Abstract
The molecular basis of tissue-specific pigmentation of maize carrying a tandemly repeated multicopy allele of pericarp color1 (p1) was examined using Mutator (Mu) transposon-mediated mutagenesis. The P1-wr allele conditions a white or colorless pericarp and a red cob glumes phenotype. However, a Mu-insertion allele, designated as P1-wr-mum6, displayed an altered phenotype that was first noted as occasional red stripes on pericarp tissue. This gain-of-pericarp-pigmentation phenotype was heritable, yielding families that displayed variable penetrance and expressivity. In one fully penetrant family, deep red pericarp pigmentation was observed. Several reports on Mu suppressible alleles have shown that Mu transposons can affect gene expression by mechanisms that depend on transposase activity. Conversely, the P1-wr-mum6 phenotype is not affected by transposase activity. The increased pigmentation was associated with elevated mRNA expression of P1-wr-mum6 copy (or copies) that was uninterrupted by the transposons. Genomic bisulfite sequencing analysis showed that the elevated expression was associated with hypomethylation of a floral-specific enhancer that is ∼4.7 kb upstream of the Mu1 insertion site and may be proximal to an adjacent repeated copy. We propose that the Mu1 insertion interferes with the DNA methylation and related chromatin packaging of P1-wr, thereby inducing expression from gene copy (or copies) that is otherwise suppressed.
WHOLE-genome amplification and tandem duplication events are the two chief mechanisms for the evolution of gene families in plants (Rizzon et al. 2006). Following duplication, many redundant genes are deleted or become pseudogenes; however, some genes have evolved specialized functions in the regulation of transcription, signal transduction, and development (Blanc et al. 2003; Maere et al. 2005). Tandem duplications are widespread among genes that have roles in disease resistance and the synthesis of secondary metabolites (Hulbert and Bennetzen 1991; Kliebenstein et al. 2001). Tandemly arranged gene copies often have specialized biological roles that may have contributed to their conservation. For example, Botrytis disease is combated in Arabidopsis by two tandemly arranged genes that encode polygalacturonase-inhibiting proteins (Ferrari et al. 2003). Both the gene copies have similar protein products but have diverged in regulatory regions so that they are activated by separate signal transduction pathways. Developmental processes can also be tightly regulated on the basis of the differential activation of gene copies. For instance, the demand for the patatin storage protein during potato tuberization is met by preferentially upregulating a subset of copies from an ∼10- to 18-copy locus (Stupar et al. 2006).
Tandem duplication can both positively and negatively affect gene expression. In barley, the resistance to powdery mildew is associated with a tandem duplication in the Mlo gene that encodes a seven-transmembrane domain protein (Piffanelli et al. 2004). In this case, an additional truncated copy functions to block the expression of wild-type transcripts. Conversely, a tandem duplication of the maize homeobox gene called knotted1 (kn1) has given rise to a mutant allele, Kn1-O, which is ectopically expressed in leaves (Veit et al. 1990; Vollbrecht et al. 1991). Aberrant expression in kn1 mutants can easily be monitored by the presence of knots that are composed of displaced ligule tissue (Smith et al. 1992). Derivative alleles of Kn1-O indicate that the presence of a third copy increases the severity of the phenotype, whereas the loss of a copy results in the restoration of wild-type function (Veit et al. 1990). Moreover, insertion of Mu transposons at the junction of the Kn1-O repeat restores the wild-type expression pattern (Lowe et al. 1992).
These studies indicate that gene copies have evolved important biological functions and can thus profoundly affect gene expression. Despite this, tandem arrays of genes encoding for transcription factors are infrequent, theoretically because of the deleterious nature of gene rearrangements (Rizzon et al. 2006). However, the presence of tandem repeats in regulatory genes should have a broader effect on the regulation of biosynthetic pathways. Herein, we have focused on a well-studied maize transcription factor called pericarp color1 (p1), which has numerous alleles that differ with respect to their copy number (Cocciolone et al. 2001). The p1 gene encodes a myb-homologous protein that regulates the transcription of structural genes required for the biosynthesis of brick-red flavonoid pigments called phlobaphenes (Grotewold et al. 1994). The tissue specificity of phlobaphene pigmentation on maize ears depends upon the allelic constitution at the p locus. Stable alleles of the p1 gene have been named according to their pericarp and cob pigmentation phenotypes: P1-wr (white pericarp, red cob), P1-rr (red pericarp, red cob), P1-rw (red pericarp, white cob), and p1-ww (white pericarp, white cob) (Anderson 1924). To understand the mechanism underlying tissue-specific patterns, many of the p1 alleles have been molecularly characterized and compared with one another (Chopra et al. 1998; Zhang and Peterson 2005a,b). For instance, molecular comparison of P1-wr and P1-rr revealed that P1-rr has a single-gene copy whereas P1-wr has a six-copy tandem-repeat structure (Chopra et al. 1998). Promoter swapping experiments indicated that the distinct expression patterns of P1-wr and P1-rr were not due to differences in their coding and proximal promoter sequences (Cocciolone et al. 2001). Rather, the DNA hypermethylation of P1-wr relative to P1-rr was associated with the absence of pericarp pigmentation (Chopra et al. 1998). In fact, the reduction of DNA methylation at P1-wr in the presence of an unlinked dominant modifier called Unstable factor for orange1 (Ufo1) results in a corresponding range of pericarp (Chopra et al. 2003) and cob glumes (Sekhon et al. 2007) pigmentation. The tandem-repeat structure of the P1-wr allele is also present in many other naturally occurring maize germplasms, some of which have pericarp pigmentation, albeit it is restricted to the kernel gown (Brink and Styles 1966; Cocciolone et al. 2001). In these instances, DNA hypomethylation is correlated with the increased gene expression (Cocciolone et al. 2001). DNA hypermethylation has also been correlated with the suppressed state of a P1-rr epiallele called P1-pr (patterned pericarp and red cob) (Das and Messing 1994). In this case, a DNAse I sensitivity assay demonstrated that the DNA hypermethylation of P1-pr correlates with chromatin condensation (Lund et al. 1995).
To identify putative cob- and pericarp-specific elements, the single-copy P1-rr allele has been extensively mutagenized using the Ac transposons, which resulted in a series of alleles showing a wide range of variegated pericarp and cob pigmentation (Athma et al. 1992).
Herein, we report the results based on 13 unique germinal Mu-insertion sites in the six-copy tandemly repeated P1-wr allele. Since P1-wr is multicopy, we knew that a mutation in any one copy (if all copies express) may not yield a phenotype. However, we also envisaged that the insertion of a Mu transposon might disrupt the epigenetic regulation of P1-wr gene expression (Barkan and Martienssen 1991; Girard and Freeling 2000; Cui et al. 2003). We recovered a single gain-of-pericarp-function allele, P1-wr-mum6, generated by a Mu1 insertion in the 5′-UTR (of one of the copies in the P1-wr array). Interestingly, P1-wr-mum6 expression is associated with the hypomethylation of a floral organ-specific enhancer sequence that is located at the 5′ end of every P1-wr gene copy. The position of this enhancer in the interrupted copy is distal from the Mu1 insertion site and may lie near an adjacent upstream copy in the P1-wr tandem gene array. We discuss a mechanism through which the Mu1 insertion in a single copy of P1-wr could lead to the increased expression.
MATERIALS AND METHODS
Maize stocks:
The P1-wr [A632] inbred line was obtained from the Germplasm Resources Information Network (U.S. Department of Agriculture, Ames, IA). p1-ww [4co63] was obtained from the National Seed Storage Laboratory (Fort Collins, CO) while P1-wr [W23] was acquired from the Maize Genetics Cooperation Stock Center (Urbana, IL). P1-rr-4B2 was obtained from Thomas Peterson (Grotewold et al. 1991a). The P1-rr-4B2 allele was introgressed into the W23 background by six generations of backcrossing. A Mu-active stock was obtained from the Maize Genetics Cooperation (University of Illinois, Urbana–Champaign, IL). A stock carrying the dominant Mu inhibitor and the Mu-suppressible Les28 reporter allele was kindly provided by Robert Martienssen, Cold Spring Harbor Laboratory (Cold Spring Harbor, NY) (Martienssen and Baron 1994). A stock heterozygous for Mu killer (Muk) was generously provided by Damon Lisch, University of California (Berkeley, CA) (Slotkin et al. 2003).
Identification of Mu-insertion lines in P1-wr:
We used the Trait Utility System for Corn (TUSC) developed by Pioneer Hi-Bred International (Meeley and Briggs 1995) for transposon-based reverse genetics of P1-wr. In this procedure, P1-wr plants from several maize inbred lines were crossed with Mu-active plants that also carry a P1-wr allele and the resulting progeny plants were screened for Mu insertions. The Mu-active plants contain the autonomous MuDR transposase that induces the excision and transposition of itself as well as other, nonautonomous Mu elements (Mu1–Mu12). To identify Mu insertions, pooled DNA of a large population of the progeny plants was screened by PCR using p1-specific primers together with the Mu-terminal inverted repeat (Mu-TIR) primer that is conserved in the border sequences of all Mu elements. Sequences of primers and their locations in P1-wr or Mu1 are listed in supplemental Table 1. Positive pools showing PCR amplification were identified and products were subcloned into the pGemT-easy TA cloning vector (Promega, Madison, WI). Subsequently, the clones were sequenced to determine the positions of Mu insertions within the P1-wr gene. The Mu-element orientation of most insertion alleles could be discerned on the basis of unique SNPs in the TIRs (Dietrich et al. 2002; R. Meeley, unpublished data).
Genetic crosses with P1-wr-mum6:
The P1-wr-mum6 insertion line was identified in the F1 of TUSC materials generated from a cross between the A632 inbred line and a stock carrying high Mu activity (see above). The F2 progeny carrying the P1-wr-mum6 insertion was screened for pericarp and cob pigmentation phenotypes. The F2 plants were pollinated with p1-ww [4co63] and the resulting plants were reciprocally testcrossed with p1-ww [4co63]. To obtain P1-wr-mum6 plants with inactive Mu elements, P1-wr-mum6/p1-ww [4co63] plants were crossed with a stock carrying the dominant Mu inhibitor and the Mu-suppressible Les28 allele (Martienssen and Baron 1994). However, it has been shown that crosses with the Mu inhibitor stock do not always dominantly inactivate Mu activity (May et al. 2003). Thus, the Mu activity was followed in F1 plants using the Les28 reporter that confers a lesion-mimic phenotype only when Mu is active. Younger leaves sometimes appeared spotted, indicating that they retained Mu activity, whereas older leaves did not have spots, indicating that Mu had been inactivated. The F2, F3, and F4 progenies also did not express the Les28 phenotype and were thus considered to be Mu inactive. In some families the Mu inhibitor stock did not completely silence Mu activity (M. Robbins and S. Chopra, unpublished data). Thus, crosses were also made using the heterozygous Mu killer (Muk) stock, which was not available when this research was started. Muk is a naturally occurring partially deleted version of MuDR that contains an inverted repeat. Muk functions dominantly and is believed to facilitate RNA-dependent chromatin remodeling and silencing of functional MuDR elements (Slotkin et al. 2005). The presence of Muk in plants carrying P1-wr-mum6 or P1-wr [A632] was determined using an established PCR-genotyping assay available at http://plantbio.berkeley.edu/∼mukiller/using.html (Slotkin et al. 2003).
DNA gel blot analysis:
Leaf genomic DNA was isolated using a modified CTAB method (Saghai-Maroof et al. 1984). DNA was digested to completion using enzymes, reagents, and incubation conditions from Promega. Digested DNA was fractionated on agarose gels and transferred to Nylon membranes, and the membranes were subsequently probed with DNA probes of interest. The DNA probes were labeled with [α-32P]dCTP through random priming, using a Prime-It RmT random primer labeling kit (Stratagene, La Jolla, CA). Membranes were prehybridized for 4 hr at 65° in buffer containing NaCl (1 m), SDS (1%), Tris-HCl (10 mm), and salmon sperm DNA (0.25 mg/ml) followed by hybridization in the same buffer containing 32P-labeled DNA probes for 16 hr at 65° (Athma and Peterson 1991). Membranes were washed twice in 0.1× SSC and 0.5% SDS at 65° for 15–30 min and exposed to X-OMAT film (Kodak, Rochester, NY). Blots were stripped of previous signal in boiling 0.1% SDS before they were reused.
RNA expression analysis:
Pericarps and cob glumes were harvested 18 days after pollination and RNA was isolated using a modified phenol–chloroform extraction protocol (Verwoerd et al. 1989). RNA gel blot analysis was performed using 10 μg of total RNA from pericarp as previously described (Chopra et al. 1996). For RT–PCR analysis, 50 μg of total RNA was treated with DNase I (GIBCO-BRL, Gaithersburg, MD). Ten micrograms of treated RNA and 0.5 μg oligo (dT)15 primer were denatured for 5 min at 70° and subsequently added to a reaction mixture containing ImProm-II reverse transcriptase (Promega). First-strand cDNA was synthesized by incubating the reaction mixture at 42° for 1 hr. The reverse transcriptase was deactivated by heating at 70° for 15 min. PCR primers of P1-wr that were used to amplify the first-stand cDNA templates are EP5-8 and SC2-2R (supplemental Table 1). EP5-8 is a forward primer that resides upstream of the Mu1 insertion in P1-wr-mum6 and was used with a reverse primer, SC2-2R, located downstream of the Mu1 insertion (Figure 1). The large size of P1-wr intron 2 does not permit genomic DNA amplification between EP5-8 and SC2-2R. The housekeeping gene α-tubulin was used as an RT–PCR control.
Figure 1.—
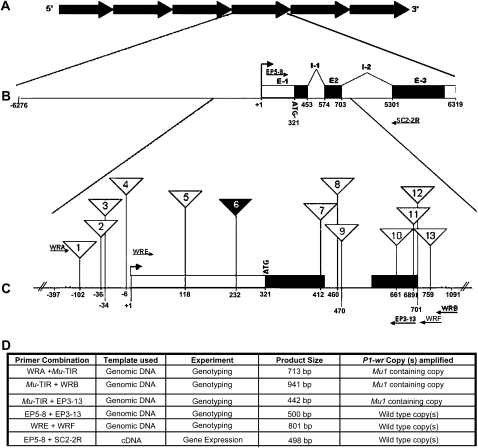
Mutator element insertion sites in P1-wr. (A) Illustration depicting the tandem repeats that make up the six-copy P1-wr complex. (B) Gene structure of one representative P1-wr copy in which exons (E) and introns (I) are shown. A bent arrow indicates the position of the transcription start site that is represented as +1. Positions of primers that were used for expression analysis of P1-wr-mum6 (see below) are represented by arrows. (C) Enlarged region of exon 1, intron 1, and the 5′ end of intron 2 showing the position of Mutator transposon insertions (triangles). Numbers inside the triangles correspond to the insertion lines (P1-wr-mum1–P1-wr-mum13) presented in Table 1. The solid triangle designates the gain-of-function mutation P1-wr-mum6. Primers that were used with genomic DNA to characterize the insertion lines are indicated as arrows. (D) Details of the PCR-based characterization of the P1-wr-mum6 allele. The amplification product size and the type of P1-wr copy amplified are listed for each experiment described in the text. P1-wr primers positioned 5′ and 3′ to the Mu1 insertion were used to amplify cDNA to determine the gene expression originating from wild-type copy (or copies).
Description of probe fragments:
The region flanking the Mu1 insertion in P1-wr-mum6 was assayed with intron 2 probe fragments 8B and 8C, and the distal enhancer was assayed with probe fragment 15 (Lechelt et al. 1989; Chopra et al. 1998; Sekhon et al. 2007). Mu activity was assayed using gel blots made from HinfI-digested genomic DNA (Chandler and Walbot 1986) and Mu1 probe fragment was obtained by the amplification of pucMuED4 plasmid using M13 forward and reverse primers. The pucMuED4 plasmid was generously provided to by David Braun, Pennsylvania State University. Probes corresponding to chalcone synthase (c2) and P1-rr cDNAs have previously been described (Paz-Ares et al. 1986; Grotewold et al. 1991a).
Genomic bisulfite sequencing:
Seedling leaf genomic DNA was extracted using a modified CTAB method (Saghai-Maroof et al. 1984). Eight micrograms of genomic DNA were restricted with suitable restriction enzymes to obtain ∼1-kb fragments containing the region of interest. The restricted DNA was purified with phenol–chloroform and treated with sodium bisulfite, using a previously standardized protocol (Jacobsen et al. 2000; Sekhon et al. 2007). The upper strand of a 387-bp region from the distal enhancer (positions −5052 to −4666 of EF165349) was amplified using PCR primers specially designed to amplify DNA modified with sodium bisulfite (supplemental Table 1). Gel-purified PCR products were cloned using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA) and sequenced using vector primers. Two plants each from the gain-of-function (i.e., showing pericarp pigmentation) and nonexpressing (i.e., with colorless pericarp) P1-wr-mum6 families were analyzed and at least 20 clones per plant were sequenced.
RESULTS
Isolation of 13 heritable Mu-insertion sites in P1-wr:
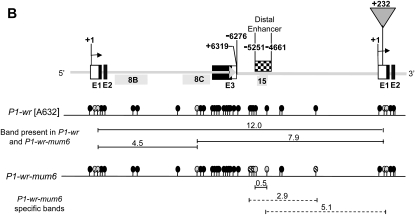
The TUSC germplasm was screened for Mu insertion in P1-wr using Mu-TIR and gene-specific primers (Figure 1). This region includes the proximal promoter and the downstream gene sequence containing exons 1 and 2, intron 1, and the 5′ end of intron 2. Thirteen heritable Mu-insertion sites were identified and these are listed 5′–3′ as P1-wr-mum1–P1-wr-mum-13 in Table 1. Of these, 10 insertion alleles were commonly identified with two or more independent primer combinations. Three of the insertion sites were associated with multiple independent Mu insertions. For example, P1-wr-mum9 (position 471) and P1-wr-mum12 (position 702) were selected twice while P1-wr-mum13 (position 760) was selected three times. Four Mu insertions were in the promoter region and three were found each in exons 1 and 2. Two insertion sites were identified in intron 1 and a single site was found in the beginning of intron 2. The types of Mu elements found in P1-wr were Mu1, Mu4, Mu8, Mu11, and MuDR (Table 1). Additionally, one Mu insertion had a TIR that resembled the published sequence of Mu1, but it contained SNPs at two positions (Barker et al. 1984). Since the region internal to the TIR was not sequenced, it is currently denoted as a Mu1-like element.
TABLE 1.
Positions of Mu insertions in P1-wr
| Insertion line | Distance from TSSa | P1-wr region | Mu element(s) types, orientationb | Target sequencec |
|---|---|---|---|---|
| P1-wr-mum1 | −102 | Promoter | Mu1, R | AATTCGGTCGGTCCGTAACGTGC |
| P1-wr-mum2 | −36 | Promoter | Mu1, F | CGTCCGCTGCTATATTATGGCCG |
| P1-wr-mum3 | −34 | Promoter | Mu11, F | TCCGCTGCTATATTATGGCCGGC |
| P1-wr-mum4 | −6 | Promoter | MuDR, ND | CGTGCCCTCTCTAGCCAGCACAG |
| P1-wr-mum5 | +118 | Exon 1 | Mu4, ND | CACCAACTCCCTTGGACGCACGC |
| P1-wr-mum6 | +232 | Exon 1 | Mu1, R | TCCGGTGTGGCCAGCGGCGGCCG |
| P1-wr-mum7 | +412 | Exon 1 | Mu1, F | TGCGGAGCACGGCGAGGGGTCC |
| P1-wr-mum8 | +460 | Intron 1 | MuDR, ND | TAAACCAAAGCCGGCCGCGCGC |
| P1-wr-mum9 | +470 | Intron 1 | Mu1, F; Mu1, F | GCCGGCCGCGCGCCATGCATCGC |
| P1-wr-mum10 | +661 | Exon 2 | Mu1, R | AGGAGGAAGAAGACATCATCATC |
| P1-wr-mum11 | +689 | Exon 2 | Mu1, F | CCACGCCACCCTCGGCAACAGGT |
| P1-wr-mum12 | +701 | Exon 2 | Mu1, R; Mu8, F | CGGCAACAGGTAACAATAAGCGC |
| P1-wr-mum13 | +759 | Inron 2 | MuDR, ND; Mu1, F; Mu1-liked, ND | TAGAGAGTAGTAGTACTACTACT |
TSS, transcription start site. The positions of insertions correspond to P1-wr sequence accession EF165349.
F, forward orientation; R, reverse orientation; ND, orientation not determinable.
The predicted 9-bp target sites of the Mu insertions are indicated in boldface type. Accession no. of P1-wr: EF165349.
Mu1-like denotes presence of two SNPs in the TIR sequence that resembles Mu1 (accession no. X00913). SNP positions are underlined in the TIR sequence: 5′-GAATCCCCTTCCCTCTTCGTCCACAATGGCAGTTATC-3′.
P1-wr-mum6 is associated with gain of function in pericarp tissue:
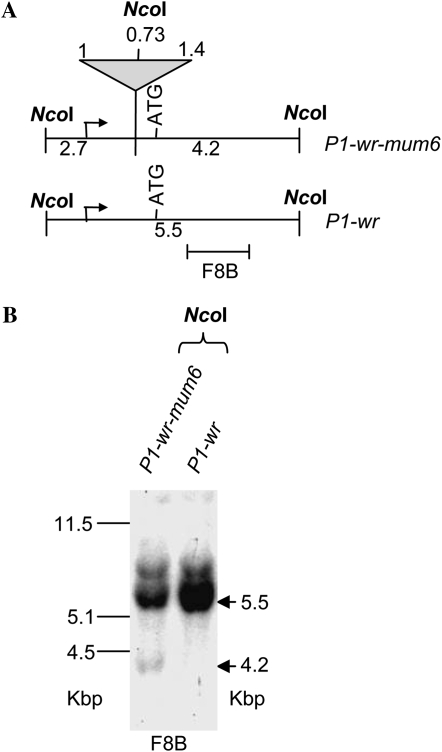
F2 progeny plants of all 13 P1-wr-specific Mu-insertion events were analyzed for altered pigmentation patterns. Loss-of-function phenotypes, such as the reduction in cob pigmentation, were not observed in any insertion line. Interestingly, one insertion line, P1-wr-mum6, exhibited a gain-of-pericarp-pigmentation phenotype. PCR amplification and sequence characterization of the P1-wr-mum6 insertion allele revealed that a Mu1 element is located in direct orientation in the 5′-UTR, 232 bp 3′ to the transcription start site of P1-wr (Figure 1; Table 1). DNA gel blot analysis was also performed to compare the structure of P1-wr with P1-wr-mum6 (Figure 2). Genomic DNA of these genotypes was digested with NcoI (Figure 2A). The p1 fragment 8B was used as a probe because it resides downstream of the Mu1 insertion site in P1-wr-mum6. In P1-wr, NcoI digestion produces a 5.5-kb fragment. Conversely, in P1-wr-mum6, NcoI cuts in both P1-wr and Mu1, yielding a 4.2-kb fragment (see Figure 2B). The weak hybridization signal of the P1-wr-mum6-specific band (4.2 kb) relative to the P1-wr-specific band (5.5 kb) strongly suggests that the Mu1 insertion is in one of the copies of P1-wr.
Figure 2.—
Structural comparison of P1-wr-mum6 and P1-wr [A632] alleles. (A) Restriction map showing the positions of NcoI sites in P1-wr-mum6. The triangle signifies the Mu1 insertion in P1-wr-mum6. Fragment sizes are indicated for both the transposon-interrupted (P1-wr-mum6) and wild-type (P1-wr) copies. (B) DNA gel blot analysis of P1-wr-mum6 showing the presence of a Mu1 insertion. The location of p1 probe fragment 8B is shown below the restriction map in A. Arrows indicate the position of expected sizes (in kilobase pairs) of specific bands, after NcoI digestion. Sizes of the molecular weight marker bands in kilobase pairs are shown on the left of the blot.
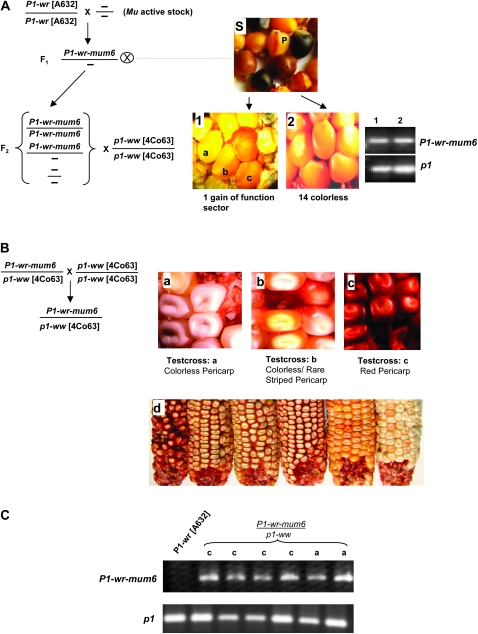
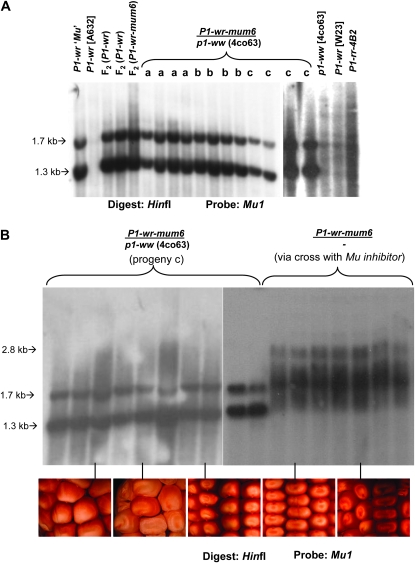
The gain-of-function allele P1-wr-mum6 was initially discovered as red stripes on colorless pericarp of ∼1 in every 10 F2 kernels (see kernel marked “P” in the section labeled “S” in Figure 3A). However, the pericarp pigmentation phenotype was present in only a single ear of a total of 15 ears recovered from the F2 plants (see Figure 3A, sections 1 and 2). Genotyping of nine individuals that had colorless pericarp revealed that eight carried the P1-wr-mum6 allele (see Figure 3A, section 2). The single gain-of-function P1-wr-mum6 ear had four kernels that displayed red sectored pericarp pigmentation (Figure 3A, section 1). In summary, the gain of pericarp phenotype of P1-wr-mum6 in early generations was associated with low expressivity and poor penetrance.
Figure 3.—
Progression of pericarp pigmentation in P1-wr-mum6. (A) The gain of pericarp pigmentation associated with P1-wr-mum6 initially had low expressivity and penetrance. The F2 source seed of P1-wr-mum6 had red phlobaphene stripes (section S, see kernel marked “p”). F2 plants grown from seeds shown in section S were crossed with p1-ww [4co63]. Phenotypes of two representative crossed ears are shown in sections 1 and 2. All F2 plants were genotyped by PCR for the presence of P1-wr-mum6, using the Mu-TIR and WRB primers (see Figure 1D). The p1 primers WRE and WRF were used to amplify regions of the P1-wr gene copies that do not contain the transposon insertion. Representative lanes of PCR-amplification products of individuals in sections 1 and 2 are shown on the right. A kernel map was constructed from the F3 ear in section 1 by lettering the kernel types a–c. (B) Plants grown from kernels marked a–c in section 1 of A were reciprocally crossed with p1-ww [4co63]. Pericarp phenotypes of representative testcross progeny ears are shown in sections a–c. Section d shows the variability in ear phenotypes that is apparent in testcross progeny c. (C) Presence of P1-wr-mum6 in the testcross progenies a and c was determined by PCR amplification of pericarp DNA using the Mu-TIR and EP3-13 primers (see Figure 1D). The p1 primers EP5-8 and EP3-13 were used to amplify regions of the uninterrupted P1-wr copies. Lanes marked a and c designate P1-wr-mum6/p1-ww [4co63] individuals obtained from test crosses a and c (see ears a and c in B), respectively.
Gain-of-pericarp-function as well as colorless-pericarp kernels carrying the P1-wr-mum6 allele were further followed to perform genetic and molecular tests. Reciprocal P1-wr-mum6/p1-ww [4co63] × p1-ww [4co63] testcross progenies were characterized from a single dark uniform kernel, two sectored kernels, and four colorless kernels. This was done to determine if there was a correlation between the pigmentation of the testcross progenies (Figure 3B, sections a–c) and that of their progenitor kernels (Figure 3A, section 1). Interestingly, the level of pericarp pigmentation in each testcross progeny (Figure 3B, sections a–c) did correspond with the level of pigmentation present on the parental P1-wr-mum6/p1-ww kernel (Figure 3A, section 1). However, the level of pericarp pigmentation did not depend on which parent (P1-wr-mum6 or p1-ww [4co63]) was used as the pollen source. The testcross progeny developed from the colorless kernels remained colorless, indicating that the suppressed state of P1-wr-mum6 had become stable (Figure 3B, section a). The sectored kernels gave rise to progeny ears either with colorless pericarp (∼70%) or with occasional red pericarp stripes (∼30%) (Figure 3A, section b). Therefore, the penetrance and expressivity of the pericarp-pigmentation phenotype associated with the progeny of the sectored kernels remained low. The fully red kernel generated a stable testcross progeny in which all P1-wr-mum6 individuals had a range of red pericarp pigmentation (Figure 3B, sections c and d). PCR genotyping of pericarp DNA from P1-wr-mum6/p1-ww testcross progenies “a” and “c” confirmed that the Mu1 insertion was present even though pericarp pigmentation was not observed (Figure 3C).
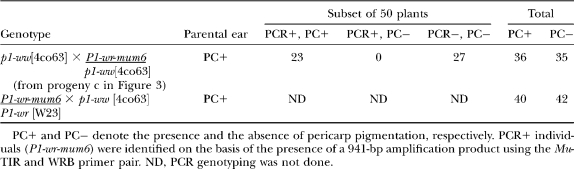
The progeny ears resulting from the dark red kernel (testcross progeny c) had the expected 1:1 ratio of red to colorless pericarp (see Table 2). This showed that the gain-of-pericarp-pigmentation phenotype was stably inherited. However, sibling plants from this population differed with respect to the level of pericarp pigmentation (Figure 3B, section d). The pericarp pigmentation was either uniformly diffused or localized to the silk attachment point or kernel gown. In addition, a small number (∼5%) of ears displayed a kernel-to-kernel variation in overall pigment accumulation. Since all pigmented individuals were heterozygous, the range in pericarp pigmentation could not be due to a dosage effect.
TABLE 2.
Analyses of testcross populations showing the linkage between the presence of the P1-wr-mum6 allele and the gain of pericarp pigmentation
To ensure that the gain of function in P1-wr-mum6 was not due to an unlinked mutation, we crossed a P1-wr-mum6/p1-ww [4co63] individual with P1-wr [W23]. The resulting F1 plant was crossed with p1-ww [4co63] to segregate P1-wr-mum6 from P1-wr [W23]. If the gain of function was due to unlinked mutations, we would expect pigmented pericarp in P1-wr [W23]/p1-ww [4co63] individuals. This cross yielded a 1:1 ratio of red to colorless pericarp, indicating that the P1-wr-mum6 stock did not contain a secondary mutation that can induce expression of naive P1-wr in pericarp (Table 2). Moreover, this result indicated that the P1-wr-mum6 allele does not interact in trans with P1-wr [W23].
The expression of linked uninterrupted gene copy (or copies) is elevated in P1-wr-mum6:
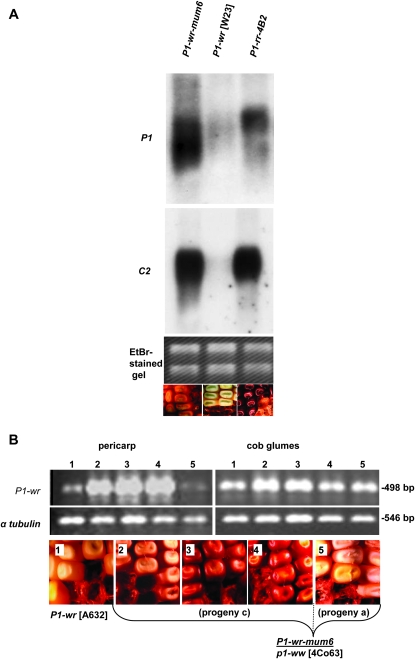
To test if the gain of pigmentation in P1-wr-mum6 was due to the increased expression of p1 and a p1-regulated structural gene, chalcone synthase (c2), we performed RNA gel blot analysis (Grotewold et al. 1991b, 1994). The C2 protein catalyzes the first committed enzymatic step in the production of phenylpropanoid compounds including flavonoid pigments (Kreuzaler and Hahlbrock 1975). As expected, when compared with P1-wr, P1-wr-mum6 had a large increase in p1 and c2 steady-state transcripts in pericarp tissue (Figure 4A). Interestingly, P1-wr-mum6 and the single-copy P1-rr-4B2 allele were expressed at nearly the same level.
Figure 4.—
Gain of pericarp pigmentation in P1-wr-mum6 results from upregulation of P1-wr. (A) RNA gel blot showing increased steady-state transcript level of p1 and c2 in P1-wr-mum6 as compared to P1-wr. The P1-rr-4B2 pericarp RNA was used as a positive control. Phenotypes of the three alleles are shown below the gel picture. (B) Reverse-transcription–PCR was used to compare the p1 expression in pericarp and cob glumes of P1-wr-mum6/p1-ww [4Co63] and homozygous P1-wr [A632] individuals. Primers EP5-8 and SC2-2R were used to amplify the first-strand cDNA (see Figure 1D). The amplified product of 498 bp results from the expression of the wild-type copy (or copies) of P1-wr-mum6 that does not contain the Mu1 insertion. Numbered lanes correspond with sections indicated below the gel picture. Section 1 is P1-wr [A632], and sections 2–4 show P1-wr-mum6/p1-ww [4co63] ears from the testcross progeny “c” (see Figure 3) that was derived from a fully red kernel. Section 5 shows a P1-wr-mum6/p1-ww [4co63] ear from the testcross progeny a (see Figure 3) that was derived from a colorless P1-wr-mum6/p1-ww [4co63] kernel.
The increased expression in P1-wr-mum6 could arise from two sources: new transcripts may originate from the gene copy containing the Mu1 insertion in the 5′-UTR, or there may be increased expression from one or more of the five other (wild-type) copies that are not interrupted by the transposon insertion. Elevated expression of the interrupted copy could be explained if the Mu1 element in the 5′-UTR functions as a cryptic promoter for the immediate downstream gene copy. For example, the suppression of the maize hcf106 mutation has been directly related to the presence of hcf106 transcripts that originate downstream of a Mu1 element (Barkan and Martienssen 1991). Several experiments were conducted to detect transcripts that may be arising from the P1-wr copy containing the Mu1 insertion. RT–PCR analysis using the Mu-TIR and EP3-13 primers did not detect any transcript originating within the Mu1 element (data not shown). Additionally, primer extension and 5′ rapid amplification of cDNA ends (RACE) PCR experiments performed using P1-wr-mum6 and P1-wr control plants also failed to detect different transcript initiation sites. All detected transcripts contained the transcription start site expected for wild-type P1-wr (data not shown). Moreover, RNA gel blots also did not reveal the presence of any aberrantly sized transcripts (Figure 4A). These results suggested that the interrupted copy is nonfunctional and that the increased expression in P1-wr-mum6 may originate from one or more of the uninterrupted copies.
To confirm that the enhanced RNA expression of P1-wr originates from wild-type copies, RT–PCR analysis was performed (Figure 4B). We used a primer EP5-8, which resides upstream of the Mu1 insertion in P1-wr-mum6, and SC2-2R, which is located downstream of the Mu1 insertion (see Figure 1B for position of primers). The presence of the Mu1 element would prohibit amplification of transcripts containing the insertion. This assay specifically yielded products with the size expected from uninterrupted P1-wr copy (or copies). Importantly, the range in pericarp pigmentation was directly proportional to the abundance of the p1 transcripts detected through RT–PCR (Figure 4B). However, the pigmentation and p1 gene expression were similar in P1-wr and P1-wr-mum6 cob glumes (Figure 4B). This suggests that the upregulation in pericarp in P1-wr-mum6 is achieved through a tissue-preferred mechanism. It is conceivable that the Mu1 insertion disrupted a suppression mechanism that is normally operative in P1-wr pericarp tissue. In summary, these results support the hypothesis that the uninterrupted copies are the source of the p1 expression in P1-wr-mum6 pericarps.
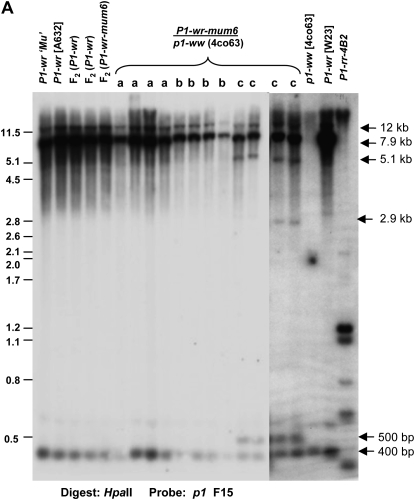
P1-wr-mum6 DNA hypomethylation correlates with pericarp pigmentation:
DNA gel blot data indicated that P1-wr-mum6 contains a six-copy structure similar to that of P1-wr, except that a single copy is interrupted by Mu1 (Figure 2 and our unpublished results). It is known that transposon insertions in genes or in their neighboring regions can affect expression and epigenetic states of such genes (Lippman et al. 2004). We therefore hypothesized that the Mu1 insertion in P1-wr-mum6 may have induced epigenetic changes of the multicopy complex, thereby altering its expression. To test if DNA methylation changes correlate with pericarp pigmentation in P1-wr-mum6, seedling leaf DNA was digested with the methylation-sensitive restriction enzyme HpaII and gel blots were hybridized with p1 probe fragment 15. The banding pattern of several genotypes was compared. First, the P1-wr sources that were used to generate P1-wr-mum6 were compared with P1-wr-mum6 and P1-wr F2 individuals of the TUSC screen that had colorless pericarp. These genotypes yielded similar ∼12.0-, 7.9-, and 0.4-kb bands, indicating that the DNA methylation was unaltered in P1-wr-mum6 plants that have colorless pericarp (Figure 5A). Second, to address whether DNA methylation changes are associated with pericarp pigmentation in P1-wr-mum6, the different p1-ww × P1-wr-mum6/p1-ww testcross progenies (see Figure 3) were also analyzed. The P1-wr-mum6 progeny that exhibited colorless pericarp (Figure 3B, section a) or possible occasional red stripes (Figure 3B, section b) had no detectable DNA methylation differences when compared with P1-wr (Figure 5; see lanes marked a or b). Only in the fully penetrant progeny with relatively high levels of pericarp pigmentation (Figure 3B, sections c and d, and Table 2) did we detect DNA hypomethylation (Figure 5, see lanes marked c). The hypomethylation consisted of three HpaII bands of ∼5.1-kb, 2.9-kb, and 500-bp sizes. However, these DNA methylation changes were not detected using intron 2 probe fragments 8B and 8C, indicating that the affected HpaII sites may reside in the upstream promoter region encompassing fragment 15 (Figure 5B). In fact, the 500-bp fragment has been previously reported to arise from hypomethylation of HpaII sites in an upstream promoter region (Chopra et al. 2003). Interestingly, this region has been shown to be part of a distal enhancer (Figure 5B) for p1 expression in pericarp tissue (Sidorenko et al. 2000; Chopra et al. 2003).
Figure 5.—
Gain of pericarp function in P1-wr-mum6 correlates with the hypomethylation of a distal enhancer sequence of P1-wr. (A) Seedling leaf DNA was digested with HpaII and gel blots were hybridized with p1 fragment 15 that corresponds to the distal enhancer element of the p1 gene (Lechelt et al. 1989; Sidorenko et al. 2000). Arrows on the right side of the blot denote the location of specific bands discussed in the text. Genotypes are shown on the top of the gel picture. These include the P1-wr parental sources used in the TUSC screen to generate P1-wr-mum6, F2 generation P1-wr and P1-wr-mum6 individuals that had colorless pericarp, and P1-wr-mum6/p1-ww [4co63] individuals derived from testcross progenies a–c (Figure 3). These testcross progenies are shown by the letters a, b, and c, respectively. The Mu-active source used to generate P1-wr-mum6 is denoted by P1-wr ‘Mu’. (B) Gene structure diagram showing two representative partial copies of the six-copy tandem gene array. The coordinates shown above the diagram correspond with the P1-wr accession EF165349. The positions of exons 1–3 (E1–E3) are given as rectangles where the open regions of exons 1 and 3 correspond with the 5′- and 3′-UTRs, respectively. The location of the Mu1 transposon in the 5′-UTR of P1-wr-mum6 is represented as a shaded inverted triangle. However, it is important to note that it is not known at this point which P1-wr copy carries the insertion. The distal enhancer that is present in each gene copy is shown as a checkered box. The p1 probe fragments used for construction of the methylation map are shown as shaded rectangles below the gene structure diagram. The DNA methylation status at HpaII sites (ovals) is based on gel blot results shown in A and in Figure 8B. Solid ovals represent hypermethylated sites, shaded ovals are partially methylated sites, and hatched ovals indicate partially methylated sites. Band sizes are shown as horizontal lines below the HpaII sites; dashed lines indicate estimated band locations because of close proximities of HpaII sites
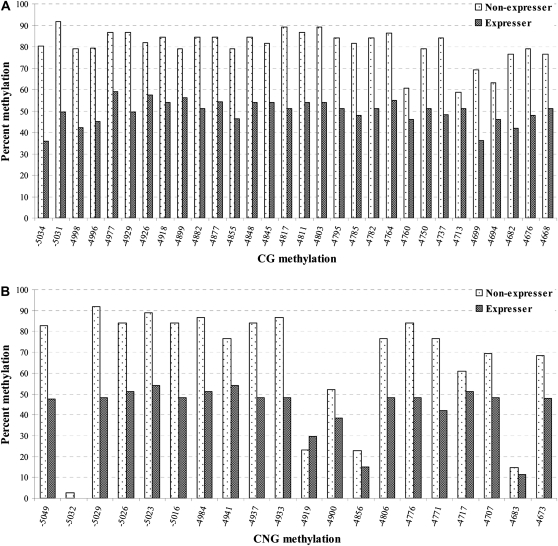
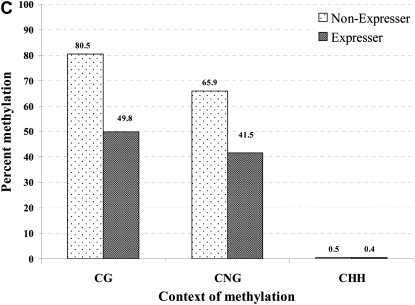
The aforementioned DNA gel blot results indicated that the distal enhancer region may be hypomethylated in P1-wr-mum6 plants that have ectopic gain-of-pericarp pigmentation. This result, although promising, reported hypomethylation of only a single HpaII site within the distal enhancer region. It therefore was hypothetically possible that the hypomethylation of this site did not reflect the DNA methylation status of the broader distal enhancer region (Figure 5B). To determine the cytosine methylation across a region encompassing the 3′ end of the distal enhancer (i.e., positions −5052 to −4666 of P1-wr accession EF165349), we used genomic bisulfite sequencing. For this analysis, we compared P1-wr-mum6 expresser plants from the fully penetrant testcross family c with nonexpresser plants from testcross family a that exhibited no gain-of-pericarp pigmentation (see Figure 3B, sections a and c). Interestingly, bisulfite sequencing results showed that the P1-wr-mum6 expresser plants were hypomethylated at all CG sites and at all but one CNG sites (Figure 6, A and B). Therefore, nearly the entire distal enhancer region tested was hypomethylated in P1-wr-mum6 expressers. The combined reduction in the assayed region was 30.3% for CG and 24.4% for CNG methylation (Figure 6C). CHH methylation levels were negligible at all sites regardless of P1-wr-mum6 expression (Figure 6C and supplemental Figure 1).
Figure 6.—
The correlation between DNA hypomethylation and pericarp pigmentation in P1-wr-mum6 was examined by genomic bisulfite sequencing. Leaf genomic DNA of P1-wr-mum6 expresser (i.e., showing red pericarp pigmentation) and nonexpresser plants (i.e., showing colorless pericarp) was used to study cytosine methylation of a distal enhancer (location shown in Figure 5B). We specifically assayed a 387-bp fragment that is located at the 3′ end of the distal enhancer (positions −5052 to −4666 of P1-wr accession EF165349). Methylation of individual CG and CNG sites in this region are shown in A and B, respectively. The position of the sites is shown on the x-axis while the percentage of methylation is presented on the y-axis. The percentage of methylation for each residue was calculated by dividing the methylated clones for that residue by the total number of clones. Two expresser and two nonexpresser plants were studied and the averages are presented here. CGG sites were counted as CG sites. (C) Cumulative methylation in CG, CNG, and CHH (H is A, C, or T) context in the genotypes studied. For each genotype, overall methylation in each context was calculated by dividing the number of methylated cytosines by the total number of cytosines in the context in all the clones. Context of methylation is shown on the x-axis and percentage of methylation is shown on the y-axis.
P1-wr-mum6 expression is not affected by Mu activity:
The DNA hypomethylation at the p1 distal enhancer may be induced by the presence of the Mu1 insertion into the 5′-UTR by at least three mechanisms: (1) the MuDR transposase could affect trans-factors that regulate gene expression mechanisms, (2) the DNA methylation at the Mu1 element could spread to the flanking P1-wr sequence, or (3) the transposon interruption itself could physically interfere with cis-regulatory regions that are important for local chromatin remodeling. We tested each of these possibilities and these are presented in the following text.
The activity of the MuDR transposase can interfere with gene expression mechanisms such as promoter function, intron splicing, and polyadenylation (Barkan and Martienssen 1991; Girard and Freeling 2000; Cui et al. 2003). To determine if such Mu suppression mechanisms were altered in P1-wr-mum6 plants with ectopic pericarp pigmentation, we tested the Mu activity status of the aforementioned p1-ww × P1-wr-mum6/p1-ww testcross progenies that had distinct levels of pericarp pigmentation (see Figure 3B, sections a–c). We used a previously described Mu activity assay that relies on the fact that all inactive Mu elements in the genome (including MuDR) are coordinately methylated (Chandler and Walbot 1986; Lisch et al. 1995; Lisch 2002). Seedling leaf genomic DNA was digested with HinfI and the resulting blot was hybridized with a Mu1 probe. HinfI sites are methylated when Mu1 is in an inactive state, which is evidenced by the loss of a 1.3-kb band and the presence of several higher-molecular-weight fragments (Chandler and Walbot 1986; Lisch et al. 1995). All testcross progeny plants showed hypomethylated Mu1 elements, indicated by the presence of the 1.3-kb band. Therefore, despite the differences in pericarp pigmentation in the testcross progenies, there was no difference in the DNA methylation of Mu1 (Figure 7A).
Figure 7.—
The gain of pericarp function associated with P1-wr-mum6 does not depend on the Mu activity. (A) A DNA gel blot containing HinfI-digested leaf genomic DNA was hybridized with a Mu1 probe. The 1.3- and 1.7-kb HinfI fragments (marked by arrows on the left) are indicative of active Mu1 elements. Genotypes studied are indicated at the top. These include the P1-wr parental sources used in the TUSC screen to generate P1-wr-mum6, F2 generation P1-wr and P1-wr-mum6 individuals that had colorless pericarp, and P1-wr-mum6/p1-ww [4co63] individuals derived from testcross progenies a, b, and c (Figure 3). These testcross progenies are shown by letters a, b, and c, respectively. The Mu-active source used to generate P1-wr-mum6 is denoted by P1-wr ‘Mu’. (B) DNA gel blot showing silencing of Mu activity in P1-wr-mum6/p1-ww by Mu inhibitor. DNA of P1-wr-mum6/− individuals derived from a cross of P1-wr-mum6 with Mu inhibitor (see materials and methods) was digested with HinfI and the resulting blot was hybridized with a Mu1 probe. Arrows to the left of the blots denote the locations of specific bands discussed in the text. Ear photos corresponding to given lanes are shown below the gel.
The idea that the DNA methylation at Mu elements can affect the DNA methylation and expression of an adjacent gene sequence was established for a Mu-insertion allele of hcf106 (Martienssen et al. 1990). In the case of the knotted1 gene, the severity of the Knotted1-mum7 mutant phenotype was directly correlated with the degree of Mu1 hypomethylation (i.e., Mu activity) (Greene et al. 1994). To test whether the presence of Mu activity positively affects pericarp pigmentation of P1-wr-mum6, we developed a Mu inactive P1-wr-mum6 stock through crosses with a stock carrying Mu inhibitor (see materials and methods). Absence of a 1.3-kb HinfI fragment and presence of higher molecular weight (e.g., 2.8 kb) demonstrated Mu elements were silenced in these individuals (Figure 7B). Ear phenotypes revealed that the pericarp pigmentation was also present in the absence of Mu activity. Furthermore, P1-wr-mum6 individuals from a testcross population that exhibited a range of pericarp pigmentation (see Figure 3B, section d) did not have a corresponding range of Mu1 methylation (See Figure 7B, bottom). In summary, these results demonstrate that the activity of MuDR does not affect the P1-wr-mum6 phenotype.
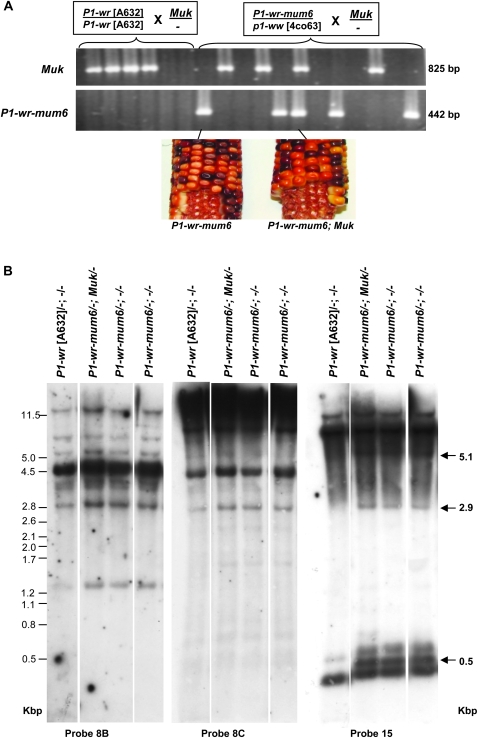
A similar genetic approach was undertaken when Mu killer (Muk) became available in the laboratory of Damon Lisch. Like Mu inhibitor, the presence of Muk dominantly silences MuDR expression albeit in a more consistent fashion (May et al. 2003; Slotkin et al. 2003, 2005). P1-wr and P1-wr-mum6 plants differing for the presence of Muk were identified using a PCR assay (Figure 8A; materials and methods). In the individuals carrying Muk, inactivity of Mu elements was confirmed by digesting leaf genomic DNA with HinfI and hybridizing the blots with a Mu1 probe (data not shown). As expected, the presence of Muk had no effect on the pericarp pigmentation in P1-wr-mum6 (Figure 8A, bottom). To test if the Mu activity has any effect on the P1-wr-mum6 epigenetic state, DNA methylation of different regions of P1-wr-mum6 was assayed by gel blot analysis. We observed that the presence of Muk does not affect the DNA methylation status of P1-wr-mum6 at p1 intron 2 and distal enhancer regions (Figure 8B). In summary, the status of Mu activity does not seem to affect P1-wr-mum6 expression and its DNA methylation status.
Figure 8.—
The presence of Muk does not affect the DNA methylation of the distal enhancer and intron 2 regions of P1-wr-mum6. (A) Ethidium bromide-stained gel picture showing PCR-based genotyping of Muk and P1-wr-mum6 individuals. P1-wr [A632] and P1-wr-mum6/ p1-ww [4co63] were crossed with a heterozygous Muk stock to obtain sibling plants with either active or inactive Mu elements. The size of the PCR products is shown on the right. P1-wr-mum6 ears that contain and lack Muk are shown below the gel. (B) DNA gel blot showing the effect of Muk on p1 methylation. A gel blot carrying HpaII-digested DNA of selected genotypes was sequentially hybridized with intron 2-specific probes 8B and 8C and the distal enhancer probe 15. Position and sizes (in kilobase pairs) of bands specific to P1-wr-mum6 are indicated with arrows on the right. Sizes of the molecular weight markers in kilobase pairs are shown on the left.
DISCUSSION
The numerous alleles of p1 that differ with respect to gene structure and tissue specificity are valuable tools for studying how copy number may regulate tissue-specific expression patterns. However, the importance of p1 gene copies in tissue-specific expression is not very well understood. Copy number does not have a clear role in governing p1 expression patterns because several p1 alleles that have similar multicopy gene structures to P1-wr have red pericarp; however, the pigmentation is not as uniform or intense as that of the single-copy P1-rr allele (Cocciolone et al. 2001). Additionally, the P1-pr epiallele of P1-rr has suppressed (patterned) pericarp pigmentation despite being single copy (Das and Messing 1994). However, it is noteworthy that there has not been a report of functional single-copy p1 alleles with colorless pericarp.
Similar to Ac transposon insertions in P1-rr (Grotewold et al. 1991b), there are specific sites within the 5′ end of P1-wr that are candidates for Mu-insertion hotspots. The identified Mu-insertion clusters even contained instances in which distinct Mu elements incorporated at the same sequence context. Since P1-wr is a multiple-copy gene and several P1-wr inbred lines were used, it could not be determined if these insertions were in the same copy of the tandem array. However, a future study might examine if and why certain gene copies are more prone to transposon insertions. Unlike the mutagenesis of P1-rr with the Ac transposons, the identification of Mu-insertion alleles in P1-wr did not result in any loss-of-function phenotypes. An obvious explanation for this result would be that more than one of the six copies of P1-wr are transcribed. Thus, the interruption of a single copy may not have a large net affect on the overall gene expression.
P1-wr-mum6 was characterized because it was the only insertion allele in which a phenotype difference was identified. We specifically investigated how ectopic pericarp pigmentation arose subsequent to a transposon insertion in the 5′-UTR. However, the specific copy of the P1-wr multicopy complex in which the Mu1 insertion resides remains unknown at this point. We showed that the P1-wr-mum6 phenotype was initially weakly penetrant and was observed as thin red stripes and small sectors on the pericarp. These results suggested that the pericarp pigmentation in P1-wr-mum6 was induced somatically until it was stably inherited germinally through a clonal sector that affected both the pericarp and the embryo tissue. After this germinal inheritance, uniformly pigmented ears were frequently observed. However, the persistence of variable or “mottled” pericarp pigmentation suggests that P1-wr-mum6 expression is often affected by somatic changes. On the basis of these observations, we strongly suggest that the presence of Mu1 in P1-wr-mum6 lifted a suppression mechanism that otherwise renders P1-wr pericarp colorless.
Since the Mu1 insertion in P1-wr-mum6 is in the 5′-UTR, it could have directed the expression of the copy in which it resides as is the case with most Mu-suppressible alleles in maize (Cui et al. 2003). However, we did not detect the presence of such ectopic transcripts. Previous studies indicated that alternate transcript initiation sites are associated with the inactivity of the MuDR transposase (Barkan and Martienssen 1991; Cui et al. 2003). However, we showed that the presence of pigmentation in P1-wr-mum6 does not depend on the DNA methylation at Mu1 or the activity of the MuDR transposase protein. Therefore, these experiments suggest that P1-wr-mum6 expression in pericarp is controlled by a mechanism that is distinct from that functioning in Mu-suppressible alleles in maize.
We considered the possibility that the Mu1 element in P1-wr-mum6 may affect its expression by physically interfering with a cis-regulatory region that affects the local chromatin structure. In Drosophila, Gypsy retrotransposons have been implicated as insulators in such chromatin alterations that disrupt the signaling between enhancers, silencers, and promoters (Kuhn and Geyer 2003; Kuhn et al. 2003; Parnell et al. 2006). In this regard, the Mu1 insertion in the 5′-UTR of P1-wr-mum6 might have affected the signaling between upstream regulatory and promoter elements. In fact, we found that P1-wr-mum6 individuals with stably expressing pericarp pigmentation have undergone hypomethylation at a floral organ-specific distal enhancer element This enhancer previously was shown to be considerably less methylated in P1-rr as compared with P1-wr (Chopra et al. 1998). Moreover, the presence of the epigenetic modifier Ufo1 reduces the DNA methylation at this enhancer, resulting in an increase in pericarp pigmentation (Chopra et al. 2003). In a parallel study from mouse, the hypomethylation of a distal enhancer element was required for the long-range (1.2-kb) activation of a downstream promoter (Forrester et al. 1999). The role of hypomethylation in distal enhancer function is putatively important because eukaryotic DNA sequences in heterochromatin do not communicate well in vivo over distances >1.5 kb (Bondarenko et al. 2003).
Bisulfite sequencing analysis revealed that P1-wr-mum6 expressers were hypomethylated at both CG and CNG sites, indicating that there was a nonselective reduction in DNA methylation. In other words, a specific class of DNA methyltransferase was not specifically inhibited (Chan et al. 2005). Rather, the perturbation of chromatin packaging, which is important for maintaining all contexts of DNA methylation, may have led to the gain of function in P1-wr-mum6 pericarps (Brzeski and Jerzmanowski 2004). The CHH methylation is a useful molecular marker in that it reports the involvement RNA-directed DNA methylation (RdDM). Hence, on the basis of the low CHH methylation levels observed, we conclude that RdDM is not required to maintain DNA methylation levels at the distal enhancer of P1-wr-mum6.
Conceivably, hypomethylation at the interrupted copy could spread to uninterrupted copies. The position of the distal enhancer in the interrupted copy is 4.9 kb upstream of the Mu1 insertion site. The distal enhancer of the interrupted copy would be 1 kb from the 3′ end of an upstream gene copy unless it is the most 5′ copy in the tandem array. If such a spread in DNA hypomethylation/euchromatin occurred, it could explain why the increased RNA expression in P1-wr-mum6 originates from the uninterrupted (wild-type) copy (or copies). Therefore, it is a distinct possibility that the hypomethylation present at the distal enhancer also affected the uninterrupted copy (or copies) of P1-wr-mum6 and led to their increased expression in pericarp. Such distal control through chromatin modification is not unprecedented. For instance, paramutation-based silencing of the anthocyanin regulatory booster1 (b1) gene of Zea mays is directed by 853-bp tandem repeats of a distal enhancer sequence, which is located ∼100 kb upstream of the transcription start site (Stam et al. 2002b). These tandem repeats also correlated with a higher order of chromatin packaging (Stam et al. 2002a). Because P1-wr is multicopy, it may be silenced in pericarp tissue by DNA–DNA interactions between copies that strengthen heterochromatin (Assaad et al. 1993; Bender 1998). In other words, the Mu1 insertion may have disrupted a critical region of a single copy that is important for copy-to-copy associations that rely on heterochromatinization. An example of this phenomenon comes from a fluorescent chromatin-tagging experiment in Arabidopsis thaliana that shows that two copies of a transgene separated by 4.2 Mbp can preferentially associate (Watanabe et al. 2005). In another example from Drosophila melanogaster, the physical pairing and silencing of tandemly repeated white (eye color) transgene copies is dependent on both a greater number of tandem repeats and their placement near heterochromatin (Gubb et al. 1990; Dorer and Henikoff 1994; Duncan 2002). The variegated eye-color phenotype was an example of position-effect variegation (PEV) in which the normally euchromatic state of the white gene is juxtaposed with the heterochromatic state (Dubinin 1936; Spofford 1961). In this regard, P1-wr-mum6 ears that show mosaicism and kernel-to-kernel differences or sectors may be the result of the juxtaposition between euchromatin and heterochromatin. Therefore, it is conceivable that the presence of tandemly repeated p1 gene copies facilitates chromatin-based gene silencing.
The interrupted copy in P1-wr-mum6 may have been accessed by such chromatin-remodeling factors on the basis of an optimal location in the tandem gene array. Alternatively, there may be subtle sequence polymorphisms in the interrupted copy that contribute to the low expression levels in P1-wr pericarp. Such a copy might be uniquely recognized by chromatin-remodeling factors. Sequences from complete single copies of P1-wr are currently available from the inbred lines W23 (accession no. EF165349; Sekhon et al. 2007) and B73 (MAGI database; Fu et al. 2005). There are several SNPs that distinguish these P1-wr copies of B73 and W23. The p1 sequences adjacent to the Mu1 insertion in P1-wr-mum6 have SNPs that resemble the W23 copy and others that resemble the B73 sequence (accession nos. EU137661 and EU137662, respectively, denote the sequences flanking the 5′ and 3′ ends of Mu1 in P1-wr-mum6). In addition, there were other putative SNPs that were not found in either sequence. Recent evidence also suggests that there are subtle sequence polymorphisms between P1-wr [W23] gene copies (P.-H. Wang, R. Sekhon and S. Chopra, personal communication). Functional characterization of these copies should be highly useful in understanding how tandem repeats may regulate tissue-specific expression patterns.
Acknowledgments
We are grateful to Thomas Peterson, Iowa State University, for his advice regarding the screening of the Mu-insertion alleles. We also acknowledge John Snyder and Catherine Svabek for their assistance with DNA isolation and field-based data collection. We thank the anonymous reviewers for their suggested changes to improve the manuscript. This research was performed under a grant from the Hatch project (no. 4154) and supported by National Science Foundation award 0619330 to S.C.
References
- Anderson, E. G., 1924. Pericarp studies in maize: II. The allelomorphism of a series of factors for pericarp color. Genetics 9 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad, F. F., K. L. Tucker and E. R. Signer, 1993. Epigenetic repeat-induced gene silencing (RIGS) in Arabidopsis. Plant Mol. Biol. 22 1067–1085. [DOI] [PubMed] [Google Scholar]
- Athma, P., and T. Peterson, 1991. Ac induces homologous recombination at the maize P locus. Genetics 128 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athma, P., E. Grotewold and T. Peterson, 1992. Insertional mutagenesis of the maize P gene by intragenic transposition of Ac. Genetics 131 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan, A., and R. A. Martienssen, 1991. Inactivation of maize transposon Mu suppresses a mutant phenotype by activating an outward-reading promoter near the end of Mu1. Proc. Natl. Acad. Sci. USA 88 3502–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, R. F., D. V. Thompson, D. R. Talbot, J. Swanson and J. L. Bennetzen, 1984. Nucleotide sequence of the maize transposable element Mul. Nucleic Acids Res. 12 5955–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, J., 1998. Cytosine methylation of repeated sequences in eukaryotes: the role of DNA pairing. Trends Biochem. Sci. 23 252–256. [DOI] [PubMed] [Google Scholar]
- Blanc, G., K. Hokamp and K. H. Wolfe, 2003. A recent polyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Res. 13 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko, V. A., Y. V. Liu, Y. I. Jiang and V. M. Studitsky, 2003. Communication over a large distance: enhancers and insulators. Biochem. Cell Biol. 81 241–251. [DOI] [PubMed] [Google Scholar]
- Brink, R. A., and E. D. Styles, 1966. A collection of pericarp factors. Maize Genet. Coop. News Lett. 40 149–160. [Google Scholar]
- Brzeski, J., and A. Jerzmanowski, 2004. Plant chromatin–epigenetics linked to ATP-dependent remodeling and architectural proteins. FEBS Lett. 567 15–19. [DOI] [PubMed] [Google Scholar]
- Chan, S. W., I. R. Henderson and S. E. Jacobsen, 2005. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 6 351–360. [DOI] [PubMed] [Google Scholar]
- Chandler, V. L., and V. Walbot, 1986. DNA modification of a maize transposable element correlates with loss of activity. Proc. Natl. Acad. Sci. USA 83 1767–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, S., P. Athma and T. Peterson, 1996. Alleles of the maize P gene with distinct tissue specificities encode Myb-homologous proteins with C-terminal replacements. Plant Cell 8 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, S., P. Athma, X. G. Li and T. Peterson, 1998. A maize Myb homolog is encoded by a multicopy gene complex. Mol. Gen. Genet. 260 372–380. [DOI] [PubMed] [Google Scholar]
- Chopra, S., S. M. Cocciolone, S. Bushman, V. Sangar, M. D. McMullen et al., 2003. The maize Unstable factor for orange1 is a dominant epigenetic modifier of a tissue specifically silent allele of pericarp color1. Genetics 163 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocciolone, S. M., S. Chopra, S. A. Flint-Garcia, M. D. McMullen and T. Peterson, 2001. Tissue-specific patterns of a maize Myb transcription factor are epigenetically regulated. Plant J. 27 467–478. [DOI] [PubMed] [Google Scholar]
- Cui, X., A. P. Hsia, F. Liu, D. A. Ashlock, R. P. Wise et al., 2003. Alternative transcription initiation sites and polyadenylation sites are recruited during Mu suppression at the rf2a locus of maize. Genetics 163 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, O. P., and J. Messing, 1994. Variegated phenotype and developmental methylation changes of a maize allele originating from epimutation. Genetics 136 1121–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, C. R., F. Cui, M. L. Packila, J. Li, D. A. Ashlock et al., 2002. Maize Mu transposons are targeted to the 5′ untranslated region of the gl8 gene and sequences flanking Mu target-site duplications exhibit nonrandom nucleotide composition throughout the genome. Genetics 160 697–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer, D. R., and S. Henikoff, 1994. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77 993–1002. [DOI] [PubMed] [Google Scholar]
- Dubinin, N. P., 1936. A new type of position effect. Biol. Zh. 5 851–874. [Google Scholar]
- Duncan, I. W., 2002. Transvection effects in Drosophila. Annu. Rev. Genet. 36 521–556. [DOI] [PubMed] [Google Scholar]
- Ferrari, S., D. Vairo, F. M. Ausubel, F. Cervone and G. De Lorenzo, 2003. Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester, W. C., L. A. Fernandez and R. Grosschedl, 1999. Nuclear matrix attachment regions antagonize methylation-dependent repression of long-range enhancer-promoter interactions. Genes Dev. 13 3003–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y., S. J. Emrich, L. Guo, T. J. Wen, D. A. Ashlock et al., 2005. Quality assessment of maize assembled genomic islands (MAGIs) and large-scale experimental verification of predicted genes. Proc. Natl. Acad. Sci. USA 102 12282–12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard, L., and M. Freeling, 2000. Mutator-suppressible alleles of rough sheath1 and liguleless3 in maize reveal multiple mechanisms for suppression. Genetics 154 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, B., R. Walko and S. Hake, 1994. Mutator insertions in an intron of the maize knotted1 gene result in dominant suppressible mutations. Genetics 138 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold, E., P. Athma and T. Peterson, 1991. a Alternatively spliced products of the maize P gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc. Natl. Acad. Sci. USA 88 4587–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold, E., P. Athma and T. Peterson, 1991. b A possible hot spot for Ac insertion in the maize P gene. Mol. Gen. Genet. 230 329–331. [DOI] [PubMed] [Google Scholar]
- Grotewold, E., B. J. Drummond, B. Bowen and T. Peterson, 1994. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76 543–553. [DOI] [PubMed] [Google Scholar]
- Gubb, D., M. Ashburner, J. Roote and T. Davis, 1990. A novel transvection phenomenon affecting the white gene of Drosophila melanogaster. Genetics 126 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert, S. H., and J. L. Bennetzen, 1991. Recombination at the Rp1 locus of maize. Mol. Gen. Genet. 226 377–382. [DOI] [PubMed] [Google Scholar]
- Jacobsen, S. E., H. Sakai, E. J. Finnegan, X. Cao and E. M. Meyerowitz, 2000. Ectopic hypermethylation of flower-specific genes in Arabidopsis. Curr. Biol. 10 179–186. [DOI] [PubMed] [Google Scholar]
- Kliebenstein, D. J., J. Kroymann, P. Brown, A. Figuth, D. Pedersen et al., 2001. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126 811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler, F., and K. Hahlbrock, 1975. Enzymic synthesis of an aromatic ring from acetate units. Partial purification and some properties of flavanone synthase from cell-suspension cultures of Petroselinum hortense. Eur. J. Biochem. 56 205–213. [DOI] [PubMed] [Google Scholar]
- Kuhn, E. J., and P. K. Geyer, 2003. Genomic insulators: connecting properties to mechanism. Curr. Opin. Cell Biol. 15 259–265. [DOI] [PubMed] [Google Scholar]
- Kuhn, E. J., M. M. Viering, K. M. Rhodes and P. K. Geyer, 2003. A test of insulator interactions in Drosophila. EMBO J. 22 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechelt, C., T. Peterson, A. Laird, J. Chen, S. L. Dellaporta et al., 1989. Isolation and molecular analysis of the maize P locus. Mol. Gen. Genet. 219 225–234. [DOI] [PubMed] [Google Scholar]
- Lippman, Z., A. V. Gendrel, M. Black, M. W. Vaughn, N. Dedhia et al., 2004. Role of transposable elements in heterochromatin and epigenetic control. Nature 430 471–476. [DOI] [PubMed] [Google Scholar]
- Lisch, D., 2002. Mutator transposons. Trends Plant Sci. 7 498–504. [DOI] [PubMed] [Google Scholar]
- Lisch, D., P. Chomet and M. Freeling, 1995. Genetic characterization of the Mutator system in maize: behavior and regulation of Mu transposons in a minimal line. Genetics 139 1777–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, B., J. Mathern and S. Hake, 1992. Active Mutator elements suppress the knotted phenotype and increase recombination at the Kn1-O tandem duplication. Genetics 132 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, G., O. P. Das and J. Messing, 1995. Tissue specific DNase I–sensitive sites of the maize P gene and their changes upon epimutation. Plant J. 7 797–807. [PubMed] [Google Scholar]
- Maere, S., S. De Bodt, J. Raes, T. Casneuf, M. Van Montagu et al., 2005. Modeling gene and genome duplications in eukaryotes. Proc. Natl. Acad. Sci. USA 102 5454–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen, R., and A. Baron, 1994. Coordinate suppression of mutations caused by Robertson's mutator transposons in maize. Genetics 136 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen, R., A. Barkan, W. C. Taylor and M. Freeling, 1990. Somatically heritable switches in the DNA modification of Mu transposable elements monitored with a suppressible mutant in maize. Genes Dev. 4 331–343. [DOI] [PubMed] [Google Scholar]
- May, B. P., H. Liu, E. Vollbrecht, L. Senior, P. D. Rabinowicz et al., 2003. Maize-targeted mutagenesis: a knockout resource for maize. Proc. Natl. Acad. Sci. USA 100 11541–11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeley, B., and S. Briggs, 1995. Reverse genetics for maize. Maize Genet. Coop. News Lett. 69 67–82. [Google Scholar]
- Parnell, T. J., E. J. Kuhn, B. L. Gilmore, C. Helou, M. S. Wold et al., 2006. Identification of genomic sites that bind the Drosophila suppressor of Hairy-wing insulator protein. Mol. Cell. Biol. 26 5983–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares, J., U. Wienand, P. A. Peterson and H. Saedler, 1986. Molecular cloning of the c locus of Zea mays: a locus regulating the anthocyanin pathway. EMBO J. 5 829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffanelli, P., L. Ramsay, R. Waugh, A. Benabdelmouna, A. D'Hont et al., 2004. A barley cultivation-associated polymorphism conveys resistance to powdery mildew. Nature 430 887–891. [DOI] [PubMed] [Google Scholar]
- Rizzon, C., L. Ponger and B. S. Gaut, 2006. Striking similarities in the genomic distribution of tandemly arrayed genes in Arabidopsis and rice. PLoS Comput. Biol. 2 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghai-Maroof, M. A., K. M. Soliman, R. A. Jorgensen and R. W. Allard, 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon, R. S., T. Peterson and S. Chopra, 2007. Epigenetic modifications of distinct sequences of the p1 regulatory gene specify tissue-specific expression patterns in maize. Genetics 175 1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenko, L. V., X. Li, S. M. Cocciolone, S. Chopra, L. Tagliani et al., 2000. Complex structure of a maize Myb gene promoter: functional analysis in transgenic plants. Plant J. 22 471–482. [DOI] [PubMed] [Google Scholar]
- Slotkin, R. K., M. Freeling and D. Lisch, 2003. Mu killer causes the heritable inactivation of the Mutator family of transposable elements in Zea mays. Genetics 165 781–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin, R. K., M. Freeling and D. Lisch, 2005. Heritable transposon silencing initiated by a naturally occurring transposon inverted duplication. Nat. Genet. 37 641–644. [DOI] [PubMed] [Google Scholar]
- Smith, L. G., B. Greene, B. Veit and S. Hake, 1992. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116 21–30. [DOI] [PubMed] [Google Scholar]
- Spofford, J. B., 1961. Parental control of position-effect variegation. II. Effect of sex of parent contributing white-mottled rearrangement in Drosophila melanogaster. Genetics 46 1151–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam, M., C. Belele, J. E. Dorweiler and V. L. Chandler, 2002. a Differential chromatin structure within a tandem array 100 kb upstream of the maize b1 locus is associated with paramutation. Genes Dev. 16 1906–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam, M., C. Belele, W. Ramakrishna, J. E. Dorweiler, J. L. Bennetzen et al., 2002. b The regulatory regions required for B′ paramutation and expression are located far upstream of the maize b1 transcribed sequences. Genetics 162 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupar, R. M., K. A. Beaubien, W. Jin, J. Song, M. K. Lee et al., 2006. Structural diversity and differential transcription of the patatin multicopy gene family during potato tuber development. Genetics 172 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit, B., E. Vollbrecht, J. Mathern and S. Hake, 1990. A tandem duplication causes the Kn1-O allele of Knotted, a dominant morphological mutant of maize. Genetics 125 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd, T. C., B. M. Dekker and A. Hoekema, 1989. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht, E., B. Veit, N. Sinha and S. Hake, 1991. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350 241–243. [DOI] [PubMed] [Google Scholar]
- Watanabe, K., A. Pecinka, A. Meister, I. Schubert and E. Lam, 2005. DNA hypomethylation reduces homologous pairing of inserted tandem repeat arrays in somatic nuclei of Arabidopsis thaliana. Plant J. 44 531–540. [DOI] [PubMed] [Google Scholar]
- Zhang, F., and T. Peterson, 2005. a Comparisons of maize pericarp color1 alleles reveal paralogous gene recombination and an organ-specific enhancer region. Plant Cell 17 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., and T. Peterson, 2005. b A segmental deletion series generated by sister-chromatid transposition of Ac transposable elements in maize. Genetics 171 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]