Abstract
The fission yeast Schizosaccharomyces pombe senses environmental glucose through a cAMP-signaling pathway. Elevated cAMP levels activate protein kinase A (PKA) to inhibit transcription of genes involved in sexual development and gluconeogenesis, including the fbp1+ gene, which encodes fructose-1,6-bisphosphatase. Glucose-mediated activation of PKA requires the function of nine glucose-insensitive transcription (git) genes, encoding adenylate cyclase, the PKA catalytic subunit, and seven “upstream” proteins required for glucose-triggered adenylate cyclase activation. We describe the cloning and characterization of the git10+ gene, which is identical to swo1+ and encodes the S. pombe Hsp90 chaperone protein. Glucose repression of fbp1+ transcription is impaired by both git10− and swo1− mutant alleles of the hsp90+ gene, as well as by chemical inhibition of Hsp90 activity and temperature stress to wild-type cells. Unlike the swo1− mutant alleles, the git10-201 allele supports cell growth at 37°, while severely reducing glucose repression of an fbp1-lacZ reporter, suggesting a separation-of-function defect. Sequence analyses of three swo1− alleles and the one git10− allele indicate that swo1− mutations alter core functional domains of Hsp90, while the git10− mutation affects the Hsp90 central domain involved in client protein binding. These results suggest that Hsp90 plays a specific role in the S. pombe glucose/cAMP pathway.
GLUCOSE signaling pathways regulate gene expression in both prokaryotic and eukaryotic cells and have been well studied in a variety of model organisms. The fission yeast Schizosaccharomyces pombe monitors glucose to regulate sexual development and metabolism. Our studies focus on the transcriptional regulation of the glucose-repressed fbp1+ gene, which encodes the gluconeogenic enzyme fructose-1,6-bisphosphatase (Vassarotti and Friesen 1985). Previously, we identified mutations in genes that confer constitutive fbp1+ transcription (Hoffman and Winston 1990). These glucose-insensitive transcription (git) genes encode the components of a protein kinase A (PKA) pathway (Hoffman 2005b), which acts antagonistically to a stress-activated MAPK (SAPK) pathway required for fbp1+ transcription (Stettler et al. 1996; Stiefel et al. 2004). The git2+/cyr1+ gene encodes adenylate cyclase (Hoffman and Winston 1991), which produces the second messenger cAMP to activate PKA, whose catalytic subunit is encoded by the pka1+/git6+ gene (Jin et al. 1995) and whose regulatory subunit is encoded by the cgs1+ gene (Devoti et al. 1991). Seven additional git genes are required for adenylate cyclase activation and form at least two functionally distinct groups. Four genes encode the Git3 G-protein-coupled receptor (Welton and Hoffman 2000) and its cognate heterotrimeric G protein composed of the Gpa2 Gα (Isshiki et al. 1992; Nocero et al. 1994), the Git5 Gβ (Landry et al. 2000), and the Git11 Gγ (Landry and Hoffman 2001). The Git3 GPCR and Git5-Git11 Gβγ dimer are required for Gpa2 Gα activation and can be bypassed by mutations that activate Gpa2 (Welton and Hoffman 2000), which directly binds and activates adenylate cyclase (Ivey and Hoffman 2005). The git1+, git7+, git10+ are required for glucose repression of fbp1+ transcription, even in a strain carrying the gpa2R176H activated allele (Welton and Hoffman 2000). Therefore, Git1, Git7, and Git10 either function independently from Gpa2 to activate adenylate cyclase or are required for Gpa2-mediated activation of adenylate cyclase. Git1 is a C2-domain protein that directly binds adenylate cyclase (Kao et al. 2006), while Git7 (Schadick et al. 2002) is a member of the Sgt1 protein family, whose Saccharomyces cerevisiae ortholog has been implicated in both adenylate cyclase function (Dubacq et al. 2002) and kinetochore assembly (Kitagawa et al. 1999).
We describe here the cloning of the git10+ gene, which is identical to the previously identified swo1+ gene encoding the only S. pombe Hsp90 heat-shock chaperone protein. (For clarity, we refer to the gene as hsp90+ and to mutant alleles as either swo1− or git10− alleles of hsp90+.) The git10− and swo1− alleles confer some overlapping phenotypes; however, the git10-201 allele is more limited to causing a severe cAMP-signaling defect without the temperature-sensitive growth defects associated with swo1− alleles. Hsp90 activity is required for proper glucose/cAMP signaling as both prolonged heat stress and Hsp90 inhibition by geldanamycin increase expression of an fbp1-lacZ reporter. As git10-201 strains display a significant defect in fbp1-lacZ regulation even at 25°, while remaining viable at 37°, it appears that git10-201 is a separation-of-function mutation specifically affecting Hsp90 function in the cAMP pathway. Consistent with this hypothesis, the git10-201 mutation alters a residue in the central domain of the protein, which is presumably involved in client protein binding, while the mutations in three swo1− mutant alleles alter residues in the N-terminal ATP-binding domain or the C-terminal dimerization domain. Thus, there appears to be a direct role for Hsp90 in the S. pombe glucose/cAMP pathway, and it is not just that mutations in the hsp90+ gene confer a general stress signal to derepress fbp1+ transcription.
MATERIALS AND METHODS
S. pombe strains and growth media:
Yeast strains used in this study are listed in Table 1. The fbp1∷ura4+ and ura4∷fbp1-lacZ reporters are translational fusions integrated at the fbp1+ and ura4+ loci, respectively (Hoffman and Winston 1990). Yeast were grown and maintained using yeast extract agar (YEA) and yeast extract liquid (YEL) (Gutz et al. 1974). Defined medium EMM (MP Biochemicals) was supplemented with required nutrients at 75 mg/liter, except for l-leucine, which was at 150 mg/liter. Sensitivity to 5-fluoroorotic acid (5-FOA) was determined on SC solid medium containing 0.4 g/liter 5-FOA and 8% glucose as previously described (Hoffman and Winston 1990). Strains were grown at 30° unless otherwise indicated. Geldanamycin (InvivoGen) was used at 2 μg/ml, 5 μg/ml, and 10 μg/ml and was dissolved in dimethyl sulfoxide (DMSO).
TABLE 1.
Strain list
| Strain | Genotype |
|---|---|
| FWP17 | mat2-102 ura4-294 lys1-131 |
| FWP72 | h−fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 |
| FWP87 | h+fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 |
| CHP567 | h+fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 ade6-M210 git10-201 |
| CHP573 | h−fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 ade6-M210 his7-366 git10-201 |
| CHP894 | h−fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 lys1-131 cdc1-P13 git10-201 |
| CHP981 | h−fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 ade6-M210 swo1-26 |
| CHP979 | h+fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 ade6-M210 his7-366 swo1-26 |
| CHP989 | h+fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 swo1-21 |
| PR164 | h−ura4-D18 leu1-32 swo1-21 |
| PR165 | h−ura4-D18 leu1-32 swo1-25 |
| CHP362 | h90leu1-32 ade6-M210 lys1-131 |
| CHP558 | h90fbp1∷ura4+leu1-32 ade6-M216 git2-1∷LEU2 |
| CHP486 | h90leu1-32 lys1-131 git5-1∷his7 |
| CHP483 | h90ura4∷fbp1-lacZ leu1-32 ade6-M216 |
| MAP1 | h90fbp1∷ura4+ura4∷fbp1-lacZ leu1-32 git10-201 |
Recombinant DNA methods:
Rescue of plasmids from S. pombe was achieved by the smash and grab method (Hoffman and Winston 1987). Yeast transformations were carried out as previously described (Bähler et al. 1998). Escherichia coli transformations were done using Ten-Blue or XL1-Blue electroporation-competent cells (Stratagene, La Jolla, CA). The S. pombe genomic DNA insert from cosmid SPAC926 was amplified by PCR using custom oligonucleotides that divided the insert into nine segments and cloned using pNMT41 TOPO cloning vector from Invitrogen (San Diego) according to the manufacturer's instructions.
Epitope tagging of Hsp90:
Oligonucleotides hsp90-for (5′ ATGTCGAACACAGAAACTTTCAAG 3′) and hsp90-revTAG (5′ ATCGACTTCCTCCATCTTGCTC 3′) were used in a PCR reaction on wild-type S. pombe genomic DNA to amplify the hsp90+ ORF. The resultant PCR product, lacking the hsp90+ STOP codon, was cloned into the TOPO cloning vector pNMT41 (Invitrogen), creating plasmid pMAR3, which expresses Hsp90 with a C-terminal V5 (Southern et al. 1991) tag followed by a hexahistidine tag (Hsp90-V5his6).
β-Galactosidase assays of fbp1-lacZ expression:
Cells were cultured for 18 hr under repressing conditions (8% glucose) in yeast extract at the indicated temperatures (YEL). Subcultures were grown to exponential phase. Soluble protein extracts were prepared by glass bead lysis and assayed to determine β-galactosidase activity. Total soluble protein was measured by BCA assay (Pierce Chemical, Rockford, IL) to calculate β-galactosidase-specific activity (Nocero et al. 1994). For temperature stress experiments, cultures were pregrown as described above and then subcultured in YEL (8% glucose) such that cell density would be ∼107 cells/ml after 6 or 24 hr incubation. Glucose concentration of the media was determined using the Sigma (St. Louis) glucose (GO) assay kit, according to manufacturer's instructions. Glucose concentrations remained >7.5% in all cultures.
DNA sequencing:
Mutant alleles of the hsp90+ gene (swo1-21, swo1-25, swo1-26, and git10-201) were PCR amplified from S. pombe strains and the PCR products were directly sequenced using custom oligonucleotides (Integrated DNA Technologies). DNA sequencing was performed using the CEQ DTCS-Quick Start kit (Beckman Coulter).
RESULTS
Genetic mapping and cloning of the S. pombe git10+ gene:
Git− mutant strains display 5-FOA-sensitive (5-FOAS) growth due to their inability to glucose repress the fbp1-ura4+ reporter (Hoffman and Winston 1990). To date, nine git genes have been shown to play a significant role in fbp1+ repression, with only git10+ remaining to be cloned. Due to the large number of multicopy suppressors encountered when screening plasmid libraries during attempts to clone genes in this pathway (Hoffman and Winston 1991; Jin et al. 1995; Dal Santo et al. 1996; Wang et al. 2005), we took a genetic mapping approach to identify the git10+ gene.
Chromosomal mapping of git10-201 by benomyl-induced haploidization of an h−/mat2-102 diploid strain (Alfa et al. 1993) was carried out with strains FWP17 and CHP573 (Table 1). This technique allows the formation of haploids from a diploid strain in the absence of meiotic recombination, such that the alleles on each of the three parental chromosomes form individual linkage groups. All 5-FOA-sensitive haploids produced this way possessed chromosome 2 from CHP573, containing the fbp1-ura4+ reporter, as well as chromosome 1 from CHP573, presumably possessing git10-201 (data not shown). The git10-201 allele was further mapped by tetrad dissection, in a cross of strain FWP87 with strain CHP894. The git10+ gene maps between lys1+ (23.2 cM with a PD:TT:NPD ratio of 45:39:0) and cdc1+ (30.4 cM with a PD:TT:NPD ratio of 38:45:1). The lys1+ and cdc1+ genes are 54.8 cM from each other with a PD:TT:NPD ratio of 22:56:6.
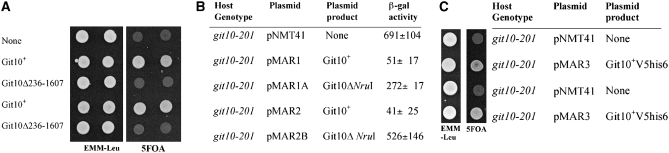
The genetic mapping data suggested that git10+ is present on cosmid SPAC926 [one of an ordered set of cosmids used in the S. pombe genome sequencing project (Wood et al. 2002)]. Insert DNA from SPAC926 was divided into nine fragments by PCR amplification and TOPO cloning into a plasmid suitable for transformation of S. pombe. Plasmids from this set of clones were used to transform S. pombe strain CHP567 (git10-201) to Leu+ and transformants were tested for restoration of 5-FOA resistance to indicate complementation of the git10− defect. Plasmids pMAR1 and pMAR2, which carry base pairs 2308–9026 in either orientation with respect to the vector, were the only clones to confer 5-FOA resistance (Figure 1A). These transformants also glucose-repress fbp1-lacZ expression as judged by β-galactosidase assays (Figure 1B). Plasmids pMAR1 and pMAR2 contain two genes, one of which is hsp90+/swo1+. Digestion with NruI followed by ligation removed a 1.4-kb fragment internal to the hsp90+ open reading frame and produced plasmids pMAR1A and pMAR2B, which lost the ability to suppress the git10-201 mutation (Figure 1, A and B). Thus, hsp90+ appears to be responsible for suppression of the git10-201 mutant allele.
Figure 1.—
Complementation of git10-201 mutation by plasmid-expressed git10+. (A) CHP567 (git10-201) cells were transformed to Leu+ with pNMT41 (empty vector), pMAR1 (git10+), pMAR1A (git10Δ236-1607), pMAR2 (git10+ cloned in the opposite orientation to that of pMAR1), and pMAR2B (git10Δ236-1607 cloned in the opposite orientation to that of pMAR1A). The git10Δ236-1607 contains a partial dropout of the git10 ORF. The two independent transformants of each plasmid indicated were spotted on EMM −leu and then replica plated after 2 days to EMM −leu and 5-FOA plates. Plates were photographed after 3 days incubation at 30°. (B) β-Galactosidase activity was determined as described in materials and methods. The values represent the average ± standard deviation of at least two independent transformants. (C) Plasmid pMAR3 carries only the hsp90 ORF, while plasmids pMAR1 and pMAR2 carry larger segments of the chromosomal DNA that include the hsp90+ gene. Plasmid pMAR3 complements the git10-201 mutation whereas pNMT41 (empty vector) does not. Transformants were spotted on EMM −leu and then replica plated after 2 days to EMM −leu and 5-FOA plates. Plates were photographed after 3 days incubation at 30°.
To confirm that hsp90+ is git10+, plasmid pMAR3 was constructed to express an epitope-tagged form of Hsp90 (see materials and methods). CHP567 (git10-201) transformants carrying pMAR3 are 5-FOA resistant (Figure 1C), proving that hsp90+ is able to suppress the git10-201 mutation. In contrast, transformation by pMAR3 fails to suppress the PKA pathway mutations git1−, git2− (cyr1−), git7−, or pka1− (data not shown). This is not surprising as Hsp90 is likely one of the most abundant proteins in S. pombe such that nmt41-driven expression of the Hsp90-V5his6 protein would not significantly increase Hsp90 activity in these strains.
Hsp90 is required for nutrient regulation of sexual development:
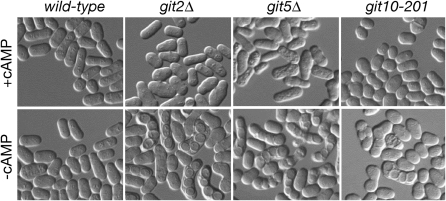
Wild-type S. pombe requires either a glucose or a nitrogen starvation signal to initiate mating and meiotic entry (Stettler et al. 1996). Consequently, mutations in genes required for glucose/cAMP signaling allow cells to mate and sporulate even in a nutrient-rich medium (Maeda et al. 1990; Isshiki et al. 1992; Jin et al. 1995; Landry et al. 2000; Welton and Hoffman 2000; Landry and Hoffman 2001; Schadick et al. 2002; Kao et al. 2006). Consistent with a role in this pathway, the git10-201 allele of hsp90+ allows homothallic (h90) cells to mate in a glucose-rich medium, as evidenced by presence of meiotic asci (Figure 2). This starvation-independent mating is similar to that conferred by deletion of the adenylate cyclase gene (git2+) or the Gβ subunit gene (git5+; Figure 2). Addition of 5 mm cAMP to the medium suppresses conjugation in all three mutant strains (Figure 2). This starvation-independent, cAMP-suppressible defect in the regulation of sexual development is another indication that Hsp90 plays a role in the S. pombe glucose/cAMP-signaling pathway.
Figure 2.—
Homothallic git10-201 cells conjugate and sporulate in nutrient-rich medium, similar to other cAMP pathway mutants. Homothallic (h90) strains CHP362 (git10+), CHP558 (git2Δ), CHP486 (git5Δ), and MAP1 (git10-201) were grown to exponential phase in liquid medium (8% glucose) at 37° (to inhibit conjugation), diluted to 106 cells/ml in liquid medium in the presence or absence of 5 mm cAMP, and incubated overnight at 30° without shaking. Starvation-independent conjugation and sporulation, which is suppressible by addition of cAMP, is observed in all three mutant strains.
Genetic, environmental, and chemical insults to Hsp90 activity derepress fbp1-lacZ expression:
To investigate the role of Hsp90 in the regulation of fbp1+ transcription, β-galactosidase activity expressed from the fbp1-lacZ reporter was measured in wild-type, git10−, and swo1− mutant strains grown at various temperatures (Table 2). Both the swo1-21 and the swo1-26 alleles confer a temperature-dependent defect in fbp1-lacZ repression, in addition to a temperature-sensitive growth defect. The git10-201 allele also confers a temperature-dependent defect in fbp1-lacZ repression; however, these cells remain viable when cultured at 37°. Surprisingly, wild-type cells display a partial defect in fbp1-lacZ repression when cultured at 37°, suggesting that temperature stress of wild-type cells leads to a reduction in PKA activity, and not simply the activation of the Spc1/Sty1 MAPK required for fbp1+ transcription (see discussion).
TABLE 2.
Glucose repression of fbp1-lacZ expression as a function of growth temperature
| β-Galactosidase activity
|
||||||
|---|---|---|---|---|---|---|
| Strain | hsp90 allele | 25° | 27° | 30° | 32° | 37° |
| FWP87 | Wild type | 15 ± 5 | 11 ± 0 | 10 ± 6 | 12 ± 4 | 392 ± 6 |
| CHP567 | git10-201 | 154 ± 20 | 252 ± 26 | 626 ± 30 | 661 ± 157 | 1336 ± 131 |
| CHP981 | swo1-26 | 54 ± 3 | 144 ± 5 | 517 ± 55 | Inviable | Inviable |
| CHP989 | swo1-21 | 157 ± 12 | 377 ± 8 | 605 ± 105 | Inviable | Inviable |
β-Galactosidase activity was measured in cells growing in YEL medium under glucose-repressing conditions (8% glucose) for 18 hr at the indicated temperature. The values given represent specific activity average ± standard deviation from two or three independent cultures.
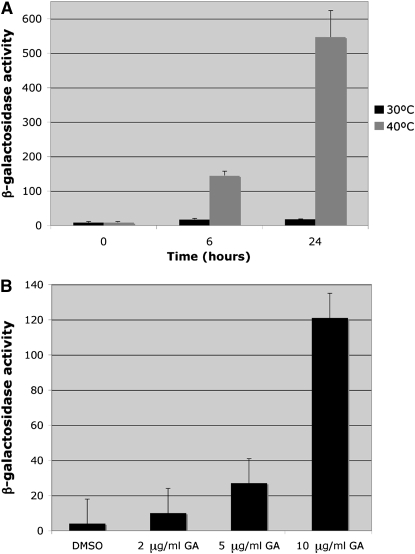
The effect of temperature stress on fbp1-lacZ repression was further examined in a time-course experiment in which wild-type cells were cultured at 30° or 40°, a temperature that does not support growth of S. pombe, but at which cells remain viable for several days (C. A. Hoffman and C. S. Hoffman, unpublished results). Increased β-galactosidase activity in response to temperature stress can be detected within 1 hr (data not shown) and remains modest even after 6 hr of incubation (Figure 3A). By 24 hr, however, the β-galactosidase activity rises to 547 ± 80 units, demonstrating that prolonged exposure to heat stress is required for significant fbp1+ derepression. As the glucose levels in the media remain >7.5% in all cultures, the increased fbp1-lacZ expression is due to heat stress and not glucose starvation.
Figure 3.—
Prolonged heat stress and chemical inhibition of Hsp90 derepress fbp1-lacZ transcription. (A) Wild-type strain FWP77 was pregrown to exponential phase at 30° and then subcultured at 30° or 40° in YEL medium under glucose-repressing conditions. β-Galactosidase activity was measured at the times indicated. The values given represent specific activity average ± standard deviation from three independent cultures. Glucose levels in the media were ≥7.5% for each culture. (B) β-Galactosidase activity was measured in cells growing in 8% glucose YEL medium for 18 hr in the presence of the Hsp90 inhibitor geldanamycin at the indicated concentrations. The values given represent specific activity average ± standard deviation from two or three independent samples.
To independently test whether Hsp90 is required for fbp1+ regulation, we examined the effect of chemical inhibition of Hsp90 on fbp1-lacZ expression by exposing cells to the Hsp90 inhibitor geldanamycin (Whitesell et al. 1994). β-Galactosidase activity was measured from wild-type strain FWP77 cells grown at 30° for 18 hr in the presence or the absence of geldanamycin (2 μg/ml, 5 μg/ml, and 10 μg/ml). There was a clear dose-dependent derepression of fbp1-lacZ expression, although the levels of expression did not reach those detected in cells subjected to prolonged heat stress (Figure 3B).
Phenotypic differences between swo1− and git10− alleles of hsp90+:
In the course of assaying β-galactosidase activity from swo1− and git10− strains, we confirmed previous observations that indicated that the swo1− alleles confer temperature-sensitive growth (Aligue et al. 1994), while the git10-201 allele does not (Hoffman and Winston 1990). For a more rigorous comparison, we carried out spot tests on hsp90+, swo1-26, swo1-21, and git10-201 strains to examine growth on rich medium at 25°, 28°, 30°, and 37°. Both swo1− mutants display a severe temperature-sensitive growth defect, even at 30°, while the git10-201 mutants display only a slow-growth phenotype at 37° rather than a loss of cell viability (Figure 4).
Figure 4.—
Temperature-dependent growth of hsp90+, swo1-26, swo1-21, and git10-201 strains. Spot tests were carried out on YEA rich medium at 25°, 28°, 30°, and 37°. Strains FWP72 (wild type), CHP567 (git10-201), CHP989 (swo1-21), and CHP979 (swo1-26) were cultured to 1 × 107 cells/ml in YEL liquid medium. Cells were washed with YEL medium and adjusted to 2 × 107 cells/ml and subjected to five 10-fold serial dilutions. Five microliters of each culture were spotted to a YEA plate and grown for 3 days at the indicated temperature before photographing.
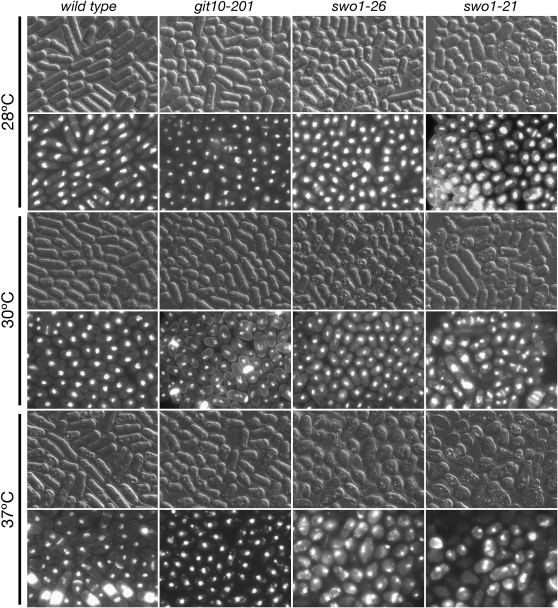
Microscopic examination of hsp90+, swo1−, and git10-201 strains growing at 28°, 30°, and 37° was carried out to examine the nature of the temperature-dependent growth defect. After 24 hr growth on EMM defined medium, the swo1-21 strain displayed abnormal cells that were lysed or binucleate or with misplaced nuclei in cultures grown at 30° and 37° (Figure 5). The swo1-26 strain appeared normal at 30°, while most cells had improperly placed nuclei at 37°. These results contrast somewhat with those from the spot test of a swo1-26 strain at 30° (Figure 4), and this appears to be a medium-specific effect with these cells displaying a more severe growth defect on YEA rich medium than on EMM defined medium. No growth defects were observed in wild-type or git10-201 cells at any temperature (Figure 6), distinguishing the cAMP pathway defect caused by the git10-201 mutation from the cell-growth defects caused by the swo1-21 and swo1-26 mutations.
Figure 5.—
Temperature-dependent morphology of hsp90+, swo1-26, swo1-21, and git10-201 strains. The same strains as shown in Figure 4 were precultured at 28° and then transferred to EMM defined medium and grown for 24 hr at 28°, 30°, and 37°. Cells were heat-fixed and stained with Hoechst 33342 and Calcofluor. Images were visualized and captured using a Zeiss Axioplan2 microscope with an Orca-ER CCD camera and Openlab software.
Figure 6.—
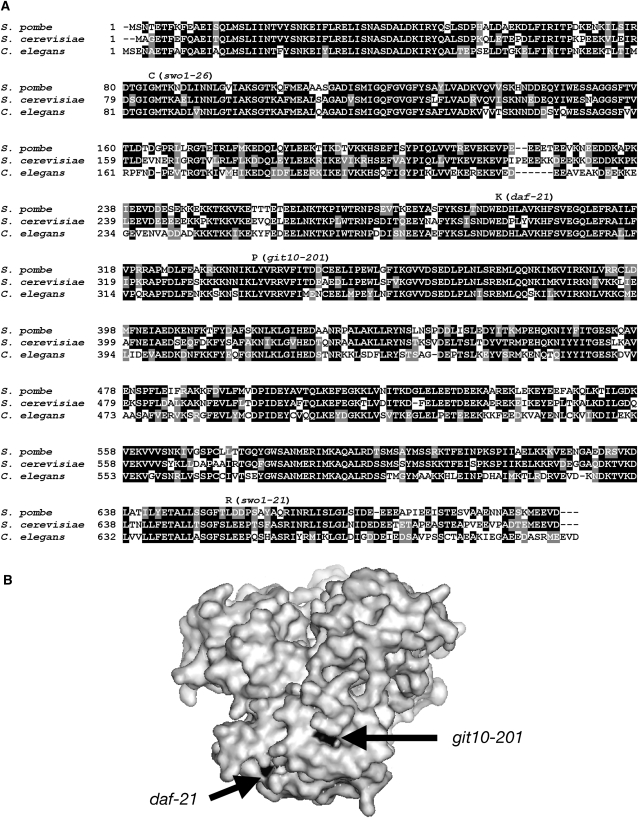
Alignment of Hsp90 proteins from S. pombe, S. cerevisiae, and C. elegans. (A) The S. pombe Hsp90 protein (accession no. CAB54152) was aligned using ClustalW (Thompson et al. 1994) with the S. cerevisiae Hsc82 protein (accession no. CAA89919) and C. elegans DAF-21 (accession no. NP_506626) and displayed using BOXSHADE 3.21. Identical residues are shaded in black, while conserved residues are shaded in gray. Amino acid changes associated with the swo1-21, swo1-26, and git10-201 mutant alleles are also indicated, as well as that of the C. elegans daf-21 mutation. (B) Crystal structure of the central domain of S. cerevisiae Hsp82 (accession no. AAA02813) showing the location of the residues altered by the S. pombe git10-201 mutation and the C. elegans daf-21 mutation. The two altered residues are on the same surface of the Hsp90 central domain. The graphic image was created using Pymol (DeLano 2002).
Sequence analysis of swo1− and git10− alleles:
The sequence of the entire hsp90+ open reading frame was determined from strains carrying the swo1-21, swo1-25, swo1-26, and git10-201 alleles. The swo1-25 and swo1-26 alleles carry the same mutation, changing residue 84 from glycine to cysteine, while the mutation in swo1-21 changes residue 654 from leucine to arginine. The git10-201 allele changes residue 338 from leucine to proline (Figure 6A). Thus, the swo1-25 and swo1-26 alleles affect the N-terminal ATP-binding domain, the swo1-21 allele affects the C-terminal dimerization domain, and the git10-201 allele affects the central, client protein-binding domain. The locations of these mutations are consistent with the observations that the swo1− mutant alleles appear to be general reduction-of-function alleles, while the git10-201 mutation appears to confer only a modest growth defect, but a significant defect in glucose/cAMP regulation of fbp1+ transcription. A similar separation-of-function allele of an Hsp90 gene has been observed in the cGMP signaling pathway of the nematode Caenorhabditis elegans. The daf-21 mutation, which allows C. elegans to enter the dauer larval form in the absence of temperature or nutritional stress signals (Birnby et al. 2000), is a missense mutation that alters a residue in the Hsp90 central domain not far from the residue altered by the S. pombe git10-201 mutation (Figure 6B). The similarity between these two mutations and their associated phenotypes suggests that Hsp90 plays a similar role in both S. pombe and C. elegans cyclic nucleotide signaling pathways to regulate metabolic pathways in response to temperature and nutritional conditions.
DISCUSSION
We have shown here that Hsp90, encoded by the hsp90+/git10+/swo1+ gene, acts in the S. pombe cAMP-signaling pathway that senses environmental glucose to repress transcription of genes involved in sexual development and gluconeogenesis, such as the fbp1+ gene (Hoffman 2005a,b). A defect in cAMP signaling in a git10-201 mutant strain was previously demonstrated (Byrne and Hoffman 1993), as was suppression of the fbp1+ regulatory defect by cAMP addition to the growth medium or by overexpression of the git2+/cyr1+ adenylate cyclase gene (Hoffman and Winston 1991). Therefore, Hsp90 activity appears to be required for cells to detect glucose and activate adenylate cyclase.
The hsp90+ gene is one of seven required for adenylate cyclase activation, which form at least two functionally distinct groups as determined by the ability of mutations to be suppressed by the mutationally activated Gpa2R176H Gα or by overexpression of the wild-type Gpa2+ protein (Welton and Hoffman 2000; Landry and Hoffman 2001). Increasing Gpa2 function bypasses the loss of the Git3p GPCR or Git5-Git11 Gβγ, but not mutations affecting the Git1 C2-domain protein (Kao et al. 2006), the Git7 Sgt1-family member protein (Schadick et al. 2002), or the Git10 Hsp90 protein. While S. cerevisiae does not encode a Git1 homolog, the S. cerevisiae Sgt1 plays an undetermined role in cAMP signaling (Dubacq et al. 2002) and functions in kinetochore assembly as an Hsp90 cochaperone or client-adaptor protein (Bansal et al. 2004; Lingelbach and Kaplan 2004; Catlett and Kaplan 2006). On the basis of our finding that Hsp90 acts in the S. pombe cAMP pathway, we presume that it is functioning together with Git7. Notably, the git7-27 and git7-235 mutant alleles confer both cell-growth and cAMP-signaling phenotypes similar to those of the swo1− mutant alleles, while git7-93 and a git7-GFP fusion allele are separation-of-function alleles similar to git10-201 as they affect cAMP signaling, but not cell growth and division. Together, the presence of such alleles for both git7+ and hsp90+ suggests that these proteins act on a distinct client protein or on the assembly of a protein complex acting in the cAMP pathway and argues against a model in which mutations that reduce Git7 and/or Hsp90 activity simply create a general stress that mimics a glucose-starvation signal.
Hsp90 is an essential molecular chaperone, which is highly conserved from bacteria to mammals (Bardwell and Craig 1987; Lindquist and Craig 1988; Spence and Georgopoulos 1989). Hsp90 possesses three domains, an N-terminal ATP-binding domain, a central regulatory domain involved in client protein binding, and a C-terminal dimerization domain (Pearl and Prodromou 2006). It is an unusual chaperone in that most of its identified substrates are signal-transduction proteins (Pearl and Prodromou 2000; Zhang and Burrows 2004; Powers and Workman 2006). Precisely how the Hsp90 machinery regulates signaling pathways in cells is not fully understood, as it appears to stabilize some client proteins and function in the assembly of protein complexes for other clients.
In S. pombe, the Hsp90 gene was first identified as swo1+, mutations in which suppress the mitotic effect of overexpression of the Wee1 kinase, which negatively regulates mitotic entry (Aligue et al. 1994). More recently, Hsp90 has been shown to play a role in myosin II function and the assembly of the S. pombe actomyosin ring involved in cytokinesis (Mishra et al. 2005). Hsp90 may be required in the glucose/cAMP pathway for the assembly of a protein complex required for signaling or may be involved in either the folding or the localization of a component of the signaling pathway. For example, Hsp90 is required together with Sgt1 in S. cerevisiae kinetochore assembly (Bansal et al. 2004; Lingelbach and Kaplan 2004), while in COS cells Hsp90 helps to localize Gα12 to lipid rafts (Waheed and Jones 2002). The failure of an activated allele of the gpa2 Gα gene to suppress the git10-201 mutation would be consistent with either of these mechanisms of action.
The central domain of Hsp90 appears to be a major site for client protein interactions (Sato et al. 2000; Fontana et al. 2002; Meyer et al. 2003). A recent report showed that this domain could also play a role in distinguishing between different types of client proteins (Hawle et al. 2006). Therefore, the git10-201 L338P mutation in the central domain might result in impairment of a client protein activity in the cAMP pathway specifically, whereas the temperature-sensitive mutants might result in a universal impairment of Hsp90 client proteins in the cell or contribute to general instability of Hsp90 itself. Thus, we have identified a separation-of-function allele of Hsp90, which confers a defect in cAMP signaling, but not other essential processes. A similar observation was reported in C. elegans; loss of Hsp90/DAF-21 involved in cGMP signaling confers an early larval lethality; however, a missense mutation affecting a residue in the middle domain produces a viable adult with a chemosensory defect (Birnby et al. 2000). Mapping of the residues altered by the daf21 mutation and by the git10-201 mutation onto the crystal structure of the S. cerevisiae Hsc82p central domain reveals that these two residues are in close proximity to each other (Figure 6B). Therefore, these two separation-of-function alleles may affect their individual cyclic nucleotide signaling pathways via the same mechanism.
These studies also revealed a new insight into heat stress in S. pombe. It has been long known that heat stress activates the Spc1/Sty1 SAPK pathway required for fbp1+ transcription, presumably by regulating the activity of the Pyp1 tyrosine phosphatase (Samejima et al. 1997). Our data further indicate that in addition to activating the SAPK pathway, heat stress reduces PKA activity. Stresses such as nitrogen starvation and osmotic stress, which activate the SAPK pathway, do not derepress fbp1+ transcription (Devoti et al. 1991; Stettler et al. 1996; Janoo et al. 2001; Yang et al. 2003; Stiefel et al. 2004), indicating that reduction of PKA activity is required for fbp1+ derepression. Therefore, we have discovered a novel link between glucose and heat sensing that appears to involve Hsp90. We speculate that heat stress redirects Hsp90 from acting in the cAMP pathway to acting upon targets that are critical to survival of heat stress. As a secondary effect, the ability of heat stress to reduce PKA activity and thus mimic glucose starvation may assist in producing a growth arrest that enhances cell survival at elevated temperatures.
In summary, we have shown that attenuating Hsp90 function by mutation, pharmacological inhibition, or temperature stress impairs cAMP-mediated glucose signaling, consistent with a specific role for Hsp90 in the glucose/cAMP pathway. Further studies will be directed to investigating the mechanism by which Hsp90 acts in this partcular pathway. Given the evidence that the Hsp90 cochaperone Git7/Sgt1 acts in both S. pombe and S. cerevisiae cAMP pathways, it is likely that Hsp90 will be found to function in the S. cerevisiae cAMP pathway as well as in cAMP pathways of other yeasts and fungi.
Acknowledgments
We thank the Sanger Centre for cosmid SPAC926 and Kazuhiro Shiozaki and Paul Russell for swo1− mutant strains. This work was supported by grant GM46226 from the National Institutes of Health to C.S.H.
References
- Alfa, C., P. Fantes, J. Hyams, M. McLeod and E. Warbrick, 1993. Experiments With Fission Yeast. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Aligue, R., H. Akhavan-Niak and P. Russell, 1994. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 13 6099–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, 3rd et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14 943–951. [DOI] [PubMed] [Google Scholar]
- Bansal, P. K., R. Abdulle and K. Kitagawa, 2004. Sgt1 associates with Hsp90: an initial step of assembly of the core kinetochore complex. Mol. Cell. Biol. 24 8069–8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell, J. C., and E. A. Craig, 1987. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc. Natl. Acad. Sci. USA 84 5177–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnby, D. A., E. M. Link, J. J. Vowels, H. Tian, P. L. Colacurcio et al., 2000. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 155 85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, S. M., and C. S. Hoffman, 1993. Six git genes encode a glucose-induced adenylate cyclase activation pathway in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 105 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett, M. G., and K. B. Kaplan, 2006. Sgt1p is a unique co-chaperone that acts as a client adaptor to link Hsp90 to Skp1p. J. Biol. Chem. 281 33739–33748. [DOI] [PubMed] [Google Scholar]
- Dal Santo, P., B. Blanchard and C. S. Hoffman, 1996. The Schizosaccharomyces pombe pyp1 protein tyrosine phosphatase negatively regulates nutrient monitoring pathways. J. Cell Sci. 109 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano, W. L, 2002. The PyMOL Molecular Graphics System. DeLano Scientific, Palo Alto, CA.
- DeVoti, J., G. Seydoux, D. Beach and M. McLeod, 1991. Interaction between ran1+ protein kinase and cAMP dependent protein kinase as negative regulators of fission yeast meiosis. EMBO J. 10 3759–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubacq, C., R. Guerois, R. Courbeyrette, K. Kitagawa and C. Mann, 2002. Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot. Cell 1 568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana, J., D. Fulton, Y. Chen, T. A. Fairchild, T. J. McCabe et al., 2002. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 90 866–873. [DOI] [PubMed] [Google Scholar]
- Gutz, H., H. Heslot, U. Leupold and N. Loprieno, 1974. Schizosaccharomyces pombe, pp. 395–446 in Handbook of Genetics, edited by R. C. King. Plenum Press, New York.
- Hawle, P., M. Siepmann, A. Harst, M. Siderius, H. P. Reusch et al., 2006. The middle domain of Hsp90 acts as a discriminator between different types of client proteins. Mol. Cell. Biol. 26 8385–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C. S., 2005. a Except in every detail: comparing and contrasting G protein signaling in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Eukaryot. Cell 4 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C. S., 2005. b Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe. Biochem. Soc. Trans. 33 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C. S., and F. Winston, 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57 267–272. [DOI] [PubMed] [Google Scholar]
- Hoffman, C. S., and F. Winston, 1990. Isolation and characterization of mutants constitutive for expression of the fbp1 gene of Schizosaccharomyces pombe. Genetics 124 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C. S., and F. Winston, 1991. Glucose repression of transcription of the Schizosaccharomyces pombe fbp1 gene occurs by a cAMP signaling pathway. Genes Dev. 5 561–571. [DOI] [PubMed] [Google Scholar]
- Isshiki, T., N. Mochizuki, T. Maeda and M. Yamamoto, 1992. Characterization of a fission yeast gene, gpa2, that encodes a Gα subunit involved in the monitoring of nutrition. Genes Dev. 6 2455–2462. [DOI] [PubMed] [Google Scholar]
- Ivey, F. D., and C. S. Hoffman, 2005. Direct activation of fission yeast adenylate cyclase by the Gpa2 Gα of the glucose signaling pathway. Proc. Natl. Acad. Sci. USA 102 6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoo, R. T., L. A. Neely, B. R. Braun, S. K. Whitehall and C. S. Hoffman, 2001. Transcriptional regulators of the Schizosaccharomyces pombe fbp1 gene include two redundant Tup1p-like corepressors and the CCAAT binding factor activation complex. Genetics 157 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, M., M. Fujita, B. M. Culley, E. Apolinario, M. Yamamoto et al., 1995. sck1, a high copy number suppressor of defects in the cAMP-dependent protein kinase pathway in fission yeast, encodes a protein homologous to the Saccharomyces cerevisiae SCH9 kinase. Genetics 140 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, R. S., E. Morreale, L. Wang, F. D. Ivey and C. S. Hoffman, 2006. Schizosaccharomyces pombe Git1 is a C2-domain protein required for glucose activation of adenylate cyclase. Genetics 173 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa, K., D. Skowyra, S. J. Elledge, J. W. Harper and P. Hieter, 1999. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol. Cell 4 21–33. [DOI] [PubMed] [Google Scholar]
- Landry, S., and C. S. Hoffman, 2001. The git5 Gβ and git11 Gγ form an atypical Gβγ dimer acting in the fission yeast glucose/cAMP pathway. Genetics 157 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry, S., M. T. Pettit, E. Apolinario and C. S. Hoffman, 2000. The fission yeast git5 gene encodes a Gβ subunit required for glucose-triggered adenylate cyclase activation. Genetics 154 1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, S., and E. A. Craig, 1988. The heat-shock proteins. Annu. Rev. Genet. 22 631–677. [DOI] [PubMed] [Google Scholar]
- Lingelbach, L. B., and K. B. Kaplan, 2004. The interaction between Sgt1p and Skp1p is regulated by HSP90 chaperones and is required for proper CBF3 assembly. Mol. Cell. Biol. 24 8938–8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, T., N. Mochizuki and M. Yamamoto, 1990. Adenylyl cyclase is dispensable for vegetative cell growth in the fission yeast Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 87 7814–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, P., C. Prodromou, B. Hu, C. Vaughan, S. M. Roe et al., 2003. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol. Cell 11 647–658. [DOI] [PubMed] [Google Scholar]
- Mishra, M., V. M. D'Souza, K. C. Chang, Y. Huang and M. K. Balasubramanian, 2005. Hsp90 protein in fission yeast Swo1p and UCS protein Rng3p facilitate myosin II assembly and function. Eukaryot. Cell 4 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocero, M., T. Isshiki, M. Yamamoto and C. S. Hoffman, 1994. Glucose repression of fbp1 transcription of Schizosaccharomyces pombe is partially regulated by adenylate cyclase activation by a G protein α subunit encoded by gpa2 (git8). Genetics 138 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl, L. H., and C. Prodromou, 2000. Structure and in vivo function of Hsp90. Curr. Opin. Struct. Biol. 10 46–51. [DOI] [PubMed] [Google Scholar]
- Pearl, L. H., and C. Prodromou, 2006. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 75 271–294. [DOI] [PubMed] [Google Scholar]
- Powers, M. V., and P. Workman, 2006. Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr. Relat. Cancer 13(Suppl. 1): S125–S135. [DOI] [PubMed] [Google Scholar]
- Samejima, I., S. Mackie and P. A. Fantes, 1997. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 16 6162–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, S., N. Fujita and T. Tsuruo, 2000. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 97 10832–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadick, K., H. M. Fourcade, P. Boumenot, J. J. Seitz, J. L. Morrell et al., 2002. Schizosaccharomyces pombe Git7p, a member of the Saccharomyces cerevisiae Sgtlp family, is required for glucose and cyclic AMP signaling, cell wall integrity, and septation. Eukaryot. Cell 1 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern, J. A., D. F. Young, F. Heaney, W. K. Baumgartner and R. E. Randall, 1991. Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J. Gen. Virol. 72 1551–1557. [DOI] [PubMed] [Google Scholar]
- Spence, J., and C. Georgopoulos, 1989. Purification and properties of the Escherichia coli heat shock protein, HtpG. J. Biol. Chem. 264 4398–4403. [PubMed] [Google Scholar]
- Stettler, S., E. Warbrick, S. Prochnik, S. Mackie and P. Fantes, 1996. The wis1 signal transduction pathway is required for expression of cAMP-repressed genes in fission yeast. J. Cell Sci. 109 1927–1935. [DOI] [PubMed] [Google Scholar]
- Stiefel, J., L. Wang, D. A. Kelly, R. T. K. Janoo, J. Seitz et al., 2004. Suppressors of an adenylate cyclase deletion in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 3 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. D., D. G. Higgins and T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassarotti, A., and J. D. Friesen, 1985. Isolation of the fructose-1,6-bisphosphatase gene of the yeast Schizosaccharomyces pombe. Evidence for transcriptional regulation. J. Biol. Chem. 260 6348–6353. [PubMed] [Google Scholar]
- Waheed, A. A., and T. L. Jones, 2002. Hsp90 interactions and acylation target the G protein Gα12 but not Gα13 to lipid rafts. J. Biol. Chem. 277 32409–32412. [DOI] [PubMed] [Google Scholar]
- Wang, L., K. Griffiths, Jr., Y. H. Zhang, F. D. Ivey and C. S. Hoffman, 2005. Schizosaccharomyces pombe adenylate cyclase suppressor mutations suggest a role for cAMP phosphodiesterase regulation in feedback control of glucose/cAMP signaling. Genetics 171 1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton, R. M., and C. S. Hoffman, 2000. Glucose monitoring in fission yeast via the gpa2 Gα, the git5 Gβ, and the git3 putative glucose receptor. Genetics 156 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell, L., E. G. Mimnaugh, B. De Costa, C. E. Myers and L. M. Neckers, 1994. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA 91 8324–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, V., R. Gwilliam, M. A. Rajandream, M. Lyne, R. Lyne et al., 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415 871–880. [DOI] [PubMed] [Google Scholar]
- Yang, P., H. Du, C. S. Hoffman and S. Marcus, 2003. The phospholipase B homolog Plb1 is a mediator of osmotic stress response and of nutrient-dependent repression of sexual differentiation in the fission yeast Schizosaccharomyces pombe. Mol. Genet. Genomics 269 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., and F. Burrows, 2004. Targeting multiple signal transduction pathways through inhibition of Hsp90. J. Mol. Med. 82 488–499. [DOI] [PubMed] [Google Scholar]