Abstract
BACKGROUND
Lentivirus vectors provide a delivery system that can both transduce nondividing cells and integrate transgenes into the genome of target cells without cytotoxicity. However, their relatively low transduction efficiency presents a significant obstacle to progress.
OBJECTIVES
In the present paper, a simple and easy method using calcium phosphate (CaPi) to enhance the efficiency of lentivirus gene transfer in both vascular smooth muscle cells and cardiac myocytes is reported.
METHODS AND RESULTS
Delivery of lentivirus vectors in the presence of CaPi coprecipitates increased vector-encoded transgene expression up to 13-fold. Of interest, the magnitudes of enhancement of transgene expression by CaPi coprecipitates in 293T cells, vascular smooth muscle cells and cardiac myocytes were greater during brief periods (10 min and 120 min) of virus-cell contact than during long periods (16 h). Moreover, with a short duration of incubation with CaPi coprecipitates (up to 120 min), there was little evidence of direct cell toxicity. CaPi coprecipitates had no effect on host range specificity of ecotropic viruses and thus appears to enhance transduction efficiency physiologically by facilitating physical interaction between virus and cell.
CONCLUSIONS
These data show that lentivirus with CaPi coprecipitates increases both the efficiency and the speed of gene transfer. These approaches provide an efficient method and an improved tool for research and possibly for therapy of cardiovascular diseases.
Keywords: Calcium phosphate, Cardiac myocytes, Gene therapy, Lentivirus, Vascular smooth muscle cells
Gene therapy by delivery of genetically modified cells has the potential to reverse genetic causes and modify the pathophysiology of many innate and acquired diseases (1–4). Both efficient delivery and long-term expression of transduced genes are required before the full benefit of such genetic manipulation strategies can be realized in the cardiovascular system. However, current methods of gene delivery each suffer major limitations.
Recent development of lentivirus-based vector systems based on a modified retroviral HIV genome has provided a delivery system that can both transduce nondividing cells and integrate transgenes into the genome of target cells (5). In addition, such lentivirus-based vector particles can be pseudotyped with the envelope of the vesicular stomatitis virus (VSV) (6), thus enabling the vector to introduce genes into a broad range of tissues (5,7,8). These features of lentivirus-based vectors, including their lack of cytotoxicity, make them potentially useful for delivery of transgenes. However, low virus titre presents a significant obstacle to progress. In 1999, we reported that sodium butyrate enhances production of lentivirus-based vectors (9). This modification of the lentivirus particle production system enabled us to increase virus titres more than 10-fold. However, once produced, the efficiency of virus gene transfer is subsequently influenced by a number of factors. Local conditions affecting the kinetics of virus adsorption to target cell membranes have a major influence on virus transduction efficiency. Polybrene is widely used to enhance the adsorption of lentivirus-based (8) and retrovirus-based vectors (6), and usually increases the titre severalfold. However, it has been reported that even with polybrene, after 2 h incubations of murine leukemia virus-derived retrovirus vectors with target cells, only approximately 5% of the infectious virions were depleted from the medium (10). This result indicates that virus adsorption is inefficient, particularly when the contact time with target cells is short.
The use of calcium phosphate (CaPi) to coprecipitate DNA was one of the first methods used for DNA transfer to cells (11). Moreover, it has been reported that incorporating recombinant adenovirus (12) or retrovirus (13) in CaPi coprecipitates enhances transduction efficiency of virus-encoded transgenes. Therefore, we tested the hypothesis that CaPi coprecipitates with lentivirus-based vectors increases the efficiency of transduction.
In the present study, we found that coprecipitation of lentivirus-based vector particles with CaPi greatly enhanced the transduction efficiency in cell culture of vascular smooth muscle cells (VSMCs), cardiac myocytes and other target cells under conditions of limited contact time, providing a convenient method to maximize the efficiency of lentiviral gene transfer.
METHODS
Cells and cell culture
293T human kidney cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) in a 37°C and 5% CO2 humidified environment (5,14). Primary cultures of neonatal rat cardiac myocytes were prepared as previously described (15,16). The population of nonmyocytes was less than 10% of the total cell population. The culture medium was DMEM/Ham’s F12 supplemented with 5% calf serum. 5-Bromodeoxyuridine (100 mM) was added during the first 24 h to prevent proliferation of nonmyocytes. Primary cultures of VSMCs isolated from the media of thoracic aortas of Sprague-Dawley rats by enzymatic digestion were kindly donated by Dr Mitsuhiro Yokoyama (Division of Cardiovascular and Respiratory Medicine, Kobe University, Japan) (17). VSMCs and NIH 3T3 cells were maintained in DMEM containing 10% FCS. Cells were routinely passaged just before reaching confluence. Experiments were performed with cultured cells between passages 5 and 10.
Virus production and transduction
Lentivirus-based vectors encoding beta-galactosidase (β-Gal) were generated by transient cotransfection of 293T cells with a three-plasmid combination, as described previously, with slight modifications (8). Briefly, 100 mm dishes of nonconfluent 293T cells were transfected with 15 μg of pCMVΔR8.2, 15 μg of pHR′-CMVLacZ, and 15 μg of pMDG (encoding the VSV-G envelope), pHIT123 (encoding the murine leukemia virus ecotropic envelope) or pHIT456 (encoding the amphotropic envelope) by the CaPi-DNA coprecipitation method (18,19). The plasmid vectors were kindly provided by Dr Luigi Naldini (University of Torino, Italy) and Dr Paula Cannon (University of Southern California, USA). Sixteen hours after transfection, the medium was adjusted to make a final concentration of 10 mM sodium butyrate and the cells were incubated for 8 h to obtain high-titre virus production as previously described (9). After the 8 h incubation, cells were washed and incubated in fresh medium without sodium butyrate. Conditioned medium was harvested 16 h later and passed through 0.45 mm filters. For transduction of 293T cells, VSMCs or cardiac myocytes, cells were seeded at 1×105 cells per well in 12-well plates (Corning, USA) and incubated at 37°C overnight in their regular medium. Transductions were carried out in 1 mL of serum-free DMEM, including serial dilutions of lentiviral vector super-natant. Increasing amounts of a 2 M CaCl2 solution were added to the lentiviral vectors suspended in DMEM to achieve the desired concentration. The solution was mixed by vortexing. Unless otherwise noted, the mixture was allowed to stand for 60 min at room temperature to form lentiviral vectors and CaPi complexes. Transductions using the coprecipitates were carried out for up to 120 min. The cells were then washed with phosphate-buffered saline (PBS) and incubated in regular medium for 48 h, after which staining with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) for detection of β-Gal activity was performed.
Analysis of transduced cells
Transduction efficiency (transducing units [TU]/mL) was calculated by multiplying the number of β-Gal-expressing cells counted per dish by the dilution factor. Cells expressing β-Gal were fixed and stained with X-Gal as described previously (20). Briefly, the cells were fixed with PBS containing 0.5% glutaraldehyde for 10 min at room temperature. After fixation, β-Gal expression was evaluated by histochemical staining with X-Gal in PBS containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)63H2O, 1 mM MgCl2 and 1 mg/mL X-Gal at 37°C for 16 h.
Cell viability assay
To measure the cellular toxicity of CaCl2, a propidium iodide (PI) exclusion assay was performed. 293T cells, VSMCs or cardiac myocytes were infected and 48 h later, 20 mg/mL PI was applied to the cells for 5 min at room temperature. The cells were then analyzed by determining the proportion of positive cells using fluorescence microscopy as described previously (21).
RESULTS AND DISCUSSION
CaPi coprecipitation enhances lentivirus-mediated gene transfer to 293T cells, VSMCs and cardiac myocytes
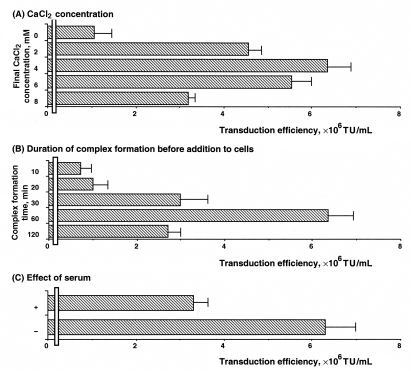
When CaCl2 was added to DMEM, into which lentivirus-based vectors encoding β-Gal had been harvested, fine precipitates of CaPi were formed. The precipitates, in concordance with a previous report (13), were visible within 60 min after the addition of CaCl2. Lentiviral vectors with CaPi coprecipitates were applied to 293T cells for up to 120 min. The cells were then washed and incubated in fresh medium for 48 h and subsequently stained with X-Gal to measure transgene expression. The data measured in Figure 1A show the effects of various concentrations of CaCl2 on the viral transduction efficiency during infection of 293T cells. Lentiviral vectors coprecipitated with 4 mM CaCl2, showing the highest transduction efficiency ([6.3±0.5]×106 TU/mL). The optimum concentration of 4 mM CaCl2 coincided with that reported for CaPi coprecipitation-mediated enhancement of adenovirus (12) and retrovirus (13) transduction. The data in Figure 1B show the duration of coprecipitate complex formation before its addition to cells. Experimental conditions used for the present study were the optimal CaCl2 concentration of 4 mM and incubation time with cells of 120 min. Complex formation time of 60 min before addition showed an optimal effect on transduction of 293T cells. It has also been reported that serum interferes with complex formation between adenoviruses and CaPi. To examine the effects of serum on lentiviral transduction efficiency on 293T cells, coprecipitates of lentiviral vectors and CaPi were formed in the presence or absence of 10% FCS. Serum diminished but did not eliminate the enhanced transduction efficiency when added to the solution before coprecipitates were formed (Figure 1C).
Figure 1.
Enhanced transduction efficiency of lentivirus-based vectors coprecipitated on 293T cells. A Effect of CaCl2 concentration. To achieve the desired concentration 2 M CaCl2 was added. Standard conditions for this study were 60 min for formation of lentivirus and calcium phosphate (CaPi) complex before addition to cells, and application of lentivirus-based vectors with or without CaPi coprecipitates to cells for 120 min. B Effect of duration of complex formation before addition to cells. Lentivirus-based vectors and CaPi complex were incubated for various durations before they were applied to cells. Standard conditions for this study were a CaCl2 concentration of 4 mM and exposure of cells to lentivirus-based vectors CaPi coprecipitates for 120 min. C Effect of serum. Lentivirus-based vectors with CaPi coprecipitates were formed in the presence (+) or absence (−) of 10% fetal calf serum. Standard conditions for this study were 4 mM CaCl2 and 60 min of complex formation. Coprecipitates were applied to cells for 120 min. Transduction efficiency was determined as described in the Methods section. Values are expressed as mean ± SE of three independent experiments. TU Tranducing units
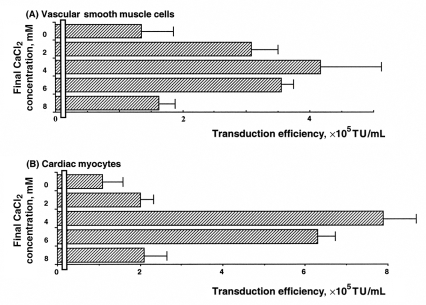
Next was to determine whether CaPi coprecipitation was a generally applicable method to enhance lentiviral vector transduction efficiency on other cell types, especially primary cultured VSMCs and cardiac myocytes (Figure 2). In VSMCs and cardiac myocytes, lentiviral vectors coprecipitated with 4 mM CaCl2 also proved to be optimal and provided transduction efficiency of (4.2±1.1)×105 TU/mL and (7.9±1.2)×105 TU/mL, respectively. In contrast, applying lentiviral vectors alone without CaPi coprecipitation showed significantly lower transduction efficiency: (1.3±0.7)×105 TU/mL and (1.1±0.5)×105 TU/mL in VSMCs and cardiac myocytes, respectively.
Figure 2.
Enhanced transduction efficiency of lentivirus-based vectors coprecipitated on vascular smooth muscle cells (A) and cardiac myocytes (B). To achieve the desired concentration 2 M CaCl2 was added. The duration of lentivirus-based vectors and calcium phosphate complex formation before its addition to cells was 60 min in the absence of serum, and the lentivirus-based vectors with or without calcium phosphate coprecipitation were applied to cells for 120 min. Transduction efficiency was determined as described in the Methods section. Values are expressed as mean ± SE of three independent experiments. TU Transducing units
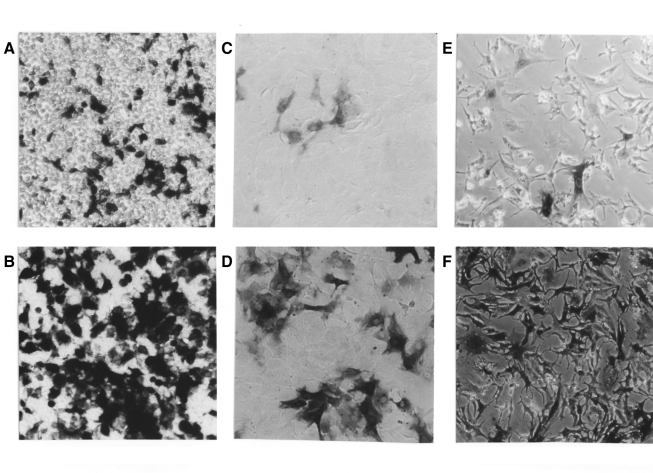
Figure 3 shows a representative example of the difference in β-Gal staining of 293T cells (A, B), VSMCs (C, D) and cardiac myocytes (E, F) after lentivirus-mediated transduction in the presence (B, D, F) or absence (A, C, E) of CaPi coprecipitates. In all cases, significant enhancement of transduction efficiency on VSMCs and cardiac myocytes, as well as on 293T cells, can be obtained after coprecipitation with 4 mM CaCl2.
Figure 3.
Beta-galactosidase (β-Gal) staining of 293T cells, vascular smooth muscle cells (VSMCs) and cardiac myocytes by lentivirus-mediated β-Gal gene transduction with or without calcium phosphate (CaPi) coprecipitates. β-Gal staining was performed as described in the Methods section. A Lentivirus-mediated β-Gal staining of 293T cells without CaPi coprecipitates. B Lentivirus-mediated β-Gal staining of 293T cells with CaPi copre-cipitates. C Lentivirus-mediated β-Gal staining of VSMCs without CaPi coprecipitates. D Lentivirus-mediated β-Gal staining of VSMCs with CaPi coprecipitates. E Lentivirus-mediated β-Gal staining of cardiac myocytes without CaPi coprecipitates. F Lentivirus-mediated β-Gal staining of cardiac myocytes with CaPi coprecipitates
The magnitude of coprecipitation-mediated enhancement of lentiviral transduction efficiency is greater at earlier time points
It is important that the incubation time (vector-cell contact time) be sufficient to allow transduction of viral DNA into target cells. Accordingly, the effect of varying the vector-cell contact time on transduction efficiency was examined with or without CaPi coprecipitates. Table 1 shows surprisingly that virus transduction efficiency was maximally enhanced in 293T cells, VSMCs and cardiac myocytes when the cells were incubated with 4 mM CaCl2 coprecipitates for only 10 min. The efficiency increased 13.1-, 5.00- and 8.71-fold, respectively.
TABLE 1.
Effect of lentiviral vector-cell contact time on transduction efficiency with or without calcium phosphate (CaPi) coprecipitates
| 10 min | 120 min | 16 h | |
|---|---|---|---|
| 293T cells | |||
| CaCI2(−), TU/mL±SE | (1.3±1.3)×105 | (1.1±0.4)×106 | (4.1±0.6)×106 |
| CaCI2(+), TU/mL±SE | (1.7±2.1)×106 | (6.4±0.5)×106 | (1.2±1.2)×107 |
| Fold change | 13.1 | 5.82 | 2.92 |
| Vascular smooth muscle cells | |||
| CaCI2(−), TU/mL±SE | (3.4±1.0)×104 | (1.3±0.7)×105 | (4.3±0.5)×105 |
| CaCI2(+), TU/mL±SE | (1.7±1.3)×105 | (4.2±1.0)×105 | (9.5±0.9)×105 |
| Fold change | 5.00 | 3.23 | 2.21 |
| Cardiac myocytes | |||
| CaCI2(−), TU/mL±SE | (3.1±2.5)×104 | (1.1±0.5)×105 | (5.5±0.9)×105 |
| CaCI2(+), TU/mL±SE | (2.7±2.4)×105 | (7.9±0.8)×105 | (1.5±1.8)×106 |
| Fold change | 8.71 | 7.18 | 2.73 |
Standard conditions for this study were CaCI2 concentrations of 0 mM (−) or 4 mM (+), and a 60 min duration of lentivirus and CaPi complex formation before addition to cells in the absence of fetal calf serum. Lentivirus with or without CaPi coprecipitates was applied to cells for the indicated times. Transduction efficiency was determined as described in the Methods section. TU Transducing units
CaPi may facilitate physical interaction between virus and cell
The enhanced efficiency of gene transfer by coprecipitated lentiviral vectors is likely due to effects on virus adsorption to target cell membranes because CaPi condenses DNA and facilitates binding to cells during plasmid transfection (22). Alternatively, coprecipitation with CaPi might facilitate a novel manner of lentivirus entry. Therefore, we tested whether the transduction efficiency of CaPilentiviral complexes altered the host range of lentiviral vector particles bearing ecotropic envelopes. Ecotropic envelopes have been known to infect only rodent species. However, amphotropic and VSV-G envelopes have been reported to infect a broad variety of cells, including humans and rodents. Table 2 shows that lentiviral vectors pseudotyped with amphotropic or VSV-G envelopes transduced both mouse NIH 3T3 and human 293T cells. CaPi coprecipitation enhanced the transduction efficiency for both cell types. In contrast, lentiviral vectors pseudotyped with an ecotropic envelope did not transduce 293T cells cultured from human kidneys, and CaPi coprecipitation had no effect. The ecotropic host range of the precipitated vectors was thus maintained in rodent species, and there was no increase in the background level of gene transfer to human cells. These results indicate that lentivirus delivered by CaPi coprecipitation maintains normal virus-cell membrane fusion triggered by appropriate envelope-receptor interactions. These results suggest that coprecipitation enhances transduction efficiency by facilitating the physical interaction of lentivirus binding and entry to cells. VSV-G interacts with the phospholipid component of the cell membrane to mediate virus entry by membrane fusion (23). Because VSV-G-mediated entry does not seem to depend on the presence of specific protein receptors, VSV-G-pseudotyped vectors have an extremely broad host cell range (24). Therefore, it seems likely that CaPi coprecipitation is a generally applicable method to enhance lentiviral titres on a broad variety of cell types. Our experiments do not address the possibility that the CaPi coprecipitates alter entry, upregulation, transcription or translation of the transgene.
TABLE 2.
Effect of various envelopes on transduction efficiency with or without calcium phosphate (CaPi) coprecipitates
| Envelope
|
||||||
|---|---|---|---|---|---|---|
| Ecotropic (MLV) | Amphotropic | Vesicular stomatitis virus G | ||||
| Final CaCI2 concentration, mM | 0 | 4 | 0 | 4 | 0 | 4 |
| NIH 3T3 cells (mouse), TU/mL±SE | (5.8±2.1)×104 | (2.2±0.7)×105 | (8.5±1.6)×104 | (3.1±1.3)×105 | (2.4±0.7)×105 | (8.3±1.4)×105 |
| 293T cells (human), TU/mL±SE | 0 | 0 | (3.3±1.5)×105 | (8.4±0.9)×105 | (1.1±0.4)×106 | (6.4±0.5)×106 |
Standard conditions for this study were CaCI2 concentrations of 0 mM or 4 mM, and a 60 min duration of lentivirus and CaPi complex formation before addition to cells in the absence of fetal calf serum. Lentivirus with various envelopes was applied to NIH 3T3 or 293T cells for 120 min. Transduction efficiency was determined as described in the Methods section. MLV Murine leukemia virus; TU Tranducing units
CaPi coprecipitation has little direct toxicity for cells during short periods of incubation
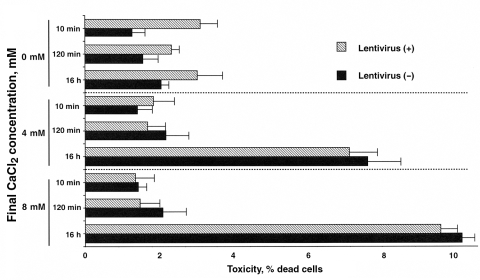
Although CaPi coprecipitation methods have been used for transfection of DNA into cells, toxicity has been observed (1). If the enhancement of transduction efficiency by CaPi coprecipitation is indirectly due to cell toxicity, there would be no improvement in the therapeutic index. Therefore, the viability of 293T cells after exposure to CaPi coprecipitates was examined. In the present study, PI exclusion was used as an assay for plasma membrane integrity, because major features discriminating dead from living cells are the loss of transport function and physical integrity of the plasma membrane. Incubation with PI results in selective labelling of dead cells, while intact membranes of living cells show exclusion of PI uptake. Figure 4 shows that when using PI staining, lentiviral vectors with CaPi coprecipitation generated little toxicity with short durations of incubation (10 min and 120 min). Furthermore, increasing CaCl2 concentration did not increase cell death. However, a 16 h exposure to CaPi either with or without lentivirus did decrease cell viability. The toxicity was believed to be generated by the CaPi coprecipitates and not by lentivirus because CaCl2 only (absence of lentivirus) generated similar levels of toxicity, while lentivirus alone did not show any toxicity (data not shown). The same results were obtained in VSMCs and cardiac myocytes (data not shown). These results indicate that a 120 min or longer exposure to CaPi coprecipitation has little direct cytotoxicity.
Figure 4.
Effects on viability of 293T cells after exposure to calcium phosphate coprecipitates. After 293T cells were transduced with lentivirus-based vectors with (+) or without (−) calcium phosphate coprecipitates, 20 mg/mL propidium iodide was applied to the cells for 5 min at room temperature. The cells were then analyzed by counting the ratio of positive cells using fluorescence microscopy. Values are expressed as mean ± SE of three independent experiments
CONCLUSIONS
We have developed a simple and effective method to enhance transduction efficiency of lentiviral vectors using CaCl2. To our knowledge, there have been no reports regarding the application of lentivirus coprecipitated with CaPi to the cardiovascular system. The system we have described has a number of advantages, and the results are encouraging. In comparison with virus alone, CaPi coprecipitation increased transduction efficiency. The enhancement of transduction efficiency by CaCl2 was greater particularly at earlier time points. Moreover, CaPi coprecipitates show little direct cell toxicity within 120 min of incubation. The efficacy of short exposure durations can prove to have potential therapeutic benefits. Ex vivo delivery of vectors to cells would shorten the time between harvest and delivery. Similarly, these results are particularly encouraging because coronary or peripheral arterial infusion of gene delivery vectors would likely entail the use of either an arterial catheter while the heart is beating or a surgical approach during time-limited cardioplegic arrest. In either case, effective gene transduction within a limited amount of time is especially important for clinical application. This method likely increases physical interaction between the vector and the target cells, and may also be generally applicable to a broad variety of cell types using VSV-G-pseudotyped lentiviral vectors combined with coprecipitation.
Acknowledgments
We are grateful to T Saluna and N Kumon for their technical assistance. We thank V Sartorelli for stimulating discussions.
REFERENCES
- 1.Crystal RG. Transfer of genes to humans: Early lessons and obstacles to success. Science. 1995;270:404–10. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 2.Leiden JM. Gene therapy – promise, pitfalls and prognosis. N Engl J Med. 1995;333:871–3. doi: 10.1056/NEJM199509283331310. [DOI] [PubMed] [Google Scholar]
- 3.Mulligan RC. The basic science of gene therapy. Science. 1993;260:926–32. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 4.Verma IM, Somia N. Gene therapy – promises, problems and prospects. Nature. 1997;389:239–42. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 5.Naldini L, Blomer U, Gally P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 6.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: Concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90:8033–7. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyake K, Suzuki N, Matsuoka H, Tohyama T, Shimada T. Stable integration of human immunodeficiency virus-based retroviral vectors into the chromosomes of nondividing cells. Hum Gene Ther. 1998;9:467–75. doi: 10.1089/hum.1998.9.4-467. [DOI] [PubMed] [Google Scholar]
- 8.Naldini L, Blömer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–8. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakoda T, Kasahara N, Hamamori Y, Kedes L. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol. 1999;31:2037–47. doi: 10.1006/jmcc.1999.1035. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Paul R, Burgeson RE, Keene DR, Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991;65:6468–77. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–67. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 12.Fasbender A, Lee JH, Walters RW, Moninger TO, Zabner J, Welsh MJ. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J Clin Invest. 1998;102:184–93. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morling FJ, Russell SJ. Enhanced transduction efficiency of retroviral vectors coprecipitated with calcium phosphate. Gene Ther. 1995;2:504–8. [PubMed] [Google Scholar]
- 14.Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987;84:156–60. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sussman MA, Hamm-Alvarez SF, Vilalta PM, Welch S, Kedes L. Involvement of phosphorylation in doxorubicin-mediated myofibril degeneration. An immunofluorescence microscopy analysis. Circ Res. 1997;80:52–61. doi: 10.1161/01.res.80.1.52. [DOI] [PubMed] [Google Scholar]
- 16.Ueyama T, Sakoda T, Kawashima S, et al. Activated RhoA stimulates c-fos gene expression in myocardial cells. Circ Res. 1997;81:672–8. doi: 10.1161/01.res.81.5.672. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Taniguchi T, Konishi H, Kikkawa U, Ishikawa Y, Yokoyama M. Activation of Akt/protein kinase B after stimulation with angiotensin II in vascular smooth muscle cells. Am J Physiol. 1999;276:H1927–34. doi: 10.1152/ajpheart.1999.276.6.H1927. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–52. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakoda T, Kaibuchi K, Kishi K, et al. smg/rap1/Krev-1 p21s inhibit the signal pathway to the c-fos promoter/enhancer from c-Kiras p21 but not from c-raf-1 kinase in NIH3T3 cells. Oncogene. 1992;7:1705–11. [PubMed] [Google Scholar]
- 20.Sanes JR, Rubenstein JL, Nicolas JF. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–42. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darzynkiewicz Z, Li X, Gong J. Assays of cell viability: Discrimination of cells dying by apoptosis. Methods Cell Bio. 1994;41:15–38. doi: 10.1016/s0091-679x(08)61707-0. [DOI] [PubMed] [Google Scholar]
- 22.Ausubel FM, Brent R, Kingston RE, et al., editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1992. [Google Scholar]
- 23.Mastromarino P, Conti C, Goldoni P, Hauttecoeur B, Orsi N. Characterization of membrane components of the erythrocyte involved in vesicular stomatitis virus attachment and fusion at acidic pH. J Gen Virol. 1987;68:2359–69. doi: 10.1099/0022-1317-68-9-2359. [DOI] [PubMed] [Google Scholar]
- 24.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–51. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]