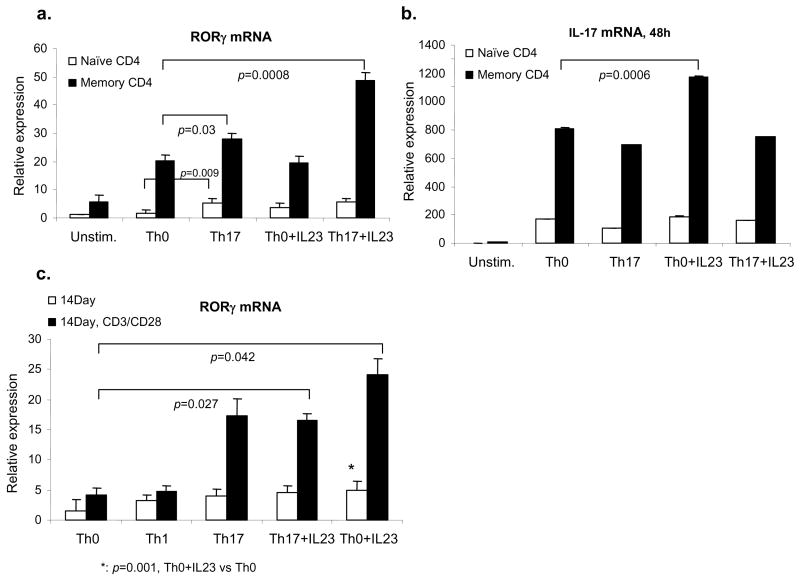
Recently RORγt has been reported as the key transcription factor regulating murine IL-17 expression (40). We therefore next investigated RORγt regulation in human CD4+ T cells. We found that RORγt mRNA levels were higher in freshly isolated memory CD4+ T cells than in naïve CD4+ T cells, but were even more highly inducible in memory cells in response to anti-CD3/CD28 (Fig 5a). Moreover, RORγt levels were slightly increased by addition of TGF-β1 and IL-6, and even greater by TGF-β1, IL-6 and IL-23 (Fig 5a), despite the fact that IL-17 levels were not affected (Fig 5b). The effects of cytokines on RORγt expression were also evident when starting with naïve CD4+ T cells, but were more pronounced at two weeks of culture, especially after CD3/CD28 restimulation (Fig 5c).
Figure 5. Regulation of RORγt in human CD4+ T cells.
Naïve and memory CD4+ T cells were cultured under Th0 or Th17 conditions with or without IL-23 for 48 h. RORγt (a) and IL-17 (b) mRNA were detected with real-time quantitative PCR. (c). Naïve CD4+ T cells were cultured under Th0, Th1, or Th17 conditions or with IL-23 and anti-IFN-γ and anti-IL-4 for 14 days as indicated. At day 14, cells were restimulated with anti-CD3 and anti-CD28 for 24 h. RORγt mRNA expression was analyzed by quantitative PCR. The figure depicts data from three separate donors assayed in duplicate. Statistical significance was calculated based on paired Student’s t-test, n=6.
Distinct regulation of IL-17 and IL-22
IL-22 is a member of IL-10 family, located on chromosome 12q15 between IFN-γ and IL-26 loci (41, 42). Recently, IL-22 has been reported to be produced by murine Th17 cells in vitro and in vivo (12, 43). We sought to determine whether the expression of IL-22 in human CD4+ T cells was regulated in the same manner as IL-17A and IL-17F. Again, we found that freshly isolated naïve CD4+ T cells did not express detectable levels of IL-22 mRNA. After TCR stimulation, IL-22 production was significantly upregulated and the induction was much greater in memory versus naïve CD4+ T cells (Fig 6a). Interestingly, IL-23 greatly enhanced IL-22 production especially in memory CD4+ T cells, contrasting sharply with its minimal effects on IL-17 production (Fig 4d). Addition of IL-23 to cultures of naïve CD4+ T cells activated by anti-CD3 and anti-CD28 also resulted in enhanced IL-22 production (Fig 6c,d). In contrast, “Th17” conditions inhibited IL-22 production (Fig 6b). Conversely though, previous studies have reported that IL-22 is produced by Th1 cells (44), and we confirmed the effects of addition of exogenous IL-12 in the experiment shown in figure 6c. Compared to anti-CD3/CD28 alone, IL-12 enhanced IL-22 production, whereas optimal “Th17” conditions attenuated IL-22 production (Fig 6b,c). However, IL-23 was clearly a more potent inducer of IL-22 (Fig 6c).
Figure 6. IL-23 enhances IL-22 production.
(a). Human naïve or memory CD4+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-23 for 24 hours. IL-22 protein in cell culture supernatants was detected by ELISA. The data represent the mean of four independent experiments. Statistical significance was calculated based on paired Student’s t-test. (b). Naïve CD4+ T cells were cultured under Th0 or Th17 conditions or with IL-23 for two days. IL-22 protein production in cell culture supernatants was measured by ELISA (n = 6) (c). Naïve CD4+ T cells were cultured under Th0, Th1, Th2 or Th17 polarizing conditions, or with IL-23 for 14 days and IL-22 mRNA was detected by Taqman at the indicated timepoints. (d). Naïve CD4+ T cells were activated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-23 and anti-IFN-γ and anti-IL-4 for two days before measuring IL-22 protein levels in culture supernatants by ELISA. The horizontal line indicates geometric mean of each stimulation. Statistical significance was calculated based on geometric means using the paired t-test.
DISCUSSION
Though the cytokine IL-17 was discovered more than 10 years ago, the existence of a new lineage of helper T cells, Th17 cells, which selectively produces IL-17 was only recently recognized (26–28). More recently, it has been argued that Th17 cells are also major producers of IL-22 (12, 43). The current view of mouse Th17 development is that TGF-β1 and IL-6 are the key cytokines that initiate the differentiation process and that IL-23 plays an essential role on the expansion and maintenance of this lineage. Support for the notion of an IL-23/IL-17 axis has been provided by a number of models of autoimmunity (6, 8–11, 26, 39, 45–47), but despite these compelling data in mice, a systematic analysis of human Th17 differentiation from naïve cells has not been previously undertaken.
In the present study, we show that regulation of IL-17 and IL-22 in human CD4+ T cells is surprisingly different from mouse T cells. In the mouse, TCR stimulation with a co-stimulatory signal alone is not sufficient to induce IL-17, especially in naïve CD4+ T cells. In contrast, in human CD4+ T cells, TCR stimulation of memory cells, and to a lesser extent naïve cells, is sufficient. Although IL-17-producing T cells are readily detectable among human memory CD4+ T cells, the cytokine cocktail (TGF-β1 and IL-6) that effectively promotes murine Th17 differentiation, was completely ineffective in driving Th17 differentiation of naïve human CD4+ T cells. Moreover, IL-23, a cytokine that does not effectively induce murine Th17 differentiation was effective in promoting IL-17 production in human T cells. One aspect of this regulation is the ability of IL-23 to induce its own receptor, which is analogous to other cytokines like IL-2, IL-4 and IL-12, which also regulate expression of their cognate receptors.
While IL-23 is an inducer of IL-17, IL-23 activates both Stat3 and Stat4 and is a potent inducer of IFN-γ and IL-22 in human CD4+ T cells. In fact, simultaneous IL-17 and IFN-γ production were readily evident in freshly isolated, CD3/CD28 activated human memory CD4+ T cells. Furthermore, in conditions that favored Th1 and Th2 differentiation, IL-17 production was not extinguished as is the case in mouse cells. Thus, while IL-23 is a regulator of Th17 differentiation, there appears to be more flexibility and less evidence of “lineage commitment” for selective cytokine secretion in human CD4+ T cells. Human memory T cells clearly maintain the capacity to promptly generate IL-17; it would expect that this locus remains accessible in memory CD4+ T cells – even under the circumstances which favor IFN-γ and IL-4 production. Remodeling of the IL-17 locus in memory cells might also allowing for efficient production of this cytokine – this possibility and others will need to be examined in the future. Additionally, we consistently observe the coexistence of IFNγ+IL17+ and IFNγ−IL17+ cells. In this regard, it is notable that IFNγ+IL17+ T cells are also evident in the mouse. Whether these two populations represent separate subsets of helper T cells or different stages of lineage polarization is also an important topic for future investigation. Although cytokine loci of opposite lineage are typically silenced in fully polarized murine Th1, Th2 and Th17 cells, this is less evident in humans CD4+ cells. Investigating the basis of these differences will be an interesting area of further study as we learn more about the transcriptional and epigenetic regulation of cytokine genes.
Stimulation of human T cells with anti-CD3/CD28 and IL-23 was associated with increased expression of the transcription factor RORγt, a key transcription factor controlling mouse Th17 differentiation (40). However, the expression of RORγt and IL-17 are not as tightly linked as they are in mouse cells suggesting that IL-17 expression is likely regulated by other transcription factors. Kinetic detection of RORγt protein expression in developing Th17 cells may give some insight into regulation of IL-17 expression, but the field is hampered by the lack of optimal reagents. Clearly in human CD4+ T cells, TCR occupancy is very important for IL-17 induction. Accordingly, the proximal promoter of the human IL17A gene contains two NFAT binding sites which appear to be important in regulation of IL-17 (35). However, our data suggest that factors other than RORγt and NFAT may be important contributors. The discordant expression of IL-17 and RORγt is also of interest when viewed in the context of the regulation of FOXP3 in human T cells. In murine T cells, Foxp3 expression is tightly controlled and correlates well with the suppressive activity of Treg cells. However, FOXP3 is more widely expressed in activated human T cells and its expression is not necessarily indicative of a population of cells with suppressive activity (48). Curiously, Foxp3+IL-17+ cells were present when T cells were stimulated with TGF-β1 and IL-6; exactly what the functional significance of such Foxp3+IL-17+ cells remains to be determined. Additionally, the mechanisms underlying the distinct regulation of IL-17 and IL-22 are not clear. IL-23-dependent IL-22 production is impaired in Stat3-deficient T cells, and this is likely due to impaired IL-23 receptor expression (data not shown). However, in preliminary experiments, IL-12-dependent IL-22 production is not impaired in Stat3-deficient mice, presumably because of the importance of Stat4.
Recent studies have argued for a role of IL-23 in autoimmune responses and a preponderance of evidence indicates that this cytokine may be more clinically relevant than its close relative IL-12 in causing autoimmunity (8–11, 46). The recent discovery of the relationship between IL-23R SNPs and the prevalence of inflammatory bowel disease patients argues for relevance of this cytokine in human autoimmune disease (49). The present data argue for the importance of an IL-23/IL-17 axis in human CD4+ T cells, consistent with what is seen in murine systems, although IL-23 seems to serve a subtly different role. As IL-23R expression is low in naïve T cells, there are likely to be other unidentified cytokines or signals that act in conjunction with IL-23 to regulate IL-17 expression. However, in human CD4+ T cells IL-23 is an also extremely potent inducer of IL-22, IFN-γ and TNF, cytokines that all participate in autoimmune diseases (12, 50, 51). Therefore, the concept that a major driver of autoimmune diseases can be explained solely by a discrete IL-23/IL-17 connection is likely an oversimplification in humans. The actions of IL-23 clearly are of interest, but this cytokine apparently has very diverse effects on human CD4+ T cells.
Supplementary Material
Acknowledgments
We are grateful for Dr. Xavier Valencia for valuable comments and providing reagents. We thank Dr. Michael Ward and Kevin Elias for assistance with statistical analysis. We thank Drs. Tom Nutman, Wendy Watford and Yuka Kanno for critically reading this manuscript.
Funded by Intramural Research Program of National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–57. [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 4.Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435(7042):637–45. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- 5.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27(1):17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351(20):2069–79. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 8.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116(5):1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–26. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203(11):2485–94. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203(11):2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2006 doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 14.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–56. [PubMed] [Google Scholar]
- 15.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–6. [PubMed] [Google Scholar]
- 16.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14(2):155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 17.Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 cytokine family. Vitam Horm. 2006;74:255–82. doi: 10.1016/S0083-6729(06)74010-9. [DOI] [PubMed] [Google Scholar]
- 18.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 19.Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176(12):7768–74. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- 20.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004;76(1):135–44. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- 23.Koenders MI, Joosten LA, van den Berg WB. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumour necrosis factor and IL-1 in experimental arthritis. Ann Rheum Dis. 2006;65(Suppl 3):iii29–iii33. doi: 10.1136/ard.2006.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204(1):41–7. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 28.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 31.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–42. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong PK, Egan PJ, Croker BA, O’Donnell K, Sims NA, Drake S, et al. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest. 2006;116(6):1571–81. doi: 10.1172/JCI25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Liu XK, Lin X, Gaffen SL. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J Biol Chem. 2004;279(50):52762–71. doi: 10.1074/jbc.M405764200. [DOI] [PubMed] [Google Scholar]
- 36.Sundrud MS, Grill SM, Ni D, Nagata K, Alkan SS, Subramaniam A, et al. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. J Immunol. 2003;171(7):3542–9. doi: 10.4049/jimmunol.171.7.3542. [DOI] [PubMed] [Google Scholar]
- 37.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 39.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17(+) T Helper Cells. Cell. 2006;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun. 2000;1(8):488–94. doi: 10.1038/sj.gene.6363716. [DOI] [PubMed] [Google Scholar]
- 42.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2–4 and IL-22R. J Biol Chem. 2000;275(40):31335–9. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 43.Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006 doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 44.Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4(5):669–77. doi: 10.1016/j.intimp.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 46.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–18. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25(2):195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science. 2006 doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21(2):241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36(5):1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.