Abstract
Fasciclin2 (Fas2) and Discslarge (Dlg) localize to the basolateral junction (BLJ) of Drosophila follicle epithelial cells and inhibit their proliferation and invasion. To identify a BLJ signaling pathway we completed a genomewide screen for mutants that enhance dlg tumorigenesis. We identified two genes that encode known BLJ scaffolding proteins, lethal giant larvae (lgl) and scribble (scrib), and several not previously associated with BLJ function, including warts (wts) and roughened eye (roe), which encode a serine–threonine kinase and a transcription factor, respectively. Like scrib, wts and roe also enhance Fas2 and lgl tumorigenesis. Further, scrib, wts, and roe block border cell migration, and cause noninvasive tumors that resemble dlg partial loss of function, suggesting that the BLJ utilizes Wts signaling to repress EMT and proliferation, but not motility. Apicolateral junction proteins Fat (Ft), Expanded (Ex), and Merlin (Mer) either are not involved in these processes, or have highly spatio-temporally restricted roles, diminishing their significance as upstream inputs to Wts in follicle cells. This is further indicated in that Wts targets, CyclinE and DIAP1, are elevated in Fas2, dlg, lgl, wts, and roe cells, but not Fat, ex, or mer cells. Thus, the BLJ appears to regulate epithelial polarity and dynamics not only as a localized scaffold, but also by communicating signals to the nucleus. Wts may be regulated by distinct junction inputs depending on developmental context.
EPITHELIA are composed of apical–basal polarized cells that contact each other and surrounding tissues through intercellular junctions. A universal theme is that each type of epithelial junction is organized by a transmembrane receptor bound to a cytoplasmic scaffolding protein, thus enabling assembly of a supermolecular complex of proteins within the cellular cortex at sites of intercellular contact (Knust 2002). Epithelial junctions are relatively stable in stationary epithelia, but they undergo extensive remodeling when epithelia transform into migratory cells during embryonic development, tissue repair and regeneration, and pathological conditions such as cancer (Grunert et al. 2003; Shook and Keller 2003; Szafranski and Goode 2004; Larue and Bellacosa 2005; Thiery and Sleeman 2006; Szafranski and Goode 2007). Understanding how epithelial junctions are used for epithelial maintenance and epithelial remodeling during normal development may allow targeting tumor invasion without perturbing normal tissue homeostasis.
We use the Drosophila follicular epithelium as a simple in vivo model to gain insight into the cellular and molecular events that initiate and drive epithelial invasion (Goode et al. 2005). A Drosophila female has a pair of ovaries. Each ovary has 30–40 tubes called ovarioles in which egg development progresses (a sequence of maturing Drosophila egg chambers within an ovariole is shown in Figure 2A). Each egg chamber is composed of 16 germ cells: 15 nurse cells and 1 oocyte (Spradling 1993). A monolayer epithelium of follicle cells surrounds the germ cells. The follicle cells proliferate during the early stages of oogenesis [stages 1–6 (s1–s6); Figure 2A], to keep pace with rapidly growing germ cells. Once follicle cell proliferation ceases, differentiation begins, setting the stage for two migration events during midoogenesis. A small cluster of cells that reside in the anterior epithelium, called border cells (BC), undergo a partial epithelial-mesenchymal transition (EMT), break free from adjacent epithelial cells, and migrate posterior between the nurse cells to the oocyte (Figure 2A) (Niewiatomska et al. 1999; Starz-Gaiano and Montell 2004; Szafranski and Goode 2007). At the same time, most follicle cells that surround the nurse cells move posterior to encapsulate the oocyte (Goode et al. 2005).
Figure 2.—
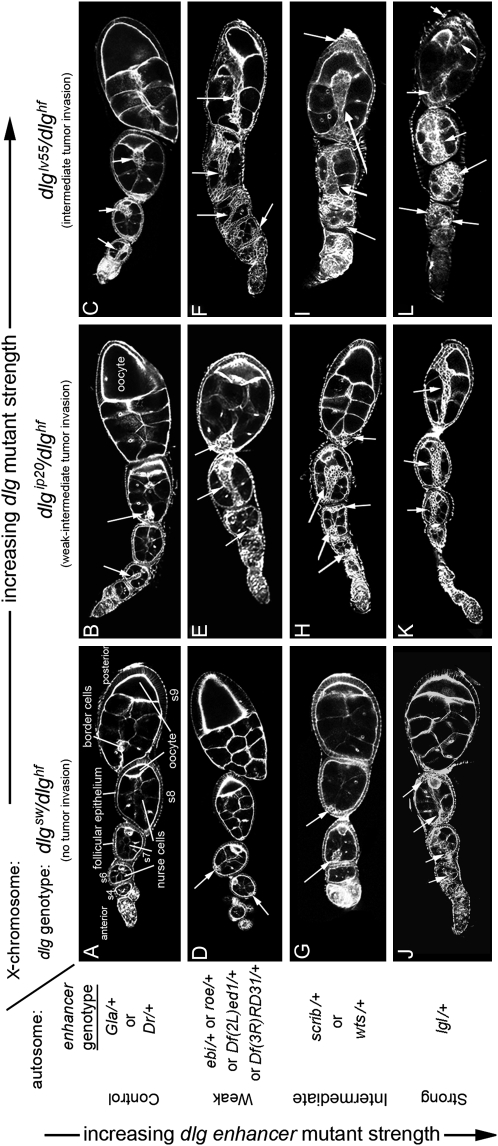
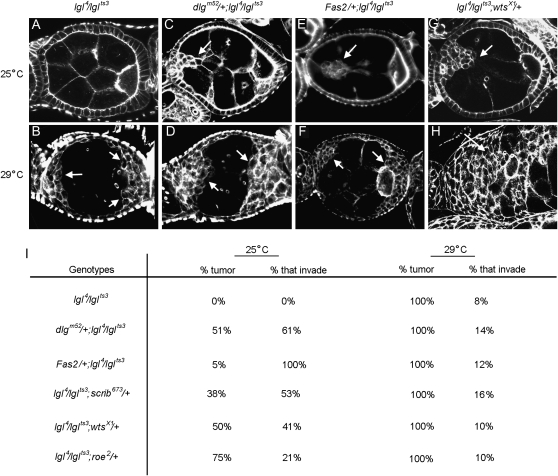
Characterization of dlg enhancer phenotypes. A–L are organized horizontally left to right by increasing dlg strength (X chromosome, dlg genotype), and vertically top to bottom by increasing dlg enhancer strength (autosome, enhancer genotype). Strings of maturing egg chambers that are representative of the indicated X chromosome; autosome genotype are shown (arrows point to tumors). (A) Control egg chambers of genotype dlgsw/dlghf; Gla/+ appear wild type. Stage 4 (s4) to s9 egg chambers are evident. Each egg chamber has 16 germ cells, 15 anterior nurse cells, and a posterior oocyte. A follicular epithelium surrounds each egg chamber. A small cluster of cells called border cells exit the anterior epithelium at stage 9 and migrates between the nurse cells to the oocyte. (B) Control egg chambers of genotype dlgip20/dlghf; Dr/+. They have small tumors that invade the germ line starting as early as stage 2 of oogenesis (arrows). (C) Control egg chambers of genotype dlglv55/dlghf; Dr/+. They have intermediate-size tumors that invade more frequently than in dlgip20/dlghf; Dr/+ animals (see text). (D, G, and J) All dlg enhancers cause tumors in dlgsw/dlghf animals that typically do not have tumors (compare to control, A). (E, H, and K) All dlg enhancers increase the frequency and size of tumors in dlgip20/dlghf animals. Whereas tumors are observed in ∼66% of dlgip20/dlghf ovarioles, they are observed in 100% of dlgip20/dlghf; enhancer/+ ovarioles, even for the weakest enhancers. (F, I, and L) In dlglv55/dlghf; enhancer/+ animals, the tumors become increasingly larger and more aggressive in appearance with increasing dlg enhancer strength, often appearing to consume the entire egg chamber in the most severe cases (L).
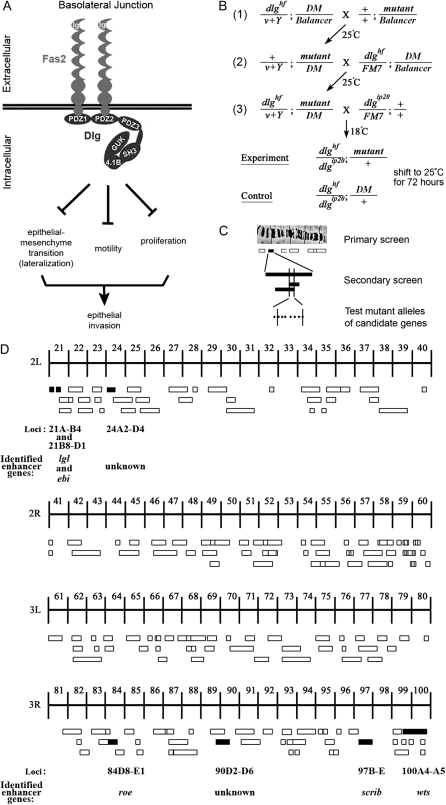
Each cell within the follicular epithelium has five junctions (Tanentzapf et al. 2000; Szafranski and Goode 2007). An apical junction connects to the germ line, while a basal junction connects to the basement membrane that surrounds each egg chamber. Three lateral junctions interconnect each follicle cell. From the apical to the basal, they are the apicolateral junction (also called the tight junction in vertebrates), the adherens junction, and the basolateral junction (BLJ). Five proteins that localize to the BLJ, Fasciclin2 (Fas2), Neuroglian (Nrg), Discslarge (Dlg), Scribble (Scrib), and Lethal-giant-larvae (Lgl), are conserved in structure and localization in vertebrate epithelia (Knust and Bossinger 2002). Fas2 is a transmembrane protein of the immunoglobulin superfamily (IgSF) (Goodman et al. 1997). Dlg is a scaffolding protein composed of three PDZ domains, an SH3 domain, a membrane protein 4.1 binding site, and a GuK domain (Woods et al. 1996). Dlg creates scaffolds by multimerizing at the BLJ and by using its multiple binding domains to recruit specific combinations of signaling, adhesion, scaffolding, and cytoskeletal molecules (Funke et al. 2005). For example, Fas2 directly binds to Dlg PDZ1+2. Nrg, another IgSF member, appears to be connected to the Fas2–Dlg–Lgl scaffold only indirectly through the actin–spectrin cortex (Wei et al. 2004; Szafranski and Goode 2007). Scrib, another multi-PDZ domain scaffolding protein, binds to the Dlg GuK domain (Mathew et al. 2002). Lgl is also a BLJ protein because it precisely colocalizes with Fas2 and Dlg in normal follicle cells or when Fas2 is expressed ectopically (Szafranski and Goode 2004), but the intermolecular interactions that bind Lgl to the Fas2–Dlg scaffold remain to be defined.
During normal egg chamber development, Fas2 is expressed at high levels in early proliferating follicle cells. Then, when follicle cells stop dividing and prepare to migrate, Fas2 levels dramatically decrease and Fas2 appears to be completely shut off in border cells, reflecting its motility repressing function. Dlg, Lgl, and Nrg, which also repress movement, are expressed ubiquitously in all follicle cells throughout oogenesis (Szafranski and Goode 2007). Thus spatiotemporal patterns of BLJ activity appear to be tightly controlled by spatiotemporal patterns of Fas2 expression, which represses cellular movement until midoogenesis. At that time, Fas2 loss in anterior follicle cells causes a dynamic rearrangement of epithelial junctions that is essential for border cells to undergo partial EMT. The partial EMT involves the process of membrane lateralization, which is constituted by a loss of apical and basal junctions and a redistribution of BLJ proteins around the circumference of the border cells (Szafranski and Goode 2007). The EMT is partial because the border cells retain apicolateral and adherens junctions, which provide the intercellular contacts that help to sculpt the stereotypical rosette pattern of the border cell cluster (Niewiadomska et al. 1999; Szafranski and Goode 2007).
Mutation of Fas2, dlg, lgl, or Nrg during early oogenesis causes follicle cells to lose epithelial polarity, undergo EMT, and to invade into the germ line, which in many ways resembles normal border cell migration (Goode et al. 2005; Szafranski and Goode 2007) (see Figure 2). The EMT in these Fas2, dlg, lgl, or Nrg tumor-like cells appears to be more complete than the partial EMT that defines border cell differentiation, because in addition to a lateralized distribution of BLJ proteins, apicolateral and adherens junction proteins also become diffusely redistributed in a lateralized pattern that overlaps with BLJ proteins (Szafranski and Goode 2007). Thus, the tumor cells do not form a rosette, but rather invade as streams and clusters of cells that appear disorganized compared to border cells. The importance of Fas2, Nrg, Dlg, and Lgl in regulating intercellular contacts and membrane and cytoskeletal dynamics, suggests that they suppress invasive tumorigenesis via contact inhibition of proliferation and movement (Szafranski and Goode 2004).
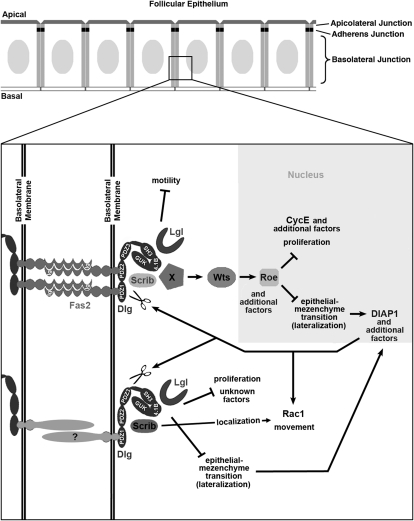
To migrate, border cells must turn on EMT and motility promoting transcription factors such as Stat and Slbo (Montell 2003). Fas2 and dlg tumor cells appear to bypass this normal migration mechanism by prematurely derepressing EMT and a motility pathway that acts through Rac1 (Szafranski and Goode 2007). In Fas2 and dlg border cells, derepression of the motility pathway causes faster migration (Szafranski and Goode 2004) and in early follicle cells, causes an increased incidence of tumor invasion (Szafranski and Goode 2007). Detailed genetic analysis of Fas2 and dlg indicates that derepression of both the EMT and motility pathways are necessary, but neither is sufficient for early tumor invasion. Whereas complete loss of dlg causes EMT and epithelial invasion, some partial loss-of-function dlg mutants cause follicle cells to accumulate in stratified layers without invading (Goode et al. 2005). In both cases follicle cells have the lateralized signature characteristic of EMT, indicating that EMT is not sufficient for invasion (Szafranski and Goode 2007). Likewise, derepression of motility is necessary but not sufficient for tumor invasion, because some partial loss-of-function Fas2 and dlg mutants cause faster border cell migration, but without early epithelial invasion. In these mutants, EMT in the early epithelium remains repressed, so tumor formation and subsequent invasion remain blocked (Szafranski and Goode 2007). Thus, the BLJ controls two pathways, one that represses EMT, the other motility, and these pathways function together to repress invasive tumorigenesis (Figure 1A).
Figure 1.—
Summary of the dlg enhancer screen. (A) Model of the BLJ. Transmembrane Fas2 and scaffolding Dlg repress tumor growth and invasion (Szafranski and Goode 2007). They repress three genetically separable activities, EMT, motility, and proliferation. EMT results in membrane lateralization (see text for discussion). (B) Cross scheme to identify dlg enhancers on the second and third chromosomes. A dominant marker (DM) gene, typically Gla on the second chromosome, or Dr on the third chromosome, was used to follow segregation of mutant chromosomes. The Balancer on the first chromosome was FM7a, on the second chromosome was Cyo, and on the third, TM3, Sb. In the first cross, v+Y is an insertion onto the Y chromosome of a fragment of the X chromosome that covers the dlg locus [10B6-10]. v+Y is introduced into the background of each mutant (cross 2, +/v+Y; mutant/DM) to rescue dlghf lethality in dlghf/v+Y; mutant/DM flies in cross 3. The third cross was completed at 18°, the dlghf/dlgip20 permissive temperature, to bypass dlg lethality. Experimental and control animals were recovered at 18°, then shifted to 25° for 72 ± 2 hr. The same scheme was used to construct dlghf/dlgsw; mutant/+ and dlghf/dlglv55; mutant/+ animals (see text). (C) The screen was completed in three phases. In the primary screen, we used a panel of deletions that uncover most autosomal regions (Bloomington deficiency kit, http://flybase.bio.indiana.edu/). In the secondary screen, we further tested each region that enhanced dlg invasion with additional deletions, to confirm or rule out the enhancing interaction and to refine the position of the interacting locus. Finally, we tested mutations located within the delineated locus to identify the enhancing gene. (D) Schematic summary of the primary screen. The figure shows 178 deficiencies assayed in the primary screen. The deficiencies uncover the genetic regions indicated by the width of the boxes. Deficiencies that scored 100% invasion are solid boxes. Deficiencies that did not enhance invasion are open boxes. The cytological limits of each enhancer locus are shown (the breakpoints are defined by the minimal region that must include the enhancing gene as determined by the pattern of overlap between all enhancing deficiencies). The gene identified as the dlg enhancer at each locus is shown.
In addition to repressing EMT and movement, BLJs also inhibit follicle cell proliferation. However, overproliferation does not appear to be essential for invasion, since small clusters of Fas2 and dlg cells that resemble a border cell rosette can completely delaminate from the follicular epithelium of early egg chambers and migrate to the oocyte (Goode et al. 2005; Szafranski and Goode 2007). These Fas2 and dlg tumor clusters indicate that the tumor cells are actively migrating, not merely pushed between germ cells as a result of excessive proliferation.
Nonetheless, the most typical pattern of Fas2 and dlg tumor invasion is large streams of cells that remain attached at their point of origin to the follicular epithelium, as the leading edge of the stream moves toward the oocyte (Goode et al. 2005; see Figure 2). The tumor stream appears to be fed by follicle cell proliferation because invasion of even very large tumor streams typically does not result in a gap in the native epithelium depleted of follicle cells or cause dramatic epithelial thinning (Figure 2; Goode et al. 2005). In contrast, follicle cells deprived of EGF signals results in epithelial thinning and gaps completely void of follicle cells (Goode et al. 1996b). Further, BrdU labeling experiments and direct cell counts indicate that excessive proliferation generates the large numbers of cells needed to create dlg tumor streams (see Figure 2) (Goode and Perrimon 1997; Goode et al. 2005). However, overproliferation is insufficient for invasion, as indicated by some hypomorphic dlg mutants that cause EMT and overproliferation, and some hypomorphic Fas2 mutants that cause derepression of motility and overproliferation, neither of which results in tumor invasion (Goode et al. 2005; Szafranski and Goode 2007). Thus, overproliferation is not essential for invasion, but facilitates the process by continually providing new cells that feed the invasive tumor stream. In summary, Fas2, Dlg, and Lgl repress proliferation, EMT, and motility (Figure 1A). Both EMT and motility are necessary, but neither are sufficient for tumor invasion, they cooperate to promote motility. In contrast, proliferation merely facilitates invasion by continually providing new cells for tumor growth.
There is considerable evidence that vertebrate orthologs of Fas2, Dlg, Lgl, and Nrg are also important for suppressing epithelial invasion in mammals and humans (Matsumine et al. 1996; Fogar et al. 1997; Roesler et al. 1997; Hoover et al. 1998; Perl et al. 1999; Huang et al. 2003; Schimanski et al. 2005; Arlt et al. 2006; Grifoni et al. 2007; Szafranski and Goode 2007). Further, mammalian and human orthologs of dlg and lgl have been shown to rescue the corresponding fly mutants (Grifoni et al. 2004). Thus, dissecting the mechanisms by which BLJ proteins suppress epithelial invasion in Drosophila may improve our understanding of the mechanisms by which metastasis is initiated during human cancer progression.
Here we aimed at identifying a signaling pathway that acts downstream of the BLJ to inhibit invasive tumorigenesis. We completed a genomewide screen to find loci that caused a dosage-sensitive enhancement of dlg invasive tumorigenesis. We identified two genes, lgl and scrib, that encode scaffolding proteins previously demonstrated to be in a BLJ complex with Dlg, thus providing proof of principle of the screen's efficacy. In addition, we identified three new genes not previously linked to BLJ function, warts (wts), roughened eye (roe), and ebi. wts encodes a serine–threonine kinase that acts as a tumor suppressor in imaginal disc tissues. wts shared several phenotypic similarities with scrib, and enhanced Fas2, dlg, and lgl invasion as strong or stronger than scrib. The unique subset of phenotypes that scrib, wts, and roe share with Fas2, dlg, and lgl indicate that they act specifically in the EMT and proliferation branches of a BLJ pathway and not in the motility-repressing branch. Further, apicolateral junction proteins Fat (Ft), Expanded (Ex), and Merlin (Mer) either are not involved in these processes or have highly spatiotemporally restricted roles, diminishing their significance as upstream inputs to Wts in follicle cells. This is further indicated in that Wts targets, CyclinE and DIAP1, are elevated in Fas2, dlg, lgl, wts, and roe cells, but not in Fat, ex, or mer cells. We propose that Fas2–Dlg–Lgl signaling through Wts to Roe defines a novel BLJ signaling pathway that represses EMT and proliferation. Thus, the BLJ appears to regulate epithelial polarity and dynamics not only by acting as a localized scaffold, but also by communicating signals to the nucleus. Wts appears to be regulated by distinct junctional inputs depending on developmental context.
MATERIALS AND METHODS
Stocks and culture conditions:
The following dlg alleles were used in this study: dlghf, dlgsw, dlgip20, dlglv55, dlgm35, and dlgm52 (Woods and Bryant 1989, 1991). For the screen and subsequent assays, temperature-sensitive, heteroallelic combinations of dlg were reared at 18° (Figure 1B). Experimental (dlg; Deficiency/+) and control (dlg; Balancer/+) animals were collected within 12 hr of eclosion. The adults were then shifted to the restrictive temperature (25°) for 72 hr to induce tumor invasion (Goode and Perrimon 1997). Other crosses were performed at 25°.
Second and third chromosome deficiency kits were obtained from the Bloomington Stock Center (http://flybase.bio.indiana.edu/). For gene identification, a search was completed in FlyBase (http://www.flybase.org/) for all gene mutations within the cytological interval defining the locus. All mutations within and just outside the cytological interval that were linked to a known transcription unit and/or that were lethal or female sterile were tested for enhancement of dlg invasion (see results for further information). Most mutations described in the text were obtained from the Bloomington Stock Center. Other mutants were obtained from the following labs: scrib mutants (Bilder lab), lgl4 and lglts3 (Knoblich lab), and wtsx1 (Halder lab).
For clonal analysis, experimental animals were generated by crossing w; dlgm52 FRT101/FM7 (Goode and Perrimon 1997) and w; Fas2B112 FRT101/FM7 to hsFLP, lacZ, FRT101 (Szafranski and Goode 2004), and yw; lgl4 FRT 42D/CyO (Manfruelli et al. 1996), w; FRT82B scrib673/TM6 (Bilder and Perrimon 2000), w; FRT82B wtsx1/TM3 (Udan et al. 2003), and FRT82B roe2 pp/TM3 (generated from Ki1 roe2 pp/TM3, see http://www.flybase.org/) to the appropriate FRT GFP chromosome stocks (see http://www.flybase.org/). Late third-instar larvae and early pupae were heat-shocked as described (Szafranski and Goode 2004). The following UAS lines were used: UAS-dlg240 (Hough et al. 1997), UAS-scrib (Mathew et al. 2002), UAS-lgl (Hutterer et al. 2004), UAS-wts37 (Lai et al. 2005), UAS-roe (St Pierre et al. 2002), and UAS-ebi (FlyBase). GR1 Gal4 (Manseau et al. 1997) is expressed in all follicle cells (Szafranski and Goode 2007).
Quantification of tumor invasion:
For the screen, 200 ovarioles were scored for the presence of invasive tumors. The percentage of ovarioles with an invasive tumor was determined. Percentage of invasion positively correlates with severity of tumor invasion in individual egg chambers and with the earliest stage at which tumor invasion is most predominant (Figure 2 and see text). The percentage-of-invasion score provided a simple, reproducible assay to quantify the severity of tumor invasion during the screen. The same assay was used for detailed characterization of some gene interactions (Figure 3A).
Figure 3.—
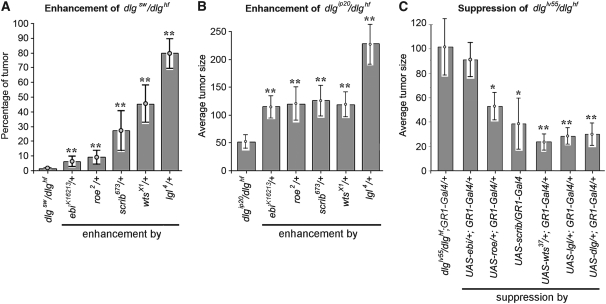
Quantitative analysis of dlg enhancement and suppression. (A) Frequency of dlgsw/dlghf; enhancer/+ tumor invasion. lgl causes the highest frequency, scrib and wts an intermediate frequency, and ebi and rn the lowest frequency of invasion. All enhancements were significant (**P < 0.01). (B) For all dlgip20/dlghf; enhancer/+ genotypes invasion occurs in 100% of ovarioles (Figure 2). To compare the strengths of enhancement we determined the average tumor size (materials and methods). Removal of one copy of lgl (dlgip20/dlghf; lgl/+) causes tumors that are four to five times the size of dlgip20/dlghf tumors, while removal of one copy of ebi, rn, scrib, or wts causes tumors that are roughly twice larger (**P < 0.01). (C) Suppression of dlglv55/dlghf tumorigenesis by dlg enhancer overexpression. The Gal4-UAS system (Brand and Perrimon 1993) was used to drive overexpression of each UAS-dlg enhancer transgene with GR1-Gal4, which is expressed in all follicle cells throughout oogenesis (Szafranski and Goode 2007). dlglv55/dlghf tumorigenesis was most significantly suppressed by dlg, lgl, and wts (**P < 0.01) and to a lesser but still significant degree by scrib and roe (*P < 0.05). ebi showed no significant suppression.
However, the percentage-of-invasion assay was inadequate for comparing Fas2 and dlg tumors, or invasive vs. noninvasive Fas2 tumors (Figures 3 and 4). For these experiments, we completed measurements to determine the average tumor size. To control for differences in tumor size due to potential differences in egg chamber size, we normalized the score as follows. A Zeiss Axioplan-2 microscope equipped with a Hamamatsu ORCA digital camera was used to capture a cross-sectional image at the center of 20–30 stage 5–8 follicles for each genotype. We then used Axiovision 3.1 (Carl Zeiss Vision) to measure (1) the area that includes all tumor cells, and (2) the egg chamber area. Dividing the average tumor area by the average egg chamber area normalized the average tumor area. The normalized, average tumor area is referred to as the average tumor size (Figures 3 and 4).
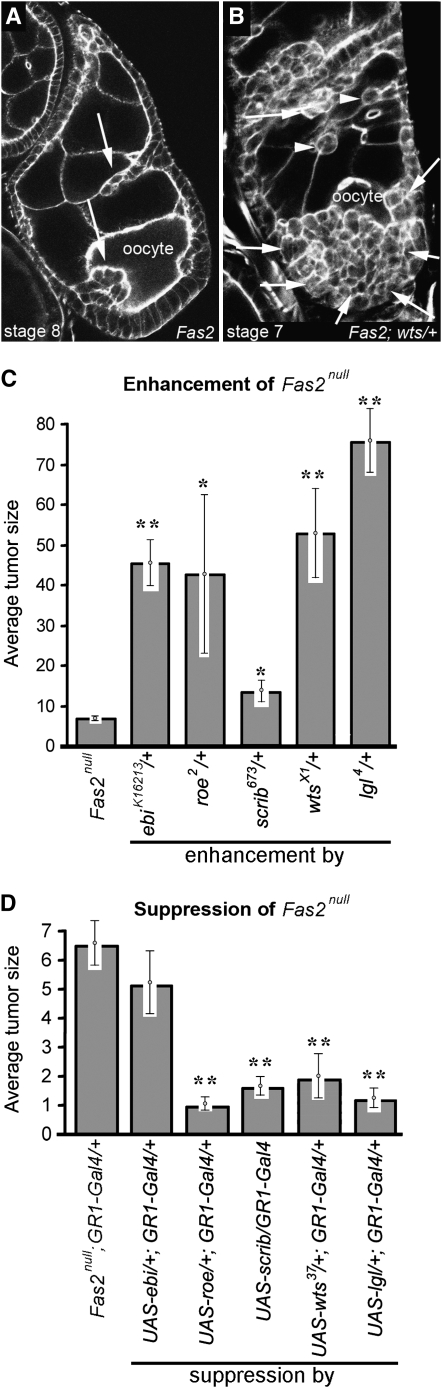
Figure 4.—
Genetic interaction of dlg enhancers with Fas2null. (A) Fas2 tumors invade into the germ line (arrows). (B) Fas2; wts/+ tumors are larger, but are typically noninvasive (arrows). All Fas2; enhancer/+ animals have the same pattern of noninvasive tumors. Occasionally a few isolated cells that have broken from the main tumor are observed between the germ cells (arrowheads). (C) Tumor scores for Fas2; enhancer/+ egg chambers. All dlg enhancers enhanced Fas2 ∼6- to 10-fold, with the exception of scrib, which showed a 2-fold enhancement (**P < 0.01; *P < 0.05). (D) Suppression of Fas2 tumorigenesis by overexpression of dlg enhancers. Overexpression was accomplished as described in Figure 3C. All but ebi showed significant suppression of Fas2 tumorigenesis.
Immunofluoresence and imaging:
Ovaries were fixed for 20 min in 4% formaldehyde in PBS. For the screen, ovarioles were teased apart using tungsten needles to keep the egg chambers within each ovariole together, so that tumor invasion could be scored as percentage of ovarioles with tumors (see above). Egg chambers were visualized by staining with Alexa488 phalloidin (1:10; Molecular Probes, Eugene, OR). The following primary antibodies were used: rabbit anti-β-gal (1:2000; Cappel), rabbit anti-PH3 (1:1000; Upstate Biotechnology, Cleveland), mouse anti-BrdU (1:250; GE Healthcare), mouse anti-CycE (1:1000; Helena Richardson lab) (Richardson et al. 1995), and mouse anti-DIAP1 (1:200; Bruce Hay lab) (Yoo et al. 2002). All images were captured using a Zeiss LSM510 laser scanning confocal microscope and processed using Adobe Photoshop.
For BrdU labeling experiments, ovaries were dissected into Shields and Sang M3 Insect Medium (Sigma, St. Louis), then transferred to the same media containing 0.5 mg/ml BrdU labeling solution (Sigma), and incubated for 1 hr. The ovaries were then fixed and processed for PI and BrdU staining as previously described (Goode and Perrimon 1997). To determine differences in the number of BrdU+ cells in clones, confocal miscroscopy was used to obtain a cross section from follicles that had an approximately equal area of clone vs. nonclone cells. The total number of propidium iodide- and BrdU-labeled cells in the clone vs. nonclone areas was ascertained. From this, the ratio of BrdU to propidium iodide positive cells was calculated. Ten or more egg chambers were assayed per genotype. The same procedure was used to measure PH3 and CycE.
Statistical analysis:
SPSS v12.0 for Windows was used for all statistical analyses. Error bars were calculated as the standard error of the mean. P-values were calculated by applying an independent sample t-test.
RESULTS
dlg enhancer screen:
Our goal was to identify a BLJ signaling pathway that controls epithelial tumorigenesis and invasion. We chose to screen for enhancers of dlg tumor invasion because Dlg is the predominant scaffolding protein that organizes the BLJ, and because dlg invasion is simple to score. To identify a genetic background to screen for enhancers of dlg invasive tumorigenesis, we characterized three dlg mutant combinations, dlghf/dlgsw, dlghf/dlgip20, and dlghf/dlglv55. dlghf is a temperature-sensitive allele that permits dlghf/dlgip20 and dlghf/dlglv55 animals to develop at 18°, thus bypassing dlg lethality at 25° (dlghf/dlgsw animals are viable at 25°) (Goode and Perrimon 1997). Once dlghf/dlgip20 and dlghf/dlglv55 animals reached adulthood, they were shifted to 25° for 48 ± 6 hr to analyze their ovarian phenotypes. To quantify the strength of invasion in dlghf/dlgsw, dlghf/dlgip20, and dlghf/dlglv55 animals, we scored the percentage of ovarioles with invasive tumors (materials and methods). Approximately 0.5% of ovarioles from dlghf/dlgsw developed very tiny tumors, most appearing wild type (Figure 2A). Sixty-three percent of ovarioles from dlghf/dlgip20 mutants had small- to intermediate-sized invasive tumors, most originated by stages 4–6 (Figure 2B). Ninety-nine percent of ovarioles from dlghf/dlglv55 mutants had large invasive tumors that originated predominantly during stages 1–3 (Figure 2C). dlgsw, dlgip20, and dlglv55 had increasingly large carboxyl-terminal deletions of the Dlg GuK domain (Woods and Bryant 1989, 1991), thus the severity of invasive tumorigenesis correlates with increasing GuK truncation. On the basis of these data, we reasoned that the weak- to intermediate-strength phenotypes observed in dlghf/dlgip20 ovaries would be readily modifiable, and thus ideal for a screen.
We used an F3 cross scheme to construct dlghf/dlgip20; Deficiency/+ animals (Figure 1B). Although the F3 cross scheme is relatively laborious to execute, the benefit of the scheme is that enhancement of invasive tumorigenesis was assayed relatively directly, as opposed to lethality, polarity, proliferation, or another phenotype that may or may not be important for invasion. For the primary screen we assayed the Bloomington deficiency kit (Figure 1C) (materials and methods). The kit uncovers ∼80% of the second and third chromosomes (Figure 1D), which corresponds to ∼64% of the Drosophila genome. We identified 18 nonoverlapping deficiencies that increased the fraction of ovarioles with egg chambers having tumors from 63 to 97–100% (materials and methods).
To determine which of these 18 loci contain an enhancer of dlg invasive tumorigenesis, we completed a secondary screen with additional deficiencies that overlap these candidate loci (Figure 1C). Ten of the 18 loci were ruled out because overlapping deficiencies scored <80%. In addition, we did not further test the region defined by Df(2L)Kr14 (60F2; 60F5), which scored 97%, because overlapping deficiencies were not available. As summarized below, at the remaining 7 loci overlapping deficiencies and mutations scored 100%. The results indicate that we could reliably identify a dlg enhancer in the primary screen when 100% of ovarioles in dlghf/dlgip20; deficiency/+ ovaries had egg chambers with invasive tumors.
To identify the enhancer genes within the seven confirmed deficiency intervals, all available lethal, female sterile, and visible mutations within each region were screened (materials and methods). Candidate enhancer genes were identified according to the following criteria: (1) gene mutations failed to complement all deficiencies defining the locus, (2) both tumor penetrance and size were quantitatively similar to deficiencies defining the locus, (3) more than one mutant allele of each enhancer gene enhanced invasion, and (4) enhancement was observed in three dlg backgrounds, dlghf/dlgsw, dlghf/dlgip20, and dlghf/dlg lv55. As described below, we used these criteria to identify a dlg enhancer gene at five of the seven enhancing loci.
Identification of dlg enhancers:
A summary of the screen that led to identification of five dlg enhancing genes is presented below. The genes are listed in order of strength with which they enhanced dlg tumorigenesis. The quantity of enhancement was assayed in several dlg backgrounds to help verify that the strength of functional interdependence between dlg and each dlg-enhancer was a consistent trait. The relative strength of the interactions is important because it provides an initial indicator of the relative participation of each interacting gene in dlg pathways. For example, a strong interaction might indicate that the gene is involved in all three dlg pathways that repress proliferation, EMT, and motility (see Introduction) (Figure 1A). A moderate or weak interaction may indicate that the gene is required either predominantly in only one or two dlg pathways or partially in all three pathways. These putative distinctions were verified by subsequent analysis.
lgl:
Nine overlapping deficiencies, spanning from 21A1 to 21B4 (Df(2L)PM4, Df(2L)net18, Df(2L)PM44, Df(2L)net62, Df(2L)PM51, Df(2L)TE21A, Df(2L)PMG, Df(2L)PM45, and Df(2L)net-PMF), caused 100% of dlghf/dlg ip20 ovarioles to have one or more egg chambers with tumors. A null mutation of lgl, lgl4 (Manfruelli et al. 1996), failed to complement all of these deficiencies. lgl4, but not mutations in five other genes uncovered by these deficiencies, enhanced dlghf/dlgip20 invasion with similar penetrance and expressivity compared to deficiencies (Figures 2K and 3, A and B). To confirm and characterize enhancement in more detail, we tested both the deficiencies and the lgl4 mutation in dlghf/dlgsw egg chambers, which typically appear like wild type (Figure 2A). dlghf/dlgsw; Df(2L)PM44/+, dlghf/dlgsw; Df(2L)PMG/+, and dlghf/dlgsw; Df(2L)PM45/+ animals produce tumors in 100, 91, and 72% of ovarioles, respectively, comparable to the 80% observed in dlghf/dlgsw; lgl4/+ animals (Figure 2J). Further, all three deficiencies and lgl4 dramatically enhanced dlghf/dlglv55 invasive tumorigenesis, causing tumors that appear to engulf the entire egg chamber, the strongest phenotype observed in the screen (Figure 2L).
To further test lgl, we assayed the temperature-sensitive mutant lglts3, and the hypomorphic mutant lglKG05323 (Manfruelli et al. 1996). Importantly, lglts3 produced tumors in 45% of dlghf/dlgsw ovarioles at the restrictive temperature, 29°, but no tumors were found at 25°, the permissive temperature. The temperature conditional enhancement of dlg invasion provides strong confirming evidence that lgl is the responsible dlg enhancer at this locus. Further, lglKG05323 enhanced invasion in all three dlg backgrounds. The penetrance in dlghf/dlgsw egg chambers was less than the null mutant lgl4 (16% of ovarioles compared to 80%), as expected for a partial loss-of-function mutation.
We conclude that enhancement of dlg invasion by several lgl alleles, and the similar enhancing scores of lgl4 compared to deficiencies of the locus, in multiple dlg genetic backgrounds, indicates that lgl is the gene primarily responsible for enhancement at this locus. Consistent with this, Lgl colocalizes with Dlg at the BLJ, and lgl has been placed in a dlg functional network on the basis of similarity of phenotype in several tissues, a common requirement for suppressing tumorigenesis, and genetic interactions with dlg (Bilder et al. 2000; Albertson and Doe 2003; Szafranski and Goode 2007). lgl was the strongest dlg enhancer that we observed, suggesting that it is involved in all three dlg functions of repressing proliferation, EMT, and motility (Figure 1A), borne out in subsequent analysis (see below). Identification of lgl as an invasion enhancer provided strong evidence for the utility of the screen and indicated that additional components of a dlg functional network could be identified using this approach.
wts:
This region was identified in the primary screen by Df(3R)tll-g/+, which caused 100% of ovarioles to have egg chambers with invasive tumors. Two small overlapping deficiencies, Df(3R)Exel8194 and Df(3R)Exel9020, confirmed and refined the position of this putative enhancer locus to 100A4–100A5 (Parks et al. 2004). These two deficiencies caused 37 and 30% of dlghf/dlgsw ovarioles to have tumors, respectively. Df(3R)Exel9020 removes 38.5 kb (Parks et al. 2004). The only genes deleted by this deficiency are zfh1, which encodes a zinc-finger homeodomain protein involved in mesodermal patterning (Postigo et al. 1999), and warts (wts), a tumor suppressor gene (Justice et al. 1995). Two wts mutations, but not zfh1, enhanced invasive tumorigenesis in all three dlg backgrounds (Figure 2, G–I, M, and N). These two mutations failed to complement all three deficiencies defining this region, demonstrating that wts was the dlg enhancer at this locus. The null mutant wtsx1, and the partial loss-of-function allele wtsP4, caused 48 and 35% of dlghf/dlgsw ovarioles to have tumors, comparable to deficiencies (Figure 3A). The strength of the wts interaction with dlghf/dlgsw was about half that of lgl, which was further verified in the dlghf/dlgip20 and dlghf/dlglv55 backgrounds (Figure 3B and data not shown). The weaker interaction of wts with dlg when compared to lgl indicates that wts is required either in all three dlg pathways, but to a lesser degree than lgl, or in only one or two dlg pathways essential for repressing invasive tumorigenesis (Figure 1A). The wts locus encodes a kinase that is a potent tumor suppressor in imaginal tissues (Justice et al. 1995), suggesting that it may relay signals from the BLJ. Below we present experiments to test this hypothesis, and to determine in which dlg pathways wts is involved in.
scrib:
This region was identified by Df(3R)Tl-1, which caused 100% of dlghf/dlgip20 ovarioles to have egg chambers with invasive tumors. No overlapping deficiencies were available. Df(3R)Tl-1 caused 32% of dlghf/dlgsw ovarioles to have invasive tumors and strongly enhanced tumor invasion in dlghf/dlglv55 egg chambers, with penetrance and phenotypic patterns most similar to wts (Figure 3, A and B). To identify the enhancer gene, six lethal mutations in five genes were analyzed. scrib673 and scrib1 (Bilder et al. 2000), but not mutations in the other genes, enhanced invasive tumorigenesis in all three dlg backgrounds (Figures 2, G–I and 3, A and B). scrib673 and scrib1 caused invasion in 27 and 30% in dlghf/dlgsw ovarioles, much like Df(3R)Tl-1 (Figure 3A), and both mutations failed to complement Df(3R)Tl-1. The similar invasion score of Df(3R)Tl-1 and scrib mutations, in all three dlg backgrounds, indicates that scrib is the dlg enhancer gene at this locus. Consistent with this, scrib alone has ovarian phenotypes that resemble dlg (Bilder et al. 2000). Further, dlg, lgl, and scrib genetically interact, indicating that they are components of functional network (Bilder et al. 2000; Peng et al. 2000; Albertson and Doe 2003). However, our analysis indicates that scrib is not functionally equivalent to lgl in regards to dlg function, since the tumor scores for scrib were roughly a third to a half of those observed for lgl (Figure 3, A and B). Thus, as for wts, the weaker interaction of scrib with dlg when compared to lgl indicates that scrib is required either for all three dlg pathways, but to a lesser degree than lgl, or in only one or two dlg pathways essential for repressing invasive tumorigenesis (Figure 1A). Scrib is a scaffolding protein that has four PDZ domains and 16 leucine-rich repeats and colocalizes with Dlg and Lgl in BLJs (Bilder and Perrimon 2000). Thus, along with lgl, identification of scrib provided proof of principle that the screen was successfully identifying crucial components of a BLJ network.
roe:
Three overlapping deficiencies, Df(3R)dsx2M, Df(3R)dsx29, and Df(3R)dsx37, caused 100% of dlghf/dlgip20 ovarioles to have egg chambers with invasive tumors. These deficiencies are predicted to remove sequences in region 84D8–84E1. Df(3R)dsx2M and Df(3R)dsx37 caused 3% of dlghf/dlgsw ovarioles to have tumors and strongly enhanced dlghf/dlglv55 invasion. To identify the enhancer gene, five mutations in four genes were analyzed. roe1 and roe2 (St Pierre et al. 2002), but not mutations in the other genes, enhanced invasion in all three dlg backgrounds (Figures 2, J–L and 3, A and B), and failed to complement all three deficiencies, indicating that roe is the gene responsible for enhancement at this locus. roe1 and roe2 caused 15 and 9% of dlghf/dlgsw ovarioles to have tumors, three to five times greater than Df(3R)dsx2M and Df(3R)dsx37. roe1 and roe2 may cause greater enhancement compared to the deficiencies if another gene resides within the deficiencies that acts as a dlg suppressor; alternatively, there may be a difference in genetic backgrounds. By several criteria, roe was a mild enhancer compared to lgl, scrib, and wts (Figure 3A and see below), but by other assays appeared similar to scrib and wts (Figure 3B and see below). Further analysis indicates that like wts and scrib, roe does not act in all branches of BLJ signaling (see below) (Figure 1A). roe encodes a Krüppel family of zinc-finger transcription factors (St Pierre et al. 2002), which combined with its functional overlap with wts and scrib in controlling EMT and proliferation, suggests that it might be a target for BLJ signals in the nucleus. However, the strength of the interaction was not as strong as for wts and scrib, indicating that it may be only one of several targets.
ebi:
One deficiency, Df(2L)al, caused 100% of dlghf/dlgip20 ovarioles to have egg chambers with invasive tumors. No overlapping deficiencies were available to confirm or refine this locus. However, Df(2L)al caused 8% of dlghf/dlgsw ovarioles to have tumors, and significantly enhanced dlghf/dlglv55 invasion, with penetrance and phenotypic patterns that resemble those seen at other mildly enhancing loci (Figures 2, D–F, and 3, A and B). Df(2L)al is predicted to remove sequences from 21B8–21D1. We examined 15 lethal mutations in 14 genes in this region. Two ebi mutations, ebik16213 and ebiWKS.24 (Tsuda et al. 2002), failed to complement Df(2L)al and both enhanced invasion in all three dlg backgrounds (Figures 2, D–F, and 3, A and B). The comparable enhancement of ebi and Df(2L)al, in multiple dlg genetic backgrounds, indicates that ebi is the gene primarily responsible for enhancement at this locus. ebi encodes for a nuclear ubiquitin ligase implicated in Notch signaling (Tsuda et al. 2002). Thus, like Roe, Ebi may be another target for BLJ signals in the nucleus. The strength of the ebi interaction with dlg was comparable to roe, but we did not observe any ebi ovarian phenotypes (see below), suggesting that ebi may only participate in one of three BLJ pathways.
Two additional dlg enhancer loci:
Df(2L)ed1 removes sequences between 24A2 and 24D4 and caused 100% of dlghf/dlgip20 ovarioles to have egg chambers with invasive tumors (Figure 1D). No overlapping deficiencies were available to confirm or refine this locus. Df(2L)ed1 did not cause invasion in dlghf/dlgsw egg chambers, but significantly enhanced invasion in the dlghf/dlglv55 background. To identify the enhancer gene at the 24A2; 24D4 locus, mutations in cappuccino, a gene required for cell polarity, were assayed (Manseau and Schüpbach 1989). No enhancement of the dlg phenotype was detected.
The other enhancing locus was located between 90D2 and 90D6 (Figure 1D), defined by overlapping deficiencies Df(3R)RD31 and Df(3R)DG4. Df(3R)RD31 caused 2% of dlghf/dlgsw ovarioles to have egg chambers with invasive tumors, but Df(3R)DG4 showed no effect. Both deficiencies caused 100% of dlghf/dlgip20 and dlghf/dlglv55 ovarioles to have tumors, with similar phenotypes to those observed for other loci. One lethal mutation was examined in the 90D2–90D6 region, but no enhancement was observed. Thus, we were unable to identify the enhancing gene at this or the Df(2L)ed1 locus, both of which were the weakest enhancers observed in the screen. For these reasons we did not analyze these loci further.
Fas2 tumorigenesis is also enhanced by lgl, scrib, wts, roe, and ebi:
Fas2 is a transmembrane protein that precisely colocalizes with Dlg in the BLJ via binding of its intracellular domain to Dlg PDZ1+2 (Szafranski and Goode 2007). Our functional analysis suggests a model in which Fas2 intercellular interactions mediate contact inhibition of proliferation and movement by regulating Dlg protein conformations in the cellular cortex, which may in turn determine which factors associate with the BLJ and thus become active. To obtain additional evidence that lgl, scrib, wts, roe, and ebi function in a BLJ pathway, we tested if they also enhance Fas2 phenotypes. For these experiments we used Fas2null, which causes invasive tumors (Figure 4A). Fas2null completely eliminates Fas2, so it might be expected that lgl, scrib, wts, roe, and ebi would not enhance Fas2null if they function in the same pathway as Fas2. However, because Dlg has multiple binding domains, putatively binding many proteins, it seemed more likely that Fas2 regulates only a fraction of Dlg activity, and thus Fas2 null mutations are functionally equivalent to a dlg hypomorph. To test this, we compared the average tumor size for Fas2null to the weak- to intermediate-strength mutant combination dlghf/dlgip20 (see materials and methods). Fas2null scored 7 (Figure 4C) and dlghf/dlgip20 scored 50 (Figure 3B). The data confirm that Fas2 can only account for a relatively small portion of Dlg tumor suppressing function. Thus, Fas2null tumorigenesis is a reasonable assay to measure enhancement by lgl, wts, scrib, roe, or ebi.
When one copy of lgl, scrib, wts, roe, and ebi is removed in Fas2null egg chambers, the resulting tumors are significantly larger compared to Fas2null alone (Figure 4B, C). lgl caused an ∼10-fold increase in Fas2null tumor size, wts a 7- to 8-fold increase, ebi and roe a 6- to 7-fold increase, and scrib a modest 2-fold increase (Figure 4C). We were surprised that scrib enhanced Fas2 tumors 3- to 5-fold less than lgl, wts, roe, or ebi, so we repeated the experiment several times with scrib673, as well as with an additional allele, scrib1, with the same result. Another surprising finding was that whereas Fas2null alone often caused invasive tumors (Figure 4A), removal of one copy of lgl, wts, scrib, roe, or ebi caused all tumors to become predominantly noninvasive (Figure 4B). We consider possible insights into BLJ function that these two surprising findings suggest in the discussion.
We conclude that lgl was the strongest Fas2 enhancer, and with the exception of scrib, the remaining enhancers showed a similar, but less severe increase in tumor size compared to lgl, much like the trend of enhancement observed for dlg in dlghf/dlgip20 egg chambers (Figure 3B). The data thus further indicate that lgl, scrib, wts, roe, and ebi are critical for mediating BLJ suppression of invasive tumorigenesis.
lgl invasive tumorigenesis is unmasked in interactions with Fas2, dlg, scrib, wts, and roe:
Lgl is another protein that is localized to the BLJ and that suppresses epithelial tumorigenesis (Szafranski and Goode 2007). To obtain further evidence that Fas2, dlg, scrib, wts, and roe function in a BLJ network, we tested if they also genetically enhance lgl. Approximately 10% of lgl tumors are invasive, while the remaining 90% have a noninvasive phenotype in which follicle cells pile up at the poles of the egg chamber (Figure 5), resembling dlg hypomorphic mutant combinations (Figure 6). The relatively low frequency of invasive tumors in lgl mutants suggests that the branch of the BLJ pathway that represses cellular motility (Figure 1A) is not as strongly derepressed as in dlg mutants. This is puzzling because lgl loss causes faster border cell migration, much like Fas2 and dlg loss (Szafranski and Goode 2004), thus suggesting the conflicting view that the branch of the BLJ pathway that represses motility has been disrupted in lgl mutants. An important observation that might help to rectify this discrepancy is that for Fas2 and dlg, loss of the motility-repressing branch of the BLJ pathway alone is not sufficient for tumor invasion, but must be lost together with EMT for invasion to occur (Figure 1A) (Szafranski and Goode 2007). Thus, lgl tumors may have a latent invasive capacity, but fail to express it more fully because some aspect of EMT crucial for movement remains repressed.
Figure 5.—
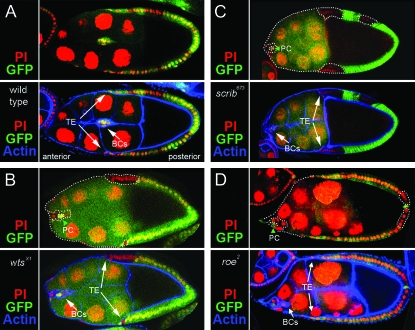
Fas2, dlg, scrib, wts, and roe enhance lgl. (A) At 25° all lgl4/lglts3 egg chambers appear wild type. (B) At 29° all lgl4/lglts3 ovarioles have one or more egg chambers with tumors. Approximately 90% of the tumors accumulate at the poles (arrows). Approximately 8% of the tumors are invasive (I). (C) dlgm52/+; lgl4/lglts3 egg chambers develop tumors at the 25° permissive temperature. Approximately 60% of these tumors are invasive (arrow, I). (D) At 29° most dlgm52/+; lgl4/lglts3 egg chambers have noninvasive tumors that are larger than the tumors in lgl4/lglts3 (arrows, compare to B). However, invasive tumors are observed at a low frequency, as for lgl4/lglts3. (E) Fas2/+; lgl4/lglts3 egg chambers develop tumors at 25°. One hundred percent of these tumors are invasive (I) and some break away from the epithelium and migrate much like a border cell cluster (arrow). (F) Fas2/+; lgl4/lglts3 egg chambers at 29° causes noninvasive tumors that are typically larger than the tumors in lgl4/lglts3 at 29° alone (arrows, compare to B). However, invasive tumors are observed at a low frequency, as in lgl4/lglts3 egg chambers. (G) lgl4/lglts3; wtsX1/+ egg chambers develop invasive tumors at 25°. Approximately 40% of tumors are invasive (arrow). (H) lgl4/lglts3; wtsX1/+ egg chambers at 29° causes noninvasive tumors that are larger than the tumors in lgl4/lglts3 at 29° alone (arrows). However, invasive tumors are observed at a low frequency, as in lgl4/lglts3 egg chambers. scrib and roe showed a similar pattern of interactions (I).
Figure 6.—
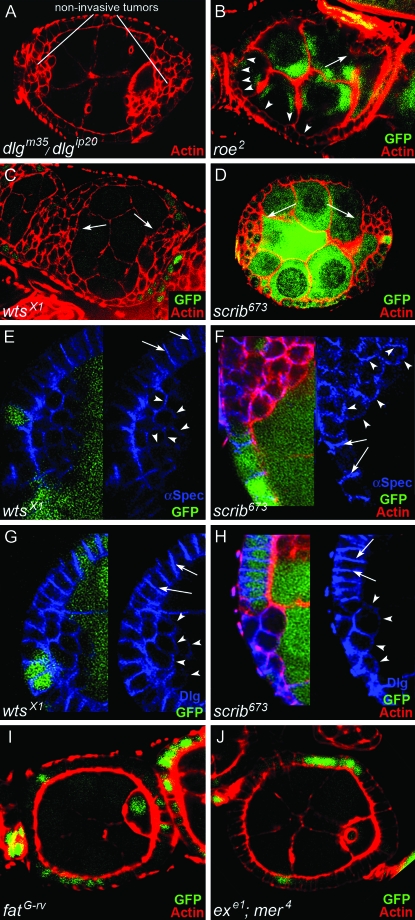
Clonal analysis of wts, scrib, roe, ft, and mer; ex. Egg chambers are stained with phalloidin to reveal actin in cellular cortexes (red). (A) Tumor cells accumulate in stratified layers at the anterior and posterior poles of dlgm35/dlgip20 egg chambers, but they do not invade. The tumor cells delaminate toward the germ line and have a rounded, mesenchymal-like morphology. (B–J) Clonal analysis of roe, wts, scrib, ft, and mer; ex. Cells without lacZ or GFP (green) are homozygous mutant for the indicated mutations. (B) roe2 causes small, noninvasive tumors that delaminate toward the germ line (arrow). Most tumors accumulate around the oocyte, but occasionally at the anterior of the egg chamber as well. More typically, anterior roe2 cells remain in the epithelium but have a rounded morphology (arrowheads), like tumor cells that have completely delaminated. (C and D) wtsX1 and scrib673 tumors are similar to dlgm35/dlgip20 tumors. (E–H) Analysis of BLJ proteins α-Spectrin and Dlg in wts and scrib follicle cells. α-Spectrin and Dlg are localized to the BLJ in the native epithelium (arrows). α-Spectrin and Dlg accumulate around the circumference of wts and scrib tumor cells (arrowheads). This lateralized phenotype is indicative of EMT (see text). (I and J) Clones of ft or mer; ex double mutant cells do not develop tumors.
To test this, we asked if removal of one copy of dlg would cause lgl tumors to become invasive. We used animals heterozygous for the temperature-sensitive mutant lglts3 and the null mutation lgl4 (Manfruelli et al. 1996). At the 25° permissive temperature, lgl4/lglts3 egg chambers were morphologically indistinguishable from wild type. At the 29° restrictive temperature, mostly noninvasive tumors accumulated at the poles of lgl4/lglts3 egg chambers (Figure 5, A and B). The phenotype was similar to the null mutant lgl4 (not shown). When one copy of dlg was removed in lgl4/lglts3 egg chambers (dlgm52/+; lgl4/lglts3) at the 25° permissive temperature, invasive tumors frequently developed (Figure 5, C and I). No tumors were observed in the control FM7/+; lgl4/lglts3 egg chambers from their sisters. When we completed the same experiment at the lgl restrictive temperature (29°), we found the tumors were larger than lgl4/lglts3 tumors, but surprisingly were mostly noninvasive, much as for lgl4/lglts3 alone (Figure 5I).
The data thus confirms that lgl cells have a latent invasive capacity. However, support for the hypothesis that lgl is continuing to repress a function crucial for EMT, thus preventing lgl cells from becoming invasive, is less clear. If the latter were true, we would expect to observe invasive tumors at both the lgl permissive and restrictive temperatures. Instead, dlg enhances lgl4/lglts3 tumor size at the restrictive temperature, without enhancement of invasion. One possibility is that lgl loss alone may cause a critical imbalance between the three BLJ pathways that repress proliferation, EMT, and motility (Figure 1A), which favors proliferation and EMT, while at the same time precluding the choice to migrate. In contrast, at the lgl permissive temperature, all three pathways are close to a threshold level, so that removal of one copy of dlg allows all three BLJ pathways to operate together.
To further test if lgl cells have a latent invasive capacity, we asked if Fas2 and scrib would also enhance lgl4/lglts3. As for dlgm52, removal of one copy of Fas2 or scrib caused de novo invasive tumors at the lgl4/lglts3 permissive temperature, but noninvasive tumors predominated at the restrictive temperature (Figure 5, E and F), further indicating that strong loss of lgl somehow precludes expression of a latent invasive capacity in lgl cells. The number of tumors observed in Fas2null/+; lgl4/lglts3 egg chambers was ∼10% of that observed in dlgm52/+; lgl4/lglts3 egg chambers, as expected because Fas2null behaves like a dlg hypomorph (see previous section). However, in contrast to dlg and scrib, 100% of Fas2null/+; lgl4/lglts3 tumors were invasive, with some breaking away from the epithelium and migrating much like a border cell cluster (Figure 5, E, F, and I). The cluster phenotype is especially interesting because Fas2 is the only epithelial junction protein that we have found to be lost in the anterior epithelium at the time of border cell migration, a morphogenetic switch that is essential for permitting border cell movement (Szafranski and Goode 2007). Thus Fas2 homophilic adhesion may be especially important for promoting follicle cell attachments that inhibit cluster delamination.
Since removal of one copy of Fas2, dlg, or scrib causes de novo invasive tumor formation in lgl4/lglts3 animals at the permissive temperature, we reasoned that this would be an excellent assay to test if wts and roe are truly components of a BLJ network. We were particularly interested in wts, because by several assays it enhanced Fas2 and dlg as strong, or stronger than, scrib (Figures 3B and 4C). Further, wts encodes a kinase known to be a potent tumor suppressor in other tissues (Justice et al. 1995). Thus one possibility was that Wts relays signals from the BLJ to Roe in the nucleus that are important for suppressing epithelial invasion. If this was true, then removal of one copy of wts or roe would be expected to cause de novo tumor formation in lgl4/lglts3 egg chambers at the permissive temperature. The data supported this hypothesis. Removal of one copy of wts or roe caused invasive tumors in lgl4/lglts3 animals at the permissive temperature (Figure 5E), and gave similar results to removal of one copy of Fas2, dlg, or scrib at the 29° restrictive temperature (Figure 5F).
The de novo generation of invasive lgl tumors by removal of one copy of scrib, wts, and roe are especially meaningful because analysis described below indicates that scrib, wts, and roe are required to repress EMT and proliferation but not motility. The data thus support the model whereby lgl cells have a latent invasive capacity that is masked by a crucial EMT function that continues to be expressed in lgl cells, but is unmasked when scrib, wts, or roe are reduced. Further, enhancement of Fas2, dlg, and lgl tumorigenesis by wts/+ and roe/+, as well as de novo formation of invasive tumors in dlghf/dlgsw; wts/+, lglts3/lgl4; wts/+, dlghf/dlgsw; roe/+, and lglts3/lgl4; roe/+ egg chambers, is consistent with the hypothesis that Wts acts to relay signals from the BLJ to Roe in the nucleus that are important for suppressing invasive tumorigenesis.
Fas2 and dlg are suppressed by overexpression of lgl, scrib, wts, and roe:
If the lgl, scrib, wts, roe, and ebi function in a BLJ pathway, then we might expect that their overexpression would have the opposite impact on Fas2 and dlg tumorigenesis as removal of one copy. To test this, we used the UAS-Gal4 system (Brand and Perrimon 1993) to overexpress lgl, scrib, wts, roe, and ebi in Fasnull and in an intermediate dlg tumor background, dlghf/dlglv55 (Figures 2C and 4A). We used GR1 Gal4 to drive UAS enhancer gene expression in all follicle cells (Szafranski and Goode 2007). We found that overexpression of all of the dlg enhancer genes except ebi suppressed Fasnull and dlghf/dlglv55 tumorigenesis (Figures 3C and 4D). UAS lgl and UAS wts were the strongest suppressors, suppressing at a level comparable to rescue by UAS dlg (Figure 3C). The results further support that lgl, scrib, wts, and roe function in a BLJ network with Fas2 and dlg to suppress tumorigenesis. ebi may function in a parallel pathway, or perhaps in only one of the three branches of the BLJ pathway (Figure 1A).
Clonal analysis:
Previous work has shown that dlg, lgl, and scrib have similar ovarian tumor phenotypes, which combined with genetic interaction data, suggested that they functionally cooperate to control polarity and proliferation (Bilder et al. 2000; Peng et al. 2000; Albertson and Doe 2003; Szafranski and Goode 2007). However, as described in the above analysis, lgl interacts with dlg stronger than scrib, suggesting that scrib either is not required in all three dlg pathways (Figure 1A) or is required to a lesser degree in all three pathways compared to lgl. Similar reasoning applies to wts, roe, and ebi. To help distinguish these alternatives, we used clonal analysis to compare the cell and tissue phenotypes of Fas2, dlg, and lgl to scrib, wts, roe, and ebi. We found that wts and scrib egg chambers had a very similar phenotype to dlg mutants that specifically disrupt the Dlg SH3 and GuK domains. Supernumerary follicle cells accumulate in stratified layers at the poles of the egg chamber, starting as early as stage 2 of oogenesis (Figure 6, A–D). Like dlg and scrib cells, wts cells often delaminate toward the germ line and have gross aberrations in epithelial polarity (Figure 6, A–D). The genetic interactions (previous sections), and similarities between the dlg and scrib phenotypes when compared to wts, indicate that these genes are functionally interdependent. However, complete loss of Fas2 or dlg not only causes overproliferation and EMT, but also invasion of follicle cells between germ cells (Figures 2 and 4), a phenotype never observed in wts or scrib clones. Thus scrib and wts appear to be crucial for the proliferation and EMT branches of the BLJ pathway, but not for repressing motility.
A similar but less severe tumor phenotype was observed in roe egg chambers. Small roe tumors accumulate predominantly at the posterior of the egg chamber, whereas in the anterior epithelium follicle cells become rounded, but do not delaminate from the epithelium (Figure 6B). We observed no morphological defects in ebi egg chambers (not shown). The clonal analysis data are thus consistent with the genetic interactions. lgl was the strongest enhancer and also caused the strongest tumor phenotype (Figure 5B and data not shown). scrib and wts were intermediate enhancers and had less severe phenotypes compared to complete loss of dlg and lgl (Figure 6, C and D). roe and ebi were mild enhancers and caused the weakest and no follicle phenotypes, respectively (Figure 6B and data not shown). These quantitative differences are most simply explained by the involvement of lgl in all three dlg pathways controlling epithelial invasion, whereas scrib, wts, and roe are only required in two branches, those that repress EMT and proliferation.
We have previously shown that both the EMT and motility branches of the BLJ pathway must be compromised for Fas2 and dlg mutant cells to invade (Figure 1A, see Introduction) (Szafranski and Goode 2007). Thus, since neither scrib nor wts appears to be required for repressing motility (confirmed for border cell analysis, see below), the data suggest that the mechanism by which scrib and wts enhance dlg invasive tumorigenesis may be by increasing EMT. To further test if scrib and wts act in a BLJ pathway crucial for repressing EMT, we asked if their tumor cells have not only lost epithelial polarity, but have also become lateralized, a process characteristic of EMT (Szafranski and Goode 2007). Lateralization is associated with a distribution of BLJ proteins around the circumference of the tumor cells, a step that not only precedes tumor invasion, but also appears to be essential for BC movement (Szafranski and Goode 2007). We thus analyzed localization of BLJ proteins Dlg and α-Spectrin in scrib and wts cells. In scrib and wts follicle cells that have not become tumorous, Dlg and α-Spectrin are localized to the BLJ, as in wild-type follicle cells. In contrast, in tumorous scrib and wts follicle cells, Dlg and α-Spectrin were localized around the circumference of the cell (Figure 6, E–H), as in dlg and Fas2 tumor cells (Szafranski and Goode 2007). Thus, scrib and wts follicle cells appear to undergo an EMT lateralization process, similar to Fas2 and dlg tumor cells.
In summary, the wts, scrib, and roe phenotypes are very similar to the noninvasive phenotype associated with dlg mutations that specifically disrupt the Dlg SH3 or GuK domains (Figure 6A). In these dlg mutants, repression of motility remains largely intact (Szafranski and Goode 2007). Thus, the specificity of the noninvasive wts, scrib, and roe phenotypes suggests that they act in a genetically separable dlg pathway that represses EMT and proliferation, but not motility (see Figure 1A and Introduction). Further support for this hypothesis is presented below.
Ft, ex, and mer; ex follicle cells do not have early wts phenotypes:
Several recent reports that use Drosophila wing and eye tissue as an experimental paradigm have provided strong evidence that the apicolateral junction provides upstream input that regulates Wts signaling. In particular, three apicolateral proteins that function as tumor suppressors appear to play an important role in Wts signaling. One is Fat (Ft), a transmembrane molecule of the Cadherin family (Hariharan 2006; Willecke et al. 2006). The other two are cytoplasmic adapter proteins, Expanded (Ex) and Merlin (Mer) (Edgar 2006; Hamaratoglu et al. 2006). The prevailing model is that activation of Ft at the cell surface recruits Ex, which together with Mer, activates Wts signaling (Edgar 2006). Nonetheless, the genetic evidence indicates that Ft-Ex-Mer cannot account for all of the upstream inputs to Wts (Hamaratoglu et al. 2006). For example, ft and ex overgrowth phenotypes are not as robust as for wts, and unlike wts, ft and ex are not required to promote developmentally regulated apoptosis (Harvey and Tapon 2007). We thus considered the possibility, on the basis of several pieces of our data, that the BLJ may be an important upstream activator of Wts signaling in follicle cells: (1) wts was one of only a handful of genes isolated in our screen that enhanced dlg tumorigenesis, (2) wts interacted with dlg and lgl as strong, or stronger than scrib, which encodes for a known component of the BLJ, and (3) clonal analysis demonstrated that wts has a similar tumor phenotype to dlg, lgl, and scrib.
To determine the relative importance of the apicolateral junction vs. the BLJ to Wts signaling in follicle cells, we asked if ft, ex, or mer had wts-like follicle cell phenotypes. We analyzed egg chambers using the null mutants ftG-rv (Willecke et al. 2006), mer4, exe1, and the double null mer4; exe1 (Hamaratoglu et al. 2006), and compared their phenotype to wtsx1. ft egg chambers were indistinguishable from wild type throughout oogenesis (Figure 6I and data not shown), while ex, mer, and mer; ex egg chambers appear wild type through stage 7 of oogenesis (Figure 6J and see below for analysis of midoogenesis phenotypes). The data thus appear to exclude, or indicate a minor role for Ft, ex, and mer in wts processes during early oogenesis, when wts is required to repress proliferation and EMT (Figure 6). The data outlined in the previous section thus strongly suggest that the BLJ provides the upstream input to Wts during early oogenesis, a hypothesis we test more extensively below.
Comparison of Fat, ex, mer, and Fas2, dlg, lgl, scrib, wts, roe, and ebi midoogenesis defects:
Stage 7 marks the beginning of midoogenesis, when follicle cells stop proliferating and start to differentiate. As the follicle cells differentiate from stages 7 to 10, they enter an endoreplication cycle that increases their ploidy from 2N to 8N or greater, evident as an increase in follicle cell nucleus size (Spradling 1993). Notch (N) is required for the proliferation to endoreplication switch (Shcherbata et al. 2004). In N mutants, supernumerary follicle cells accumulate that have a conspicuously small nucleus during stages 7–10, indicating that they have failed to differentiate and enter the endoreplication cycle.
Two recent reports show that ex and wts have a small nucleus phenotype similar to the N phenotype (supplemental Figure 1, A and B) (Meignin et al. 2007; Polesello and Tapon 2007). The phenotype is restricted predominantly to posterior cells, but is also sometimes observed in anterior cells, even though all ex or wts follicle cells are mutant (supplemental Figure 1, A and B). We found that neither Ft nor mer expressed the small nucleus phenotype and found that wts expressed the small nucleus phenotype with higher penetrance and expressivity compared to ex (supplemental Figure 1, A and B) (Meignin et al. 2007; Polesello and Tapon 2007). A similar difference in penetrance and expressivity between wts and ex is observed for tumor growth in eye, wing, and other imaginal disc-derived tissues (Hamaratoglu et al. 2006; Harvey and Tapon 2007). The difference in imaginal tissues appears to result from functional redundancy between Ex and Mer, both membrane protein 4.1 family members. Whereas mer or ex alone cause relatively mild overgrowth phenotypes in imaginal tissue, mer; ex double mutant overgrowth is much more dramatic and is quantitatively similar to wts (Hamaratoglu et al. 2006; Harvey and Tapon 2007). To test if Mer-Ex functional redundancy may also be important in follicle cells, we analyzed mer; ex double mutant clones. The small nucleus phenotype was observed in ∼90% of ex clones (n = 16), but only 30% of mer; ex clones (n = 14), suggesting that Mer may have an opposing role to Ex in regulating this aspect of follicle cell differentiation. Although further work will be needed to test this hypothesis, we can conclude that for the endocycle switch in follicle cells, Wts appears to be regulated in a fundamentally different manner than in imaginal tissues, both in the lack of a requirement for Ft, and in the differing roles of Ex and Mer.
The ex small nucleus phenotype is not as penetrant as the wts phenotype, suggesting that additional regulatory inputs must be needed for Wts activation during midoogenesis. To test if the BLJ may also regulate Wts at this time, we asked if Fas2, dlg, lgl, scrib, roe, and ebi also have a small nucleus phenotype. We observed this phenotype for all of these mutants (supplemental Figure 1, C–G; data for ebi not shown), consistent with the notion that the BLJ is also important for regulating Wts during midoogenesis. The phenotype was highly penetrant for all mutants except Fas2, indicating that although Fas2 appears to be involved, it may not be the predominant BLJ ligand that regulates the endocycle switch.
To further analyze the putative roles of the apicolateral vs. BLJ in Wts regulation at midoogenesis, we examined an additional wts phenotype. The wts small nucleus phenotype is typically observed in posterior populations of follicle cells that accumulate in two or more disorganized layers, in contrast to the ordered monolayer of follicle cells characteristic of wild type (supplemental Figure 1B) (Meignin et al. 2007; Polesello and Tapon 2007). The same disorganized phenotype is observed in ex, and to a much lesser degree in mer follicle cells (MacDougall et al. 2001; Meignin et al. 2007; Polesello and Tapon 2007), but in both mutants the phenotype is typically much less expressive compared to wts (supplemental Figure 1A and data not shown). Further, as for the small nucleus phenotype, there is no dramatic increase in expressivity of the disorganized epithelium phenotype in mer; ex double mutants, further indicating an absence of Mer-Ex synergism in follicle cells, and further indicating that they are not likely to collaborate in Wts activation by the same mechanism as in other tissues. In contrast, in Fas2, dlg, lgl, scrib, roe, and ebi mutants, the disorganized epithelium is observed with an expressivity equal to or greater than that seen for wts, consistent with the importance of the BLJ in regulating Wts during midoogenesis (supplemental Figure 1, B–G; data for ebi not shown). Thus, the data are consistent with the BLJ playing an important role in Wts regulation during both early and midoogenesis, but as follicle cells differentiate at midoogenesis, we suggest that Wts in addition may receive input from Ex to regulate the endocycle switch and possibly from both Mer and Ex to maintain epithelial integrity. To further test this model we compared the role of the BLJ and apicolateral junctions in positioning the germinal vesicle.
Mer and Ex, like Wts, are required in follicle cells at stages 7–8 of oogenesis for sending a signal to the oocyte that permits migration of the germinal vesicle from the posterior to the presumptive anterior–dorsal corner of the oocyte (MacDougall et al. 2001; Meignin et al. 2007; Polesello and Tapon 2007). In mer, ex, and wts mutants the germinal vesicle fails to migrate, causing it to be mislocalized at the posterior pole of the ooctye starting as early as stage 8. However, the phenotype is not observed in Ft egg chambers and is much more penetrant in wts compared to mer or ex (Meignin et al. 2007; Polesello and Tapon 2007) (this study). In addition, as for the small nucleus and epithelial integrity defects, we see no dramatic enhancement in the germinal vesicle defects in mer; ex double mutants, further indicating that Fat, Mer, and Ex do not have the same collaborative role in Wts activation in follicle cells as they do in other tissues. Since the germinal vesicle defect is observed in ∼40% of mer; ex double mutants, but in >80% of wts egg chambers (supplemental Figure 1), there must be additional inputs to Wts in controlling this process. We thus analyzed the germinal vesicle defect in BLJ mutants. We observed this phenotype in Fas2, dlg, scrib, but only rarely in lgl, and not in roe or ebi egg chambers (supplemental Figure 1, H and I). The data are thus consistent with a role for the BLJ junction in regulating Wts signaling that is important for germinal vesicle localization, through a branch of the BLJ signaling in which Lgl and Roe play no role or a relatively minor role.
Our data suggest that during midoogenesis Ex and Mer cannot regulate Wts in the same manner as in other tissues and most importantly cannot account for all if any input to Wts. Further, Ex and Mer are not likely to be involved in Wts regulation during early oogenesis, because wts tumors are observed starting at stage 3, whereas Ex and Mer phenotypes are not observed until after stage 7. Rather, our data are most consistent with the BLJ playing the predominant role in Wts regulation throughout oogenesis, with Ex playing an accessory role in Wts regulation starting at stage 7. Additional experiments described below further test the relative importance of components of the BLJ vs. the apicolateral junction in regulating Wts signaling. Further, our data do not exclude the possibility that in follicle cells Ex may function independently of the apicolateral junction. For example, Ex is membrane protein 4.1 family member, and membrane protein 4.1 binds and colocalizes with hDlg in human cells (Lue et al. 1994). This suggests the possibility that Dlg may function independently of Ex during early oogenesis, then collaborate with Ex at midoogenesis to increase activation of Wts important for driving differentiation.
wts, scrib, roe, and mer, but not ft or ex, are required for border cell migration:
Fas2, dlg, and lgl all cause invasive tumors as well as faster border cell migration. The faster border cell migration phenotype is caused by derepression of a branch of the BLJ motility pathway that is also essential for tumor invasion (Szafranski and Goode 2007). In contrast, in dlg mutants that specifically disrupt the Dlg SH3 and/or GuK domains, such as dlgip20/dlgm35, noninvasive tumors predominate (Figure 6A), and border cells migrate only slightly faster than wild type (Szafranski and Goode 2007). Thus, the branch of the BLJ pathway that represses motility appears to be only slightly derepressed in dlgip20/dlgm35 cells.
The noninvasive phenotypes of scrib, wts, and most roe tumors are similar to dlgip20/dlgm35 (Figure 6), suggesting that they act predominantly within a branch of the BLJ pathway that is essential for repressing EMT and proliferation. To further test if scrib, wts, and roe are required in a branch of BLJ signaling essential for repressing motility, we analyzed their border cell migration phenotypes. In contrast to Fas2, dlg, and lgl, which cause faster migration, we found that scrib, wts, and roe border cells have dramatically delayed, or completely blocked movement (Figure 7). In combination with their noninvasive tumor phenotypes, the data further indicate that scrib, wts, and roe do not act in the motility-repressing branch of the BLJ pathway (Figure 1A), but rather act specifically in branches of the BLJ pathway that controls EMT and proliferation. Further, because both the EMT and motility branches of the BLJ pathway must be lost together for tumor invasion (Szafranski and Goode 2007), the simplest explanation for how scrib, wts, and roe enhance dlg invasive tumorigenesis is that they increase the rate at which follicle cells undergo EMT, not by increasing motility.
Figure 7.—
Analysis of wts, scrib, and roe border cell migration. (A) A stage-9 egg chamber from the wtsx1 clonal analysis experiment without a clone (GFP is expressed in all follicle cells). The follicle cell nuclei are marked with propidium iodide (red), and the actin cortexes with phalloidin (blue). The border cell cluster (arrow) migrates in sync with the trailing edge of the follicular epithelium (TE, arrows). (B) wtsx1 clone in a stage-9 egg chamber (follicle cells without GFP, white dotted outline). The border cell cluster (arrow) remains at the anterior of the egg chamber, while the TE has almost completed migration (arrows). The two nonclone cells marked with GFP at the center of the border cell cluster are the polar cells, which help to organize the cluster and are carried along by the border cells, but do not actively migrate (Szafranski and Goode 2004). (C and D) scrib673 and roe2 clones in stage-9 egg chambers. scrib673 border cell clusters (arrow) remain at the anterior of the egg chamber, while the TE has almost completed migration (arrows). roe2 has a similar, but less severe phenotype, in which the border cells typically start to migrate when the TE has moved about halfway to the oocyte.
To further test if ft, ex, and mer might be involved in wts functions in follicle cells, we analyzed their border cell migration phenotypes. ft and ex have no defects in border cell movement, but mer loss blocked migration. The data thus exclude a role for Ft and Ex but not Mer in Wts activation in border cells. If Mer plays a role in Wts activation in border cells, then it must be through a distinct mechanism from interaction with Fat and Ex. One possibility is that Mer interacts with components of the BLJ to activate Wts. For example, Mer may directly interact with Dlg since Mer is homologous to membrane protein 4.1, which binds and colocalizes with hDlg in human cells (Lue et al. 1994).
Fas2, dlg, lgl, scrib, and wts, but not ft, ex, or mer; ex, repress cellular proliferation:
To further test if wts and roe act with Fas2, dlg, scrib, and lgl to repress cellular proliferation, we completed a detailed analysis to determine if they have similar proliferation phenotypes. BrdU is incorporated into newly synthesized DNA and serves to mark cells that have recently entered, or are in S phase. Although follicle cells stop proliferating at stage 6, they continue in S phase via cycles of endoreplication (Shcherbata et al. 2004). Thus, to utilize BrdU as a specific marker for proliferation, we analyzed stage-5 to -6 egg chambers.
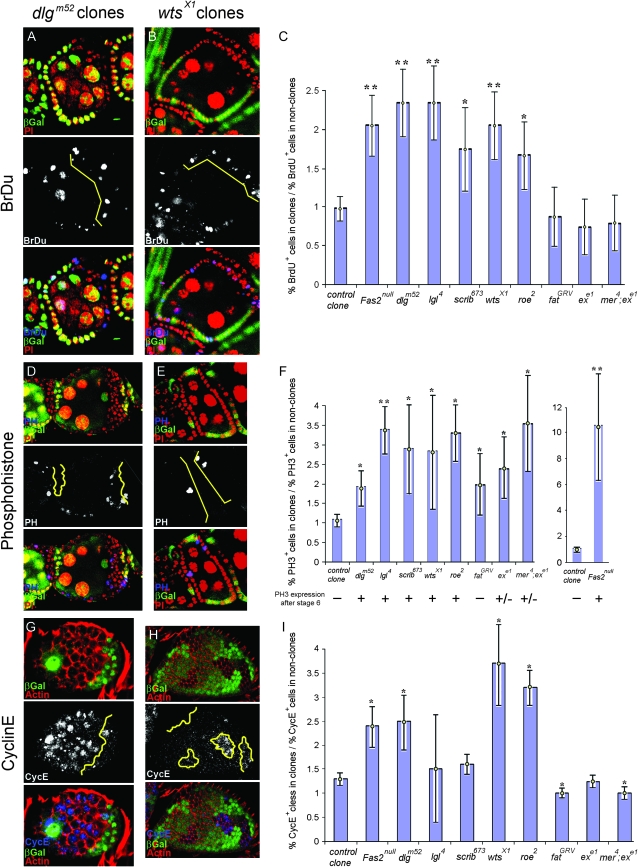
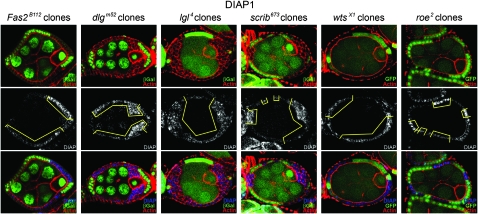
We made follicle cell clones for each mutant and compared BrdU incorporation in homozygous mutant cells to nonclone cells. As shown for dlg and wts, the typical pattern is greater BrdU incorporation in the homozygous mutant cells (Figure 8, A and B). To quantify the difference we compared the ratio of BrdU positive cells in homozygous mutant clones to nonclones (materials and methods). In control clones there was no significant change in BrdU incorporation (Figure 8C). We found a significant, roughly twofold increase in BrdU incorporation in Fas2, dlg, lgl, scrib, wts, and roe cells, compared to adjacent nonclone cells (Figure 8C). Ft, ex, and mer; ex cells showed a modest decrease in BrdU incorporation, further establishing that wts does not function downstream of Ft or mer; ex in early follicle cells. The data thus suggest an approximately twofold increase in the number of S-phase follicle cells for Fas2, dlg, lgl, scrib, wts, and roe cells. A similar result was obtained for dlg using an independent approach (Goode and Perrimon 1997).
Figure 8.—
Proliferation of Fas2, dlg, lgl, scrib, wts, roe, ft, and mer; ex epithelial cells. (A and B) BrdU incorporation in dlgm52 and wtsX1 clones. dlgm52 cells are marked by absence of β-galactosidase (β-gal) and wtsX1 cells by absence of GFP. PI stains all nuclei, while BrdU labels nuclei that are in or have entered S phase during the 1-hour incubation period. A greater number of BrdU+ cells are observed in dlgm52 and wtsX1 cells compared to nonclone cells. (C) Quantitative analysis of BrdU in Fas2, dlg, lgl, scrib, wts, roe, ft, ex, and mer; ex clones. The bar graph shows the percentage of BrdU+ cells in clones divided by the percentage of BrdU+ cells in nonclones. Control clones were generated with y w P[w+, FRT 101], the parent chromosome for Fas2null and dlgm52. Control clones have a ratio of ∼1, indicating no difference in the percentage of BrdU+ cells in GFP− clone vs. GFP+ nonclone cells. In contrast, the ratio of BrdU+ cells is ∼2-fold greater for Fas2, dlg, lgl, scrib, wts, and roe cells compared to nonclone cells (**P < 0.01; *P < 0.05). ft, ex, and mer; ex cells showed a slight, insignificant decrease in the number of BrdU+ cells. (D and E) PH3 in dlgm52 and wtsX1 clones. dlgm52 cells are marked by absence of β-gal and wtsX1 cells by absence of GFP. PI stains all nuclei, while PH3 labels nuclei that are in mitosis. A greater number of PH3+ cells are observed in dlgm52 and wtsX1 cells compared to nonclone cells. (F) Quantitative analysis of PH3 in Fas2, dlg, lgl, scrib, wts and roe, ft, ex, and mer; ex clones. The bar graph shows the percentage of PH3+ cells in clones divided by the percentage of PH3+ cells in nonclones. Control clones have a ratio of ∼1, indicating no difference in the percentage of PH3+ cells in GFP− clone vs. GFP+ nonclone cells. The ratio of PH3+ cells is ∼2- to 3-fold greater for dlg, lgl, scrib, wts, roe, ft, ex, and mer; ex cells compared to nonclone cells (**P < 0.01; *P < 0.05). Fas2 showed a 10-fold increase in PH3+ cells. Only Fas2, dlg, lgl, scrib, wts, and roe cells showed highly expressive and penetrant PH3 staining after s6, when follicle cells normally stop dividing. (G and H) CycE in dlgm52 and wtsX1 clones. dlgm52 cells are marked by absence of β-gal and wtsX1 cells by absence of GFP. PI stains all nuclei, while CycE labels nuclei that are in mitosis. A greater number of CycE+ cells are observed in dlgm52 and wtsX1 cells compared to nonclone cells. (I) Quantitative analysis of CycE in Fas2, dlg, lgl, scrib, wts, and roe, ft, ex, and mer; ex clones. The bar graph shows the percentage of CycE+ cells in clones divided by the percentage of CycE+ cells in nonclones. Control clones have a ratio of ∼1.2, indicating little difference in the percentage of CycE+ cells in GFP− clone vs. GFP+ nonclone cells. The ratio of CycE+ cells is ∼2- to 3-fold greater for Fas2, dlg, wts, and roe compared to nonclone cells, while lgl and scrib showed a trend toward increased CycE expression. ft, ex, and mer; ex cells showed a significant decrease in CycE expression (**P < 0.01; *P < 0.05).
As a second test for increased proliferation, we analyzed phospho-histone-3 (PH3), a marker for cells in mitosis. Phosphorylation of H3 is normally turned off after stage 6, when follicle cells stop dividing. In contrast, in stage 7–8 Fas2, dlg, lgl, scrib, wts, and roe egg chambers, PH3 presence persisted, indicating a loss of proliferation control (Figure 8F). Persistence in PH3 staining was never observed in ft, and is only occasionally seen in ex and mer; ex egg chambers (Figure 8F), further indicating that Ft is not important for Wts signaling at this time, and that Mer and Ex play a relatively minor role compared to members of the BLJ. In addition to the stage specificity of PH3 expression, we compared the penetrance of PH3 in clone vs. nonclone cells in early egg chambers. Like BrdU, the typical pattern was a higher ratio of PH3 positive cells in homozygous mutant clones compared to nonclones (Figure 8, D and E). A significant, 2- to 3 fold increase of H3 phosphorylation was observed in each mutant compared to nonclone cells, except for Fas2 (Figure 8F). For Fas2, we observe a 10-fold increase in PH3 expression (Figure 8F). In contrast, in control clones there was no significant difference in H3 phosphorylation between clone and nonclone cells (Figure 8C).
Although ft and mer; ex caused some early increase in PH3 phosphorylation, similar to the BLJ mutants (Figure 8C), phosphorylation of H3 after stage 6 is a more robust assay because it demonstrates loss of proliferation control in cells that otherwise do not proliferate. Thus, the persistence of PH3 after s6 in wts, but not in ft, and only occasionally in ex or mer; ex follicle cells, provides further evidence that Ft is functionally independent of wts in follicle cells, and that mer and ex play relatively minor roles, consistent with their relatively mild morphological defects (see above). Further, the similar increase in incorporation of BrdU and H3 phosphorylation in Fas2, dlg, lgl, scrib, and wts cells, along with the persistence of H3 phosphorylation past stage 6, indicates that these genes share a common function in repressing cellular proliferation. The only significant difference that we could detect was a threefold greater number of PH3 positive cells in Fas2 compared to the other mutants. Since Fas2 showed similar increases in proliferation compared to dlg, scrib, lgl, wts, and roe when measured by BrdU, the data suggest that Fas2 may function in an additional pathway that acts independent of dlg, lgl, scrib, wts, and roe, and that impinges on histone phosphorylation.
Fas2, dlg, lgl, scrib, wts, and roe, but not ft, ex, mer, or mer; ex, repress Cyclin E expression:
The Fas2, dlg, lgl, scrib, wts, and roe tumor phenotypes, along with BrdU and PH3 analysis, indicate that they share an essential requirement in repressing proliferation. The data do not indicate if they repress the same cell cycle pathways. Cyclin E (CycE) expression, which drives entry of cells into S phase, is a commonly used target to assay for Wts pathway activity (Harvey et al. 2003). To further test if Wts might be a target for BLJ signals, we analyzed CycE expression in Fas2, dlg, lgl, scrib, wts, and roe follicle cells. The typical pattern for Fas2, dlg, wts, scrib, lgl, and roe cells was a greater number of CycE positive cells compared to nonclone cells (Figure 8, G and H, and data not shown). Quantification showed roughly two- to threefold increase in the number of CycE postive cells for Fas2, dlg, wts, and roe, and a very modest but nonetheless significant increase for scrib, with a similar trend for lgl (Figure 8I). In contrast, ft, mer, ex, and mer; ex cells had significantly lower CycE expression compared to control clones (Figure 8I). The data provide additional, more specific, evidence that ft and mer; ex function in a pathway independent of wts in early follicle cells.
Fas2, dlg, lgl, scrib, wts, and roe, but not ft, mer, ex, or mer; ex repress DIAP1 expression:
DIAP1 expression is another commonly used assay for Wts pathway activity (Harvey et al. 2003). DIAP1 protects cells from apoptosis in eye tissue (Hay et al. 1995) and is important for border cell migration in the fly ovary (Silver et al. 2005). To further test if Wts might be a target for BLJ signals, we analyzed DIAP1 expression in Fas2, dlg, lgl, scrib, wts, and roe follicle cells. We observe a dramatic upregulation of DIAP1 in Fas2, dlg, lgl, scrib, wts, and roe follicle cells (Figure 9). We saw no upregulation of DIAP1 in Ft, mer, ex, or mer; ex cells (not shown). Thus, standard assays for Wts function, CycE, and DIAP1 expression, further suggested that ft, mer, and mer; ex function in a wts-independent pathway in follicle cells.
Figure 9.—
Elevated DIAP1 levels in Fas2, dlg, lgl, scrib, wts, and roe cells. Follicle cell clones are marked by the absence of β-gal or GFP. Egg chambers were stained with phalloidin to mark the cellular cortexes and with DIAP1 antibodies. DIAP1 is dramatically elevated in most mutant clone cells compared to nonclone cells. Increased DIAP1 was not observed in ft, ex, mer, or mer; ex clones (not shown).
DISCUSSION
The purpose of this work was to gain greater insight into how the BLJ suppresses epithelial tumorigenesis and invasion by identifying and understanding the function of new genes important for BLJ function. To do so, we completed a genomewide screen for enhancers of dlg, which encodes a scaffolding protein that is a crucial organizer of the BLJ and is a potent repressor of follicle epithelial cell tumorigenesis and invasion. We systematically screened deficiencies that cumulatively span ∼80% of the autosomes, or 64% of the Drosophila genome. We identified a relatively small number of enhancers, ∼1 per 1000 genes screened, indicating that our screen selected for loci specifically required for dlg function. This is further validated in that two of the enhancers, lgl and scrib, encode proteins that localize to the BLJ, and genetically interact with dlg in other tissues (Albertson and Doe 2003; Bilder et al. 2000; Peng et al. 2000). Thus, the novel dlg enhancer genes that we identified, wts, roe, ebi, as well as at least two genes yet to be identified, are likely to be key collaborators with dlg in suppressing epithelial invasion. The specificity of the interactions between dlg and these enhancers is further indicated in that more than one allele of each gene showed an interaction, in several dlg backgrounds, and the strengths of enhancement were similar to deficiencies defining each locus. wts, roe, and ebi also enhanced Fas2 and lgl (Figures 4 and 5), indicating that they are not just important for dlg function, but for the function of the BLJ as a whole. In addition, overexpression of all enhancers except ebi suppressed dlg and Fas2 tumorigenesis (Figures 3C and 4D), further confirming that the identified genes function in a BLJ network.
BLJ pathway components in the nucleus and their putative relationship to Notch:
ebi encodes an F-box protein with WD repeats that promotes protein degradation of specific targets (Dong et al. 1999; Boulton et al. 2000). The failure of ebi overexpression to suppress Fas2 or dlg, and the relatively mild ebi phenotypes (midoogenesis small-nucleus and epithelial-organization defects, but no defects in germinal vesicle localization), suggest that ebi may function in only one of the three branches of BLJ signaling (Figure 1A) or in a parallel pathway to the BLJ. In the eye, ebi is important for promoting differentiation and inhibiting proliferation, which appear to be separable functions (Boulton et al. 2000). Thus ebi could enhance Fas2 and dlg tumorigenesis by functioning within the proliferation-repressing branch of the BLJ, or the importance of ebi for differentiation suggests that it could function in the EMT branch of the BLJ (Figure 1A) or both. On the other hand, ebi promotes protein degradation in response to Notch (N) and Drosophila EGF receptor (EgfR) signals (Dong et al. 1999; Boulton et al. 2000; Tsuda et al. 2002), suggesting that it may act in a parallel pathway. Both Ebi and its mammalian homolog, TBL1, function in a corepressor complex through association with nuclear hormone transcriptional corepressor SMRTER/SMRT (Guenther et al. 2000; Tsuda et al. 2002).
Interestingly, although most N appears to be localized on the apical surface of follicle cells, some N is also localized in BLJs (Goode et al. 1996a). Thus, it is possible that N localized to the BLJ may signal directly to Ebi. Consistent with this possibility, we found that all of the genes in the BLJ network share some midoogenesis defects with N, including the small nucleus phenotype, epithelial stratification defects (Goode et al. 1996a), and mislocalization of the germinal vesicle (supplemental Figure 1). The epithelial defects are also reminiscent of N-pathway mutants brainiac and egghead, which are required in the germ line for regulating N that is localized on the apical surface of the follicle cells abutting the germ line (Goode et al. 1996a). Thus one possibility is that N signaling activity is regulated by its localization to apical vs. basolateral junctions in response to several signaling pathways acting during midoogenesis.
The other modest dlg enhancer that we identified, roe, encodes a Krüppel-family zinc-finger protein that appears to be a transcription factor. Roe is also implicated in Notch signaling (Brand and Campos-Ortega 1990; St Pierre et al. 2002) and thus may function with Ebi in N-dependent processes as proposed above. However, in contrast to ebi, roe loss caused follicle cell tumors (Figure 6), suggesting that roe may function more directly in a BLJ pathway than ebi. Consistent with a direct role for Roe in BLJ signaling, we found that roe overexpression suppressed Fas2 and dlg tumorigenesis (Figures 3C and 4D). Further, as for Fas2, dlg, and wts, roe represses CycE and DIAP1 expression (Figures 8 and 9). We propose that Roe is a downstream nuclear target of the BLJ and Wts (see our working model, Figure 10), where it may directly participate in repressing CycE and DIAP1 expression. The combined data for Ebi and Roe suggest for the first time that the BLJ may signal to the nucleus.
Figure 10.—
Working model for Wts signaling from the BLJ. We propose that Dlg organizes two or more signaling complexes to repress invasive tumorigenesis. One complex includes Fas2 as a Dlg PDZ ligand. This complex includes an unknown activator of Wts (X) that represses EMT and proliferation, through regulation of Roe, CycE, DIAP1, and other unknown factors. DIAP1 promotes movement as part of a complex with Rac1, and by triggering Dlg cleavage (scissors, see text). The complex also includes Lgl and does not or only weakly interacts with Scrib (represented as a reduced intensity). Fas2–Dlg–Lgl also repress motility through an undefined pathway. A second complex has an unknown Dlg PDZ ligand (?). This complex is associated predominantly with Scrib, also includes Lgl, and represses EMT and proliferation. In addition to repressing DIAP1, Scrib may promote border cell movement by localizing Rac1, as in humans. Ex and Mer may also be involved in Wts signaling starting during midoogenesis (not shown, see text).
Wts may collaborate with distinct epithelial junctions depending on developmental context:
We were especially interested in wts because of the many similarities we observed in the quality and strength of wts and scrib phenotypes, suggesting that they are components in a BLJ signaling pathway, rather than a parallel pathway that cross talks with BLJ signaling. wts encodes a serine/threonine kinase that is an ortholog of human tumor suppressors Lats1 and Lats2, both of which have been linked to highly aggressive breast cancers (Takahashi et al. 2005). The prevailing model for Wts signaling in Drosophila is based on signaling in eye and wing tissue. Wts appears to relay signals from apicolateral junction proteins Ft, Ex, and Mer in wing and eye tissues (Edgar 2006; Hamaratoglu et al. 2006; Willecke et al. 2006). However, the results from almost every assay, including early tumor formation, border cell migration, BrdU, PH3, CycE, and DIAP1 expression, indicated little functional overlap between Ft, ex, mer, or mer; ex and wts, thus diminishing the importance of apicolateral Ft-Ex-Mer for Wts activation in follicle cells. The exceptions were that during midoogenesis, Mer is required for border cell migration and Ex is required for the endocycle switch, while both are required for maintenance of epithelial integrity and positioning of the germinal vesicle. However, the involvement of Ex and Mer in these processes are fundamentally distinct from how they act in Wts-dependent processes in other tissues. First, Ft is not involved; second, we did not observe any indication of Ex-Mer synergism; third, ex, mer, and mer; ex phenotypes are relatively mild when compared to wts. We conclude that the model for Wts activation in which apicolateral junction proteins Ft, Ex, and Mer play the predominant role cannot be universally applicable in all cell types. Rather, the relative importance of Ex and Mer for Wts regulation appears to depend on developmental context.
Consistent with this proposal, we found strong functional interdependence and phenotypic similarities between Fas2, dlg, lgl, scrib, and wts, thus indicating that the BLJ, not the apicolateral junction, plays the predominant role in Wts regulation during oogenesis (summarized in supplemental Table 1). Although genetic evidence alone cannot completely rule out that Wts may act in a parallel pathway to the BLJ and impinge on a set of downstream targets that overlap with those targeted by the BLJ, the following observations favor a model in which the BLJ is more directly involved in Wts regulation (we note that these are not mutually exclusive alternatives): (1) over 50 tumor suppressor genes have been identified in Drosophila (Watson et al. 1994), but lgl, scrib, and wts were the only strong dlg enhancers identified in our genomewide screen; (2) wts showed strong genetic interactions with Fas2, dlg, and lgl, similar to or stronger than scrib, which encodes a known BLJ protein; (3) wts has early tumor phenotypes similar to dlg partial loss of function and to scrib (Figure 6); (4) wts has the same border cell migration phenotype as scrib (Figure 7); (5) wts has similar small nucleus, epithelial stratification, and germinal vesicle defects as Fas2, dlg, lgl, and scrib (supplemental Figure 1); (6) like lgl and scrib, wts overexpression suppressed Fas2 and dlg tumorigenesis (Figures 3 and 4); (7) Fas2, dlg, and wts have similar proliferation defects (Figure 8); and (8) Fas2, dlg, and wts similarly repress CycE and DIAP1 expression (Figures 8 and 9), which is especially crucial, because CycE and DIAP1 are downstream targets of Wts signaling, and ex and mer had no impact on their expression, contrary to results in other tissues. Thus, the data strongly indicate that the BLJ signals through Wts, and may impinge on Roe in the nucleus, thus suggesting the first BLJ signaling pathway in animal cells. This implies that the BLJ not only acts as a localized scaffold, but also signals to the nucleus to control gene expression, both of which cooperate to regulate epithelial polarity and dynamics.
How can our results in follicle cells, which suggest that Wts acts predominantly downstream of the BLJ, be reconciled with findings in eye tissue, which indicate that Wts acts downstream of the apicolateral junction? Interestingly, the genetic data in the eye suggest that Ft, Ex, and Mer cannot account for all of the signals that activate Wts, because wts overgrowth and tissue disorganization phenotypes are more severe than ft or mer; ex (Cho et al. 2006; Hamaratoglu et al. 2006; Willecke et al. 2006). On the basis of our findings in follicle cells, it is possible that Wts activation in the eye requires additional input from the BLJ. This possibility may have been overlooked thus far because dlg does not appear to have an overgrowth phenotype in the eye (Pagliarini and Xu 2003). dlg may be essential for additional functions in the eye that are epistatic to its tumor suppressor function, thus preventing loss of cells from the epithelium that could mask an overgrowth phenotype. Consistent with this, when activated Rasv12 is combined with dlg loss, dramatic tumors develop that are larger and more invasive than those produced by Rasv12 alone (Pagliarini and Xu 2003).
On the other hand, Dlg may have a diminished role in Wts signaling in the eye, much as our evidence indicates a diminished role for Ex and Mer in Wts signaling in the ovary. According to this model, Wts receives predominant input from distinct lateral junctions depending on tissue context. One distinction is that ovarian follicle cells are derived from a mesodermal lineage, while the eye and wing tissues are from ectodermal lineages. Further, many genes that disrupt apical–basal polarity and epithelial morphology have only subtle phenotypes in the eye by comparison to the ovary or embryo (Nam et al. 2007). Finally, the follicular epithelium requires input from junctions on all three follicle cell surfaces, lateral, apical, and basal, whereas most epithelia require only two, lateral and apical or basal (Tanentzapf et al. 2000). Thus, ovarian and imaginal tissues are likely to organize signaling pathways acting downstream of epithelial junctions in similar, yet fundamentally different ways to meet the unique organizational requirements of their cell-tissue morphologies. Some or all of these differences may contribute to the suggested specificity that we observe in Wts signaling downstream of BLJs in follicle cells. In general, our findings raise the possibility for future investigation that depending on the cell-tissue morphologies of a given organ, one lateral junction may play a predominant organizational role, and Wts signaling may act as a universal signaling adapter for mediating contact inhibition from that junction.
An especially interesting aspect of Mer and Ex function that we uncovered in follicle cells is that it appears to be restricted to predominantly postmitotic, differentiated cells, in contrast to the role of Mer and Ex in other tissues. Further, given the absence of an involvement of Ft and lack of Mer-Ex synergism we conclude that if Mer and Ex would be involved in Wts activation in follicle cells, they would have to function via a fundamentally distinct mechanism than in other tissues. We proposed that during early oogenesis, the BLJ alone may provide the predominant input to Wts. Then, during midoogenesis, Ex and Mer may become involved in novel interactions with Dlg or other components of the BLJ to activate Wts in spatiotemporally distinct populations of differentiating cells to help achieve their unique developmental functions.
Scrib, Wts, and Roe appear to be crucial for the EMT branch of BLJ signaling:
How do wts, scrib, and roe promote motility? We propose that Scrib, Wts, and Roe are all crucially involved in EMT. In EMT, cells (1) loose apical–basal polarity and become mesenchymal-like, and (2) adopt a polarity conducive to movement. scrib, wts, and roe cells clearly lose epithelial polarity and become mesenchymal-like as indicated by their rounded morphology and lateralized phenotype (Figure 6). However, scrib, wts, and roe tumors do not invade, and scrib, wts, and roe border cells do not move, suggesting that the second aspect of EMT, adoption of a polarity conducive to movement, is defective. Consistent with this, mammalian Scrib is required for migration and epithelial wound healing of cultured human breast epithelial cells, and is also required in vivo for wound healing in mice (Dow et al. 2007). Human Scrib directs migration by organizing several polarities crucial for migration, including the orientation of the microtubule and Golgi networks and the localization of Cdc42 and Rac1 to the cell's leading edge (Dow et al. 2007). Thus Scrib has a conserved function in directed cell migration by organizing a polarity conducive to movement. In mammalian PC12 cells Scrib is in complex with Rac1 (Audebert et al. 2004). Fly Rac1 is essential for border cell migration (Geisbrecht and Montell 2004) and invasion of Fas2 and dlg tumors (Szafranski and Goode 2007), suggesting that an essential role of Scrib in Rac1 function may be of crucial importance for movement. The apparent conserved role of BLJ proteins in organizing EMT, and both promoting and repressing movement, reemphasizes our previous suggestion that BLJ proteins do more than merely maintain apical–basal polarity, but rather repress a cellular transformation from epithelial polarity to a mesenchymal, lateralized signature conducive to movement (Szafranski and Goode 2007).
How is the function of scrib, wts, and roe in promoting border cell movement consistent with the requirement of Fas2, dlg, and lgl in repressing border cell movement? Further, how do scrib and wts act as enhancers of dlg tumor invasion even though scrib and wts tumors are noninvasive? For border cell movement, Fas2 and dlg mutations not only accelerate movement, but also delay border cell delamination. The delay in border cell delamination suggests that the BLJ normally promotes motility, but this promoting function can be bypassed when the repression of motility branch of the BLJ pathway is simultaneously lost (Szafranski and Goode 2004). Our cumulative data indicate that scrib, wts, and roe act predominantly within the EMT and proliferation branches of the BLJ pathway, and not the repression of motility branch. We suggest that without simultaneous loss of the repression of motility branch of the BLJ pathway, scrib and wts border cells cannot bypass the essential requirement for the second step of EMT, thus border cell motility is blocked.
This interpretation is also consistent with the seemingly paradoxical function of scrib and wts as enhancers of dlg tumor invasion, even though Scrib and Wts promote rather than repress border cell movement. The noninvasive scrib and wts tumor phenotypes indicate that they are crucial for repressing the first step of EMT, loss of epithelial polarity and adoption of a lateralized, mesenchymal-like phenotype. We suggest that scrib and wts enhance dlg invasive tumorigenesis by increasing the rate at which dlg mutant follicle cells undergo EMT and further facilitate invasion by depressing proliferation control and increasing the number of follicle cells available for movement. Thus, even though scrib and wts are required to promote movement, we suggest that in dlg; scrib/+ or dlg; wts/+ tumors this requirement can be bypassed because the branch of the BLJ pathway that represses motility is simultaneously disrupted.
The noninvasive tumor phenotypes of scrib and wts are very similar to the phenotypes of dlg mutants that specifically disrupt Dlg SH3 and GuK domains (Figure 6). Thus Scrib and Wts may act specifically downstream of the Dlg SH3 and GuK domains. Consistent with this, Scrib appears to associate with the Dlg GuK domain in neuronal synapses via the linker protein GuK-holder (Mathew et al. 2002). Further, whereas Fas2, dlg, and lgl cause faster border cell migration, border cell migration is very similar to wild type in the dlg SH3/GuK-specific mutants, suggesting that Dlg SH3/GuK predominantly represses the first step of EMT and proliferation but not motility. On the basis of this specificity, we suggest that one reason that lgl may be a stronger dlg enhancer than scrib and wts is that lgl represses motility in addition to EMT and proliferation. For example, the de novo tumor formation observed when one copy of lgl, scrib, or wts is removed in dlghf/dlgsw ovaries suggests that a threshold level of BLJ activity essential for maintenance of polarity has been lost. However, the lgl interaction may be much stronger than scrib and wts because lgl additionally represses motility.
We observed increased expression of CycE and DIAP1, known Wts targets, in Fas2, dlg, lgl, scrib, wts, and roe cells. Thus the importance of CycE for proliferation control, and DIAP1 for control of EMT and motility, suggests that part of the mechanism by which Fas2–Dlg represses tumorigenesis is through activating Wts signaling. DIAP1 is in a complex with Rac1 and Profilin and enables border cell motility apparently by promoting actin turnover (Geisbrecht and Montell 2004). Further, in the embryo, DIAP1 loss leads to Dlg cleavage and cellular rounding and dispersal (Chandraratna et al. 2007). Too much DIAP1 also appears to be deleterious to movement, because targeted overexpression of DIAP1 specifically in border cells slows their migration (data not shown). Thus maintaining the proper balance of DIAP1 is critical for directed movement, and it may be part of the mechanism by which Scrib and Wts influence border cell movement (Figure 10). In mammalian PC12 cells Scrib is in a complex with Dlg and Rac1 (Audebert et al. 2004), suggesting that interaction with Dlg and Rac1 may be another level at which Scrib regulates EMT and movement (Figure 10). In flies, as in humans, Rac1 is also required for proliferation (Brumby et al. 2004; Dipersio 2007), consistent with the possibility that it functions downstream of Scrib and Wts in follicle cells to repress both EMT and proliferation.
Genetic evidence for distinct BLJ complexes:
In contrast to the strong enhancement of dlg by scrib, Fas2 was only weakly enhanced by scrib (Figure 4). We propose that this difference suggests the existence of two distinct Dlg complexes within the BLJ. One complex uses Fas2 as a Dlg ligand, as well as Lgl and an unknown protein that activates Wts, but little or no Scrib (Figure 10). A second complex has an unknown Dlg ligand and includes both Scrib and Lgl (Figure 10). In support of this working model, biochemical evidence favors the existence of mutually exclusive Dlg complexes (Qian and Prehoda 2006). Given the complexity of coordinating EMT, proliferation, and motility within an epithelial field, perhaps the simplest model is that multiple Dlg complexes reside within the BLJ, each with a distinct set of ligands that control one or more morphogenetic activities.
Another interesting difference in the enhancement of dlg and Fas2 by lgl, scrib, wts, and roe was that they all enhanced both dlg tumorigenesis and invasion, but only enhanced Fas2 tumorigenesis, without invasion (Figure 4). An important difference between these experiments may be that in Fas2null follicle cells, Dlg is missing Fas2 as a ligand, whereas in dlghf/dlgsw, dlghf/dlgip20, and dlghf/dlglv55 follicle cells, Fas2 is localized at sites of contact between follicle cells in both the native epithelium and in streams of invading cells (data not shown), suggesting that Fas2 continues to act as a Dlg ligand in these cells. This is probably an important difference because Fas2–Dlg binding is expected to control the conformation of Dlg. Dlg conformations in turn may specify Dlg intra- and intermolecular interactions that determine the relative balance of EMT, proliferation, and invasion factors that associate with the BLJ scaffold. For example, in neuronal cells intramolecular interactions between Dlg SH3 and GuK domains regulate the strength of intermolecular binding of GuK-holder, which binds Scrib (Qian and Prehoda 2006). The SH3–GuK intramolecular interaction is further modulated by intramolecular interactions with PDZ3, which are regulated by intermolecular interactions with neurolignin, a transmembrane ligand for PDZ3 (Qian and Prehoda 2006).
On the basis of this molecular model, we propose that in the absence of Fas2, Dlg has a distinct conformation that tilts the balance toward EMT and proliferation over invasion, when Lgl, Scrib, Wts, or Roe are reduced. As described above and in the results, lgl, scrib, wts, and roe are expected to act predominantly downstream of Dlg SH3 and GuK domains to repress EMT and proliferation (Figures 5 and 6 and see above discussion). Thus, removal of one copy of lgl, scrib, wts, or roe in Fas2 cells may tip the ratio of factors controlling EMT, motility, and proliferation toward derepression of EMT and proliferation, masking the Fas2 requirement for invasion. One possibility is that lgl, scrib, wts, or roe are especially important for expression of a protein in the apicolateral junction, such as Par-3/Bazooka, which is essential for dlg invasion (Abdelilah-Seyfried et al. 2003). Consistent with this, we see Ex upregulation in both dlg and wts clones (data not shown). Further, as discussed in the results, lgl enhancement at the lglts permissive temperature showed essentially the opposite trend from Fas2. Rather than enhance tumorigenesis over invasion, removal of one copy of Fas2, dlg, scrib, wts, or roe in lgl egg chambers favored invasion. Thus, we suggest that tumor invasiveness associated with particular combinations of mutated BLJ proteins may be masked or unmasked on the basis of the balance of activities that are disrupted, rather than disruption of particular activities per se.
In summary, we appear to have identified the first signaling pathway that acts downstream of the BLJ that specifically controls EMT and proliferation, and we have gained important clues as to how this signaling may be organized. Like the Drosophila follicular epithelium, the human ovarian surface epithelium, which is thought to be the site of origin of most ovarian cancers, is derived from a mesodermal lineage (Ozols 2003). Our data suggest that the BLJ plays an especially crucial role in the follicle cells compared to ectodermal lineages in repressing epithelial invasion and that the follicular epithelium appears to organize signaling from epithelial junctions in distinct ways compared to other epithelia. Given the conservation in the lineage of the fly and human epithelia, and the sensitivity of our screen for detecting molecules important for invasive carcinogenesis, we propose that the fly egg chamber may serve as a prototype for identifying early molecular events that are crucial for invasion of human ovarian cancer and possibly other malignancies that remain undetected before they start to invade.
Acknowledgments
We thank Jun Hui Bian and Sirish Kishore for help with the primary screen for dlg enhancers. David Bilder sent scrib stocks, Jürgen Knoblich sent lgl4, and George Halder provided wtsx1 flies. We thank Helena Richardson for sending cyclin E antibodies and Bruce Hay for sending DIAP1 antibodies. We thank Rick Kelley and anonymous reviewers for helpful comments. The following grants to S.G. supported this work: National Institutes of Health (NIH) 2R01 CA087751-06A2, Mary Kay Ash 038-03, and NIH Breast Cancer Specialized Program of Research Excellence P50CA58183.
References
- Abdelilah-Seyfried, S., D. N. Cox and Y. N. Jan, 2003. Bazooka is a permissive factor for the invasive behavior of discs large tumor cells in Drosophila ovarian follicular epithelia. Development 130 1927–1935. [DOI] [PubMed] [Google Scholar]
- Albertson, R., and C. Q. Doe, 2003. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat. Cell. Biol. 5 166–170. [DOI] [PubMed] [Google Scholar]
- Arlt, M. J., I. Novak-Hofer, D. Gast, V. Gschwend, G. Moldenhauer et al., 2006. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 66 936–943. [DOI] [PubMed] [Google Scholar]
- Audebert, S., C. Navarro, C. Nourry, S. Chasserot-Golaz, P. Lecine et al., 2004. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 14 987–995. [DOI] [PubMed] [Google Scholar]
- Bilder, D., and N. Perrimon, 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403 676–680. [DOI] [PubMed] [Google Scholar]
- Bilder, D., M. Li and N. Perrimon, 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289 113–116. [DOI] [PubMed] [Google Scholar]
- Boulton, S. J., A. Brook, K. Staehling-Hampton, P. Heitzler and N. Dyson, 2000. A role for Ebi in neuronal cell cycle control. EMBO J. 19 5376–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401–415. [DOI] [PubMed] [Google Scholar]
- Brand, M., and J. A. Campos-Ortega, 1990. Second-site modifiers of the split mutation of Notch define genes involved in neurogenesis in Drosophila melanogaster. Roux's Arch. Dev. Biol. 198 275–285. [DOI] [PubMed] [Google Scholar]
- Brumby, A., J. Secombe, J. Horsfield, M. Coombe, N. Amin et al., 2004. A genetic screen for dominant modifiers of a cyclin E hypomorphic mutation identifies novel regulators of S-phase entry in Drosophila. Genetics 168 227–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandraratna, D., N. Lawrence, D. P. Welchman and B. Sanson, 2007. An in vivo model of apoptosis: linking cell behaviours and caspase substrates in embryos lacking DIAP1. J. Cell Sci. 120 2594–2608. [DOI] [PubMed] [Google Scholar]
- Cho, E., Y. Feng, C. Rauskolb, S. Maitra, R. Fehon et al., 2006. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 38 1142–1150. [DOI] [PubMed] [Google Scholar]
- DiPersio, C. M., 2007. Double duty for Rac1 in epidermal wound healing. Sci. STKE 2007 pe33. [DOI] [PubMed] [Google Scholar]
- Dong, X., L. Tsuda, K. H. Zavitz, M. Lin, S. Li et al., 1999. ebi regulates epidermal growth factor receptor signaling pathways in Drosophila. Genes Dev. 13 954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow, L. E., J. S. Kauffman, J. Caddy, A. S. Peterson, S. M. Jane et al., 2007. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26 2272–2282. [DOI] [PubMed] [Google Scholar]
- Edgar, B. A., 2006. From cell structure to transcription: Hippo forges a new path. Cell 124 267–273. [DOI] [PubMed] [Google Scholar]
- Fogar, P., D. Basso, C. Pasquali, M. De Paoli, C. Sperti et al., 1997. Neural cell adhesion molecule (N-CAM) in gastrointestinal neoplasias. Anticancer Res. 17 1227–1230. [PubMed] [Google Scholar]
- Funke, L., S. Dakoji and D. S. Bredt, 2005. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu. Rev. Biochem. 74 219–245. [DOI] [PubMed] [Google Scholar]
- Geisbrecht, E. R., and D. J. Montell, 2004. A role for Drosophila IAP1-mediated caspase inhibition in Rac-dependent cell migration. Cell 118 111–125. [DOI] [PubMed] [Google Scholar]
- Goode, S., and N. Perrimon, 1997. Inhibition of patterned cell shape change and cell invasion by Discs large during Drosophila oogenesis. Genes Dev. 11 2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, S., M. Melnick, T. B. Chou and N. Perrimon, 1996. a The neurogenic genes egghead and brainiac define a novel signaling pathway essential for epithelial morphogenesis during Drosophila oogenesis. Development 122 3863–3879. [DOI] [PubMed] [Google Scholar]
- Goode, S., M. Morgan, Y. P. Liang and A. P. Mahowald, 1996. b Brainiac encodes a novel, putative secreted protein that cooperates with Grk TGF alpha in the genesis of the follicular epithelium. Dev. Biol. 178 35–50. [DOI] [PubMed] [Google Scholar]
- Goode, S., J. Wei and S. Kishore, 2005. Novel spatiotemporal patterns of epithelial tumor invasion in Drosophila discs large egg chambers. Dev. Dyn. 232 855–864. [DOI] [PubMed] [Google Scholar]
- Goodman, C. S., G. W. Davis and K. Zito, 1997. The many faces of fasciclin II: genetic analysis reveals multiple roles for a cell adhesion molecule during the generation of neuronal specificity. Cold Spring Harbor Symp. Quant. Biol. 62 479–491. [PubMed] [Google Scholar]
- Grifoni, D., F. Garoia, C. C. Schimanski, G. Schmitz, E. Laurenti et al., 2004. The human protein Hugl-1 substitutes for Drosophila lethal giant larvae tumour suppressor function in vivo. Oncogene 23 8688–8694. [DOI] [PubMed] [Google Scholar]
- Grifoni, D., F. Garoia, P. Bellosta, F. Parisi, D. De Biase et al., 2007. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene 26 5960–5965. [DOI] [PubMed] [Google Scholar]
- Grunert, S., M. Jechlinger and H. Beug, 2003. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 4 657–665. [DOI] [PubMed] [Google Scholar]
- Guenther, M. G., W. S. Lane, W. Fischle, E. Verdin, M. A. Lazar et al., 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 14 1048–1057. [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu, F., M. Willecke, M. Kango-Singh, R. Nolo, E. Hyun et al., 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell. Biol. 8 27–36. [DOI] [PubMed] [Google Scholar]
- Hariharan, I. K., 2006. Growth regulation: a beginning for the hippo pathway. Curr. Biol. 16 R1037–R1039. [DOI] [PubMed] [Google Scholar]
- Harvey, K., and N. Tapon, 2007. The Salvador-Warts-Hippo pathway–an emerging tumour-suppressor network. Nat. Rev. Cancer 7 182–191. [DOI] [PubMed] [Google Scholar]
- Harvey, K. F., C. M. Pfleger and I. K. Hariharan, 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114 457–467. [DOI] [PubMed] [Google Scholar]
- Hay, B. A., D. A. Wassarman and G. M. Rubin, 1995. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83 1253–1262. [DOI] [PubMed] [Google Scholar]
- Hoover, K. B., S. Y. Liao and P. J. Bryant, 1998. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am. J. Pathol. 153 1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. H., A. Rajkovic, P. Szafranski, S. Ochsner, J. Richards et al., 2003. Expression of Drosophila neoplastic tumor suppressor genes discslarge, scribble, and lethal giant larvae in the mammalian ovary. Gene Expr. Patterns 3 3–11. [DOI] [PubMed] [Google Scholar]
- Hutterer, A., J. Betschinger, M. Petronczki and J. A. Knoblich, 2004. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell 6 845–854. [DOI] [PubMed] [Google Scholar]
- Justice, R. W., O. Zilian, D. F. Woods, M. Noll and P. J. Bryant, 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9 534–546. [DOI] [PubMed] [Google Scholar]
- Knust, E., 2002. Regulation of epithelial cell shape and polarity by cell-cell adhesion. Mol. Membr. Biol. 19 113–120. [DOI] [PubMed] [Google Scholar]
- Knust, E., and O. Bossinger, 2002. Composition and formation of intercellular junctions in epithelial cells. Science 298 1955–1959. [DOI] [PubMed] [Google Scholar]
- Lai, Z. C., X. Wei, T. Shimizu, E. Ramos, M. Rohrbaugh et al., 2005. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120 675–685. [DOI] [PubMed] [Google Scholar]
- Larue, L., and A. Bellacosa, 2005. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24 7443–7454. [DOI] [PubMed] [Google Scholar]
- Lue, R. A., S. M. Marfatia, D. Branton and A. H. Chishti, 1994. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc. Natl. Acad. Sci. USA 91 9818–9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall, N., Y. Lad, G. S. Wilkie, H. Francis-Lang, W. Sullivan et al., 2001. Merlin, the Drosophila homologue of neurofibromatosis-2, is specifically required in posterior follicle cells for axis formation in the oocyte. Development 128 665–673. [DOI] [PubMed] [Google Scholar]
- Manfruelli, P., N. Arquier, W. P. Hanratty and M. Semeriva, 1996. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development 122 2283–2294. [DOI] [PubMed] [Google Scholar]
- Manseau, L., A. Baradaran, D. Brower, A. Budhu, F. Elefant et al., 1997. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev. Dyn. 209 310–322. [DOI] [PubMed] [Google Scholar]
- Manseau, L. J., and T. Schüpbach, 1989. cappuccino and spire: two unique maternal-effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes Dev. 3 1437–1452. [DOI] [PubMed] [Google Scholar]
- Mathew, D., L. S. Gramates, M. Packard, U. Thomas, D. Bilder et al., 2002. Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr. Biol. 12 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumine, A., A. Ogai, T. Senda, N. Okumura, K. Satoh et al., 1996. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 272 1020–1023. [DOI] [PubMed] [Google Scholar]
- Meignin, C., I. Alvarez-Garcia, I. Davis and I. M. Palacios, 2007. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 17 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell, D. J., 2003. Border-cell migration: the race is on. Nat. Rev. Mol. Cell Biol. 4 13–24. [DOI] [PubMed] [Google Scholar]
- Nam, S. C., B. Mukhopadhyay and K. W. Choi, 2007. Antagonistic functions of Par-1 kinase and protein phosphatase 2A are required for localization of Bazooka and photoreceptor morphogenesis in Drosophila. Dev. Biol. 306 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska, P., D. Godt and U. Tepass, 1999. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J. Cell Biol. 144 533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols, R. F., 2003. Ovarian Cancer. BC Decker, London.
- Pagliarini, R. A., and T. Xu, 2003. A genetic screen in Drosophila for metastatic behavior. Science 302 1227–1231. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36 288–292. [DOI] [PubMed] [Google Scholar]
- Peng, C. Y., L. Manning, R. Albertson and C. Q. Doe, 2000. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408 596–600. [DOI] [PubMed] [Google Scholar]
- Perl, A. K., U. Dahl, P. Wilgenbus, H. Cremer, H. Semb et al., 1999. Reduced expression of neural cell adhesion molecule induces metastatic dissemination of pancreatic beta tumor cells. Nat. Med. 5 286–291. [DOI] [PubMed] [Google Scholar]
- Polesello, C., and N. Tapon, 2007. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17 1864–1870. [DOI] [PubMed] [Google Scholar]
- Postigo, A. A., E. Ward, J. B. Skeath and D. C. Dean, 1999. zfh-1, the Drosophila homologue of ZEB, is a transcriptional repressor that regulates somatic myogenesis. Mol. Cell. Biol. 19 7255–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y., and K. E. Prehoda, 2006. Interdomain interactions in the tumor suppressor discs large regulate binding to the synaptic protein GukHolder. J. Biol. Chem. 281 35757–35763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, H., L. V. O'Keefe, T. Marty and R. Saint, 1995. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development 121 3371–3379. [DOI] [PubMed] [Google Scholar]
- Roesler, J., E. Srivatsan, F. Moatamed, J. Peters and E. H. Livingston, 1997. Tumor suppressor activity of neural cell adhesion molecule in colon carcinoma. Am. J. Surg. 174 251–257. [DOI] [PubMed] [Google Scholar]
- Schimanski, C. C., G. Schmitz, A. Kashyap, A. K. Bosserhoff, F. Bataille et al., 2005. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene 24 3100–3109. [DOI] [PubMed] [Google Scholar]
- Shcherbata, H. R., C. Althauser, S. D. Findley and H. Ruohola-Baker, 2004. The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development 131 3169–3181. [DOI] [PubMed] [Google Scholar]
- Shook, D., and R. Keller, 2003. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev. 120 1351–1383. [DOI] [PubMed] [Google Scholar]
- Silver, D. L., E. R. Geisbrecht and D. J. Montell, 2005. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 132 3483–3492. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C., 1993. Developmental Genetics of Oogenesis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- St Pierre, S. E., M. I. Galindo, J. P. Couso and S. Thor, 2002. Control of Drosophila imaginal disc development by rotund and roughened eye: differentially expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development 129 1273–1281. [DOI] [PubMed] [Google Scholar]
- Starz-Gaiano, M., and D. J. Montell, 2004. Genes that drive invasion and migration in Drosophila. Curr. Opin. Genet. Dev. 14 86–91. [DOI] [PubMed] [Google Scholar]
- Szafranski, P., and S. Goode, 2004. A Fasciclin 2 morphogenetic switch organizes epithelial cell cluster polarity and motility. Development 131 2023–2036. [DOI] [PubMed] [Google Scholar]
- Szafranski, P., and S. Goode, 2007. Basolateral junctions are sufficient to suppress epithelial invasion during Drosophila oogenesis. Dev. Dyn. 236 364–373. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y., Y. Miyoshi, C. Takahata, N. Irahara, T. Taguchi et al., 2005. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin. Cancer Res. 11 1380–1385. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G., C. Smith, J. McGlade and U. Tepass, 2000. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151 891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery, J. P., and J. P. Sleeman, 2006. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7 131–142. [DOI] [PubMed] [Google Scholar]
- Tsuda, L., R. Nagaraj, S. L. Zipursky and U. Banerjee, 2002. An EGFR/Ebi/Sno pathway promotes delta expression by inactivating Su(H)/SMRTER repression during inductive notch signaling. Cell 110 625–637. [DOI] [PubMed] [Google Scholar]
- Udan, R. S., M. Kango-Singh, R. Nolo, C. Tao and G. Halder, 2003. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5 914–920. [DOI] [PubMed] [Google Scholar]
- Watson, K. L., R. W. Justice and P. J. Bryant, 1994. Drosophila in cancer research: the first fifty tumor suppressor genes. J. Cell Sci. Suppl. 18 19–33. [DOI] [PubMed] [Google Scholar]
- Wei, J., M. Hortsch and S. Goode, 2004. Neuroglian stabilizes epithelial structure during Drosophila oogenesis. Dev. Dyn. 230 800–808. [DOI] [PubMed] [Google Scholar]
- Willecke, M., F. Hamaratoglu, M. Kango-Singh, R. Udan, C. L. Chen et al., 2006. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 16 2090–2100. [DOI] [PubMed] [Google Scholar]
- Woods, D. F., and P. J. Bryant, 1989. Molecular cloning of the lethal(1)discs large-1 oncogene of Drosophila. Dev. Biol. 134 222–235. [DOI] [PubMed] [Google Scholar]
- Woods, D. F., and P. J. Bryant, 1991. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66 451–464. [DOI] [PubMed] [Google Scholar]
- Woods, D. F., C. Hough, D. Peel, G. Callaini and P. J. Bryant, 1996. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 134 1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, S. J., J. R. Huh, I. Muro, H. Yu, L. Wang et al., 2002. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 4 416–424. [DOI] [PubMed] [Google Scholar]