Abstract
Different animal models of pulmonary fibrosis have been developed to investigate potential therapies for idiopathic pulmonary fibrosis (IPF). The most common is the bleomycin model in rodents (mouse, rat and hamster). Over the years, numerous agents have been shown to inhibit fibrosis in this model. However, to date none of these compounds are used in the clinical management of IPF and none has shown a comparable antifibrotic effect in humans. We performed a systematic review of publications on drug efficacy studies in the bleomycin model to evaluate the value of this model regarding transferability to clinical use. Between 1980 and 2006 we identified 246 experimental studies describing beneficial antifibrotic compounds in the bleomycin model. In 221 of the studies we found enough details about the timing of drug application to allow inter-study comparison. 211 of those used a preventive regimen (drug given ≤ day 7 after last bleomycin application), only 10 were therapeutic trials (> 7 days after last bleomycin application). It is critical to distinguish between drugs interfering with the inflammatory and early fibrogenic response from those preventing progression of fibrosis, the latter likely much more meaningful for clinical application. All potential antifibrotic compounds should be evaluated in the phase of established fibrosis rather than in the early period of bleomycin-induced inflammation for assessment of its antifibrotic properties. Further care should be taken in extrapolation of drugs successfully tested in the bleomycin model due to partial reversibility of bleomycin induced fibrosis over time. The use of alternative and more robust animal models, which better reflect human IPF, is warranted.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive and ultimately fatal lung disease of unknown etiology. Its prognosis is poor and the outcome even worse than in many malignant diseases. IPF is one of the most frequent interstitial lung diseases and is characterized by the histological pattern of usual interstitial pneumonia (UIP) (ATS, 2000). The natural history of IPF is unknown and the onset of symptoms is gradual, starting usually with non-productive cough and exertional dyspnea. With involvement of larger areas of the lung, severe dyspnea at rest and signs of right heart failure develop (ATS, 2002). In some cases the clinical state is preserved for a period of several years, but the majority of patients deteriorate more rapidly. Mortality during acute exacerbation is high. The prevalence of IPF is estimated at 20/100,000 for males and 13/100,000 for females, and survival time from diagnosis ranges from 2 to 4 years (D. S. Kim, Collard, & King, 2006). Histological characteristics of UIP include remodeling of lung architecture with fibroblastic foci and “honeycombing”. The lung involvement is patchy with a predominantly basal and subpleural pattern of matrix deposition and tissue distortion (ATS, 2002). Most patients present at an advanced stage of disease. Treatment options for pulmonary fibrosis are limited. The clinical management focuses on treatment of complications (e.g. right heart failure, infections, etc.), supportive care and in few cases involves lung transplantation. Anti-inflammatory drugs such as prednisone may carry symptomatic relief, but they do not appear to halt progression of fibrosis, and their beneficial effects in IPF remain in question. Cytotoxic drugs (cyclophosphamide, azathioprin, etc) have not been shown to improve lung function or life expectancy and may be associated with harmful side effects.
The last two decades have markedly improved the knowledge about underlying mechanisms of pulmonary fibrosis and helped to identify potential targets for novel therapies. However, despite the large number of anti-fibrotic drugs being described in experimental pre-clinical studies, the translation of these findings into clinical practice has not been accomplished yet. This review will focus on the bleomycin model of pulmonary fibrosis, highlight its undisputable contribution to investigation of basic pathomechanism of disease and critically reflect its usefulness in determining efficacy of antifibrotic drugs.
Animal models of pulmonary fibrosis
Animal models play an important role in the investigation of diseases, and many models are established to examine pulmonary pathobiology. Chronic diseases are more difficult to model. The situation with IPF is even more complicated, since the etiology and natural history of the disease is unclear and no single trigger is known that is able to induce “IPF” in animals. Different models of pulmonary fibrosis have been developed over the years. Most of them mimic some, but never all features of human IPF, especially the progressive and irreversible nature of the condition. Common methods include radiation damage, instillation of bleomycin, silica or asbestos, and transgenic mice or gene transfer employing fibrogenic cytokines. So far, the standard agent for induction of experimental pulmonary fibrosis in animals is bleomycin.
Bleomycin
Bleomycin is a chemotherapeutic antibiotic, produced by the bacterium “Streptomyces verticillus” (Adamson, 1976; Umezawa, 1967). Its use in animal models of pulmonary fibrosis is based on the fact that fibrosis is one of the major adverse drug effects of bleomycin in human cancer therapy. Bleomycin plays an important role in the treatment of lymphoma, squamous cell carcinomas, germ cell tumors and malignant pleural effusion, where it is injected intrapleurally. It is believed that bleomycin acts by causing single and double-strand DNA breaks in tumor cells and thereby interrupting the cell cycle. This happens by chelation of metal ions, and reaction of the formed pseudoenzyme with oxygen, which leads to production of DNA-cleaving superoxide and hydroxide free radicals (Claussen & Long, 1999). An overproduction of reactive oxygen species can lead to an inflammatory response causing pulmonary toxicity, activation of fibroblasts and subsequent fibrosis (Chaudhary, Schnapp, & Park, 2006; Grande NR, 1998). Bleomycin hydrolase, a bleomycin-inactivating enzyme, critically influences the effects of this drug on different tissues. The lungs maintain low levels of the enzyme and therefore are more susceptible to bleomycin-induced tissue injury(Sebti, Mignano, Jani, Srimatkandada, & Lazo, 1989). Pulmonary side effects in patients are dose-dependent, age-related and occur more often in the presence of pre-existing pulmonary diseases or smoking. Lung toxicity develops in ∼10% of patients receiving bleomycin, and is clinically associated with cough, dyspnea, fever, cyanosis, and deterioration of lung function parameters. Within weeks to months this response might progress to pulmonary fibrosis in ∼1% of patients (“Compendium of Pharmaceuticals and Specialties. Blenoxane®. Canadian Pharmacists Association.” 2006).
The bleomycin animal model
Bleomycin as an agent to induce experimental lung fibrosis was first described in dogs (Fleischman et al., 1971), later in mice (Adamson & Bowden, 1974), hamsters (Snider, Celli, Goldstein, O'Brien, & Lucey, 1978), and rats (Thrall, McCormick, Jack, McReynolds, & Ward, 1979). It causes inflammatory and fibrotic reactions within a short period of time, even more so after intratracheal instillation. The initial elevation of pro-inflammatory cytokines (interleukin-1, tumor necrosis factor-α, interleukin-6, interferon-γ) is followed by increased expression of pro-fibrotic markers (transforming growth factor-β1, fibronectin, procollagen-1), with a peak around day 14. The “switch” between inflammation and fibrosis appears to occur around day 9 after bleomycin (Chaudhary, Schnapp, & Park, 2006). Notable in the murine model are remarkable strain differences in susceptibility to develop fibrosis following bleomycin, with CBA and C57Bl/6 mice being strong responders and Balb/c mice relatively fibrosis-resistant. These differences are likely due to different expression patterns of cytokines and proteases/ anti-proteases (Phan & Kunkel, 1992).
It has been reported that histological hallmarks, such as intra-alveolar buds, mural incorporation of collagen and obliteration of the alveolar space, are present in bleomycin-treated animals similar to IPF patients (Usuki K, 1995). This observation has led to the assumption, that bleomycin reproduces typical features of the human disease and hence, the use of this model has become very popular. Further, the bleomycin model has the advantage that it is quite easy to perform, widely accessible and reproducible, and therefore fulfills important criteria expected from a good animal model. Fairly consistent dosages have been established for each species to achieve a fibrotic response, and, dependent on the route of administration, different fibrotic patterns develop. Intratracheal instillation of bleomycin, the standard route of administration, results in bronchiocentric accentuated fibrosis, whereas intravenous or intraperitoneal administration induces subpleural scarring similar to human disease (Chua, Gauldie, & Laurent, 2005). The bleomycin model has contributed tremendously to elucidate the roles of cytokines, growth factors and signaling pathways involved in pulmonary fibrosis. For instance, it has helped to determine transforming growth factor (TGF) β as one of the key factors in the development of pulmonary fibrosis (J. Zhao et al., 2002). A number of novel (e.g. TGFβ antagonists) and not so novel (e.g. ACE-inhibitors) drugs interfering with TGFβ have been investigated in the bleomycin model, some of them quite promising. One of them is decorin, an endogenous proteoglycan and known TGFβ inhibitor. It has been shown that intratracheal administration of Decorin using an adenovirus vector leads to a substantial reduction of the fibrotic response to bleomycin (Kolb et al., 2001).
However, despite undisputed qualities and some similarities in histological alterations, the bleomycin model has significant limitations in regard to understanding the progressive nature of human IPF. As mentioned, bleomycin causes an inflammatory response, triggered by overproduction of free radicals, with induction of pro-inflammatory cytokines and activation of macrophages and neutrophils, thus resembling acute lung injury in some way. The subsequent development of fibrosis, however, is at least partially reversible, independent from any intervention (Izbicki, Segel, Christensen, Conner, & Breuer, 2002). The aspect of slow and irreversible progression of IPF in patients is not reproduced in the bleomycin model (Chua, Gauldie, & Laurent, 2005). One of the most critical hallmarks of human IPF is therefore not present in animals, which has to be considered when this model is used for drug intervention studies.
Drug intervention studies in the bleomycin model
The bleomycin animal model is widely used in the assessment of potential antifibrotic agents. A large number of compounds have been shown to prevent fibrotic progression in this model and have been suggested to qualify for clinical use. We performed a Pub Med search and identified 232 papers published between 1980 and 2006 which discuss antifibrotic compounds in the bleomycin model. All these compounds were reported to be successful and antifibrotic, either as “preventive treatment” (that means given early, ≤ day 7 after last bleomycin application), and/or therapeutically (> 7 days after last bleomycin application) (figure 1). Some authors compared the effects of several compounds, increasing the total number to 246 experimental attempts (table 1).
Figure 1. Sequence of events in Bleomycin-induced pulmonary fibrosis.
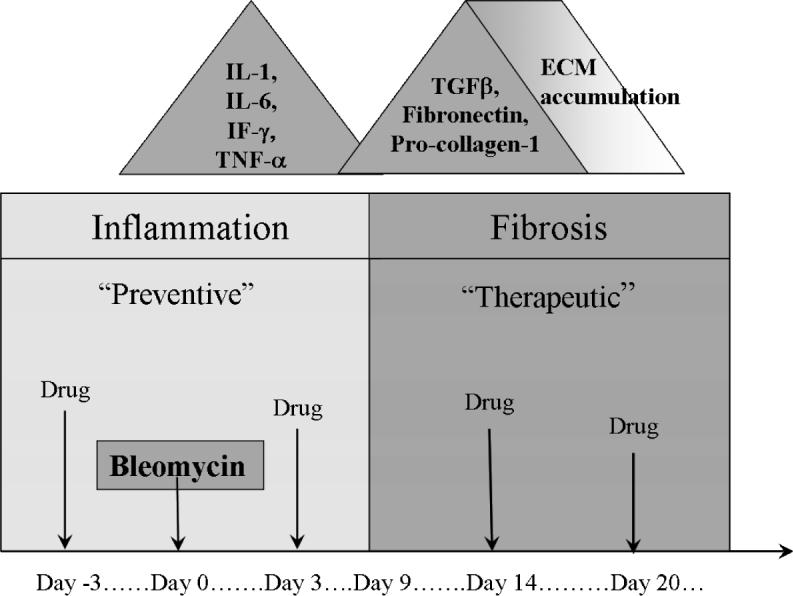
After administration of bleomycin, there is the onset of an acute inflammatory response lasting up to 8 days, followed by fibrogenic changes resulting in deposition of matrix and distortion of lung structure out to 28 or 35 days. Treatments during the first seven days would be considered “preventive” while treatments during the later stages after day 7−10 would be considered “therapeutic”.
Table 1. Preventive and therapeutic agents showing beneficial antifibrotic effects in the bleomycin model of pulmonary fibrosis.
“Early” application means ≤ day 7 after last bleomycin application, “late” application > 7 days after bleomycin; “unclear” means that the exact information about the application schedule was not easily available and/or not specified in the article.
In most of the studies bleomycin was given by a single intratracheal instillation in weight adjusted dosages. We defined the day of bleomycin administration as day 0, allowing the association of this time point with the schedule of compound administration. This is important in order to distinguish between anti-inflammatory and antifibrotic drug effects, as the interpretation of drug effects is crucially dependent on timing of compound administration. Compounds administered during the early phase may predominantly act as anti-inflammatory agents and should be considered as “preventive treatment”, whereas “true” antifibrotic agents might be effective irrespective of timing, particularly if administered during the “fibrotic” phase of the model (Chaudhary, Schnapp, & Park, 2006). In the vast majority of the reviewed studies, the compound was given as preventive treatment, thus confounding the designation as anti-inflammatory or anti-fibrotic.
Different routes of drug administration were applied, including oral, subcutaneous, intraperitoneal, or intravenous injections. Further, gene modifying techniques were used, such as adenoviral or HVJ (hemagglutinating virus of Japan) envelope vector mediated gene transfer, intramuscular gene transfection, or gene knock out. Mice and rats were by far the most common species, followed by hamsters, rabbits and dogs. The endpoints were variable, ranging from day 1 to day 80 after bleomycin, most frequently between day 14 and day 28. Choosing the correct endpoint in the bleomycin model is critical, especially as it has been shown that the standard outcome parameters are highly variably after day 21 and may even return back to baseline (histomorphomerty and hydroxyproline lung content) (Izbicki, Segel, Christensen, Conner, & Breuer, 2002).
For the assessment of fibrosis several common methods were used, including semi-quantitative histological analysis, sometimes based on the scoring system by Ashcroft (Ashcroft, Simpson, & Timbrell, 1988), and quantification of hydroxyproline and/or collagen content. Broncho-alveolar lavage fluid (BALF) was often analyzed for changes in total cell count, differential count of leucocytes, and measurement of tumor necrosis factor alpha (TNF) α or TGFβ levels. The expression of other pro-inflammatory mediators, e.g. monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-2 (MIP-2) was quantified in some cases. Additional parameters such as weight, lung index (lung wet weight in mg versus body weight in g) and survival time were not always, but frequently assessed. Measurement of enzyme activities was performed, including superoxide dismutase (SOD) and catalase (CAT), as indicators of the generation of free radicals, myeloperoxidase (MPO), as a marker of neutrophil influx and malondialdehyde (MDA), as an index of oxidative stress. Occasionally the TUNEL (terminal deoxynucleotidyl transferase mediated dUTP nick end labeling) assay was applied to identify apoptotic cells in situ.
A great variety of compound classes showing apparent antifibrotic effects in the bleomycin model have been identified. Those that appear to have a major effect include: Antioxidants (Ambroxol (Pozzi et al., 1989; Pozzi et al., 1987), Niacin (A. Nagai et al., 1994; O'Neill & Giri, 1994; Q. J. Wang, Giri, Hyde, Nakashima, & Javadi, 1990), Taurin (Blaisdell & Giri, 1995; Giri & Wang, 1992; Gurujeyalakshmi, Hollinger, & Giri, 1998; Gurujeyalakshmi, Iyer, Hollinger, & Giri, 1996; Gurujeyalakshmi, Wang, & Giri, 2000; Q. Wang, Hyde, & Giri, 1992; Q. J. Wang, Giri, Hyde, & Nakashima, 1989), N-Acetylcysteine (Cortijo et al., 2001; Hagiwara, Ishii, & Kitamura, 2000; Mata et al., 2003; Serrano-Mollar et al., 2003; Shahzeidi, Sarnstrand, Jeffery, McAnulty, & Laurent, 1991; Yildirim et al., 2005), Vitamin E (Kilinc et al., 1993), Curcumin (Punithavathi, Venkatesan, & Babu, 2000), Aminoguanidine (X. L. Chen, Huang, Li, Wang, & Wang, 2001; X. L. Chen, Li, Zhou, Ai, & Huang, 2003; de Rezende, Martinez, Capelozzi, Simoes, & Beppu, 2000; Giri, Biring, Nguyen, Wang, & Hyde, 2002; Hu, Xu, & Li, 1999; Yildirim et al., 2004), Melatonin (Arslan, Zerin, Vural, & Coskun, 2002; Genovese, Di Paola et al., 2005; Yildirim et al., 2006), Bilirubin (H. D. Wang et al., 2002), CAPE = caffeic acid phenethyl ester (Ozyurt et al., 2004), Erdosteine (Boyaci et al., 2006; Sogut et al., 2004; Yildirim et al., 2005; Yildirim et al., 2004) etc.), Angiotensin converting enzyme inhibitors (Captopril (R. Wang, Ibarra-Sunga, Verlinski, Pick, & Uhal, 2000), Ramipril (Marshall et al., 2004) etc.), Angiotensin receptor blockers (Losartan (Fang, Zhu, Hu, & Liu, 2002; Marshall et al., 2004; Yao, Zhu, Zhao, & Lu, 2006), Candesartan (Otsuka, Takahashi, Shiratori, Chiba, & Abe, 2004), Valsartan (F. Liu, Xu, & Ye, 2005; Mancini & Khalil, 2005) etc.), Anticoagulants (TFPI = tissue factor pathway inhibitor (Kijiyama et al., 2006), Urokinase (Hart, Whidden, Green, Henkin, & Woods, 1994; Hattori et al., 2004; Howell et al., 2001; Howell, Laurent, & Chambers, 2002; Ikeda, Hirose, Koto, Hirano, & Shigematsu, 1989), Heparin (Gunther et al., 2003; Piguet, Van, & Guo, 1996), APC = anticoagulant protein C (S. Shimizu et al., 2003; H. Yasui et al., 2001), Thromboxane synthetase inhibitor (Sato et al., 2004) etc.), Macrolide antibiotics (Erythromycin (Azuma et al., 1998; B. Chen, Jiang, Zhao, Yu, & Hou, 1997; Y. Li, He, & Wang, 1999; Tan, Liu, He, & Xu, 1999), Azithromycin (J. Chen, He, Li, Wang, & Zhang, 1999; Ma, He, Li, & Zhang, 2002), Clarithromycin (Azuma et al., 2001; Kawashima et al., 2002; Y. Li, Azuma, Takahashi et al., 2002), Roxithromycin (Azuma et al., 2001; Kawashima et al., 2002; Y. Li, Azuma, Takahashi et al., 2002) etc.), Cytokines (Interferon-β (Azuma, Li et al., 2005), Interferon-γ (Gurujeyalakshmi & Giri, 1995; Hyde, Henderson, Giri, Tyler, & Stovall, 1988; Okada, Sugie, & Aisaka, 1993), Interleukin (IL)-1beta (M. Yasui et al., 1991), IL-10 (Arai et al., 2000), IL-18 (Nakatani-Okuda et al., 2005), Keratinocyte growth factor (Deterding et al., 1997; Sugahara, Iyama, Kuroda, & Sano, 1998; Yi et al., 1996), Hepatocyte growth factor (Dohi, Hasegawa, Yamamoto, & Marshall, 2000; Mizuno, Matsumoto, Li, & Nakamura, 2005; Umeda et al., 2004; Yaekashiwa et al., 1997), Chemokine ligand (CXCL)-10 (Tager et al., 2004),CXCL11 (Burdick et al., 2005), CD (cluster of differentiation)-36 (Yehualaeshet et al., 2000) etc.), Cytokine antibodies (Transforming growth factor-β (Giri, Hyde, & Hollinger, 1993), Tumor necrosis factor-α (Fichtner-Feigl, Strober, Kawakami, Puri, & Kitani, 2006; Fujita et al., 2003; Piguet & Vesin, 1994), Connective tissue growth factor (Matsuoka et al., 2002), IL-12 (Maeyama et al., 2001), IL-13 (Fichtner-Feigl, Strober, Kawakami, Puri, & Kitani, 2006), Platelet derived growth factor (Aono et al., 2005; Chaudhary, Schnapp, & Park, 2006; Daniels et al., 2004; Yoshida et al., 1999), Vascular endothelial growth factor (Hamada et al., 2005), CCR-1 (Tokuda et al., 2000), CCR-3 (Huaux et al., 2005), CCL-11 (Huaux et al., 2005), CD-11 (Piguet, Rosen, Vesin, & Grau, 1993), MCP-1 (Inoshima, Kuwano, Hamada, Hagimoto et al., 2004) etc.), Chinese herbs (Feitai (Gong et al., 2004; Gong et al., 2005; Shen et al., 2005), Salviae miltiorrhizae (Hua, Cui, & Liu, 1994; J. Liu, 1992; J. Liu et al., 1993), Ginkgo biloba (Daba et al., 2002; Iraz et al., 2006), Moxibustion (R. Li et al., 2005), Fufang (Kong et al., 2005) etc.), Immunosuppressants (Cyclosporin-A (Lossos, Or, Goldstein, Conner, & Breuer, 1996), Rapamycin analogue SDZ RAD (Simler et al., 2002)), Corticosteroids (Dexamethasone (F. Chen et al., 2006; Dik et al., 2003; H. P. Li, Li, He, Yi, & Kaplan, 2004), Methylprednisolone (Phan, Thrall, & Williams, 1981), Prednisolone (Chaudhary, Schnapp, & Park, 2006; Entzian et al., 1998) etc.), Chelating agents (D-Penicillamine (Geismar, Hennessey, Reiser, & Last, 1986) etc.), and Pirfenidone (Ammar et al., 2006; Gurujeyalakshmi, Hollinger, & Giri, 1999; Iyer, Gurujeyalakshmi, & Giri, 1999; Iyer, Hyde, & Giri, 2000; Iyer, Margolin, Hyde, & Giri, 1998; Iyer et al., 1995; Kakugawa et al., 2004; Mansoor, Chen, Schelegle, & Giri, 1999; Schelegle, Mansoor, & Giri, 1997). Considering the extensive number of compounds we will discuss only a few representative examples in greater detail. The full list is provided in table 1.
Selected examples of preventive compounds
Ginkgo biloba
is a flavonoid-rich antioxidant, containing ginkgolides extracted from Ginkgo leaves. Clinically, this substrate is used as a memory enhancer, anti-vertigo agent and for intermittent claudication. Evidence exists that Gingko biloba improves blood flow, protects from free radicals and blocks platelet aggregation and blood clotting (Dubey, Shankar, Upadhyaya, & Deshpande, 2004; Ernst, 2002; Mahady, 2002). These properties appear to be potentially antifibrotic, and for that reason Gingko biloba has been examined in the bleomycin model. Investigators administered Ginkgo biloba orally from day −1 to day 14, which led to a lower degree of fibrosis in treated animals compared to bleomycin controls. Fibrosis was assessed by using Ashcroft score, hydroxyproline content, BALF total cell count, nitrite levels, and enzyme activities (Iraz et al., 2006).
Losartan
is an angiotensin II receptor antagonist, clinically used for the treatment of systemic hypertension, diabetic nephropathy and for prevention of cardiovascular events. Several reports have promoted Losartan as inhibitor of fibrotic progression in the bleomycin model (Fang, Zhu, Hu, & Liu, 2002; Marshall et al., 2004; Molina-Molina et al., 2006), and a recent report confirmed these findings (Yao, Zhu, Zhao, & Lu, 2006). Losartan was given by daily gavage from day 0 to day 14 or to day 21. Alveolitis and fibrosis scores were significantly lower, hydroxyproline content reduced and TGF-β1 levels lower compared to untreated control rats, which had received bleomycin only. The drug treated animals also lost less weight and had lower indices of lung fibrosis. The antifibrotic effect of Losartan in the context of pulmonary fibrosis might be associated with antioxidant activity and reduction in TGF-β1 levels (Yao, Zhu, Zhao, & Lu, 2006).
EM703
is a derivate of erythromycin, a macrolide antibiotic, which is first line therapy for community-acquired pneumonia. Erythromycin is produced from a strain of actinomyces and contains a 14-membered lactone ring. It prevents growth of typical and atypical bacteria by interfering with their protein synthesis. EM703 is a derivate of erythromycin and has been reported to exhibit anti-inflammatory activity independent from anti-bacterial activity by suppressing nuclear factor-κ B and inhibiting interleukin-8 expression (Y. Li et al., 2006). Previously, 14-membered macrolides have been shown to attenuate leukocyte migration in the early inflammatory phase and thereby prevent bleomycin induced lung fibrosis (Y. Li, Azuma, Takahashi et al., 2002). A recent study confirms the preventive effect of EM703 when orally administered starting three days prior to bleomycin. The degree of fibrosis was assessed by Ashcroft score, hydroxyproline content, and BALF cell counts. The authors concluded that EM703 improves bleomycin-induced pulmonary fibrosis in mice by acting as an anti-inflammatory agent and regulating TGF-β signaling (Y. Li et al., 2006).
Selected examples of therapeutic compounds
Pirfenidone
is an orally active small molecule drug with anti-inflammatory, antioxidant and antifibrotic effects. It is known that Pirfenidone modifies the regulation of cytokines, including PDGF, and thereby inhibits fibroblast proliferation and extracellular matrix synthesis. It has also been shown to reduce the increase in TGF-β levels after bleomycin administration. The exact mechanism for the antifibrotic effect is not yet fully understood (Gurujeyalakshmi, Hollinger, & Giri, 1999). Therapeutic antifibrotic effects have been observed in the bleomycin model, when animals received Pirfenidone starting 14 days after bleomycin administration. The authors concluded that this drug may have antifibrotic potential, since it had been administered after the inflammatory phase had subsided. They speculated that this might be based on inhibition of heat shock protein 47 positive cells and α−smooth muscle actin positive myofibroblasts (Kakugawa et al., 2004). Pirfenidone has recently been tested in multiple clinical trials, showing some promising results such as improvement of vital capacity and reduction of acute exacerbations (Azuma, Li et al., 2005). To further clarify the safety of this drug, a large phase III clinical trial (CAPACITY) has been initiated in 2006.
Hepatocyte growth factor (HGF)
is a multifunctional growth factor produced by mesenchymal cells such as fibroblasts, macrophages and endothelial cells. HGF acts on epithelial cells as a mitogen, stimulating migration and morphogenesis (Dohi, Hasegawa, Yamamoto, & Marshall, 2000; Yaekashiwa et al., 1997). It is described as potent inducer of matrix metalloproteinases (MMPs), which degrade extracellular matrix and are overexpressed during progression of myofibroblast apoptosis (Mizuno, Matsumoto, Li, & Nakamura, 2005). HGF levels are increased in the BALF of IPF patients (Dohi, Hasegawa, Yamamoto, & Marshall, 2000) and evidence exists that its function is protective against lung damage, preventing subsequent fibrogenesis (Mizuno, Matsumoto, Li, & Nakamura, 2005). Similar antifibrotic properties were demonstrated in an animal study, where bleomycin was administered by intraperitoneal infusion from day 0 to day 7, followed by infusion of recombinant HGF from day 7 to day 14, day 14 to day 21, or day 21 to day 28. Fibrosis scores as well as hydroxyproline levels were markedly reduced in the different treatment times, even in the later time points where hydroxyproline levels fell to normal after one week of therapy, implying that recombinant HGF is effective in diminishing fibrotic changes even in established fibrosis (Yaekashiwa et al., 1997) (Mizuno, Matsumoto, Li, & Nakamura, 2005)
Most recently BIBF 1000, a selective inhibitor of the group of vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF) and fibroblast growth factor (FGF) receptor tyrosine kinases, has been tested as an anti-fibrotic agent. These growth factors are known as fibrogenic mediators promoting fibroblast proliferation and matrix contraction. BIBF has been applied orally from day 10 to day 21 in the bleomycin treated rat model leading to reduced gene expression of transforming growth factor (TGF)-β1, procollagen-1, fibronectin and connective tissue growth factor (CTGF), as well as less collagen staining in treated animals compared to bleomycin controls (Chaudary NI, 2007). This compound is currently entering clinical trials in IPF.
Clinical trials
Only a relatively small number of compounds considered as having “promising antifibrotic properties” in the bleomycin model were or currently are tested in clinical trials (Walter, Collard, & King, 2006). Some of them were retrospective analyses or case series only. Among the drugs tried or on trial are Etanercept, Imatinib, Prednisone (Daniil et al., 1999; Douglas et al., 1997; Douglas, Ryu, & Schroeder, 2000; Douglas et al., 1998; Nicholson AG, 2000; Riha et al., 2002; Ziesche, Hofbauer, Wittmann, Petkov, & Block, 1999), N-Acetylcysteine (Demedts et al., 2005), TGF-β antibody (Genzyme, 2007), Interferon-γ (Antoniou et al., 2006; Raghu et al., 2004; Raghu R, 2001), Interferon-β (Raghu, Bozic, & Brown, 2001), Pirfenidone (Azuma, Nukiwa et al., 2005; S. Nagai et al., 2002; Raghu, Johnson, Lockhart, & Mageto, 1999), Colchicine (Douglas, Ryu, & Schroeder, 2000; Douglas et al., 1998; Selman et al., 1998), Bosentan, Cyclosporin-A (Alton, Johnson, & Turner-Warwick, 1989; Moolman, Bardin, Rossouw, & Joubert, 1991), D-Penicillamin (Chapela, Zuniga, & Selman, 1986; Selman et al., 1998), Heparin (Kubo et al., 2005), Relaxin (ATS, 2002), Angiotensin converting enzyme (ACE) inhibitors (Nadrous, Ryu, Douglas, Decker, & Olson, 2004), and CTGF antibodies (Mageto Y, 2004). Interestingly, azathioprine and cyclophosphamide, two drugs that are still in the current ATS/ERS guidelines for IPF treatment (ATS, 2002), have never been evaluated in the bleomycin model as far as we know. However, to date none of these drugs have shown comparable success in patients as seen in the bleomycin model. One major issue is the fact that most agents were given to the animals in a preventive regimen, prior to or simultaneous with bleomycin. As discussed earlier, effectiveness in this setting may reflect more anti-inflammatory action by blocking the early response without influencing the subsequent events causing progressive fibrosis. This type of activity can hardly be considered as novel or sufficient, since other potent anti-inflammatory drugs, such as corticosteroids, have failed to improve the course of IPF in patients. Theoretically, compounds which are successfully administered as “therapeutic treatments” in animal models should be much more promising candidates for clinical use.
Selected examples of compounds in clinical trials
N-Acetylcysteine (NAC)
is a precursor in the formation of the antioxidant glutathione and possesses the ability of reducing free radicals. This drug has been in clinical use as mucolytic therapy in a variety of respiratory diseases, in the management of acetaminophen overdose, and in the prevention of radiocontrast-induced nephropathy by augmenting glutathione reserves for binding of toxic metabolites. In the context of pulmonary fibrosis, is has shown effectiveness as preventive medication in the bleomycin animal model (Yildirim et al., 2005). The IFIGENIA study, a double-blind, randomized, placebo-controlled trial enrolling 182 IPF patients, tested NAC in combination with prednisone and azathioprine. Treatment with NAC compared to placebo resulted in a small, but significant delay of functional deterioration over one year (forced vital capacity and diffusion capacity for CO), but there was no improvement in survival. The interpretation of this trial is difficult, because NAC was used in a triple therapy and may have helped to tolerate the cytotoxic drugs better without having an impact on fibrogenesis. The NIH-IPF network has announced the intention to perform a trial of monotherapy with NAC versus placebo to clarify this issue.
Bosentan (Tracleer®)
is an endothelin (ET) receptor antagonist, which blocks ETA and ETB receptors in endothelium and vascular smooth muscle and thereby prevents the hormone endothelin-1 (ET-1) from binding to these receptors. ET-1 causes vasoconstriction of pulmonary blood vessels leading to pulmonary artery hypertension. Currently, Bosentan is in clinical use for treatment of pulmonary hypertension, given orally in increasing dosages. Bosentan has been investigated in the bleomycin animal model as a preventive drug showing increased volumes of total air and decreased volumes of connective tissue. This led to the assumption that Bosentan might be a useful medication for IPF patients, even more so for those with superimposed pulmonary hypertension (Park, Saleh, Giaid, & Michel, 1997). A series of clinical trials has already been carried out. The outcomes of BUILD-1, a double-blind randomized, placebo-controlled study enrolling 158 patients with IPF showed no effect on functional parameters but positive trends on survival at 12 months. BUILD-2 showed similar effects in scleroderma patients with secondary interstitial lung disease. Currently, BUILD-3 is underway, expecting the enrollment of ∼400 IPF patients with time to disease worsening or death as primary outcomes.
Etanercept (Enbrel®)
is an antagonist of tumor necrosis factor alpha (TNF-α) receptor. TNF-α is a cytokine, produced by monocytes and macrophages, which acts as inflammatory mediator by stimulation of leukocytes. Etanercept is a soluble TNF-α receptor, which inhibits TNF-α and thereby inhibits the inflammatory response. Clinically, Etanercept is classified as immunosuppressant and plays a role in the treatment of inflammatory immune diseases such as rheumatoid arthritis, psoriasis or psoriatic arthritis, and ankylosing spondylitis. Anti-fibrotic properties have been found in a recent bleomycin animal study (Fichtner-Feigl, Strober, Kawakami, Puri, & Kitani, 2006). Despite TNF-α being a prototype inflammatory molecule, the benefits in the bleomycin model have been interpreted as result of preventing IL-13-R-α2 expression by TNF-α blockage, leading to reduction of TGF-β expression and consequently to less inflammation and fibrosis (Fichtner-Feigl, Strober, Kawakami, Puri, & Kitani, 2006). Antagonism of TNF-α has previously been described as a method to prevent fibrosis (Piguet, Collart, Grau, Kapanci, & Vassalli, 1989) and showed some effects on established fibrosis in the bleomycin model (Piguet & Vesin, 1994). Recently, a double-blind, placebo-controlled, randomized, phase II study in IPF patients has been initiated. The outcomes will be analyzed regarding safety and efficacy, quality of life and pharmacokinetics comparing Etanercept treatment vs. no treatment (Wyeth, 2007).
None of the other drugs mentioned above in the experimental animal model have been able to qualify for clinical use, due to lack of beneficial outcome, adverse drug effects or deficits in study design. Other clinical trials, for instance a phase II study, enrolling 120 IPF patients treated with the tyrosine kinase inhibitor Imatinib (Gleevec®), are yet to be analyzed. Further trials in progress are CAPACITY, investigating the clinical usefulness of Pirfenidone in ∼600 patients, as well as a recently initiated TGF-β antibody (GC1008®) study. Unfortunately, the largest trial in IPF to date, a study investigating the effect of interferon-1β (Actimmune®) treatment in more than 800 patients (INSPIRE), was terminated in early 2007 because of inefficacy in an interim analysis.
Summary
Major discrepancies between drug effects in animal models and in human trials have recently been pointed out, which may be due to design of the models, assessment tools for determination of drug efficacy, and timing of drug application (Perel et al., 2007). These facts have to be taken into consideration, especially for long term drug intervention studies. In the context of pulmonary fibrosis and the bleomycin model it means that experimental findings have to be interpreted carefully, with the knowledge that bleomycin fibrosis in the animal lacks important features of the human disease. Further, the assessment tools used to determine drug efficacy may need to be re-evaluated. Finally, drug intervention studies in the bleomycin model employing a preventive strategy appear to be difficult to translate to human disease. Using a therapeutic strategy will likely have more validity in determining “real” antifibrotic drug effects.
In conclusion, this review suggests that the bleomycin model of pulmonary fibrosis is very helpful to illustrate pathobiology in vivo and to identify new targets for medication, and it is a good tool to assess efficacy of potential compounds in general as proof of principle. However, the bleomycin model may be of limited valid to detailed assess and evaluate these novel drugs for clinical use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson IY. Pulmonary toxicity of bleomycin. Environ Health Perspect. 1976;16:119–125. doi: 10.1289/ehp.7616119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77(2):185–197. [PMC free article] [PubMed] [Google Scholar]
- Alton EW, Johnson M, Turner-Warwick M. Advanced cryptogenic fibrosing alveolitis: preliminary report on treatment with cyclosporin A. Respir Med. 1989;83(4):277–279. doi: 10.1016/s0954-6111(89)80196-9. [DOI] [PubMed] [Google Scholar]
- Ammar YA, Ismail MM, El-Sehrawi HM, Noaman E, Bayomi AH, Shawer TZ. Novel pirfenidone analogues: synthesis of pyridin-2-ones for the treatment of pulmonary fibrosis. Arch Pharm (Weinheim) 2006;339(8):429–436. doi: 10.1002/ardp.200600017. [DOI] [PubMed] [Google Scholar]
- Antoniou KM, Nicholson AG, Dimadi M, Malagari K, Latsi P, Rapti A, et al. Long-term clinical effects of interferon gamma-1b and colchicine in idiopathic pulmonary fibrosis. Eur Respir J. 2006;28(3):496–504. doi: 10.1183/09031936.06.00032605. [DOI] [PubMed] [Google Scholar]
- Aoki F, Kurabayashi M, Hasegawa Y, Kojima I. Attenuation of bleomycin-induced pulmonary fibrosis by follistatin. Am J Respir Crit Care Med. 2005;172(6):713–720. doi: 10.1164/rccm.200412-1620OC. [DOI] [PubMed] [Google Scholar]
- Aono Y, Nishioka Y, Inayama M, Ugai M, Kishi J, Uehara H, et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2005;171(11):1279–1285. doi: 10.1164/rccm.200404-531OC. [DOI] [PubMed] [Google Scholar]
- Arai T, Abe K, Matsuoka H, Yoshida M, Mori M, Goya S, et al. Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278(5):L914–922. doi: 10.1152/ajplung.2000.278.5.L914. [DOI] [PubMed] [Google Scholar]
- Arslan SO, Zerin M, Vural H, Coskun A. The effect of melatonin on bleomycin-induced pulmonary fibrosis in rats. J Pineal Res. 2002;32(1):21–25. doi: 10.1034/j.1600-079x.2002.10796.x. [DOI] [PubMed] [Google Scholar]
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATS American Thoracic Society. Idiopathic Pulmonary Fibrosis: Diagnosis and Treatment International Consensus Statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- ATS American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- Atzori L, Chua F, Dunsmore SE, Willis D, Barbarisi M, McAnulty RJ, et al. Attenuation of bleomycin induced pulmonary fibrosis in mice using the heme oxygenase inhibitor Zn-deuteroporphyrin IX-2,4-bisethylene glycol. Thorax. 2004;59(3):217–223. doi: 10.1136/thx.2003.008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2006;174(4):420–427. doi: 10.1164/rccm.200603-333OC. [DOI] [PubMed] [Google Scholar]
- Azuma A, Furuta T, Enomoto T, Hashimoto Y, Uematsu K, Nukariya N, et al. Preventive effect of erythromycin on experimental bleomycin-induced acute lung injury in rats. Thorax. 1998;53(3):186–189. doi: 10.1136/thx.53.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma A, Li Y, Usuki J, Aoyama A, Enomoto T, Kudoh S. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule-1 messenger RNA induction preventing neutrophil-induced lung injury and fibrosis in bleomycin-challenged mice. Chest. 2001;120(1 Suppl):20S–22S. doi: 10.1378/chest.120.1_suppl.s20-a. [DOI] [PubMed] [Google Scholar]
- Azuma A, Li YJ, Abe S, Usuki J, Matsuda K, Henmi S, et al. Interferon-{beta} inhibits bleomycin-induced lung fibrosis by decreasing transforming growth factor-{beta} and thrombospondin. Am J Respir Cell Mol Biol. 2005;32(2):93–98. doi: 10.1165/rcmb.2003-0374OC. [DOI] [PubMed] [Google Scholar]
- Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- Blaisdell RJ, Giri SN. Mechanism of antifibrotic effect of taurine and niacin in the multidose bleomycin-hamster model of lung fibrosis: inhibition of lysyl oxidase and collagenase. J Biochem Toxicol. 1995;10(4):203–210. doi: 10.1002/jbt.2570100404. [DOI] [PubMed] [Google Scholar]
- Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L719–726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- Boyaci H, Maral H, Turan G, Basyigit I, Dillioglugil MO, Yildiz F, et al. Effects of erdosteine on bleomycin-induced lung fibrosis in rats. Mol Cell Biochem. 2006;281(1−2):129–137. doi: 10.1007/s11010-006-0640-3. [DOI] [PubMed] [Google Scholar]
- Breuer R, Lossos IS, Or R, Krymsky M, Dagan A, Yedgar S. Abatement of bleomycin-induced pulmonary injury by cell-impermeable inhibitor of phospholipase A2. Life Sci. 1995;57(16):PL237–240. doi: 10.1016/0024-3205(95)02116-z. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Dick R, Ullenbruch MR, Jin H, Phan SH. Inhibition of key cytokines by tetrathiomolybdate in the bleomycin model of pulmonary fibrosis. J Inorg Biochem. 2004;98(12):2160–2167. doi: 10.1016/j.jinorgbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Ullenbruch MR, Dick R, Olivarez L, Phan SH. Tetrathiomolybdate therapy protects against bleomycin-induced pulmonary fibrosis in mice. J Lab Clin Med. 2003;141(3):210–216. doi: 10.1067/mlc.2003.20. [DOI] [PubMed] [Google Scholar]
- Burdick MD, Murray LA, Keane MP, Xue YY, Zisman DA, Belperio JA, et al. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171(3):261–268. doi: 10.1164/rccm.200409-1164OC. [DOI] [PubMed] [Google Scholar]
- Chandler DB, Fulmer JD. The effect of deferoxamine on bleomycin-induced lung fibrosis in the hamster. Am Rev Respir Dis. 1985;131(4):596–598. doi: 10.1164/arrd.1985.131.4.596. [DOI] [PubMed] [Google Scholar]
- Chandler DB, Young K. The effect of diclofenac acid (Voltaren) on bleomycin-induced pulmonary fibrosis in hamsters. Prostaglandins Leukot Essent Fatty Acids. 1989;38(1):9–14. doi: 10.1016/0952-3278(89)90141-5. [DOI] [PubMed] [Google Scholar]
- Chapela R, Zuniga G, Selman M. D-penicillamine in the therapy of fibrotic lung diseases. Int J Clin Pharmacol Ther Toxicol. 1986;24(1):16–17. [PubMed] [Google Scholar]
- Chaudary NI, R. G, Hilberg F, Mueller-Quernheim J, Prasse A, Zissel G, Schnapps A, Park JE. Inhibitions of PDGF VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007 2007 May;29(5):976–85. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- Chaudhary NI, Schnapp A, Park JE. Pharmacologic Differentiation of Inflammation and Fibrosis in the Rat Bleomycin Model. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200505-717OC. [DOI] [PubMed] [Google Scholar]
- Chen B, Jiang L, Zhao W, Yu R, Hou XM. Prevention of bleomycin-induced on bleomycin-induced pulmonary fibrosis: role of alveolar macrophage activation and cytokine release. Respirology. 1997;2(2):151–155. doi: 10.1111/j.1440-1843.1997.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Chen F, Gong L, Zhang L, Wang H, Qi X, Wu X, et al. Short courses of low dose dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur J Pharmacol. 2006;536(3):287–295. doi: 10.1016/j.ejphar.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Chen J, He B, Li Y, Wang G, Zhang W. An experimental study on the effect of azithromycin treatment in bleomycin-induced pulmonary fibrosis of rats. Zhonghua Nei Ke Za Zhi. 1999;38(10):677–680. [PubMed] [Google Scholar]
- Chen XL, Huang SS, Li WB, Wang DH, Wang XL. Inhibitory effect of aminoguanidine on bleomycin-induced pulmonary toxicity in rat. Acta Pharmacol Sin. 2001;22(8):711–715. [PubMed] [Google Scholar]
- Chen XL, Li WB, Zhou AM, Ai J, Huang SS. Role of endogenous peroxynitrite in pulmonary injury and fibrosis induced by bleomycin A5 in rats. Acta Pharmacol Sin. 2003;24(7):697–702. [PubMed] [Google Scholar]
- Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33(1):9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- Claussen CA, Long EC. Nucleic Acid recognition by metal complexes of bleomycin. Chem Rev. 1999;99(9):2797–2816. doi: 10.1021/cr980449z. [DOI] [PubMed] [Google Scholar]
- Compendium of Pharmaceuticals and Specialties. Blenoxane®. Canadian Pharmacists Association. 2006.
- Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol. 2001;193(4):538–545. doi: 10.1002/path.826. [DOI] [PubMed] [Google Scholar]
- Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, et al. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J. 2001;17(6):1228–1235. doi: 10.1183/09031936.01.00049701. [DOI] [PubMed] [Google Scholar]
- Daba MH, Abdel-Aziz AA, Moustafa AM, Al-Majed AA, Al-Shabanah OA, El-Kashef HA. L-carnitine and ginkgo biloba extract (EG b 761) in experimental bleomycin-induced lung fibrosis. Pharmacol Res. 2002;45(6):461–467. doi: 10.1006/phrs.2002.0985. [DOI] [PubMed] [Google Scholar]
- Dai L, Hou J, Cai H. Using ligustrazini and angelica sinensis treat the bleomycin-induced pulmonary fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi. 1996;19(1):26–28. [PubMed] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114(9):1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniil ZD, Gilchrist FC, Nicholson AG, Hansell DM, Harris J, Colby TV, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 1999;160(3):899–905. doi: 10.1164/ajrccm.160.3.9903021. [DOI] [PubMed] [Google Scholar]
- de Rezende MC, Martinez JA, Capelozzi VL, Simoes MJ, Beppu OS. Protective effect of aminoguanidine in a murine model of pulmonary fibrosis induced by bleomycin. Fundam Clin Pharmacol. 2000;14(6):561–567. doi: 10.1111/j.1472-8206.2000.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- Deterding RR, Havill AM, Yano T, Middleton SC, Jacoby CR, Shannon JM, et al. Prevention of bleomycin-induced lung injury in rats by keratinocyte growth factor. Proc Assoc Am Physicians. 1997;109(3):254–268. [PubMed] [Google Scholar]
- Dik WA, McAnulty RJ, Versnel MA, Naber BA, Zimmermann LJ, Laurent GJ, et al. Short course dexamethasone treatment following injury inhibits bleomycin induced fibrosis in rats. Thorax. 2003;58(9):765–771. doi: 10.1136/thorax.58.9.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi M, Hasegawa T, Yamamoto K, Marshall BC. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am J Respir Crit Care Med. 2000;162(6):2302–2307. doi: 10.1164/ajrccm.162.6.9908097. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ryu JH, Bjoraker JA, Schroeder DR, Myers JL, Tazelaar HD, et al. Colchicine versus prednisone as treatment of usual interstitial pneumonia. Mayo Clin Proc. 1997;72(3):201–209. doi: 10.4065/72.3.201. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: Impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1172–1178. doi: 10.1164/ajrccm.161.4.9907002. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ryu JH, Swensen SJ, Offord KP, Schroeder DR, Caron GM, et al. Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis. A randomized prospective study. Members of the Lung Study Group. Am J Respir Crit Care Med. 1998;158(1):220–225. doi: 10.1164/ajrccm.158.1.9709089. [DOI] [PubMed] [Google Scholar]
- Dubey AK, Shankar PR, Upadhyaya D, Deshpande VY. Ginkgo biloba--an appraisal. Kathmandu Univ Med J (KUMJ) 2004;2(3):225–229. [PubMed] [Google Scholar]
- Ekimoto H, Takada K, Takahashi K, Matsuda A, Takita T, Umezawa H. Effect of oxygen concentration on pulmonary fibrosis caused by peplomycin in mice. J Antibiot (Tokyo) 1984;37(6):659–663. doi: 10.7164/antibiotics.37.659. [DOI] [PubMed] [Google Scholar]
- El-Medany A, Hagar HH, Moursi M, At Muhammed R, El-Rakhawy FI, El-Medany G. Attenuation of bleomycin-induced lung fibrosis in rats by mesna. Eur J Pharmacol. 2005;509(1):61–70. doi: 10.1016/j.ejphar.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Entzian P, Gerlach C, Gerdes J, Schlaak M, Zabel P. Pentoxifylline inhibits experimental bleomycin-induced fibrosing alveolitis. Pneumologie. 1997;51(4):375–380. [PubMed] [Google Scholar]
- Entzian P, Zahringer U, Schlaak M, Gerlach C, Galle J, Zabel P. Comparative study on effects of pentoxifylline, prednisolone and colchicine in experimental alveolitis. Int J Immunopharmacol. 1998;20(12):723–735. doi: 10.1016/s0192-0561(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John's Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann Intern Med. 2002;136(1):42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- Fang X, Zhu Y, Hu X, Liu Y. Losartan in the rat model of bleomycin-induced pulmonary fibrosis and its impact on the expression of monocyte chemoattractant protein-1 and basic fibroblast growth factor. Zhonghua Jie He He Hu Xi Za Zhi. 2002;25(5):268–272. [PubMed] [Google Scholar]
- Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- Fleischman RW, Baker JR, Thompson GR, Schaeppi UH, Illievski VR, Cooney DA, et al. Bleomycin-induced interstitial pneumonia in dogs. Thorax. 1971;26(6):675–682. doi: 10.1136/thx.26.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SB, Rakieten N, Raisfeld-Danse IH. Studies of the effects of alpha-difluoromethylornithine and ethanol on the pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Toxicol Appl Pharmacol. 1983;71(3):307–315. doi: 10.1016/0041-008x(83)90017-0. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Gabazza EC, Taguchi O, Nishii Y, Nakahara H, Bruno NE, et al. Thrombin-activatable fibrinolysis inhibitor deficiency attenuates bleomycin-induced lung fibrosis. Am J Pathol. 2006;168(4):1086–1096. doi: 10.2353/ajpath.2006.050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Shannon JM, Morikawa O, Gauldie J, Hara N, Mason RJ. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol. 2003;29(6):669–676. doi: 10.1165/rcmb.2002-0046OC. [DOI] [PubMed] [Google Scholar]
- Fujita M, Ye Q, Ouchi H, Harada E, Inoshima I, Kuwano K, et al. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob Agents Chemother. 2006;50(2):739–743. doi: 10.1128/AAC.50.2.739-743.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeng DP, Geiser M, Cruz-Orive LM, Larsen SE, Schaffner T, Laissue JA, et al. Paradoxical effects of bleomycin and heavy water (D2O) in mice. Int J Cancer. 1995;62(6):784–790. doi: 10.1002/ijc.2910620623. [DOI] [PubMed] [Google Scholar]
- Gao JM, Lu B. The pivotal role of CXCR3 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28(1):28–32. [PubMed] [Google Scholar]
- Geismar LS, Hennessey S, Reiser KM, Last JA. D-penicillamine prevents collagen accumulation in lungs of rats given bleomycin. Chest. 1986;89(3 Suppl):153S–154S. doi: 10.1378/chest.89.3_supplement.153s. [DOI] [PubMed] [Google Scholar]
- Genovese T, Cuzzocrea S, Di Paola R, Mazzon E, Mastruzzo C, Catalano P, et al. Effect of rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur Respir J. 2005;25(2):225–234. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- Genovese T, Di Paola R, Mazzon E, Muia C, Caputi AP, Cuzzocrea S. Melatonin limits lung injury in bleomycin treated mice. J Pineal Res. 2005;39(2):105–112. doi: 10.1111/j.1600-079X.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- Genzyme Phase I, Open-Label, Multi-Center, Single-Dose, Dose-Escalating, Safety, Tolerability and Pharmacokinetic Study of GC1008 in Patients With IPF. 2007.
- Giri SN, Biring I, Nguyen T, Wang Q, Hyde DM. Abrogation of bleomycin-induced lung fibrosis by nitric oxide synthase inhibitor, aminoguanidine in mice. Nitric Oxide. 2002;7(2):109–118. doi: 10.1016/s1089-8603(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Giri SN, Hyde DM. Ameliorating effect of an interferon inducer polyinosinic-polycytidylic acid on bleomycin-induced lung fibrosis in hamsters. Morphologic and biochemical evidence. Am J Pathol. 1988;133(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54(11):1205–1216. doi: 10.1016/s0006-2952(97)00343-2. [DOI] [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48(10):959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SN, Sharma AK, Hyde DM, Wild JS. Amelioration of bleomycin-induced lung fibrosis by treatment with the platelet activating factor receptor antagonist WEB 2086 in hamsters. Exp Lung Res. 1995;21(2):287–307. doi: 10.3109/01902149509068833. [DOI] [PubMed] [Google Scholar]
- Giri SN, Wang Q. Taurine and niacin offer a novel therapeutic modality in prevention of chemically-induced pulmonary fibrosis in hamsters. Adv Exp Med Biol. 1992;315:329–340. doi: 10.1007/978-1-4615-3436-5_39. [DOI] [PubMed] [Google Scholar]
- Gong LK, Li XH, Wang H, Zhang L, Cai Y, Qi XM, et al. Feitai attenuates bleomycin-induced pulmonary fibrosis in rats. Biol Pharm Bull. 2004;27(5):634–640. doi: 10.1248/bpb.27.634. [DOI] [PubMed] [Google Scholar]
- Gong LK, Li XH, Wang H, Zhang L, Chen FP, Cai Y, et al. Effect of Feitai on bleomycin-induced pulmonary fibrosis in rats. J Ethnopharmacol. 2005;96(3):537–544. doi: 10.1016/j.jep.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Grande NR, P. M, de Sá CM, Águas AP. Lung fibrosis induced by bleomycin: structural changes and overview of recent advances. Scanning Microscopy. 1998;12(3):487–494. [Google Scholar]
- Greco MJ, Kemnitzer JE, Fox JD, Choe JK, Kohn J, Riley DJ, et al. Polymer of proline analogue with sustained antifibrotic activity in lung fibrosis. Am J Respir Crit Care Med. 1997;155(4):1391–1397. doi: 10.1164/ajrccm.155.4.9105084. [DOI] [PubMed] [Google Scholar]
- Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, et al. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med. 2003;168(11):1358–1365. doi: 10.1164/rccm.2201082. [DOI] [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21(5):791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Hollinger MA, Giri SN. Regulation of transforming growth factor-beta1 mRNA expression by taurine and niacin in the bleomycin hamster model of lung fibrosis. Am J Respir Cell Mol Biol. 1998;18(3):334–342. doi: 10.1165/ajrcmb.18.3.2867. [DOI] [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. 1999. Am J Physiol. 276(2 Pt 1):L311–318. doi: 10.1152/ajplung.1999.276.2.L311. [DOI] [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Iyer SN, Hollinger MA, Giri SN. Procollagen gene expression is down-regulated by taurine and niacin at the transcriptional level in the bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1996;277(2):1152–1157. [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Wang Y, Giri SN. Taurine and niacin block lung injury and fibrosis by down-regulating bleomycin-induced activation of transcription nuclear factor-kappaB in mice. J Pharmacol Exp Ther. 2000;293(1):82–90. [PubMed] [Google Scholar]
- Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med. 2000;162(1):225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, et al. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol. 2005;175(2):1224–1231. doi: 10.4049/jimmunol.175.2.1224. [DOI] [PubMed] [Google Scholar]
- Hart DA, Whidden P, Green F, Henkin J, Woods DE. Partial reversal of established bleomycin-induced pulmonary fibrosis by rh-urokinase in a rat model. Clin Invest Med. 1994;17(2):69–76. [PubMed] [Google Scholar]
- Hattori N, Mizuno S, Yoshida Y, Chin K, Mishima M, Sisson TH, et al. The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism. Am J Pathol. 2004;164(3):1091–1098. doi: 10.1016/S0002-9440(10)63196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman EH, Hasinoff BB, Zhang J, Raley LG, Zhang TM, Fukuda Y, et al. Morphologic and morphometric evaluation of the effect of ICRF-187 on bleomycin-induced pulmonary toxicity. Toxicology. 1995;98(1−3):163–175. doi: 10.1016/0300-483x(94)02987-6. [DOI] [PubMed] [Google Scholar]
- Honore S, Attalah HL, Azoulay E, Soussy CJ, Saudubray F, Harf A, et al. Beneficial effect of an inhibitor of leukocyte elastase (EPI-hNE-4) in presence of repeated lung injuries. Shock. 2004;22(2):131–136. doi: 10.1097/01.shk.0000126861.77543.d0. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Nakamura H, Okamoto M, Kato S, Araya S, Nomiyama K, et al. Redox-active protein thioredoxin prevents proinflammatory cytokine- or bleomycin-induced lung injury. Am J Respir Crit Care Med. 2003;168(9):1075–1083. doi: 10.1164/rccm.200209-982OC. [DOI] [PubMed] [Google Scholar]
- Howell DC, Goldsack NR, Marshall RP, McAnulty RJ, Starke R, Purdy G, et al. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2001;159(4):1383–1395. doi: 10.1016/S0002-9440(10)62525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, et al. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166(5):1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC, Laurent GJ, Chambers RC. Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochem Soc Trans. 2002;30(2):211–216. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Hu J, Xu Q, Li B. The effect of aminoguanidine, a nitric oxide synthase inhibitor, on bleomycin-induced lung injury in rat. Zhonghua Jie He He Hu Xi Za Zhi. 1999;22(1):51–53. [PubMed] [Google Scholar]
- Hua G, Cui Y, Liu J. Bleomycin-induced fibroblast proliferation is inhibited by IH764−3. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1994;16(5):390–393. [PubMed] [Google Scholar]
- Huaux F, Gharaee-Kermani M, Liu T, Morel V, McGarry B, Ullenbruch M, et al. Role of Eotaxin-1 (CCL11) and CC chemokine receptor 3 (CCR3) in bleomycin-induced lung injury and fibrosis. Am J Pathol. 2005;167(6):1485–1496. doi: 10.1016/S0002-9440(10)61235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DM, Giri SN. Polyinosinic-polycytidylic acid, an interferon inducer, ameliorates bleomycin-induced lung fibrosis in mice. Exp Lung Res. 1990;16(5):533–546. doi: 10.3109/01902149009068825. [DOI] [PubMed] [Google Scholar]
- Hyde DM, Giri SN, Schiedt MJ, Krishna GA. Effects of three cysteine pro-drugs on bleomycin-induced lung fibrosis in hamsters. Pathology. 1990;22(2):93–101. doi: 10.3109/00313029009063787. [DOI] [PubMed] [Google Scholar]
- Hyde DM, Henderson TS, Giri SN, Tyler NK, Stovall MY. Effect of murine gamma interferon on the cellular responses to bleomycin in mice. Exp Lung Res. 1988;14(5):687–704. doi: 10.3109/01902148809087837. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hirose N, Koto H, Hirano H, Shigematsu N. Fibrin deposition and fibrinolysis in the pathogenesis of pulmonary fibrosis. Nihon Kyobu Shikkan Gakkai Zasshi. 1989;27(4):448–451. [PubMed] [Google Scholar]
- Ikezaki S, Nishikawa A, Enami T, Furukawa F, Imazawa T, Uneyama C, et al. Inhibitory effects of the dietary antioxidants butylated hydroxyanisole and butylated hydroxytoluene on bronchioloalveolar cell proliferation during the bleomycin-induced pulmonary fibrosing process in hamsters. Food Chem Toxicol. 1996;34(4):327–335. doi: 10.1016/0278-6915(96)00127-5. [DOI] [PubMed] [Google Scholar]
- Inayama M, Nishioka Y,, Azuma M, Muto S, Aono Y, Makino H, et al. A Novel I (kappa)B kinase-{beta} Inhibitor Ameliorates Bleomycin-induced Pulmonary Fibrosis in Mice. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200506-947OC. [DOI] [PubMed] [Google Scholar]
- Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, Maeyama T, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L1038–1044. doi: 10.1152/ajplung.00167.2003. [DOI] [PubMed] [Google Scholar]
- Inoshima I, Kuwano K, Hamada N, Yoshimi M, Maeyama T, Hagimoto N, et al. Induction of CDK inhibitor p21 gene as a new therapeutic strategy against pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L727–733. doi: 10.1152/ajplung.00209.2003. [DOI] [PubMed] [Google Scholar]
- Iraz M, Erdogan H, Kotuk M, Yagmurca M, Kilic T, Ermis H, et al. Ginkgo biloba inhibits bleomycin-induced lung fibrosis in rats. Pharmacol Res. 2006 doi: 10.1016/j.phrs.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Fujimoto S, Fukuda T. Gefitinib prevents bleomycin-induced lung fibrosis in mice. Am J Respir Crit Care Med. 2006;174(5):550–556. doi: 10.1164/rccm.200509-1534OC. [DOI] [PubMed] [Google Scholar]
- Ito M, Suwabe A, Suzuki T, Tominaga M, Takahashi K. The effects of surfactant-TA on bleomycin-induced lung injury and lung fibroblast proliferation. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35(11):1163–1172. [PubMed] [Google Scholar]
- Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291(1):367–373. [PubMed] [Google Scholar]
- Iyer SN, Hyde DM, Giri SN. Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflammation. 2000;24(5):477–491. doi: 10.1023/a:1007068313370. [DOI] [PubMed] [Google Scholar]
- Iyer SN, Margolin SB, Hyde DM, Giri SN. Lung fibrosis is ameliorated by pirfenidone fed in diet after the second dose in a three-dose bleomycin-hamster model. Exp Lung Res. 1998;24(1):119–132. doi: 10.3109/01902149809046058. [DOI] [PubMed] [Google Scholar]
- Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med. 1995;125(6):779–785. [PubMed] [Google Scholar]
- Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83(3):111–119. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankov RP, Luo X, Demin P, Aslam R, Hannam V, Tanswell AK, et al. Hepoxilin analogs inhibit bleomycin-induced pulmonary fibrosis in the mouse. J Pharmacol Exp Ther. 2002;301(2):435–440. doi: 10.1124/jpet.301.2.435. [DOI] [PubMed] [Google Scholar]
- Jiang L, Chen B, Li Z. Studies on the role of colchicine in bleomycin-induced pulmonary fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi. 1998;21(6):340–343. [PubMed] [Google Scholar]
- Kakugawa T, Mukae H, Hayashi T, Ishii H, Abe K, Fujii T, et al. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. Eur Respir J. 2004;24(1):57–65. doi: 10.1183/09031936.04.00120803. [DOI] [PubMed] [Google Scholar]
- Kawashima M, yatsunami J, Fukuno Y, Nagata M, Tominaga M, Hayashi S. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung. 2002;180(2):73–89. doi: 10.1007/pl00021246. [DOI] [PubMed] [Google Scholar]
- Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L92–97. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- Kelley J, Newman RA, Evans JN. Bleomycin-induced pulmonary fibrosis in the rat. Prevention with an inhibitor of collagen synthesis. J Lab Clin Med. 1980;96(6):954–964. [PubMed] [Google Scholar]
- Kennedy JI,, Jr., Chandler DB, Fulmer JD, Wert MB, Grizzle WE. Dietary fish oil inhibits bleomycin-induced pulmonary fibrosis in the rat. Exp Lung Res. 1989;15(2):315–329. doi: 10.3109/01902148909087861. [DOI] [PubMed] [Google Scholar]
- Kijiyama N, Ueno H, Sugimoto I, Sasaguri Y, Yatera K, Kido M, et al. Intratracheal gene transfer of tissue factor pathway inhibitor attenuates pulmonary fibrosis. Biochem Biophys Res Commun. 2006;339(4):1113–1119. doi: 10.1016/j.bbrc.2005.11.127. [DOI] [PubMed] [Google Scholar]
- Kilinc C, Ozcan O, Karaoz E, Sunguroglu K, Kutluay T, Karaca L. Vitamin E reduces bleomycin-induced lung fibrosis in mice: biochemical and morphological studies. J Basic Clin Physiol Pharmacol. 1993;4(3):249–269. doi: 10.1515/jbcpp.1993.4.3.249. [DOI] [PubMed] [Google Scholar]
- Kim DS, Collard HR, King TE,, Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim HY, Kim S, Chung JH, Park WS, Chung DH. Natural killer T (NKT) cells attenuate bleomycin-induced pulmonary fibrosis by producing interferon-gamma. Am J Pathol. 2005;167(5):1231–1241. doi: 10.1016/s0002-9440(10)61211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Ishii Y, Morishima Y, Shibuya A, Shibuya K, Taniguchi M, et al. Treatment with alpha-galactosylceramide attenuates the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2004;172(9):5782–5789. doi: 10.4049/jimmunol.172.9.5782. [DOI] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Galt T, Sime PJ, Xing Z, Schmidt M, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med. 2001;163(3 Pt 1):770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- Kong L, Wang JF, Niu JZ, Xia ZG, Qiao C, Wei YF, et al. The protective effects of Fufang Biejiafang on bleomycin-induced pulmonary fibrosis in rats. Zhongguo Zhong Yao Za Zhi. 2005;30(3):204–207. [PubMed] [Google Scholar]
- Krishna G, Liu K, Shigemitsu H, Gao M, Raffin TA, Rosen GD. PG490−88, a derivative of triptolide, blocks bleomycin-induced lung fibrosis. Am J Pathol. 2001;158(3):997–1004. doi: 10.1016/S0002-9440(10)64046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128(3):1475–1482. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, et al. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L316–325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- Ledwozyw A. The effect of colchicine and vinblastine on bleomycin-induced lung fibrosis in rats. Acta Physiol Hung. 1994;82(4):383–389. [PubMed] [Google Scholar]
- Ledwozyw A. The influence of proline analogs on bleomycin-induced lung injury in rats. Acta Physiol Hung. 1995;83(2):195–202. [PubMed] [Google Scholar]
- Li HP, Li X, He GJ, Yi XH, Kaplan AP. The influence of dexamethasone on the proliferation and apoptosis of pulmonary inflammatory cells in bleomycin-induced pulmonary fibrosis in rats. Respirology. 2004;9(1):25–32. doi: 10.1111/j.1440-1843.2003.00523.x. [DOI] [PubMed] [Google Scholar]
- Li R, Li WJ, Cai YN, Li ZG, Luo Q, Zhou MJ, et al. Effects of moxibustion at Feishu (BL 13) and Gaohuang (BL 43) on expression of TGF-beta1 in the bleomycin-induced pulmonary fibrosis. Zhongguo Zhen Jiu. 2005;25(11):790–792. [PubMed] [Google Scholar]
- Li X, Cui S. The mechanisms and effects of panax notoginside and methylprednisolone in a rat model of pulmonary fibrosis. Zhonghua Jie He He Hu Xi Za Zhi. 2002;25(9):520–523. [PubMed] [Google Scholar]
- Li Y, Azuma A, Takahashi S, Usuki J, Matsuda K, Aoyama A, et al. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migration: role in preventing lung injury and fibrosis in bleomycin-challenged mice. Chest. 2002;122(6):2137–2145. doi: 10.1378/chest.122.6.2137. [DOI] [PubMed] [Google Scholar]
- Li Y, Azuma A, Usuki J, Abe S, Matsuda K, Sunazuka T, et al. EM703 improves bleomycin-induced pulmonary fibrosis in mice by the inhibition of TGF-beta signaling in lung fibroblasts. Respir Res. 2006;7(1):16. doi: 10.1186/1465-9921-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Azuma A, Usuki J, Matsuda K, Aoyama A, Kudoh S. Attenuated mRNA induction of molecules associated with neutrophil migration by 14-membered ring macrolides inhibits bleomycin induced acute lung injury in mice. J Nippon Med Sch. 2002;69(3):252–261. doi: 10.1272/jnms.69.252. [DOI] [PubMed] [Google Scholar]
- Li Y, He B, Wang H. The effect of erythromycin on NF-kappa B activation and cytokines mRNA expression in bleomycin-induced pulmonary fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi. 1999;22(12):725–727. [PubMed] [Google Scholar]
- Lindenschmidt RC, Witschi H. Attenuation of pulmonary fibrosis in mice by aminophylline. Biochem Pharmacol. 1985;34(24):4269–4273. doi: 10.1016/0006-2952(85)90283-7. [DOI] [PubMed] [Google Scholar]
- Liu F, Xu QY, Ye YQ. Effects of valsartan on bleomycin-induced pulmonary fibrosis in rats and the expression of hepatocyte growth factor in lung tissue. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28(7):479–483. [PubMed] [Google Scholar]
- Liu J. Experimental study of the effect of IH764−3, a potent component isolated from salviae milltiorrhize, against pulmonary fibrosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1992;14(4):250–256. [PubMed] [Google Scholar]
- Liu J, Hua G, Wang H, Cui Y, Liu Y, Chu Y, et al. Experimental study of the effect of IH764−3 on pulmonary fibrosis. Chin Med Sci J. 1993;8(1):9–14. [PubMed] [Google Scholar]
- Lossos IS, Or R, Goldstein RH, Conner MW, Breuer R. Amelioration of bleomycin-induced pulmonary injury by cyclosporin A. Exp Lung Res. 1996;22(3):337–349. doi: 10.3109/01902149609031779. [DOI] [PubMed] [Google Scholar]
- Ma J, He B, Li N, Zhang H. Intervention by azithromycin on bleomycin-induced lung injury in rats and its mechanisms. Zhonghua Jie He He Hu Xi Za Zhi. 2002;25(7):392–395. [PubMed] [Google Scholar]
- Maeyama T, Kuwano K, Kawasaki M, Kunitake R, Hagimoto N, Hara N. Attenuation of bleomycin-induced pneumopathy in mice by monoclonal antibody to interleukin-12. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1128–1137. doi: 10.1152/ajplung.2001.280.6.L1128. [DOI] [PubMed] [Google Scholar]
- Mageto Y, K. F, Brown K, Fong A, Raghu G. Safety and Tolerability of Human Monoclonal Antibody FG-3019, Anti-connective Tissue Growth Factor, in Patients with Idiopathic Pulmonary Fibrosis. CHEST Meeting Abstracts. 2004;126:773. [Google Scholar]
- Mahady GB. Ginkgo biloba for the prevention and treatment of cardiovascular disease: a review of the literature. J Cardiovasc Nurs. 2002;16(4):21–32. doi: 10.1097/00005082-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Mall G, Zimmermann P, Siemens I, Burkhardt A, Otto HF. Prevention of bleomycin-induced fibrosing alveolitis with indomethacin: stereological studies on rat lungs. Virchows Arch A Pathol Anat Histopathol. 1991;419(4):339–347. doi: 10.1007/BF01606525. [DOI] [PubMed] [Google Scholar]
- Mancini GB, Khalil N. Angiotensin II type 1 receptor blocker inhibits pulmonary injury. Clin Invest Med. 2005;28(3):118–126. [PubMed] [Google Scholar]
- Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, et al. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6(1):11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor JK, Chen AT, Schelegle ES, Giri S. Effect of diet-ingested pirfenidone on pulmonary function, cardiovasculature and blood gas measurements in rats. Res Commun Mol Pathol Pharmacol. 1999;103(3):260–268. [PubMed] [Google Scholar]
- Marshall RP, Gohlke P, Chambers RC, Howell DC, Bottoms SE, Unger T, et al. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286(1):L156–164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- Mata M, Ruiz A, Cerda M, Martinez-Losa M, Cortijo J, Santangelo F, et al. Oral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in rats. Eur Respir J. 2003;22(6):900–905. doi: 10.1183/09031936.03.00018003. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Arai T, Mori M, Goya S, Kida H, Morishita H, et al. A p38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;283(1):L103–112. doi: 10.1152/ajplung.00187.2001. [DOI] [PubMed] [Google Scholar]
- Matsuyama W, Watanabe M, Shirahama Y, Hirano R, Mitsuyama H, Higashimoto I, et al. Suppression of discoidin domain receptor 1 by RNA interference attenuates lung inflammation. J Immunol. 2006;176(3):1928–1936. doi: 10.4049/jimmunol.176.3.1928. [DOI] [PubMed] [Google Scholar]
- McLaughlin GE, Frank L. Effects of the 21-aminosteroid, U74389F, on bleomycin-induced pulmonary fibrosis in rats. Crit Care Med. 1994;22(2):313–319. doi: 10.1097/00003246-199402000-00024. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi H, Asano S, Nonaka T, Hamamura I, Masuda K, Kiyoki M. Administration of truncated secretory leukoprotease inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am J Respir Crit Care Med. 1996;153(1):369–374. doi: 10.1164/ajrccm.153.1.8542145. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. Faseb J. 2005;19(6):580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- Molina-Molina M, Serrano-Mollar A, Bulbena O, Fernandez-Zabalegui L, Closa D, Marin-Arguedas A, et al. Losartan attenuates bleomycin induced lung fibrosis by increasing prostaglandin E2 synthesis. Thorax. 2006;61(7):604–610. doi: 10.1136/thx.2005.051946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolman JA, Bardin PG, Rossouw DJ, Joubert JR. Cyclosporin as a treatment for interstitial lung disease of unknown aetiology. Thorax. 1991;46(8):592–595. doi: 10.1136/thx.46.8.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Tanaka H, Kawada K, Nagai H, Koda A. Suppressive effects of tranilast on pulmonary fibrosis and activation of alveolar macrophages in mice treated with bleomycin: role of alveolar macrophages in the fibrosis. Jpn J Pharmacol. 1995;67(4):279–289. doi: 10.1254/jjp.67.279. [DOI] [PubMed] [Google Scholar]
- Murakami S, Nagaya N, Itoh T, Fujii T, Iwase T, Hamada K, et al. C-type natriuretic peptide attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1172–1177. doi: 10.1152/ajplung.00087.2004. [DOI] [PubMed] [Google Scholar]
- Murakami S, Nagaya N, Itoh T, Kataoka M, Iwase T, Horio T, et al. Prostacyclin agonist with thromboxane synthase inhibitory activity (ONO-1301) attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L59–65. doi: 10.1152/ajplung.00042.2005. [DOI] [PubMed] [Google Scholar]
- Nadrous HF, Ryu JH, Douglas WW, Decker PA, Olson EJ. Impact of angiotensin-converting enzyme inhibitors and statins on survival in idiopathic pulmonary fibrosis. Chest. 2004;126(2):438–446. doi: 10.1378/chest.126.2.438. [DOI] [PubMed] [Google Scholar]
- Nagai A, Aoshiba K, Ishihara Y, Inano H, Sakamoto K, Yamaguchi E, et al. Administration of alpha 1-proteinase inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am Rev Respir Dis. 1992;145(3):651–656. doi: 10.1164/ajrccm/145.3.651. [DOI] [PubMed] [Google Scholar]
- Nagai A, Matsumiya H, Hayashi M, Yasui S, Okamoto H, Konno K. Effects of nicotinamide and niacin on bleomycin-induced acute injury and subsequent fibrosis in hamster lungs. Exp Lung Res. 1994;20(4):263–281. doi: 10.3109/01902149409064387. [DOI] [PubMed] [Google Scholar]
- Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002;41(12):1118–1123. doi: 10.2169/internalmedicine.41.1118. [DOI] [PubMed] [Google Scholar]
- Nagler A, Firman N, Feferman R, Cotev S, Pines M, Shoshan S. Reduction in pulmonary fibrosis in vivo by halofuginone. Am J Respir Crit Care Med. 1996;154(4 Pt 1):1082–1086. doi: 10.1164/ajrccm.154.4.8887611. [DOI] [PubMed] [Google Scholar]
- Nakatani-Okuda A, Ueda H, Kashiwamura S, Sekiyama A, Kubota A, Fujita Y, et al. Protection against bleomycin-induced lung injury by IL-18 in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L280–287. doi: 10.1152/ajplung.00380.2004. [DOI] [PubMed] [Google Scholar]
- Nicholson AG, C. T, Dubois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. . Am J Respir Crit Care Med. 2000;162:2213–2217. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- Nici L, Santos-Moore A, Kuhn C, Calabresi P. Modulation of bleomycin-induced pulmonary toxicity in the hamster by the antioxidant amifostine. Cancer. 1998;83(9):2008–2014. [PubMed] [Google Scholar]
- O'Neill CA, Giri SN. Biochemical mechanisms for the attenuation of bleomycin-induced lung fibrosis by treatment with niacin in hamsters: the role of NAD and ATP. Exp Lung Res. 1994;20(1):41–56. doi: 10.3109/01902149409064372. [DOI] [PubMed] [Google Scholar]
- O'Neill CA, Giri SN, Wang Q, Perricone MA, Hyde DM. Effects of dibutyrylcyclic adenosine monophosphate on bleomycin-induced lung toxicity in hamsters. J Appl Toxicol. 1992;12(2):97–111. doi: 10.1002/jat.2550120206. [DOI] [PubMed] [Google Scholar]
- Okada T, Sugie I, Aisaka K. Effects of gamma-interferon on collagen and histamine content in bleomycin-induced lung fibrosis in rats. Lymphokine Cytokine Res. 1993;12(2):87–91. [PubMed] [Google Scholar]
- Osanai K, Takahashi K, Suwabe A, Takada K, Ikeda H, Sato S, et al. The effect of cigarette smoke on bleomycin-induced pulmonary fibrosis in hamsters. Am Rev Respir Dis. 1988;138(5):1276–1281. doi: 10.1164/ajrccm/138.5.1276. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Takahashi H, Shiratori M, Chiba H, Abe S. Reduction of bleomycin induced lung fibrosis by candesartan cilexetil, an angiotensin II type 1 receptor antagonist. Thorax. 2004;59(1):31–38. doi: 10.1136/thx.2003.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25(2):164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- Ozyurt H, Sogut S, Yildirim Z, Kart L, Iraz M, Armutcu F, et al. Inhibitory effect of caffeic acid phenethyl ester on bleomycine-induced lung fibrosis in rats. Clin Chim Acta. 2004;339(1−2):65–75. doi: 10.1016/j.cccn.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156(2 Pt 1):600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. Bmj. 2007;334(7586):197. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan SH, Fantone JC. Inhibition of bleomycin-induced pulmonary fibrosis by lipopolysaccharide. Lab Invest. 1984;50(5):587–591. [PubMed] [Google Scholar]
- Phan SH, Kunkel SL. Inhibition of bleomycin-induced pulmonary fibrosis by nordihydroguaiaretic acid. The role of alveolar macrophage activation and mediator production. Am J Pathol. 1986;124(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992;18(1):29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Phan SH, Thrall RS. Inhibition of bleomycin-induced pulmonary fibrosis by cobra venom factor. Am J Pathol. 1982;107(1):25–28. [PMC free article] [PubMed] [Google Scholar]
- Phan SH, Thrall RS, Williams C. Bleomycin-induced pulmonary fibrosis. Effects of steroid on lung collagen metabolism. Am Rev Respir Dis. 1981;124(4):428–434. doi: 10.1164/arrd.1981.124.4.428. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Grau GE, de Kossodo S. Role of granulocyte-macrophage colony-stimulating factor in pulmonary fibrosis induced in mice by bleomycin. Exp Lung Res. 1993;19(5):579–587. doi: 10.3109/01902149309031729. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Rosen H, Vesin C, Grau GE. Effective treatment of the pulmonary fibrosis elicited in mice by bleomycin or silica with anti-CD-11 antibodies. Am Rev Respir Dis. 1993;147(2):435–441. doi: 10.1164/ajrccm/147.2.435. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Van GY, Guo J. Heparin attenuates bleomycin but not silica-induced pulmonary fibrosis in mice: possible relationship with involvement of myofibroblasts in bleomycin, and fibroblasts in silica-induced fibrosis. Int J Exp Pathol. 1996;77(4):155–161. doi: 10.1046/j.1365-2613.1996.d01-214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J. 1994;7(3):515–518. doi: 10.1183/09031936.94.07030515. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Vesin C, Grau GE, Thompson RC. Interleukin 1 receptor antagonist (IL-1ra) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine. 1993;5(1):57–61. doi: 10.1016/1043-4666(93)90024-y. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Vesin C, Thomas F. Bombesin down modulates pulmonary fibrosis elicited in mice by bleomycin. Exp Lung Res. 1995;21(2):227–237. doi: 10.3109/01902149509068829. [DOI] [PubMed] [Google Scholar]
- Poiani GJ, Greco M, Choe JK, Fox JD, Riley DJ. Liposome encapsulation improves the effect of antifibrotic agent in rat lung fibrosis. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1623–1627. doi: 10.1164/ajrccm.150.6.7524983. [DOI] [PubMed] [Google Scholar]
- Pozzi E, Luisetti M, Spialtini L, Coccia P, Rossi A, Donnini M, et al. Relationship between changes in alveolar surfactant levels and lung defence mechanisms. Respiration. 1989;55(Suppl 1):53–59. doi: 10.1159/000195752. [DOI] [PubMed] [Google Scholar]
- Pozzi E, Salmona M, Masturzo P, Genghini M, Scelsi M, Spialtini L, et al. Role of alveolar phospholipids in bleomycin-induced pulmonary fibrosis in the rat. Respiration. 1987;51(Suppl 1):23–32. doi: 10.1159/000195271. [DOI] [PubMed] [Google Scholar]
- Punithavathi D, Venkatesan N, Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol. 2000;131(2):169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Bozic C, Brown K. Feasibility of a trial of interferon beta-1a in the treatment pulmonary fibrosis. Am J Respir Crit Care Med. 2001;163:A707. [Google Scholar]
- Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350(2):125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061–1069. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- Raghu R, B. C, Brown K, Lynch D, Center D, Aguayo SMK, Lloyd K, Lull J, Kervitsky D, Schwartz DA, et al. Feasibility of a trial of interferon-gamma-1a (IFN-gamma-1a) in the treatment of idiopathic pulmonary fibrosis (IPF) [abstract]. . Am J Respir Crit Care Med. 2001;163:A707. [Google Scholar]
- Riha RL, Duhig EE, Clarke BE, Steele RH, Slaughter RE, Zimmerman PV. Survival of patients with biopsy-proven usual interstitial pneumonia and nonspecific interstitial pneumonia. Eur Respir J. 2002;19(6):1114–1118. doi: 10.1183/09031936.02.00244002. [DOI] [PubMed] [Google Scholar]
- Riley DJ, Kerr JS, Berg RA, Ianni BD, Pietra GG, Edelman NH, et al. Prevention of bleomycin-induced pulmonary fibrosis in the hamster by cis-4-hydroxy-l-proline. Am Rev Respir Dis. 1981;123(4 Pt 1):388–393. doi: 10.1164/arrd.1981.123.4.388. [DOI] [PubMed] [Google Scholar]
- Riley DJ, Kerr JS, Berg RA, Ianni BD, Pietra GG, Edelman NH, et al. beta-Aminopropionitrile prevents bleomycin-induced pulmonary fibrosis in the hamster. Am Rev Respir Dis. 1982;125(1):67–73. doi: 10.1164/arrd.1982.125.1.67. [DOI] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33(2):145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M, Takai S, Jin D, Okamoto Y, Muramatsu M, Kim S, et al. A specific chymase inhibitor, NK3201, suppresses bleomycin-induced pulmonary fibrosis in hamsters. Eur J Pharmacol. 2004;493(1−3):173–176. doi: 10.1016/j.ejphar.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kitasato H, Murakami Y, Hashimoto A, Endo H, Kondo H, et al. Down-regulation of lipoxin A4 receptor by thromboxane A2 signaling in RAW246.7 cells in vitro and bleomycin-induced lung fibrosis in vivo. Biomed Pharmacother. 2004;58(6−7):381–387. doi: 10.1016/j.biopha.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed MM, Mansour HH, Gharib OA, Hafez HF. Acetyl-L-carnitine modulates bleomycin-induced oxidative stress and energy depletion in lung tissues. J Egypt Natl Canc Inst. 2004;16(4):237–243. [PubMed] [Google Scholar]
- Schelegle ES, Mansoor JK, Giri S. Protective effect of liposomal changes in pulmonary functions in hamsters. Proc Soc Exp Biol Med. 1997;216(3):392–397. doi: 10.3181/00379727-216-44187. [DOI] [PubMed] [Google Scholar]
- Sebti SM, Mignano JE, Jani JP, Srimatkandada S, Lazo JS. Bleomycin hydrolase: molecular cloning, sequencing, and biochemical studies reveal membership in the cysteine proteinase family. Biochemistry. 1989;28(16):6544–6548. doi: 10.1021/bi00442a003. [DOI] [PubMed] [Google Scholar]
- Selman M, Carrillo G, Salas J, Padilla RP, Perez-Chavira R, Sansores R, et al. Colchicine, D-penicillamine, and prednisone in the treatment of idiopathic pulmonary fibrosis: a controlled clinical trial. Chest. 1998;114(2):507–512. doi: 10.1378/chest.114.2.507. [DOI] [PubMed] [Google Scholar]
- Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, et al. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br J Pharmacol. 2003;138(6):1037–1048. doi: 10.1038/sj.bjp.0705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzeidi S, Sarnstrand B, Jeffery PK, McAnulty RJ, Laurent GJ. Oral N-acetylcysteine reduces bleomycin-induced collagen deposition in the lungs of mice. Eur Respir J. 1991;4(7):845–852. [PubMed] [Google Scholar]
- Sharma SK, MacLean JA, Pinto C, Kradin RL. The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury. Am J Respir Crit Care Med. 1996;154(1):193–200. doi: 10.1164/ajrccm.154.1.8680680. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhao HL, Du J, Li YT, Tan F, Huang CG, et al. Feitai, a Chinese herbal medicine, reduces transforming growth factor-beta1 and monocyte chemoattractant protein-1 expression in bleomycin-induced lung fibrosis in mice. Clin Exp Pharmacol Physiol. 2005;32(12):1071–1077. doi: 10.1111/j.1440-1681.2005.04314.x. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Gabazza EC, Taguchi O, Yasui H, Taguchi Y, Hayashi T, et al. Activated protein C inhibits the expression of platelet-derived growth factor in the lung. Am J Respir Crit Care Med. 2003;167(10):1416–1426. doi: 10.1164/rccm.200206-515OC. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Dobashi K, Iizuka K, Horie T, Suzuki K, Tukagoshi H, et al. Contribution of small GTPase Rho and its target protein rock in a murine model of lung fibrosis. Am J Respir Crit Care Med. 2001;163(1):210–217. doi: 10.1164/ajrccm.163.1.2001089. [DOI] [PubMed] [Google Scholar]
- Shimizukawa M, Ebina M, Narumi K, Kikuchi T, Munakata H, Nukiwa T. Intratracheal gene transfer of decorin reduces subpleural fibroproliferation induced by bleomycin. Am J Physiol Lung Cell Mol Physiol. 2003;284(3):L526–532. doi: 10.1152/ajplung.00131.2002. [DOI] [PubMed] [Google Scholar]
- Simler NR, Howell DC, Marshall RP, Goldsack NR, Hasleton PS, Laurent GJ, et al. The rapamycin analogue SDZ RAD attenuates bleomycin-induced pulmonary fibrosis in rats. Eur Respir J. 2002;19(6):1124–1127. doi: 10.1183/09031936.02.00281602. [DOI] [PubMed] [Google Scholar]
- Snider GL, Celli BR, Goldstein RH, O'Brien JJ, Lucey EC. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Lung volumes, volume-pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis. 1978;117(2):289–297. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- Sogut S, Ozyurt H, Armutcu F, Kart L, Iraz M, Akyol O, et al. Erdosteine prevents bleomycin-induced pulmonary fibrosis in rats. Eur J Pharmacol. 2004;494(2−3):213–220. doi: 10.1016/j.ejphar.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol. 1998;186(1):90–98. doi: 10.1002/(SICI)1096-9896(199809)186:1<90::AID-PATH137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Suntres ZE, Shek PN. Protective effect of liposomal alpha-tocopherol against bleomycin-induced lung injury. Biomed Environ Sci. 1997;10(1):47–59. [PubMed] [Google Scholar]
- Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31(4):395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- Tamagawa K, Taooka Y, Maeda A, Hiyama K, Ishioka S, Yamakido M. Inhibitory effects of a lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1279–1284. doi: 10.1164/ajrccm.161.4.9906099. [DOI] [PubMed] [Google Scholar]
- Tan W, Liu X, He B, Xu S. The mechanism and effect of erythromycin in development of pulmonary fibrosis. Zhonghua Jie He He Hu Xi Za Zhi. 1999;22(1):46–47. [PubMed] [Google Scholar]
- Taooka Y, Maeda A, Hiyama K, Ishioka S, Yamakido M. Effects of neutrophil elastase inhibitor on bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 1997;156(1):260–265. doi: 10.1164/ajrccm.156.1.9612077. [DOI] [PubMed] [Google Scholar]
- Thrall RS, McCormick JR, Jack RM, McReynolds RA, Ward PA. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979;95(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- Tokuda A, Itakura M, Onai N, Kimura H, Kuriyama T, Matsushima K. Pivotal role of CCR1-positive leukocytes in bleomycin-induced lung fibrosis in mice. J Immunol. 2000;164(5):2745–2751. doi: 10.4049/jimmunol.164.5.2745. [DOI] [PubMed] [Google Scholar]
- Tomimori Y, Muto T, Saito K, Tanaka T, Maruoka H, Sumida M, et al. Involvement of mast cell chymase in bleomycin-induced pulmonary fibrosis in mice. Eur J Pharmacol. 2003;478(2−3):179–185. doi: 10.1016/j.ejphar.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Tsuburai T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Hasiba T, et al. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lung prevents bleomycin-induced pulmonary fibrosis via a Fas-Fas ligand-independent pathway. Hum Gene Ther. 2002;13(16):1945–1960. doi: 10.1089/10430340260355356. [DOI] [PubMed] [Google Scholar]
- Umeda Y, Marui T, Matsuno Y, Shirahashi K, Iwata H, Takagi H, et al. Skeletal muscle targeting in vivo electroporation-mediated HGF gene therapy of bleomycin-induced pulmonary fibrosis in mice. Lab Invest. 2004;84(7):836–844. doi: 10.1038/labinvest.3700098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H. Studies on bleomycin. Cancer. 1967;20:891. doi: 10.1002/1097-0142(1967)20:5<891::aid-cncr2820200550>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Underwood DC, Osborn RR, Bochnowicz S, Webb EF, Rieman DJ, Lee JC, et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L895–902. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Pickford LB, Salles AL, Piercy CE, Grove BH, Erikson ME, et al. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest. 1996;98(12):2739–2745. doi: 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuki K, F. Y. Evolution of three patterns of intra-alveolar fibrosis produced by bleomycin in rats. Pathol Int. 1995;45:552–564. doi: 10.1111/j.1440-1827.1995.tb03503.x. [DOI] [PubMed] [Google Scholar]
- Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166(2):367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N, Collard HR, King TE,, Jr. Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3(4):330–338. doi: 10.1513/pats.200602-016TK. [DOI] [PubMed] [Google Scholar]
- Wang CM, He QZ, Zhang RX. Effects of tanshionone to bleomycin induced pulmonary fibrosis of rats on histological changes and production of lipid peroxides and hydroxyproline. Zhonghua Jie He He Hu Xi Za Zhi. 1994;17(5):308–310, 320. [PubMed] [Google Scholar]
- Wang HD, Yamaya M, Okinaga S, Jia YX, Kamanaka M, Takahashi H, et al. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2002;165(3):406–411. doi: 10.1164/ajrccm.165.3.2003149. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hyde DM, Giri SN. Abatement of bleomycin-induced increases in vascular permeability, inflammatory cell infiltration, and fibrotic lesions in hamster lungs by combined treatment with taurine and niacin. Lab Invest. 1992;67(2):234–242. [PubMed] [Google Scholar]
- Wang Q, Hyde DM, Gotwals PJ, Giri SN. Effects of delayed treatment with transforming growth factor-beta soluble receptor in a three-dose bleomycin model of lung fibrosis in hamsters. Exp Lung Res. 2002;28(6):405–417. doi: 10.1080/01902140290096700. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang Y, Hyde DM, Gotwals PJ, Koteliansky VE, Ryan ST, et al. Reduction of bleomycin induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax. 1999;54(9):805–812. doi: 10.1136/thx.54.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang Y, Hyde DM, Gotwals PJ, Lobb RR, Ryan ST, et al. Effect of antibody against integrin alpha4 on bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol. 2000;60(12):1949–1958. doi: 10.1016/s0006-2952(00)00491-3. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Giri SN, Hyde DM, Nakashima JM. Effects of taurine on bleomycin-induced lung fibrosis in hamsters. Proc Soc Exp Biol Med. 1989;190(4):330–338. doi: 10.3181/00379727-190-42868. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Giri SN, Hyde DM, Nakashima JM, Javadi I. Niacin attenuates bleomycin-induced lung fibrosis in the hamster. J Biochem Toxicol. 1990;5(1):13–22. doi: 10.1002/jbt.2570050104. [DOI] [PubMed] [Google Scholar]
- Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal BD. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L143–151. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- Wild JS, Hyde DM, Hubbell HR, Giri SN. Dose-related effects of Ampligen (poly(I).poly(C12U)), a mismatched double-stranded RNA, in a bleomycin-mouse model of pulmonary fibrosis. Exp Lung Res. 1996;22(3):375–391. doi: 10.3109/01902149609031781. [DOI] [PubMed] [Google Scholar]
- Wyeth Study Evaluating the Safety and Efficacy of Etanercept in Patients With Idiopathic Pulmonary Fibrosis Clinical Trial in Progress. 2007.
- Xiao JH, Zhang JH, Chen HL, Feng XL, Wang JL. Inhibitory effects of isoliensinine on bleomycin-induced pulmonary fibrosis in mice. Planta Med. 2005;71(3):225–230. doi: 10.1055/s-2005-837821. [DOI] [PubMed] [Google Scholar]
- Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, et al. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. A morphologic study. Am J Respir Crit Care Med. 1997;156(6):1937–1944. doi: 10.1164/ajrccm.156.6.9611057. [DOI] [PubMed] [Google Scholar]
- Yamazaki C, Hoshino J, Hori Y, Sekiguchi T, Miyauchi S, Mizuno S, et al. Effect of lecithinized-superoxide dismutase on the interstitial pneumonia model induced by bleomycin in mice. Jpn J Pharmacol. 1997;75(1):97–100. doi: 10.1254/jjp.75.97. [DOI] [PubMed] [Google Scholar]
- Yao HW, Zhu JP, Zhao MH, Lu Y. Losartan Attenuates Bleomycin-Induced Pulmonary Fibrosis in Rats. Respiration. 2006 doi: 10.1159/000090140. [DOI] [PubMed] [Google Scholar]
- Yara S, Kawakami K, Kudeken N, Tohyama M, Teruya K, Chinen T, et al. FTS reduces bleomycin-induced cytokine and chemokine production and inhibits pulmonary fibrosis in mice. Clin Exp Immunol. 2001;124(1):77–85. doi: 10.1046/j.1365-2249.2001.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H, Gabazza EC, Tamaki S, Kobayashi T, Hataji O, Yuda H, et al. Intratracheal administration of activated protein C inhibits bleomycin-induced lung fibrosis in the mouse. Am J Respir Crit Care Med. 2001;163(7):1660–1668. doi: 10.1164/ajrccm.163.7.9911068. [DOI] [PubMed] [Google Scholar]
- Yasui M, Yoshida T, Nomura M, Kurashima K, Shintani H, Nakatsumi Y, et al. Inhibitory effects of rhIL-1 beta pretreatment on bleomycin-induced pneumonitis in mice. Nihon Kyobu Shikkan Gakkai Zasshi. 1991;29(11):1450–1456. [PubMed] [Google Scholar]
- Yehualaeshet T, O'Connor R, Begleiter A, Murphy-Ullrich JE, Silverstein R, Khalil N. A CD36 synthetic peptide inhibits bleomycin-induced pulmonary inflammation and connective tissue synthesis in the rat. Am J Respir Cell Mol Biol. 2000;23(2):204–212. doi: 10.1165/ajrcmb.23.2.4089. [DOI] [PubMed] [Google Scholar]
- Yi ES, Williams ST, Lee H, Malicki DM, Chin EM, Yin S, et al. Keratinocyte growth factor ameliorates radiation- and bleomycin-induced lung injury and mortality. Am J Pathol. 1996;149(6):1963–1970. [PMC free article] [PubMed] [Google Scholar]
- Yildirim Z, Kotuk M, Erdogan H, Iraz M, Yagmurca M, Kuku I, et al. Preventive effect of melatonin on bleomycin-induced lung fibrosis in rats. J Pineal Res. 2006;40(1):27–33. doi: 10.1111/j.1600-079X.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Yildirim Z, Kotuk M, Iraz M, Kuku I, Ulu R, Armutcu F, et al. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: erdosteine and N-acetylcysteine. Pulm Pharmacol Ther. 2005;18(5):367–373. doi: 10.1016/j.pupt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Yildirim Z, Turkoz Y, Kotuk M, Armutcu F, Gurel A, Iraz M, et al. Effects of aminoguanidine and antioxidant erdosteine on bleomycin-induced lung fibrosis in rats. Nitric Oxide. 2004;11(2):156–165. doi: 10.1016/j.niox.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Yamamoto T, Ueta E, Osaki T. Azelastine hydrochloride (Azeptin) inhibits peplomycin (PLM)-induced pulmonary fibrosis by contradicting the up-regulation of signal transduction. Anticancer Drug Des. 1997;12(7):577–590. [PubMed] [Google Scholar]
- Yoshida M, Sakuma-Mochizuki J, Abe K, Arai T, Mori M, Goya S, et al. In vivo gene transfer of an extracellular domain of platelet-derived growth factor beta receptor by the HVJ-liposome method ameliorates bleomycin-induced pulmonary fibrosis. Biochem Biophys Res Commun. 1999;265(2):503–508. doi: 10.1006/bbrc.1999.1647. [DOI] [PubMed] [Google Scholar]
- Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, et al. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol. 2005;33(5):447–454. doi: 10.1165/rcmb.2004-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Wang JF, Niu JZ, Yang MJ, Wang LQ, Jin H, et al. Influence of compound biejia ruangan prescription on extracelluar matrix in bleomycin induced pulmonary fibrosis rats. Zhongguo Zhong Yao Za Zhi. 2004;29(1):62–66. [PubMed] [Google Scholar]
- Zhang L, Zhu Y, Luo W, Xi P, Yan Y. The protective effect of colchicine on bleomycin-induced pulmonary fibrosis in rats. Chin Med Sci J. 1992;7(1):58–60. [PubMed] [Google Scholar]
- Zhang XY, Shimura S, Masuda T, Saitoh H, Shirato K. Antisense oligonucleotides to NF-kappaB improve survival in bleomycin-induced pneumopathy of the mouse. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1561–1568. doi: 10.1164/ajrccm.162.4.9908093. [DOI] [PubMed] [Google Scholar]
- Zhao J, Shi W, Wang YL, Chen H, Bringas P,, Jr., Datto MB, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L585–593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhao M, Fang Q. Spironolactone ameliorates rat pulmonary fibrosis induced by bleomycin A5. Zhonghua Jie He He Hu Xi Za Zhi. 1998;21(5):300–302. [PubMed] [Google Scholar]
- Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, et al. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol. 2005;166(1):27–37. doi: 10.1016/S0002-9440(10)62229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia S, Hyde DM, Giri SN. Effects of an interferon inducer bropirimine on bleomycin-induced lung fibrosis in hamsters. Pharmacol Toxicol. 1992;71(1):11–18. doi: 10.1111/j.1600-0773.1992.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Ziboh VA, Yun M, Hyde DM, Giri SN. Gamma-Linolenic acid-containing diet attenuates bleomycin-induced lung fibrosis in hamsters. Lipids. 1997;32(7):759–767. doi: 10.1007/s11745-997-0097-x. [DOI] [PubMed] [Google Scholar]
- Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341(17):1264–1269. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]


