Abstract
CD4+ helper T cells can differentiate into several possible fates including: Th1, Th2, Treg, and Th17 cells. Although, cytokine production by non-T cells is an important factor in helper T cell differentiation, a characteristic feature of both Th1 and Th2 lineages is their ability to secrete cytokines that promote their respective differentiation. However, cytokines produced by T cells that help to sustain Th17 cells have not yet been identified. Here we show that IL-21 is a product of Th17 cells, which is induced in a Stat3-dependent manner. Additionally, Stat3 can directly bind the Il21 promoter. IL-21 also induces IL-17 production and expression of the transcription factor, RORγt. Furthermore, generation of Th17 cells in the conventional manner is attenuated by blocking IL-21. IL-21 is known to activate Stat3 and its ability to induce Th17 differentiation is abrogated in the absence of Stat3. These data argue that IL-21 serves as an autocrine factor secreted by Th17 cells that promotes or sustains Th17 lineage commitment.
CD4+ helper T (Th) cells shape immune responses by differentiating into discrete subsets that secrete distinctive cytokines. These patterns are thought to determine the success of the immune system, protect against a particular pathogen, and limit damage to host tissues (1). Until recently, differentiating Th cells were thought to have one of two possible fates defined by their cytokine secretion, namely Th1 and Th2 (2).
Intracellular pathogens promote the production of IL-12 by dendritic cells (DC). In concert, with antigen stimulation, IL-12 acting via the transcription factor Stat4, induces the development of a Th1 cell that produces the signature cytokine IFN-γ. Furthermore, IFN-γ acting via Stat1 also promotes the expression of the transcription factor T-bet, which is important for Th1 differentiation (3). In contrast, helminthic pathogens promote the generation Th2 cells that produce the key cytokine IL-4, which activates Stat6 (4). IL-4 has pleiotropic effects, but a key effect is the promotion of Th2 differentiation and antagonism of IFN-γ production. Thus, a classic feature of Th cells is their production of cytokines that promote their own differentiation and antagonize the differentiation to the other lineage.
This simplistic dichotomotous view of T cell differentiation has recently been challenged by the discovery of a new lineage of T cells characterized by the ability to preferentially secrete a proinflammatory cytokine, IL-17 and thus designated Th17 cells (5). These T cells have been implicated in protection from extracellular bacteria (6–8) and in autoimmune diseases in animals and humans (9,10).
Initially, an IL-12-related cytokine also produced by DC, IL-23, was thought to be the main driver of Th17 differentiation. However, it was later recognized that IL-23 does not act on naïve CD4+ T cells to induce Th17 differentiation. Rather, other inflammatory cytokines produced by DC, IL-1 and IL-6 in conjunction with transforming growth factor-beta 1 (TGFβ-1) were found to be efficient inducers of Th17 differentiation from naïve CD4+ T cell precursors. IL-23 was therefore thought to be more critical for in vivo maintenance of Th17 cells (11). Both IL-6 and IL-23 activate Stat3, which was found to be critical for Th17 differentiation (12,13). Another key transcription factor associated with the Th17 lineage, the orphan retinoid receptor, RORγt, is critical for Th17 differentiation and is induced by the aforementioned cytokines in a Stat3-dependent manner (14).
As indicated, a characteristic feature of both Th1 and Th2 cells is the production of cytokines that sustain their respective differentiation while simultaneously antagonizing differentiation to the opposing lineage. However, the potential cytokine that might play a similar sustaining role in Th17 cells has not been identified.
In the present study, we investigated cytokines produced by Th17 cells with the goal of identifying candidates that would promote/sustain Th17 differentiation. We found that Th17 cells selectively produce IL-21. Additionally, IL-21 induces expression of RORγt, IL-17A, and IL-17F. Thus IL-21 appears to serve as an autocrine regulator of IL-17 production and serves to promote/sustain Th17 lineage commitment.
EXPERIMENTAL PROCEDURES
Mice
Mice bearing loxP-flanked conditional (fl/fl) alleles of STAT3 on a C57BL/6J inbred background were kindly provided by David Levy (NYU). Stat3fl/fl mice were bred with mice expressing Cre under the control of either the CD4 promoter (CD4-Cre) to produce Stat3fl/fl; CD4-Cre mice or the MMTV-LTR (MMTV-Cre) to produce Stat3fl/fl; MMTV-Cre mice. Animals were handled and housed in accordance with the guidelines of the NIH Animal Care and Use Committee.
Isolation of cells and cell culture
Splenocytes were obtained by disrupting organs of 10- to 13-week-old mice. Unless stated otherwise, all cell cultures were performed in RPMI supplemented with 10% fetal calf serum, 2 mM glutamine, 100 IU/mL penicillin, 0.1 mg/mL streptomycin (Invitrogen, Calsbad, CA), and 2 mM β-mercaptoethanol. T cells were enriched with a Mouse CD4+ T Cell Kit using AutoMacs isolator (Miltenyi Biotec, Bergisch Gladbach, Germany). Naive T cells were obtained by surface staining with anti-CD62L, anti-CD44, and anti-CD25. The CD62L+CD44−CD25− population was isolated by flow cytometry cell sorting with a Mo-Flo cell sorter (Dako, Carpinteria, CA). Cells were activated by plate-bound anti-CD3 (5 μg/mL) and anti-CD28 (5 μg/mL) (BD PharMingen, San Diego, CA) for 3 days. Th0 conditions indicate the neutral conditions (no exogenous cytokines and anti-cytokine antibodies). Th1 conditions indicate addition of IL-12 (10 ng/mL) and anti-IL-4 (10 μg/mL). Th2 conditions indicate addition of IL-4 (10 ng/mL) and anti-IL-12 (10 μg/mL). Th17 conditions indicate addition of IL-6 (10 ng/mL), TGFβ-1 (5 ng/mL), anti-IFN-γ (10 μg/mL), and anti-IL-4 (10 μg/mL). Where indicated, IL-21 (100 ng/mL) and anti-IL-21 antibody (4 μg/mL) were added to the cell cultures. All cytokines are from R&D Systems (Minneapolis, MN), except for IL-21, which is from PeproTech (Rocky Hill, NJ). All anti-cytokine antibodies are from BD PharMingen, except for anti-IL-21 antibody, which is from R&D Systems.
Measurement of cytokines
Peripheral naïve CD4+ lymphocytes were isolated as described above. Detection of IFN-γ and IL-17-producing cells was determined by intracellular cytokine staining with anti-IFN-γ-FITC and anti-IL-17-phycoerythrin (BD Biosciences, San Jose, CA). Briefly, cells were stimulated for 4 h with PMA and ionomycin. GolgiStop (BD Biosciences) was added after 2 h and cell was fixed in 4% formyl saline. Fixed cells were stained with fluorescent antibodies in 0.1% saponin permeabilization buffer and analyzed on a FACSCalibur (BD Biosciences). Events were collected and analyzed using FLOWJO software (Tree Star, Ashland, OR). Cytokine production in cell culture supernatants was analyzed using mouse IL-17A Quantikine assay kits (R&D Systems), IL-17F DuoSet assay kit (R&D Systems), and mouse IL-21 DuoSet assay kit (R&D Systems) according to the manufacturer’s instructions.
RNA Preparation and Microarray Analysis
Total cellular RNA from cells cultured under Th0 and optimal Th17 conditions was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Approximately 10 lg of RNA was labeled and hybridized to GeneChip Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA) according to the manufacturer’s protocols. Expression values were determined using GeneChip Operating Software (GCOS) v1.1.1 software. All data analysis was performed using GeneSpring software GX 7.3.1 (Agilent Technologies, Santa Clara, CA). Gene expression values were normalized as described previously (15). Fold induction of individual gene between Th0 and Th17 cells is represented by the ratio of normalized gene expression value in Th17 cells to value in Th0 cells.
Quantitative real-time PCR
Total RNA was extracted by RNeasy kit (Qiagen, Valencia, CA). cDNA was synthesized with Reverse Transcription kit (Applied Biosystems, Foster City, CA) using random hexamers as primers according to the manufacturer’s instruction. Actin was used as endogenous control. TaqMan primers and probes for murine IL-21, IL-17A, IL-17F, RORγt and Actin were purchased from Applied Biosystems, and samples were analyzed using the ABI PRISM 7500 Sequence Detection System (Applied Biosystems).
Chromatin immunoprecipitation (ChIP)
ChIP experiments were performed using the ChIP-IT™ Chromatin Immunoprecipitation Kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. CD4+ T cells were polarized under neutral condition (Th0) or Th17 conditions for 72 h. Anti-Stat3 antibody (sc7179, Santa Cruz Biotechnology, Santa Cruz, CA) was used. ChIP samples were analyzed by q-PCR using primers targeting the Il21 promoter as showed below: 5′-TGCCGCTGCTTTACTCATTG -3′; 5′-GCACCGTCAGCTTTCAGAGA -3′.
RESULTS
Selective production of IL-21 by Th17 cells
To survey cytokines potentially produced by Th17 cells, we initially employed microarray analysis, comparing differentiated Th17 cells to Th0 cells. Among the genes selectively expressed by Th17 cells were Rorc (RORγt), Rora, Ccr6, Ccl20, and Il1r1, genes now known to be expressed by Th17 cells (Table 1). In addition, we noted that the gene encoding IL-21 was also selectively expressed in Th17 cells. To verify and quantify IL-21 production by Th17 cells, we sorted naïve CD4+CD62L+CD44−CD25− peripheral T cells from wild type mice and cultured the cells under optimal Th0, Th1, Th2, and Th17 conditions for 72 h. As shown in Figure 1, IL-21 mRNA (Fig 1A) and protein (Fig 1B) was expressed at low levels in Th0, Th1 and Th2 cells. By comparison, very high levels of IL-21 were generated under Th17 conditions. These results suggested that IL-21 is a product of Th17 cells, which produce much greater levels than other differentiated T cell subsets.
Table 1. Selected genes induced in Th17 cells.
Total cellular RNA from Th0 and Th17 cells were subjected to microarray analysis. Fold induction was represented by the ratio of normalized gene expression in Th17 cells versus the expression in Th0 cells.
| Gene Symbol | Fold Induction | Description | |
|---|---|---|---|
| Cytokines | Il17 | 244 | interleukin 17 |
| Il21 | 6 | interleukin 21 | |
| Chemokines | Ccl20 | 33 | chemokine (C-C motif) ligand 20 |
| Xcl1 | 33 | chemokine (C motif) ligand 1 | |
| Ccl9 | 8 | chemokine (C-C motif) ligand 9 | |
| Ccl6 | 3 | chemokine (C-C motif) ligand 6 | |
| Cell Surface Receptors | Il1r1 | 129 | interleukin 1 receptor, type I |
| Tnfrsf12a | 56 | tumor necrosis factor receptor superfamily, member 12a | |
| Gpr15 | 49 | G protein-coupled receptor 15 | |
| Itgae | 28 | integrin, alpha E, epithelial-associated | |
| Stab1 | 27 | stabilin 1 | |
| Fcgr2b | 23 | Fc receptor, IgG, low affinity IIb | |
| Ccr6 | 23 | chemokine (C-C motif) receptor 6 | |
| Cx3cr1 | 19 | chemokine (C-X3-C) receptor 1 | |
| Lgr4 | 16 | leucine-rich repeat-containing G protein-coupled receptor 4 | |
| Nuclear Receptors | Rorc | 999 | RAR-related orphan receptor gamma |
| Rora | 45 | RAR-related orphan receptor alpha | |
| Ahr | 13 | aryl-hydrocarbon receptor | |
| Vdr | 7 | vitamin D receptor | |
| Thra | 5 | thyroid hormone receptor alpha |
Figure 1. IL-21 is selectively produced by Th17 cells and is regulated by Stat3.

(A) Total RNA was isolated from wild-type naïve CD4+ T cells cultured under Th0, Th1, Th2, or Th17 conditions. IL-21 mRNA level was detected by quantitative real-time PCR using Taqman assay kit from ABI. Gene expression was normalized to actin mRNA level in each sample. (B) ELISA was performed to detect IL-21 production in supernatants from wild-type naïve CD4+ T cells cultured under Th0, Th1, Th2, or Th17 conditions. (C) Total RNA was isolated from wild-type (WT) or CD4-Cre Stat3fl/fl (Stat3 KO) naïve CD4+ T cells cultured under Th0 or Th17 conditions. IL-21 mRNA levels were detected by quantitative real-time PCR using Taqman assay kit from ABI. (D) ELISA was performed to detect IL-21 production in supernatants from wild-type (WT) or CD4-Cre Stat3fl/f (Stat3 KO) naïve CD4+ T cells cultured under Th0 or Th17 conditions. (E) Peripheral CD4+ T cells were polarized under Th0 or Th17 conditions for 72 h. ChIP assays were performed using anti-Stat3 antibody.
Regulation of IL-21 by Stat3
We and others have previously shown that IL-17 production is Stat3 dependent (12,13). To determine whether IL-21 production might also be dependent upon this transcription factor, we analyzed IL-21 production in Stat3-deficient CD4+ T cells. Indeed, Stat3-deficient T cells cultured under standard Th17 conditions failed to produce IL-17 mRNA or protein (see below). Additionally, IL-21 mRNA (Fig 1C) and protein (Fig 1D) was also undetectable in Stat3-deficient cells even though wild-type cells cultured under the same conditions produced comparatively high levels of this cytokine.
To determine if Stat3 might play a direct role in regulating IL-21 in Th17 cells, we sought to determine if Stat3 can bind the Il21 promoter using chromatin immunoprecipitation assays. As shown in Fig 1E, Stat3 binding to the Il21 promoter was significantly enriched under Th17 culture conditions comparing to Th0 conditions. Taken together, these results argue that Th17 cells selectively produce high levels of IL-21, which appears to be induced directly in a Stat3-dependent manner.
IL-21 promotes IL-17 production by naïve CD4+ T helper cells
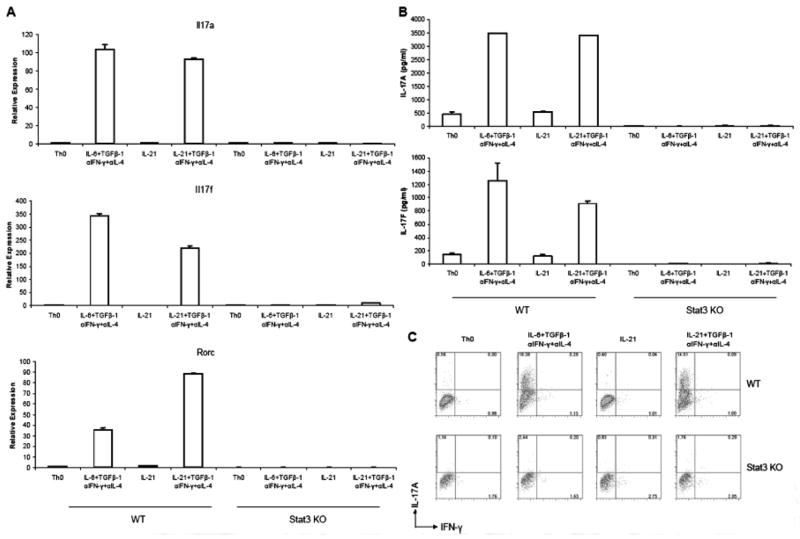
Given the critical role of Stat3 in Th17 differentiation and given that IL-21 has been reported to selectively induce phosphorylation of Stat3 (16), we next examined whether IL-21 could itself promote Th17 differentiation. Peripheral naïve CD4+ T cells were cultured under optimal Th17 conditions or under conditions in which IL-6 was omitted and replaced with IL-21. As shown in Figure 2A–B, optimal Th17 conditions (IL-6 + TGFβ-1 + αIFN-γ + αIL-4) induced high levels of IL-17A, IL-17F mRNA and proteins, as well as high proportion of IL-17A-producing cells (Fig 2C). Interestingly, replacement of IL-6 with IL-21 was a very effective means of inducing IL-17-producing cells. Like IL-6 alone, IL-21 alone in the absence of TGFβ-1 had no ability to induce Th17 differentiation.
Figure 2. IL-21 can replace IL-6 to promote Th17 differentiation.
Peripheral naïve CD4+ T cells from wild-type mice (WT) or CD4-Cre Stat3fl/fl (Stat3 KO) mice were cultured under the indicated conditions for 72 h. (A) Levels of mRNA for IL-17A, IL-17F, and Rorc were measured by quantitative real-time PCR using Taqman assay kits from ABI. Gene expression was normalized to Actin mRNA level in each sample. (B) ELISA was performed to detect IL-17A and IL-17F production in cell culture supernatants. (C) Proportions of IL-17A- and IFN-γ-producing cells in Stat3fl/f (WT) and MMTV-Cre Stat3fl/fl (Stat3 KO) cell cultures were determined by intracellular cytokine staining and flow cytometry.
The transcription factor RORγt is essential for Th17 differentiation (14). Culture of naïve CD4+ T cells under standard Th17 conditions markedly upregulates expression of this transcription factor (Fig 2A). Importantly, under conditions in which IL-21 replaced IL-6, RORγt expression was markedly induced.
As noted, like IL-6, IL-21 is a potent activator of Stat3. We therefore next assessed whether the ability of IL-21 to induce Th17 differentiation was also Stat3 dependent. Peripheral naïve CD4+ T cells from Stat3-deficient mice were cultured under optimal Th17 conditions or under conditions in which IL-6 was omitted and replaced with IL-21. As shown in Figure 2A–B, very low level of, if any, IL-17A and IL-17F mRNA as well as proteins were detected under all four culture conditions. In addition, very few IL-17A-producing cells were found in Stat3-deficient mice (Fig 2C). These results indicated that induction of IL-17 by IL-21 and TGFβ-1 depends on functional Stat3, which is analogous to stimulation in the presence of TGFβ-1 and IL-6.
Neutralization of IL-21 interferes with the formation of Th17 cells
Our data demonstrate that Th17 cells produce significant amount of IL-21 and IL-21 can promote Th17 lineage differentiation. We therefore considered possibility that with the conventional means of generating Th17, autocrine IL-21 was an important, but previously overlooked contributor. We reasoned that if IL-21 was an important factor, neutralizing IL-21 produced by T cells would inhibit the formation of Th17 cells. Indeed, antibody blockade of IL-21 in the Th17 cultures drastically reduced IL-17A and IL-17F mRNA expression (Fig 3A) as well as protein production detected by ELISA (Fig 3B). Intracellular staining of IL-17A also indicated that neutralization of IL-21 partially blocked differentiation of Th17 cells (Fig 3C). These results indicate that autocrine IL-21 production contributes to the optimal in vitro differentiation of Th17 cells.
Figure 3. Conventional Th17 differentiation is dependent upon IL-21.

Peripheral naïve CD4+ T cells from wild type mice were cultured in neutral (Th0), optimal Th17, or Th17 conditions with anti-IL-21 antibody for 72 h. (A) Levels of mRNA for IL-17A and IL-17F were assayed by quantitative real-time PCR using Taqman assay kits from ABI. Gene expression was normalized to Actin mRNA level in each sample. (B) ELISA was performed to detect IL-17A and IL-17F production in cell culture supernatants. (C) Proportions of IL-17A- and IFN-γ-producing cells were determined by intracellular cytokine staining and flow cytometry.
DISCUSSION
In this study, we report that IL-21 is a functionally important product of Th17 cells; and compared to other T cells subsets, Th17 cells selectively produce this cytokine. The induction of IL-21 is Stat3 dependent, and Stat3 directly binds the Il21 promoter, indicating that it may be a direct regulator. While products of antigen presenting cells, including IL-6 and IL-23, are important for promoting and maintaining Th17 cells, antibody blocking experiments indicate that autocrine production of IL-21 is another, previously unrecognized factor that contributes to Th17 differentiation.
IL-21 is a member of the common gamma chain family of cytokines, which binds to a heterodimeric receptor complex consisting of IL-21R and the common gamma chain receptor subunit (17). Like other members of the common gamma chain family, IL-21 signaling is dependent upon Janus kinase 3 (Jak3). Although other common gamma chain cytokines like IL-2 and IL-7 are major activators of Stat5, IL-21 mainly signals through Stat3 (16).
This is notable because both IL-6 and IL-23 activate Stat3 in T cells and it is now well established that Stat3 is a critical factor in Th17 differentiation. Deficiency of Stat3 abrogates Th17 differentiation (13), whereas, deficiency of Socs3, which enhances Stat3 activation, promotes Th17 differentiation (12). The latter is associated with widespread autoimmune disease characterized by overproduction of IL-17 and increased expression of IL-17 targets (12). Our results demonstrated that IL-21 induction in Th17 cells also depends upon Stat3. Moreover, IL-21 activates Stat3 to promote Th17 differentiation, adding more evidence that Stat3 plays a critical role in Th17 differentiation. With respect to the importance of the different Stat3-activating cytokines, it should be noted that our results indicate that blocking IL-21 reduced, but did not abrogate IL-17 production. Additionally, one action of IL-17 is to induce IL-6 production in target cells (18), so the relative roles of IL-6, IL-23 versus IL-21 in Th17 lineage differentiation will need to be carefully examined in the appropriate cytokine knockout mice.
IL-21 is known to be a product of activated CD4+ T cells (19). A major in vivo effect of IL-21 is the regulation of B cells, especially the transition of B cells to plasma cells (20,21). IL-21 has also been reported to be important for the growth of CD8+ T cells (22). In the classical view, IL-21 has been reported to be preferentially produced by Th2 cells, although IL-21 protein was not measured (23–25). Prior to the present study, the production of IL-21 in Th1 and Th2 cells was not compared to Th17 cells. We now find that Th1 and Th2 cells make little IL-21 compared to developing Th17 cells. Moreover, IL-21 promotes Th17 differentiation and seems to function analogously to the effects of Th1 and Th2 cytokines on their respective lineages. IL-21 is capable of acting on Th17 cells in an autocrine manner in response to antigen stimulation. Thus IL-21 serves as a factor that is produced by Th17 cells to promote or sustain their differentiation.
A recent report has suggested exposure of naïve CD4+ T cells to IL-21 inhibits Th1 differentiation and IFN-γ production (26). Therefore, IL-21 production by Th17 cells may also be analogous to other lineage-specific cytokines (IL-4 and IFN-γ), which not only promote their respective lineage but also antagonize differentiation to other cell fates.
Blocking IL-21/IL-21R system has been reported to reduce disease progression in murine lupus (27) and rheumatoid arthritis (28) models. Our finding that IL-21 promotes Th17 differentiation may explain the underlining mechanism of the efficacy of this therapy. It will be of great interest to determine the importance of IL-21 in human Th17 differentiation and to assess whether blocking of IL-21 signaling is effective in human diseases associated with overproduction of IL-17.
Acknowledgments
We would like to thank David Levy for providing the Stat3fl/fl mice, Marko Pesu and Yuka Kanno for critical review of the manuscript, as well as Wendy Watford for discussion. L.W. is supported by National Research Council Research Associate Fellowship. K.M.E. is a Howard Hughes Medical Institute – National Institutes of Health Research Scholar.
The abbreviation used are
- IL
interleukin
- Stat
signal transducer and activator of transcription
- Treg
T regulatory cells
- IFN
interferon
- T-bet
T-box expressed in T cells
- RORγt
RAR-related orphan receptor gamma T
- IL-21R
IL-21 receptor
- Socs3
suppresser of cytokine signaling 3
- NRS
normal rabbit serum
References
- 1.O’Garra A, Vieira P. Nat Med. 2004;10(8):801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 3.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 4.Ansel KM, Djuretic I, Tanasa B, Rao A. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 5.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Immunity. 2006;24(6):677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Fedele G, Stefanelli P, Spensieri F, Fazio C, Mastrantonio P, Ausiello CM. Infect Immun. 2005;73(3):1590–1597. doi: 10.1128/IAI.73.3.1590-1597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. J Exp Med. 2005;202(6):761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 9.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 11.Weaver CT, Hatton RD, Mangan PR, Harrington LE. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Proc Natl Acad Sci U S A. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer LM, Kim JG, Pfeffer SR, Carrigan DJ, Baker DP, Wei L, Homayouni R. J Biol Chem. 2004;279(30):31304–31311. doi: 10.1074/jbc.M308975200. [DOI] [PubMed] [Google Scholar]
- 16.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. Blood. 2007;109(10):4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovanen PE, Leonard WJ. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 18.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Immunity. 1995;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim HP, Korn LL, Gamero AM, Leonard WJ. J Biol Chem. 2005;280(26):25291–25297. doi: 10.1074/jbc.M501459200. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. Science. 2002;298(5598):1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. J Immunol. 2005;175(12):7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 22.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. J Exp Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. J Exp Med. 2002;196(7):969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. J Clin Invest. 2006;116(7):2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, Kopf M. Blood. 2007;109(5):2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 26.Suto A, Wurster AL, Reiner SL, Grusby MJ. J Immunol. 2006;177(6):3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 27.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. J Immunol. 2007;178(6):3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 28.Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, Senices M, Wu PW, Sibley B, Leathurby Y, Brown TP, Nickerson-Nutter C, Keith JC, Jr, Collins M. Arthritis Rheum. 2007;56(4):1152–1163. doi: 10.1002/art.22452. [DOI] [PubMed] [Google Scholar]