Abstract
The Vasa DEAD-box helicases are widespread markers of germ cells across species, and in some organisms have been shown to be essential for germ-cell formation and development. In contrast to the single Vasa gene in most systems analyzed, Caenorhabditis elegans has four Vasa family members, the germline helicases GLH-1, GLH-2, GLH-3, and GLH-4. Our analysis of deletion alleles of each glh gene demonstrates that GLH-1 is the key member of the family: loss of GLH-1 function causes sterility that is mainly maternal effect, is manifested predominantly at elevated temperature, and is due to reduced germ-cell proliferation and impaired formation of both sperm and oocytes. The other GLHs are not essential. However, GLH-4 serves redundant roles with GLH-1: loss of both genes' function causes glh-1-like sterility at all temperatures. Molecular epistasis analysis demonstrates that GLH-1 and GLH-4 are required for proper association of the PGL family of proteins with P granules, suggesting a pathway of P-granule assembly in which the GLHs are upstream of the PGL proteins and the mRNA cap-binding protein IFE-1. While loss of some P-granule components causes worms to be defective in RNA interference, loss of GLH-1 and GLH-4 does not compromise RNAi. Thus, RNAi likely does not require intact P granules but instead relies on particular P-granule factors. We discuss the evolution of the Vasa/GLH genes and current views of their functions and the assembly and roles of germ granules among species.
HOW germ cells are distinguished from somatic cells is a critical issue in developmental biology (e.g., see Strome and Lehmann 2007). One unique feature of germ cells is their possession of specialized cytoplasm called germ plasm, which contains distinctive non-membrane-bound organelles generically called germ granules (reviewed in Wylie 2000; Santos and Lehmann 2004; Zhou and King 2004; Strome 2005; Seydoux and Braun 2006). Germ granules are widespread in nature. In several model systems, including Caenorhabditis, Drosophila, and Xenopus, germ granules are maternally loaded into the embryo and segregated to the primordial germ cells (PGCs) during the initial embryonic divisions, and PGC formation and/or function relies on germ-plasm components (reviewed in references above). In mammals, PGC formation does not depend on segregated germ plasm; instead PGCs are formed by a different mechanism, induction, at a later stage of embryonic development (reviewed in Saffman and Lasko 1999; Hayashi et al. 2007). Nevertheless, mammalian embryos contain germ-granule components, which display specific protein–protein interactions and subcellular localization in nascent germ cells, suggesting that even mammalian PGCs may rely on assembled germ plasm for proper development (Fox et al. 2007).
The composition and functions of germ granules are being actively investigated. Perhaps surprisingly, many germ-granule components appear to be species specific; for example, PGL-1 is unique to worm germ granules (Kawasaki et al. 1998), while Oskar is unique to flies (Ephrussi et al. 1991; Ephrussi and Lehmann 1992). The best-studied germ-granule component that is shared among species is Vasa and its homologs. First discovered in Drosophila, Vasa is a DEAD (Asp-Glu-Ala-Asp) family RNA helicase that has an RGG (Arg-Gly-Gly)-rich domain (Hay et al. 1988a,b; Lasko and Ashburner 1988). Vasa is maternally supplied and required to assemble germ plasm at the posterior pole of the fly oocyte; embryos lacking germ plasm fail to bud PGCs from the posterior end of the embryo and develop into sterile adults (Lasko and Ashburner 1988; Hay et al. 1990; Lehmann 1992; Liang et al. 1994). Strong Vasa alleles result in defects in oocyte growth, patterning, and differentiation (Lasko and Ashburner 1988; Liang et al. 1994). Among the targets of Vasa translational regulation are gurken, oskar, and nanos mRNA (Dahanukar and Wharton 1996; Markussen et al. 1997; Styhler et al. 1998). Recent reports implicate Vasa and germ granules in microRNA control of gene expression in flies as well as in mice (Kotaja et al. 2006; Megosh et al. 2006).
The reported Caenorhabditis elegans homologs of Vasa are the four GLH germline helicases (Roussell and Bennett 1993; Gruidl et al. 1996; Kuznicki et al. 2000). All four GLH proteins associate with C. elegans germ granules or “P granules,” named for their segregation to the germline P blastomeres P1, P2, P3, and P4. Like Vasa, each of the four GLHs contains a DEAD box helicase domain. Also like Vasa, GLH-1, GLH-2, and GLH-4 contain a Gly-rich domain. However, while Vasa contains RGG repeats, these three GLHs contain FGG repeats. This repeat domain of the GLHs differs in overall charge from that in Vasa and may serve a different role; RGG repeats are an established RNA-binding domain (Kiledjian and Dreyfuss 1992; Zanotti et al. 2006), whereas FG repeats are common to nuclear pore proteins (Suntharalingam and Wente 2003). In fact, P granules are perinuclearly localized during most of the worm's life cycle (Strome and Wood 1982), and they overlie nuclear pores (Pitt et al. 2000). All four GLH proteins additionally contain CCHC-type zinc-finger motifs not shared by Vasa. Previous RNAi analysis (Kuznicki et al. 2000) suggested that among the GLHs, GLH-1 and GLH-2 are the most important GLH family members; depletion of either caused sterility at elevated temperature. Concomitant RNAi depletion of GLH-1 and GLH-4 caused sterility even at lower temperature, suggesting that GLH-1 and GLH-4 function redundantly. RNAi depletion of GLH-3 did not cause notable defects and did not enhance the glh-1(RNAi) or glh-2(RNAi) sterile phenotype, suggesting that GLH-3 serves only minor roles in the germline. Because of the uncertain effectiveness of RNAi depletion of gene product, the potential for dsRNAs to cross-hybridize and reduce expression of multiple glh genes, and the difficulty discriminating between maternal-effect and zygotic sterility by RNAi, we set out to isolate and study bona fide mutations in the four glh genes.
This article describes detailed genetic analysis of glh mutants. Two severe deletion alleles of glh-1 reveal that the sterile phenotype is mainly maternal effect, is very sensitive to temperature, and results from defects in germline proliferation and in formation of gametes. Deletion alleles of glh-2, glh-3, and glh-4 do not cause dramatic phenotypes on their own, but a glh-4 mutation strongly enhances the glh-1 mutant phenotype, by abolishing the sensitivity to temperature. Several mutant forms of GLH-1 protein do not assemble into P granules; probably as a result, the PGL proteins also show defects in their association with P granules. Although pgl-1 mutants are defective in RNAi, glh-1 mutants and glh-4 glh-1 double mutants are not, revealing that different P-granule components serve different roles in RNAi.
MATERIALS AND METHODS
Strains:
C. elegans strains were maintained as described in Brenner (1974). Strains used were wild-type variety Bristol strain N2, LGI glh-1(bn103, bn125, gk100, ok439), glh-2(um2), glh-3(um1), glh-4(gk225), dpy5(e61), unc-13(e1091), hDf8, the GFP-tagged balancer hT2[bli-4(e937) let-?(q782) qIs48], LGIII pgl-2(bn123), LGIV pgl-1(ct131, bn102), unc-24(e138), LGV pgl-3(bn104), and dpy-11(e224). Strains were usually kept at 20° as the permissive temperature unless otherwise noted.
Isolation and sequencing of glh mutant alleles:
glh-1(gk100), glh-1(ok439), and glh-4(gk225) were isolated by the C. elegans Gene Knockout Consortium. Worm libraries that had been mutagenized with trimethylpsoralen and UV irradiation were screened by PCR for deletions in glh-1 (T21G5.3) or glh-4 (T12F5.3) (http://celeganskoconsortium.omrf.org/). A similar approach was used to isolate glh-2(um2) and glh-3(um1) (this work and Kuznicki et al. 2000). Deletion mutants were outcrossed 6–10 times before analysis. The Tc1 insertion allele glh-1(bn103) was isolated from an rde-3 (formerly called mut-2) mutator background. The rde-3/mut-2 mutation r459 was subsequently shown to map <1 cM from glh-1 (Chen et al. 2005) and is present in glh-1(bn103). glh-1(bn125) was isolated in a screen of EMS-mutagenized worms for those with diffuse GFP∷PGL-1 (as described in detail by C. Spike et al. 2008). Sequencing was performed using ABI Prism Big Dye mix and an ABI 3700 Prism automated fluorescent sequencer.
Generation of glh-1/deficiency and glh-1 trans-heterozygous worms:
To generate glh-1(gk100)/hDf8 and glh-1(bn125)/hDf8, homozygous glh-1 males were crossed to dpy-5 unc-13/hDf8 hermaphrodites. Among F1 progeny, L4 stage non-Dpy non-Unc worms were picked to individual plates at 25°, allowed to lay progeny for 24 hr, and assayed by single-worm PCR for the presence of each glh-1 allele. Among F2 progeny lacking Dpys and Uncs, worms were scored for sterility as described below. To generate glh-1(bn125)/glh-1(gk100) and glh-1(bn125)/glh-1(ok439), glh-1(gk100)/hT2 or glh-1(ok439)/hT2 males were crossed to glh-1(bn125) hermaphrodites. Among F1 progeny, L4-stage worms lacking the balancer (GFP−) were picked to individual plates, allowed to lay progeny for 24 hr at 25°, and analyzed by PCR for the presence of the deletion allele. F2 progeny were scored for sterility.
Generation of a glh-4(gk225) glh-1(ok439) double mutant:
glh-1(ok439)/hT2-GFP hermaphrodites were crossed with glh-4(gk225) homozygous males. Non-GFP F1 hermaphrodites were individually mated with GFP+ F1 males. One hundred twenty-five GFP+ F2 hermaphrodites were individually plated and tested by PCR. The progeny from two plates were verified for deletions in both glh-1 and glh-4 (which are 4.2 map units apart) and for the ability to produce non-GFP double homozygotes. The double glh-4(gk225) glh-1(ok439) is maintained over the hT2-GFP balancer.
Analysis of sterility:
L4-stage glh-1/hT2-GFP or glh-4 glh-1/hT2-GFP hermaphrodites were picked to individual plates and incubated at 16°, 20°, 24.5°, or 26°. F1 M+Z− homozygous glh adult hermaphrodite progeny were scored for sterility at each temperature. Worms that lacked embryos in their uterus were visually scored as sterile. Worms that contained material in their uterus were transferred to individual plates, to assess whether they produced viable offspring. Fertile F1 M+Z− hermaphrodites were picked to new plates and placed back at each temperature to lay progeny. F2 M−Z− hermaphrodites were scored for sterility at each temperature as described for the F1 worms.
Germ-cell counts and analysis of gametogenesis:
glh-1 M−Z− and glh-4 glh-1 M+Z− animals were shifted to 26° and their progeny fixed and stained with the DNA dye Hoechst (Shim et al. 2002) as adults, ∼24 hr after they were identified as midstage L4s. All germlines were scored for the presence of sperm and oocyte nuclei; animals used for precise germ-cell counts are a subset of the total and were selected at random. All identifiable germ cells other than sperm were counted.
DAPI staining of dissected germlines:
glh/hT2-GFP mothers were shifted from 20° to 26°, their homozygous glh/glh offspring worms (M+Z−) picked to individual plates at 26°, and their M−Z− hermaphrodite progeny analyzed as young adults, ∼1 day beyond the L4 stage. Worms were picked into 10 μl of M9 containing 5 mm levamisole on a slide and cut, and their gonads extruded using a 15-gauge needle. Samples were overlaid with a coverslip, frozen on dry ice, fixed in formaldehyde fixation solution (3.73 ml 1 m K2HPO4, 1.47 ml 1 m KH2PO4, 10 ml 16% formaldehyde, 36.8 ml ddH2O) for 1 hr at room temperature, rinsed, fixed in methanol at −20° for 5 min, washed, stained with 0.1 μg/ml DAPI, mounted in gelutol (Dupont, Wilmington, DE), and observed with a Zeiss Axioplan microscope and a Spot CCD camera.
RNAi analysis:
The glh-1 RNAi construct was generated by cloning a 1.3-kb XmaI-NcoI genomic fragment from glh-1 into L4440 (Timmons and Fire 1998). Other RNAi clones (Kamath et al. 2003) were purchased (Geneservice, Cambridge, UK). RNAi was essentially as described (Kamath et al. 2001) but used plates containing 0.2% lactose to induce dsRNA expression (E. Lambie, personal communication).
Western blot analysis:
Twenty-five glh-1(bn103), glh-1(bn125), glh-1(gk100), glh-1(ok439), and wild-type adult hermaphrodites were picked into SDS sample buffer containing protease inhibitors (2 mm PMSF, 20 μg/ml pepstatin A, 20 μg/ml leupeptin, and 5 mm aprotinin), boiled, electrophoresed on a 10% SDS–PAGE gel, and transferred to nitrocellulose membrane. After blocking in 3% nonfat dry milk, 0.1% Triton X-100 in PBS, the membrane was incubated with rabbit anti-GLH-1 at 1:5000 and with JLA20 mouse monoclonal anti-actin at 1:1000 as a loading control. After washing, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse each at 1:10,000. After washing, signal was detected using an enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Biotech, Piscataway, NJ).
Immunofluorescence microscopy:
Cut worms and embryos were fixed and stained as described in Strome and Wood (1983). Primary antibodies used were rabbit anti-GLH-1 at 1:1000 (Gruidl et al. 1996; Kawasaki et al. 2004), chicken anti-GLH-2 at 1:100 (Gruidl et al. 1996), rabbit anti-GLH-3 at 1:50 (Kuznicki et al. 2000), rabbit anti-GLH-4 at 1:50 (Kuznicki et al. 2000), rabbit anti-PGL-1 at 1:10,000 (Kawasaki et al. 1998), rabbit anti-PGL-2 at 1:500 (Kawasaki et al. 2004), rat anti-PGL-3 at 1:5000 (Kawasaki et al. 2004), rabbit anti-histone H3 phosphorylated on Ser10 at 1:200 (Upstate Biotechnology, Lake Placid, NY), rabbit anti-hyperacetylated histone H4 at 1:500 (Molecular Probes, Eugene, OR), and mouse PA3 anti-chromatin antibody at 1:500 (gift from M. Monestier; Monestier et al. 1994). Secondary antibodies used were FITC, TRITC, and Alexa488-conjugated goat anti-rabbit, anti-chicken, and anti-mouse at 1:250 (Jackson ImmunoResearch, West Grove, PA; Molecular Probes). Images were collected as single sections or Z-series projections using a Perkin Elmer Ultraview LC130E spinning-disc confocal microscope and either Ultraview software (Perkin Elmer Life Sciences, Downers Grove, IL) or MetaMorph 7 software (Molecular Devices, Sunnyvale, CA). Images were processed using Photoshop 7.0 (Adobe Systems, Palo Alto, CA) or ImageJ 1.38x (Wayne Rasband, NIH, http://rsb.info.nih.gov/ij/).
RESULTS
Molecular characterization of glh-1 mutant alleles:
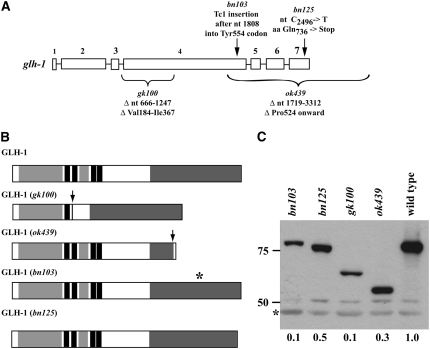
Previous RNAi depletion of each of the four GLH proteins suggested a hierarchy of GLH function, with GLH-1 being most important for germline development (Kuznicki et al. 2000). To enable genetic analysis of the roles of GLH-1, we isolated mutant alleles (Figure 1) by a combination of approaches. The C. elegans Gene Knockout Consortium isolated two deletion alleles, gk100 and ok439, by performing gene-specific PCR on DNA from collections of UV-TMP-mutagenized worms. The Tc1 insertion allele, bn103, was obtained by screening through a collection of rde-3 (formerly called mut-2) worms in which transposons had mobilized in the germline. The missense allele, bn125, was isolated in a screen of EMS-mutagenized worms (8500 haploid genomes) for mutants that cause GFP∷PGL-1 to lose its P-granule association and be distributed diffusely in the cytoplasm Spike et al. 2008. Molecular analysis of the genetic lesions and their effects on protein production are shown in Figure 1.
Figure 1.—
Mutant alleles of glh-1. (A) Schematic of the glh-1 gene, showing the positions and molecular lesions of the four mutant alleles. Coding regions are shown as boxes and introns as lines. (B) Graph of the GLH-1 polypeptide predicted to be produced by each mutant. Box with light shading is a Gly-rich region. Solid box is a CCHC zinc finger. Dark shading is a DEAD-box helicase domain. The arrows in gk100 and ok439 indicate the positions of missing amino acids. The ok439 lesion is predicted to fuse a novel 10-amino-acid stretch to the carboxy terminus of GLH-1. The asterisk in bn103 shows the position of the Tc1 insertion. bn125 removes the last 28 amino acids. (C) Western blot analysis of the GLH-1 polypeptide produced by each mutant. The blot was reacted with affinity-purified rabbit anti-GLH-1 and mouse anti-actin as a loading control (shown by an asterisk). Values beneath the lanes are the levels of GLH-1, normalized to the actin level and expressed relative to wild type.
gk100:
This allele deletes 582 nucleotides from intron 3 and exon 4 of the glh-1 gene. RT–PCR analysis of mRNA products revealed that the remaining 15 nucleotides in intron 3 are not spliced out. The predicted protein product contains a 6-amino-acid insertion and lacks 184 central amino acids (Val184–Ile367), which comprise three CCHC zinc fingers and a Gly-rich segment. Indeed, by Western blot analysis, mutant protein migrates at ∼62 kDa, consistent with its predicted size of 585 amino acids.
ok439:
In this allele, a 1594-nucleotide deletion removes the 3′ segment of coding sequence, starting in exon 4, and extends 3′ of the gene. By RT–PCR, the resulting mRNA would encode a protein product that lacks the last 240 amino acids of the protein (Pro524 onward), and thus lacks much of the DEAD-box helicase domain, and contains a novel 10-amino acid C-terminal extension. On Western blots, mutant protein migrates at ∼57 kDa, close to its predicted size of 533 amino acids.
bn103:
This allele is an insertion of a 1.6-kb Tc1 transposon after nucleotide 1808 in exon 4. RT–PCR analysis revealed several different mRNA products, all of which lacked the transposon and would be predicted to produce protein products with small deletions or insertions in the middle (near Tyr554) of the DEAD-box helicase domain. By Western blot analysis, this allele produces a reduced level (∼10% of wild type) of approximately normal-sized GLH-1 protein.
bn125:
This allele is a base-pair substitution that changes Gln736 to a premature stop codon. This would delete 28 amino acids from the carboxyl terminus of the protein. On Western blots, protein from this allele is reduced in level (∼50% of wild type) and migrates at a slightly smaller size than wild-type GLH-1.
glh-1 mutations cause temperature-sensitive sterility:
All four mutations in glh-1 cause the same spectrum of phenotypes (Figure 2 and Table 1): temperature-sensitive embryonic lethality and sterility that display a strong maternal effect. The mutations form an allelic series: strongest–gk100, ok439, bn125–weakest. We did not include bn103 in this series and in fact discontinued detailed analysis when sequencing revealed that the bn103-bearing chromosome contains a closely linked (<1 cM) rde-3/mut-2 mutation (r459; Chen et al. 2005) that was present in the parental strain in which we screened for a transposon insertion into glh-1. The rde-3/mut-2 mutation on its own causes 100% sterility at 26° (Q. Boese and J. Collins, personal communication; C. Spike, unpublished results), making it difficult to assess the impact of bn103 on germline development. One common feature of all glh-1 alleles is the variable severity of germline defects among worms and sometimes between experiments. As discussed later, we think that this is due to the sensitivity of glh-1 mutants to even slight variations in temperature and perhaps to the involvement of GLH-1 in regulation of stochastic events. Detailed phenotypic analysis is presented below.
Figure 2.—
glh-1 mutations result in sterility that is sensitive to maternal genotype and temperature and is enhanced by a glh-4 mutation. (A) Analysis of zygotic (M+Z−) and maternal-effect (M−Z−) sterility at different temperatures. The number of worms analyzed ranged from 10 to >200. (B) Graph showing the generation of M+Z− and M−Z− worms. (C) Graphical display of sterility as a function of temperature for N2 (wild-type) and M−Z− glh-1, glh-4, and glh-4 glh-1 double mutants. glh-1 sterility is relatively low at 16°, 20°, and 24.5° and high at 26°. glh-4 glh-1 sterility is high at all temperatures.
TABLE 1.
Analysis of embryos and broods produced by fertile M+Z− glh hermaphrodites at 26°
| Maternal genotype | Average % embryonic lethality (range) | Average brood size (range) |
|---|---|---|
| N2 | 0 | 168 (109–211) |
| glh-1(ok439) | 17 (0–83) | 48 (1–114) |
| glh-1(bn125) | 15 (0–94) | 49 (1–132) |
| glh-4(gk225)a | 8 (0–40) | 45 (9–80) |
| glh-4(gk225) glh-1(ok439) | 53 (0–98) | 21 (1–93) |
glh/balancer mothers were shifted from 20° to 26°, and 20 of their glh/glh offspring worms (M+Z−) were picked for analysis. M+Z− worms that produced live progeny were analyzed for percentage of embryos laid that did not hatch (% embryonic lethality) and number of live progeny produced (brood size). gk100 was not included, as M+Z− worms did not produce live progeny.
glh-4 worms are maintained as homozygotes. glh-4/glh-4 mothers were shifted from 20° to 26°, and their glh-4/glh-4 offspring worms (M−Z−) were analyzed as described above.
The glh-1 sterile phenotype shows a strong dependence on maternal genotype and on temperature. glh-1 mutants produced by heterozygous mothers inherit a maternal load of glh-1(+) product but do not synthesize zygotic product (called M+Z−, see Figure 2B). Such M+Z− hermaphrodites appear healthy but display temperature-sensitive sterility: 6–15% are sterile at 16°–20°, and 29–84% are sterile at 26° (Figure 2A). glh-1 mutants produced by homozygous mutant mothers, which lack both maternal and zygotic glh-1(+) product (M−Z−), display a similar but more severe temperature-sensitive phenotype: 6–14% are sterile at 16°–20°, and 94–100% are sterile at 26° (Figure 2, A and C). Thus, loss of maternal and zygotic glh-1(+) function results in high-penetrance (100%) sterility at elevated temperature and low-penetrance (∼10%) sterility at lower temperatures. A maternal load of glh-1(+) product can rescue the fertility of a substantial fraction of ok439 and bn125 M+Z− mutants even at elevated temperature.
The transition temperature for worms developing into fertile vs. sterile is 24°–25°. At 24.5°, the majority of M+Z− and M−Z− glh-1 mutants are fertile, whereas just 1.5° higher, the majority of M+Z− gk100 and of M−Z− ok439 and bn125 are sterile (Figure 2, A and C). Twenty-five degrees, a typically used “restrictive temperature” for many temperature-sensitive mutations in C. elegans, yielded variable results (but mainly sterile worms) from experiment to experiment. The basis for the temperature sensitivity of all glh-1 alleles is unknown but is shared by mutations in another P-granule component, PGL-1 (Kawasaki et al. 1998, 2004; see discussion).
At elevated temperature, even “fertile” glh-1 worms have severely reduced fertility. A significant percentage of their embryos fail to hatch and the number of viable offspring is small (Table 1). For example, at 26° fertile M+Z− ok439 hermaphrodites produced an average of 17% (range of 0–83%) dead embryos and an average of 48 (range of 1–114) viable progeny. Values for fertile M+Z− bn125 hermaphrodites were similar. For comparison, values for wild type were 0% dead embryos and 168 (range of 109–211) viable progeny. These results suggest that 100% of M+Z− glh-1 mutants have defective germlines at 26°. Many of those animals do not produce any viable progeny and hence are scored as sterile. The others (approximately half of the animals) produce some dead embryos and some viable embryos that develop into sterile adults.
All of the glh-1 alleles produce some protein product (Figure 1C). To address whether their phenotypes represent the null phenotype for the locus, we placed two of the glh-1 alleles over a deficiency for the region (hDf8) and placed a weak allele over the two stronger alleles and analyzed fertility/sterility among their progeny (M−Z− generation) at a borderline temperature (25°) (Table 2). gk100/hDf8 hermaphrodites, like gk100/gk100 homozygotes, displayed 100% sterility. bn125/hDf8 hermaphrodites displayed higher sterility than bn125/bn125, consistent with bn125 being a weak allele. bn125/gk100 and bn125/ok439 resembled bn125/hDf8 in displaying a higher percentage of sterility than bn125/bn125. Thus, gk100 and ok439 both behave as strong loss-of-function alleles.
TABLE 2.
Phenotypic analysis of glh-1/hDf8 and glh-1 heteroallelic combinations
| Maternal genotype | % sterile progeny (n) |
|---|---|
| N2 | 0.5 (1216) |
| glh-1(gk100) | 100 (144) |
| glh-1(ok439) | 100 (216) |
| glh-1(bn125) | 33 (601) |
| glh-1(gk100)/hDf8 | 100 (160) |
| glh-1(bn125)/hDf8 | 63 (236) |
| glh-1(bn125)/glh-1(gk100) | 72 (253) |
| glh-1(bn125)/glh-1(ok439) | 57 (443) |
L4-stage M+Z− hermaphrodites were picked to individual plates at 25° and allowed to produce progeny for 24 hr. M−Z− progeny were scored as fertile or sterile by visual examination.
Causes of sterility in glh-1 mutants:
To investigate why M−Z− animals develop into sterile adults at elevated temperature (26°), we Hoechst stained DNA in intact hermaphrodites, counted germ nuclei, and examined germlines for the presence of gametes. We observed dramatic decreases in both germ-cell counts and presence of gametes and also considerable variation from worm to worm (Table 3). In contrast to the ∼800 germ nuclei observed in wild-type gonad arms, gonad arms in glh-1(gk100) hermaphrodites contained from 0 to ∼400 germ nuclei (mean = 193). The extreme variation in severity of germline proliferation defects is exemplified by the fact that 38% of gonad arms contained 0 or very few germ nuclei, and 31% contained >250 germ nuclei. Thirty percent of gonad arms contained oocytes, but only 2% contained sperm, and none of the worms examined contained embryos. ok439 and bn125 mutants displayed similar but slightly milder defects. These results demonstrate that glh-1 germlines are defective in germ-cell proliferation and in production of gametes. Figure 3 shows representative images of glh mutant germlines stained with DAPI. Table 4 documents that, while 100% of wild-type gonads contained mitotically dividing cells (judged by staining with antibody to phosphorylated histone H3 Ser10; Hendzel et al. 1997) in their distal stem-cell region, many glh-1 mutant gonads with reasonable numbers of germ nuclei lacked phosphorylated H3 Ser10-marked mitotic cells. This supports the notion that glh-1 mutant germ nuclei are impaired in their stem-cell division capacity.
TABLE 3.
Germline development in glh mutant hermaphrodites
| Maternal genotype | Temperature | Average no. of germ nuclei per gonad arm (range)a | % nearly empty gonad arms | % gonad arms lacking oocytesb | % gonad arms lacking spermb | % worms lacking embryos |
|---|---|---|---|---|---|---|
| N2 | 26° | 787 (691–872) | 0 | 2 | 6 | 0 |
| glh-1(gk100) | 26° | 193 (0–407)c | 38c | 70 | 98 | 100 |
| glh-1(ok439) | 26° | 287 (0–525)c | 20c | 43 | 68 | 86 |
| glh-1(bn125) | 26° | NDd | 14 | 43 | 33 | 82 |
| glh-4(gk225) | 26° | NDd | 0 | 4 | 7 | 0 |
| glh-4(gk225) glh-1(ok439) | 26° | 127 (0–667)ce | 68ce | 74e | 80e | 92 |
| glh-4(gk225) glh-1(ok439) | 20° | NDd | 39e | 50e | 42e | 63 |
L4-stage M−Z− worms from glh-1/balancer grandmothers were picked to individual plates and shifted from 20° to 26°. Their adult progeny were analyzed (24 hr after L4).
Number of gonad arms examined was 8–30. Germ-cell counts included oocytes.
Number of gonad arms examined was 42–98 for mutants, 184 for N2.
In gk100 worms, 13% of gonad arms examined had 0 germ nuclei; 31% had >250 germ nuclei. In ok439 worms, 14% of gonad arms examined had 0 germ nuclei; 64% had >250 germ nuclei. In glh-4 glh-1 doubles, 63% of gonad arms examined had 0 germ nuclei; 27% had >250 germ nuclei.
Not done.
L4-stage M+Z− progeny from glh-4 glh-1/balancer mothers were picked to individual plates and shifted from 20° to 26°. Their adult progeny were analyzed (24 hr after L4).
Figure 3.—
glh-1 mutants display a range of germline sizes and gamete defects. glh/hT2∷gfp mothers were shifted from 20° to 26°. Homozygous glh/glh M−Z− hermaphrodites were DAPI stained ∼1 day beyond the L4 stage. Images represent a common phenotype for each strain. (A) Wild type. (B) glh-1(bn125) large germline. (C) glh-1(ok439) large germline containing endomitotically replicating oocytes (arrows). (D) glh-1(ok439) small germline. (E) glh-1(gk100) small germline. (F) glh-4(gk225) “Y”-shaped gonad observed in 1–15% of gonad arms. (G) glh-4(gk225) glh-1(ok439) very small germline. Bars for A–F and G, 50 μm.
TABLE 4.
Analysis of mitotic germ cells in glh mutants at 25°
| Maternal genotype | % gonad arms lacking phospho-H3 Ser10 staining (n = 40) |
|---|---|
| N2 | 0 |
| glh-1(gk100) | 36 |
| glh-1(ok439) | 70 |
| glh-1(bn125) | 18 |
| glh-4(gk225) glh-1(ok439) | 92 |
L4-stage N2 and glh-1 M−Z− mutants were picked to individual plates and shifted from 20° to 25°. The germlines of their adult progeny were analyzed. N2 germlines contained an average of six stained nuclei.
glh-1 mutants show defects in assembly or stability of P granules:
Since each of the glh-1 mutant alleles produces protein that is detectable by Western blot analysis, we investigated the distributions of the various mutant forms of protein. In wild-type worms, GLH-1 is concentrated on P granules in larval and adult germlines and in embryos (Gruidl et al. 1996). In contrast, in gk100, ok439, and bn103 mutant hermaphrodites, the majority of GLH-1 is distributed uniformly in the cytoplasm in germlines and embryos (Figure 4 and data not shown). The weak allele bn125 causes a mild elevation in the level of diffuse cytoplasmic GLH-1, but much of the mutant protein remains associated with P granules. The altered distributions of mutant forms of GLH-1 are observed at low as well as high temperatures (Figure 4 shows M−Z− worms grown at 20°). As described above, low-temperature-grown animals generally develop into fertile worms. Thus, dissociation of the majority of GLH-1 from P granules does not necessarily lead to sterility, although it may be a contributing factor.
Figure 4.—
Two mutant forms of GLH-1 are not detectably associated with P granules. glh M−Z− hermaphrodites derived from glh/hT2∷gfp grandmothers at 20° were cut, fixed, and stained with mouse PA3 to stain DNA (A, C, E, G, and I) and with rabbit anti-GLH-1 (B, D, F, H, and J) and imaged by confocal microscopy. Each panel shows a single ∼0.5-μm optical section in the pachytene region of the germline. (A and B) Wild type. (C and D) glh-1(gk100). (E and F) glh-1(ok439). (G and H) glh-1(bn125). (I and J) glh-4(gk225) glh-1(ok439). The alleles gk100 and ok439 cause dispersal of GLH-1 from P granules (D, F, and J). The bn125 allele causes only modest dispersal (H). Bar, 10 μm.
To determine the impact of glh-1 mutations on P-granule integrity, we investigated the distributions of several other P-granule proteins in glh-1 M−Z− mutants grown at 20° (Figures 5 and 6). GLH-4 is concentrated in granules in glh-1 mutants. However, in gk100 and ok439 mutant germlines, many of the GLH-4-containing granules have lost their perinuclear association (Figure 5). The distributions of PGL-1, PGL-2, and PGL-3 are more dramatically altered in glh-1 mutants. PGL-1 and PGL-3 were studied in detail in gk100 and ok439 mutants. Both PGL-1 and PGL-3 appear to be completely dispersed in the cytoplasm in some regions of the germline (e.g., the pachytene region) but partially concentrated on P granules in other regions of the germline (e.g., mitotic region and diplotene) (Figure 6 and data not shown). In early-stage mutant embryos, a fraction of PGL-1 and PGL-3 is associated with P granules while a higher-than-normal fraction is dispersed in the cytoplasm. Later-stage embryos show diminished staining of PGL-1 and PGL-3, but display clear concentration of both proteins on P granules (Figure 6 and data not shown). bn125 displays some dissociation of PGL-1 and PGL-3 from P granules, but generally resembles wild type more than the other three alleles. Thus, all alleles of glh-1 cause PGL-1 and PGL-3 to be dissociated from P granules, to a greater or lesser extent depending on the stage of development.
Figure 5.—
GLH-4 granules are not properly concentrated around nuclei in fixed glh-1 mutants. Worms were prepared as for Figure 4, but stained with mouse PA3 to stain DNA (A, C, and E) and with rabbit anti-GLH-4 (B, D, and F). Each panel shows a single ∼0.5-μm confocal section of the pachytene region of the germline. (A and B) Wild type. (C and D) glh-1(gk100). (E and F) glh-1(ok439). The alleles gk100 and ok439 cause some GLH-4 granules to lose their perinuclear localization (D and F). Bar, 10 μm.
Figure 6.—
PGL-1 is not properly concentrated on P granules in fixed glh-1 and glh-4 glh-1 mutants. Worms were prepared as for Figure 4, but stained with mouse PA3 to stain DNA (A, C, E, and G) and with rabbit anti-PGL-1 (B, D, F, and H). Each panel shows a single ∼0.5-μm confocal section of the distal tip plus pachytene region of the germline, and two panels include a 100- to 200-cell embryo as well. (A and B) Wild type. (C and D) glh-1(gk100). (E and F) glh-1(ok439). (G and H) glh-4(gk225) glh-1(ok439). The alleles gk100 and ok439 cause some PGL-1 to become dispersed in the cytoplasm, especially in the pachytene region (D and F). PGL-1 is completely dispersed in glh-4 glh-1 double mutants (H). Bar, 10 μm.
To assess PGL-1 association with P granules in living glh-1 mutant worms, we used a transgenic strain that expresses GFP∷PGL-1 in the maternal germline and in early embryos (using pie-1 regulatory sequences) (Cheeks et al. 2004). The distribution of GFP, which is bright in worms grown at ≥24.5°, recapitulates the subcellular distribution of PGL-1 observed by fixing and staining samples with anti-PGL-1 antibody: concentrated on P granules at all stages examined. Screening in this strain for mutants with dispersed GFP∷PGL-1 was the method used to isolate bn125 (Figure 7). After introducing the ok439 and bn103 mutations into the GFP∷PGL-1 line, we observed GFP∷PGL-1 mainly dispersed in the cytoplasm (Figure 7 and data not shown). To investigate the temporal requirement for GLH-1, we used RNAi to deplete GLH-1 from GFP∷PGL-1-expressing worms. RNAi was performed by feeding worms bacteria expressing glh-1 dsRNA starting at the L4 stage. Within 24 hr, GFP∷PGL-1 was notably dispersed in the cytoplasm in adult germline tissue and in embryos (not shown). This suggests an ongoing requirement for the presence of adequate levels of GLH-1 in P granules for PGL-1 to be efficiently recruited and/or retained on granules.
Figure 7.—
GFP∷PGL-1 is not properly concentrated on P granules in living glh-1 mutants. Live worms containing a GFP∷PGL-1 transgene were imaged by confocal microscopy. Images represent single ∼0.5-μm optical sections. (A and B) Wild type. (C and D) glh-1(bn103). (E and F) glh-1(bn125). The majority of GFP∷PGL-1 is dispersed in the cytoplasm of glh-1(bn103) embryos (C) and germlines (D). glh-1(bn125) worms display less dramatic dispersal of GFP∷PGL-1. Bars, 10 μm.
glh-2 and glh-3 mutants do not display dramatic phenotypes on their own, while glh-4 mutants show striking pleiotropic effects at high temperature:
In previous RNAi studies, depletion of GLH-2 caused significant levels of sterility while depletion of GLH-3 or GLH-4 did not cause notable germline defects (Kuznicki et al. 2000). Because of the uncertainties associated with RNAi, we isolated mutant alleles of each gene (Figure 8), to enable genetic analysis.
Figure 8.—
Mutant alleles of glh-2, glh-3, and glh-4. A schematic of each glh gene shows the position and extent of each deletion allele. Coding regions are shown as boxes and introns as lines.
glh-2(um2):
This 2873-nucleotide deletion extends from exon 2 through exon 8. The sequence remains in frame and if translated would produce a small protein of ∼130 amino acids. In fact, a chicken antibody generated against an N-terminal GLH-2 peptide does show some residual P-granule staining in fixed glh-2 mutant worms (not shown). The deletion removes all six CCHC zinc fingers and the DEAD-box helicase domain, which are likely essential for protein function.
glh-3(um1):
This 1811-nucleotide deletion extends from exon 2 through exon 4. If translated, the resultant protein would contain only the first 90 amino acids and would lack both the two CCHC zinc fingers and the DEAD-box helicase domain. An affinity-purified rabbit antibody raised against a C-terminal peptide does not recognize any GLH-3 protein in the mutant background (Kuznicki et al. 2000).
glh-4(gk225):
This allele deletes 590 nucleotides, including the first 280 nucleotides of coding sequence in exon 1. A rabbit anti-GLH-4 antibody raised against both an N-terminal peptide and a full-length GLH-4 fusion protein does not recognize GLH-4 protein in the mutant strain by immunocytochemistry or by Western blot analysis (supplemental Figure 1 and data not shown). Western blots with the anti-GLH-4 antibody also verify that the glh-4-like gene on cosmid T08D2 (Kuznicki et al. 2000) does not produce detectable protein, as predicted if T08D2.3 is a pseudogene.
All three of these strains were examined for sterility and brood size after being raised at 15°, 20°, and 26°. None of the three mutants displayed significant sterility at any temperature. glh-2(um2) and glh-3(um1) worms produced modestly reduced broods at 15° (25% below wild type for glh-2 and 30% below wild type for glh-3) and close to wild-type broods at 20° and 26°. glh-4(gk225) worms grown at 26° for two generations have reduced broods (Table 1) and consistently show a remarkable phenotype in a small percentage (1–15%) of the worms examined: the distal gonad is bifurcated, either slightly or completely (Figure 3F). Bifurcated gonad arms were observed to have a distal tip cell on each tip (two of seven gonads) or only on one tip (five of seven gonads). The mechanism by which bifurcations arise is being investigated. Bifurcations are occasionally seen in glh-1(RNAi) worms. Thus, gonad bifurcation appears to be a mutant phenotype shared by multiple glh genes.
glh-1 and glh-4 function redundantly:
In previous RNAi studies, simultaneous depletion of GLH-1 and GLH-4 caused enhanced sterility compared to depletion of GLH-1 alone (Kuznicki et al. 2000). Analysis of whether sterility was zygotic or maternal effect was not done. To further investigate a potential genetic interaction between glh-1 and glh-4, we generated a glh-4(gk225) glh-1(ok439) double-mutant strain. Compared to glh-1(ok439) alone, the double mutant showed enhanced zygotic and maternal-effect sterility at all temperatures tested (Figure 2). The enhancement was most dramatic at low temperatures. For example, at 16° and 20° zygotic sterility was elevated from 11–15% in glh-1 alone to 25–38% in glh-4 glh-1 double mutants. More dramatically, maternal-effect sterility was elevated from 10–11% in glh-1 alone to 97–100% in glh-4 glh-1 double mutants. At high temperature (26°) M+Z− glh-4 glh-1 double-mutant hermaphrodites produced a higher percentage of dead embryos and smaller broods than glh-1 alone (Table 1), and M−Z− glh-4 glh-1 double-mutant hermaphrodites displayed more severe germline defects than glh-1 alone (Figure 3, Tables 3 and 4). For example, 63% of M−Z− glh-4 glh-1 double mutants lacked germ cells altogether, compared to 14% of M−Z− glh-1 alone (Table 3 footnote). Thus, on the basis of analyzing both percentage of sterile worms and germline development in sterile worms, glh-4 glh-1 double mutants show more severe defects than glh-1 alone. This finding suggests that GLH-1 and GLH-4 function redundantly. In the absence of GLH-1, GLH-4(+) provides sufficient GLH function to render most worms fertile at 16°–24.5°; GLH-4(+) is not sufficient for fertility at 26°. We note that glh-1(ok439) mutant worms containing a gfp∷glh-4 transgene were easily propagated at 26°. This result suggests that boosting the level of GLH-4 can compensate for the loss of GLH-1 function at 26° and is consistent with the two GLH proteins being functionally redundant.
The distributions of PGL-1 and PGL-3 were examined in the germlines of sterile M−Z− glh-4 glh-1 double mutants. Both proteins were completely dispersed in the cytoplasm (Figure 6 and data not shown). Thus, loss of GLH-4 enhances the P-granule phenotype observed in glh-1 single mutants. It is likely that dispersal of P-granule proteins is a primary defect in double mutants and not a secondary consequence of other defects in those sterile germlines (see discussion).
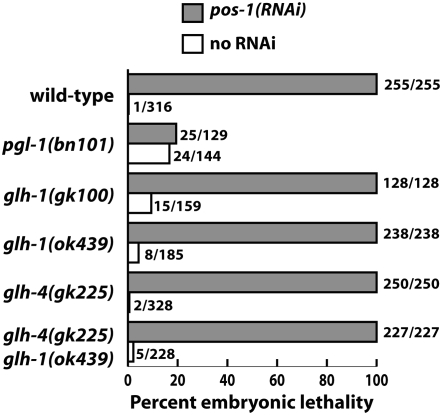
glh mutants are not defective in RNAi:
pgl-1 mutant worms are defective in RNAi (Robert et al. 2005; Figure 9). This finding raised the question whether it is PGL-1 in particular or P granules in general that are critical for efficient RNAi. To address that, we tested whether glh-1 mutant worms are also defective in RNAi. Neither gk100 nor ok439 mutants displayed resistance to pos-1 RNAi (Figure 9) or skn-1 RNAi (data not shown). Similarly, glh-4 single mutants and glh-4 glh-1 double mutants did not display resistance to RNAi (Figure 9). These results suggest that particular components of P granules are critical for RNAi and others are not. Furthermore, loss of PGL-1 (in pgl-1 mutants) produces much more severe effects on RNAi than dissociation of the majority of PGL-1 from P granules (in glh-1 mutants).
Figure 9.—
glh-1 mutants and glh-4 glh-1 double mutants are not resistant to RNAi. Worms of the genotypes shown were fed bacteria expressing dsRNA against pos-1 and allowed to produce embryos at 20°. The following strains and generations were used: glh-1(ok439), glh-4(gk225), and pgl-1(bn101), M−Z− generation; glh-1(gk100) and glh-4(gk225) glh-1(ok439), M+Z− generation. pgl-1 mutants were resistant to pos-1 RNAi, as reported by Robert et al. (2005). The other strains were sensitive. glh-1(gk100) M−Z− animals also appear to be sensitive to pos-1(RNAi) (95% lethality), but glh-1(gk100) M−Z− animals not exposed to pos-1 dsRNA also laid a significant percentage of eggs that died (51%).
DISCUSSION
glh mutant phenotypes:
Genetic analysis of the GLH family of genes has revealed a hierarchy of gene importance. GLH-1 is critical for fertility in worms grown at elevated temperature (26°): loss of zygotically encoded GLH-1 function results in significant sterility, and loss of both maternally supplied and zygotically encoded GLH-1 results in 100% sterility. Lower-temperature growth (16°–24.5°) rescues the fertility of most glh-1 mutant animals. Worms lacking GLH-2, GLH-3, or GLH-4 function are generally fertile at all temperatures. Concomitant loss of both GLH-1 and GLH-4 function causes the same strong maternal, weaker zygotic sterility as described for GLH-1, but independent of growth temperature. Thus, in the absence of GLH-1 function, GLH-4 promotes healthy germline development in worms grown at lower temperatures. GLH-2 and GLH-3 may contribute as well but are not sufficient to confer fertility in the absence of both GLH-1 and GLH-4.
A hierarchy of gene importance is also observed for the PGL family of P-granule proteins. PGL-1 is the most important. pgl-1 mutants display phenotypes very similar to glh-1 mutants: strong maternal and weaker zygotic sterility, predominantly at elevated temperature. Worms lacking the related proteins PGL-2 or PGL-3 are fertile at all temperatures. PGL-3 is the key redundant PGL factor: concomitant loss of both PGL-1 and PGL-3 function causes strong maternal and weaker zygotic sterility independent of growth temperature (Kawasaki et al. 2004).
One noteworthy aspect of the glh-1 (and pgl-1) mutant phenotypes is the extreme variability. For example, glh-1(ok439) M+Z− hermaphrodites grown at 26° produce broods that display from 0 to 83% embryo lethality, and the number of viable offspring ranges from 1 to >100. Although all of the viable M−Z− offspring develop into sterile adults, their germlines contain widely varying numbers of germ cells, from 0 to >500. Such variability is observed even in glh-4 glh-1 double mutants. We envision that this variability is due to variable accumulation of defects caused by gradual loss of P-granule function in M+Z− and then in M−Z− worms. In the absence of zygotically expressed GLH-1, which normally commences in late embryogenesis (Y. Kohara, personal communication), the maternal load of P granules must retain enough function to enable M+Z− worms to develop well-proliferated germlines, but not enough function to ensure production of uniformly good gametes and embryos. In the next generation of worms that lack both maternal and zygotic GLH-1, germ-cell proliferation is also compromised. To get around the complications of gradual loss of wild-type protein in first-generation homozygous mutants, it would be useful to have a thermolabile version of GLH-1 that would allow rapid protein inactivation. Such an allele of dynein heavy-chain DHC-1 was valuable in dissecting the requirement for dynein function at different stages of development (Schmidt et al. 2005).
Another noteworthy feature of the glh-1 and pgl-1 mutant phenotypes is the sensitivity to temperature. Instead of being due to temperature-sensitive lesions, it appears that strong loss-of-function and null alleles cause mild phenotypes at low temperatures and much more severe phenotypes at elevated temperature. For glh-1 and pgl-1, the mild-to-severe transition temperature is 24°–25°. Although wild-type animals are fully fertile at 26°, that temperature is likely to stress worms' ability to reproduce, such that worms are especially sensitive to loss of important factors such as GLH-1 and PGL-1. At lower temperatures, other GLH and PGL family members provide sufficient function for good fertility. However, loss of both GLH-1 and GLH-4 or loss of both PGL-1 and PGL-3 at low temperatures recapitulates the defects observed in GLH-1 and PGL-1 single mutants at high temperature. In fact, elevated temperature enhances the phenotypes of many germline-required genes, such as him-17 (Reddy and Villeneuve 2004), hpl-2 (Couteau et al. 2002), rha-1 (Walstrom et al. 2005), mes-1 (Strome et al. 1995), deps-1 (C. Spike et al. 2008), prg-1 (Yigit et al. 2006), several eri genes (Duchaine et al. 2006), kgb-1 (Smith et al. 2002; Orsborn et al. 2007), vbh-1 (Salinas et al. 2007), and mex-3 gld-1 double mutants (Ciosk et al. 2006; R. Ciosk, personal communication), and enhances the expression of transgenes in the germline (Strome et al. 2001). Performing screens for germline mutants at high temperature may be analogous to screening in a sensitized background and may identify genes and gene families that would have been missed at lower temperatures.
The defects observed in M−Z− glh-1 mutants at elevated temperature and in glh-4 glh-1 double mutants at all temperatures suggest that the GLHs and probably germ granules in general serve diverse roles in the germline: promotion of stem cell divisions, production of adequate numbers of gametes, and production of high-quality gametes capable of successful embryogenesis after fertilization. RNAi depletion of GLH family members caused a similar range of defects but with a different gamete bias than observed in mutants. While RNAi-induced sterile worms usually contained sperm and only rarely contained oocytes (Kuznicki et al. 2000), M−Z− glh-1(gk100) and glh-1(ok439) single mutants and glh-4 glh-1(ok439) double mutants infrequently (2–32%) contained sperm, and a significant percentage (26–57%) contained oocytes (Table 3). The reason for this difference is not known. Now that glh-4 glh-1 double mutants exist, in-depth analysis and efforts to identify specific RNA targets of GLH regulation can be undertaken (see below).
Vasa regulates the translation of several mRNAs (Dahanukar and Wharton 1996; Markussen et al. 1997; Styhler et al. 1998) and is implicated in miRNA control of gene expression (Kotaja et al. 2006). Evidence from C. elegans suggests that P-granule components could be involved in mRNA trafficking, translation, stability and RNAi-related processes (Schisa et al. 2001, Seydoux and Braun 2006, C. Spike et al. 2008). Since glh-1(ok439) germlines have P-granule assembly defects even at low temperatures (this work), we performed a genomewide microarray analysis to look at mRNA accumulation patterns in glh-1(ok439) M−Z− germlines at 20° (C. Spike and V. Reinke, unpublished results). Our preliminary analysis of those data indicates that few mRNAs are misregulated in glh-1 mutant germlines (only eight genes were misregulated ≥1.8-fold relative to wild type), suggesting that GLH-1 and normally assembled P granules do not generally regulate mRNA stability. These conclusions are consistent with a similar analysis performed on the M−Z− germlines of another mutant required for P-granule assembly (deps-1; C. Spike et al. 2008). We note, however, that microarray analysis of glh-1(ok439) germlines at low temperature would not be expected to reveal mRNAs/genes that are redundantly regulated by both GLH-1 and GLH-4. Such targets remain to be identified and may shed light on the diverse roles of the GLHs and germ granules.
P-granule assembly and roles:
Molecular epistasis results place the GLH proteins “upstream” of the PGL proteins in a P-granule assembly pathway. pgl-1; pgl-2; pgl-3 triple mutants display an apparently normal concentration of GLH-1 in perinuclear P granules (Kawasaki et al. 2004), revealing that the PGL proteins are not required for GLH-1 recruitment or retention in P granules. In contrast, glh-1 mutants display partial dispersal of the PGL proteins, and glh-4 glh-1 double mutants display full dispersal. We think the latter is probably a direct effect of loss of both GLH-1 and GLH-4 function and not an indirect effect of germline sickliness, as analysis of many sterile mutants, including pgl-1; pgl-2; pgl-3 triple mutants, demonstrates that P granules retain their granular nature even in severely compromised germlines (Kawasaki et al. 2004; S. Strome, unpublished results). The finding that RNAi depletion of GLH-1 from L4-stage worms results in dispersed GFP∷PGL-1 within 24 hr (C. Spike, unpublished results) suggests that P granules are likely to be dynamic structures and that GLH-1 is continuously required for PGL-1 retention. In glh-1 single mutants, at least some PGL-1 remains associated with granules in the distal region but not in the pachytene region (this study). In the future, electron microscopy may shed light on stage-specific variations in P-granule structure and perhaps function in the germline.
As suggested above, GLH-1 and GLH-4 may participate directly or indirectly in the recruitment or retention of PGL proteins on P granules. Another possibility is that only functional GLH and PGL proteins associate with P granules. Mutant forms of GLH-1 may be nonfunctional and therefore dispersed in the cytoplasm. Our analysis of glh-1 mutants indicates that two different regions of GLH-1 are required for its association with P granules: the DEAD-box helicase domain and a Gly-rich region containing 3 CCHC zinc fingers. DEAD-box helicase and CCHC domains are able to bind RNA (Dannull et al. 1994; Sengoku et al. 2006), suggesting that the association of GLH-1 with RNA might be critical for its association with RNA-rich P granules. Alternatively, GLH-1 proteins lacking the helicase domain or zinc fingers might fail to localize to P granules because they are misfolded. If GLH-1 is required for proper PGL protein function, then the dispersal of PGL proteins in glh-1 mutant germlines may be a result of loss of PGL function. This scenario resembles one model emerging for P-body assembly and function (Eulalio et al. 2007b). P bodies are cytoplasmic RNP particles that are the probable sites of mRNA decapping and decay; they were first described in yeast and subsequently observed in mammals, flies, and worms (reviewed in Eulalio et al. 2007a). In Drosophila tissue culture cells, the formation of P bodies appears to be the consequence of active processes, such as RNAi, in which the components of P bodies participate (Eulalio et al. 2007b).
Intriguingly, mutations in pgl-1 and in another P-granule gene deps-1 lead to a germline RNAi-defective (Rde) phenotype (Robert et al. 2005; C. Spike et al. 2008; this study), suggesting potential links between P granules and RNAi. However, since (1) glh-1 mutants and glh-4 glh-1 double mutants do not show defects in RNAi and (2) PGL-1 and PGL-3 are completely dispersed from perinuclear granules in glh-4 glh-1 double mutants, localized PGL-1 and intact P granules do not appear to be essential for RNAi. This scenario also resembles what is currently known about the relationship between RNAi and P bodies. Although P bodies require active processes such as RNAi to form, intact P bodies in Drosophila tissue culture cells are not required for effective RNAi (Eulalio et al. 2007b).
Because P granules share many components with P bodies, including the C. elegans proteins CGH-1 and CAR-1, it is not surprising that several reports have suggested that C. elegans P granules are related to P bodies (Wickens and Goldstrohm 2003; Seydoux and Braun 2006; Strome and Lehmann 2007). P bodies can store mRNAs that are silenced by microRNAs, and they contain RNA-induced silencing complex (RISC) proteins, including ALG-1/PIWI/Argonaute (Chan and Slack 2006; Jakymiw et al. 2007). Another P-body-associated protein in fission yeast is Dicer, the RNAseIII enzyme essential for creating siRNAs used for RNAi and for processing microRNAs, the noncoding, small RNAs important in development (Carmichael et al. 2006). Dicer is also in the chromatoid bodies of mouse male germ cells and physically associates with the mouse Vasa homolog MVH (Kotaja et al. 2006). Another study, using Drosophila protein lysates enriched for polar granules, recently reported that PIWI, a fly RISC protein, forms a complex with DICER-1 to regulate levels of Vasa (Megosh et al. 2006). Thus, RISC proteins with microRNA functions are associated with germ granules and interact with Vasa homologs in flies and mice. GLH-1 also has recently been found to have both a physical and a genetic relationship to DCR-1 (E. Racen and K. Bennett, unpublished results). Since glh-1 and glh-4 glh-1 mutants are not RNAi resistant (this work), these GLHs could have a relationship related to microRNAs rather than to RNAi.
Germline-required DEAD-box helicases:
Drosophila Vasa was the first protein component of germ plasm to be molecularly identified (Hay et al. 1988b). Vasa homologs were subsequently identified in numerous species, including worms, mice, chickens, frogs, zebrafish, leeches, sea urchins, brine shrimp, and water fleas and, in fact, are widely used markers of germ cells (reviewed in Raz 2000). Many of these homologs have been shown to be enriched in germ plasm and important for germline development (Extavour and Akam 2003). While Vasa is a single-copy gene in Drosophila and in numerous other systems, in C. elegans Vasa-related proteins are encoded by the four glh genes described here and a second family of genes (vbh-1, Y71H2AM.19) related both to Vasa and to the DEAD-box RNA helicase known as Belle (Johnstone et al. 2005; Salinas et al. 2007). Both gene families are required for normal germ-cell function and have members that localize to P granules (Gruidl et al. 1996; Kuznicki et al. 2000; Salinas et al. 2007). Two of the C. elegans GLHs (GLH-1 and GLH-4) and D. melanogaster Vasa have roles in germ-granule assembly (Breitwieser et al. 1996; Schisa et al. 2001; this work).
The similarity between the GLHs and Vasa with respect to germ-granule assembly is striking considering the protein features that distinguish Caenorhabditis GLH proteins from most other Vasa homologs. First, the Caenorhabditis GLH Gly-rich N termini are in the form of FG and FGG repeats, in contrast to RG and RGG repeats found in Drosophila and vertebrate Vasa homologs. FG repeats may mediate the association of FG-rich Vasa homologs with each other (Smith et al. 2002) or with FG-rich nuclear pore proteins (Frey et al. 2006), whereas RGG repeats probably mediate the association of RG-rich Vasa homologs with RNAs (Kiledjian and Dreyfuss 1992). Second, all of the Caenorhabditis GLH proteins possess several (two to six) CCHC-type zinc fingers not found in Drosophila and vertebrate Vasa homologs (see phylogenetic analysis in Salinas et al. 2007). Perhaps the CCHC zinc fingers participate in RNA binding and compensate for the lack of an RGG domain in GLH family members. Interestingly, Ciona Vasa possesses a mix of FG and RG repeats, and the Vasa homologs in Ciona, Daphnia, Hydra, Artemia, and several other marine invertebrates contain CCHC zinc fingers (Fujimura and Takamura 2000; Sagawa et al. 2005).
The closely related nematode C. briggsae contains a glh-1-related gene and a glh-4-related gene Stein et al. 2003. The predicted GLH-1 protein in C. briggsae shows highest sequence identity in the helicase domain to CeGLH-1 (82%), followed by CeGLH-2 (81%), CeGLH-3 (71%), and CeGLH-4 (36%). CbGLH-1 and CeGLH-1 also share a 240- to 270-aa N-terminal FG-rich domain. We speculate that C. elegans glh-2 and glh-3 arose from glh-1 by gene duplication events followed by expansion of the FG domain in the case of glh-2 and loss of the FG domain in the case of glh-3. C. elegans and perhaps C. briggsae rely on a division of labor between the two most divergent GLHs, GLH-1 and GLH-4, for normal germ-granule assembly and germ-cell proliferation (this work). It is not clear what roles the other C. elegans GLHs play during germ-cell development, but GLH-2 was recently identified as a factor that associates with sperm chromatin (Chu et al. 2006), suggesting that one of the GLHs may function in the nucleus as well as on P granules.
Acknowledgments
We thank Emily Gressman-Coberly for helping isolate the glh-2(um2) and glh-3(um1) alleles and Steve Dunkelbarger for generating the glh-1(RNAi) construct. This work was supported by Ruth Kirchstein National Research Service Award postdoctoral fellowship GM69084 (C.S.), American Cancer Society postdoctoral fellowship PF-04-034-01-DDC (C.S.), a University of Missouri Life Science Fellowship (A.O.), National Institutes of Health (NIH) training grant GM008396 (A.O. and E.R.), NIH grant GM34059 (S.S.), and National Science Foundation grants IBN 0078169 and IBN 0415699 (K.B.). Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources.
References
- Breitwieser, W., F. H. Markussen, H. Horstmann and A. Ephrussi, 1996. Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev. 10 2179–2188. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael, J. B., C. Stoica, H. Parker, J. M. McCaffery, A. J. Simmonds et al., 2006. RNA interference effector proteins localize to mobile cytoplasmic puncta in Schizosaccharomyces pombe. Traffic 7 1032–1044. [DOI] [PubMed] [Google Scholar]
- Chan, S. P., and F. J. Slack, 2006. MicroRNA-mediated silencing inside P-bodies. RNA Biol. 3 97–100. [DOI] [PubMed] [Google Scholar]
- Cheeks, R. J., J. C. Canman, W. N. Gabriel, N. Meyer, S. Strome et al., 2004. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr. Biol. 14 851–862. [DOI] [PubMed] [Google Scholar]
- Chen, C. C., M. J. Simard, H. Tabara, D. R. Brownell, J. A. McCollough et al., 2005. A member of the polymerase beta nucleotidyltransferase superfamily is required for RNA interference in C. elegans. Curr. Biol. 15 378–383. [DOI] [PubMed] [Google Scholar]
- Chu, D. S., H. Liu, P. Nix, T. F. Wu, E. J. Ralston et al., 2006. Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature 443 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosk, R., M. DePalma and J. R. Priess, 2006. Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science 311 851–853. [DOI] [PubMed] [Google Scholar]
- Couteau, F., F. Guerry, F. Muller and F. Palladino, 2002. A heterochromatin protein 1 homologue in Caenorhabditis elegans acts in germline and vulval development. EMBO Rep. 3 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar, A., and R. P. Wharton, 1996. The Nanos gradient in Drosophila embryos is generated by translational regulation. Genes Dev. 10 2610–2620. [DOI] [PubMed] [Google Scholar]
- Dannull, J., A. Surovoy, G. Jung and K. Moelling, 1994. Specific binding of HIV-1 nucleocapsid protein to PSI RNA in vitro requires N-terminal zinc finger and flanking basic amino acid residues. EMBO J. 13 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine, T. F., J. A. Wohlschlegel, S. Kennedy, Y. Bei, D. Conte, Jr. et al., 2006. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell 124 343–354. [DOI] [PubMed] [Google Scholar]
- Ephrussi, A., and R. Lehmann, 1992. Induction of germ cell formation by oskar. Nature 358 387–392. [DOI] [PubMed] [Google Scholar]
- Ephrussi, A., L. K. Dickinson and R. Lehmann, 1991. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66 37–50. [DOI] [PubMed] [Google Scholar]
- Eulalio, A., I. Behm-Ansmant and E. Izaurralde, 2007. a P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell. Biol. 8 9–22. [DOI] [PubMed] [Google Scholar]
- Eulalio, A., I. Behm-Ansmant, D. Schweizer and E. Izaurralde, 2007. b P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 27 3970–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extavour, C. G., and M. Akam, 2003. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130 5869–5884. [DOI] [PubMed] [Google Scholar]
- Fox, M. S., A. T. Clark, M. El Majdoubi, J. L. Vigne, J. Urano et al., 2007. Intermolecular interactions of homologs of germ plasm components in mammalian germ cells. Dev. Biol. 301 417–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, S., R. P. Richter and D. Gorlich, 2006. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314 815–817. [DOI] [PubMed] [Google Scholar]
- Fujimura, M., and K. Takamura, 2000. Characterization of an ascidian DEAD-box gene, Ci-DEAD1: specific expression in the germ cells and its mRNA localization in the posterior-most blastomeres in early embryos. Dev. Genes Evol. 210 64–72. [DOI] [PubMed] [Google Scholar]
- Gruidl, M. E., P. A. Smith, K. A. Kuznicki, J. S. McCrone, J. Kirchner et al., 1996. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93 13837–13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, B., L. Ackerman, S. Barbel, L. Y. Jan and Y. N. Jan, 1988. a Identification of a component of Drosophila polar granules. Development 103 625–640. [DOI] [PubMed] [Google Scholar]
- Hay, B., L. Y. Jan and Y. N. Jan, 1988. b A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 55 577–587. [DOI] [PubMed] [Google Scholar]
- Hay, B., L. Y. Jan and Y. N. Jan, 1990. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development 109 425–433. [DOI] [PubMed] [Google Scholar]
- Hayashi, K., S. M. de Sousa Lopes and M. A. Surani, 2007. Germ cell specification in mice. Science 316 394–396. [DOI] [PubMed] [Google Scholar]
- Hendzel, M. J., Y. Wei, M. A. Mancini, A. Van Hooser, T. Ranalli et al., 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106 348–360. [DOI] [PubMed] [Google Scholar]
- Jakymiw, A., K. M. Pauley, S. Li, K. Ikeda, S. Lian et al., 2007. The role of GW/P-bodies in RNA processing and silencing. J. Cell Sci. 120 1317–1323. [DOI] [PubMed] [Google Scholar]
- Johnstone, O., R. Deuring, R. Bock, P. Linder, M. T. Fuller et al., 2005. Belle is a Drosophila DEAD-box protein required for viability and in the germ line. Dev. Biol. 277 92–101. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser and J. Ahringer, 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2 research0002.1–0002.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231–237. [DOI] [PubMed] [Google Scholar]
- Kawasaki, I., Y. H. Shim, J. Kirchner, J. Kaminker, W. B. Wood et al., 1998. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell 94 635–645. [DOI] [PubMed] [Google Scholar]
- Kawasaki, I., A. Amiri, Y. Fan, N. Meyer, S. Dunkelbarger et al., 2004. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics 167 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiledjian, M., and G. Dreyfuss, 1992. Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J. 11 2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaja, N., S. N. Bhattacharyya, L. Jaskiewicz, S. Kimmins, M. Parvinen et al., 2006. The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc. Natl. Acad. Sci. USA 103 2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznicki, K. A., P. A. Smith, W. M. Leung-Chiu, A. O. Estevez, H. C. Scott et al., 2000. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development 127 2907–2916. [DOI] [PubMed] [Google Scholar]
- Lasko, P. F., and M. Ashburner, 1988. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335 611–617. [DOI] [PubMed] [Google Scholar]
- Lehmann, R., 1992. Germ-plasm formation and germ-cell determination in Drosophila. Curr. Opin. Genet. Dev. 2 543–549. [DOI] [PubMed] [Google Scholar]
- Liang, L., W. Diehl-Jones and P. Lasko, 1994. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development 120 1201–1211. [DOI] [PubMed] [Google Scholar]
- Markussen, F. H., W. Breitwieser and A. Ephrussi, 1997. Efficient translation and phosphorylation of Oskar require Oskar protein and the RNA helicase Vasa. Cold Spring Harbor Symp. Quant. Biol. 62 13–17. [PubMed] [Google Scholar]
- Megosh, H. B., D. N. Cox, C. Campbell and H. Lin, 2006. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr. Biol. 16 1884–1894. [DOI] [PubMed] [Google Scholar]
- Monestier, M., K. E. Novick and M. J. Losman, 1994. D-penicillamine- and quinidine-induced antinuclear antibodies in A.SW (H-2s) mice: similarities with autoantibodies in spontaneous and heavy metal-induced autoimmunity. Eur. J. Immunol. 24 723–730. [DOI] [PubMed] [Google Scholar]
- Orsborn, A. M., W. Li, T. J. McEwen, T. Mizuno, E. Kuzmin et al., 2007. GLH-1, the C. elegans P granule protein, is controlled by JNK KGB-1 and by the COP9 subunit CSN-5. Development 134 3383–3392. [DOI] [PubMed] [Google Scholar]
- Pitt, J. N., J. A. Schisa and J. R. Priess, 2000. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol. 219 315–333. [DOI] [PubMed] [Google Scholar]
- Raz, E., 2000. The function and regulation of vasa-like genes in germ-cell development. Genome Biol 1 reviews1017.1–1017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, K. C., and A. M. Villeneuve, 2004. C. elegans HIM-17 links chromatin modification and competence for initiation of meiotic recombination. Cell 118 439–452. [DOI] [PubMed] [Google Scholar]
- Robert, V. J., T. Sijen, J. van Wolfswinkel and R. H. Plasterk, 2005. Chromatin and RNAi factors protect the C. elegans germline against repetitive sequences. Genes Dev. 19 782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussell, D. L., and K. L. Bennett, 1993. glh-1, a germ-line putative RNA helicase from Caenorhabditis, has four zinc fingers. Proc. Natl. Acad. Sci. USA 90 9300–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman, E. E., and P. Lasko, 1999. Germline development in vertebrates and invertebrates. Cell. Mol. Life Sci. 55 1141–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa, K., H. Yamagata and Y. Shiga, 2005. Exploring embryonic germ line development in the water flea, Daphnia magna, by zinc-finger-containing VASA as a marker. Gene Expr. Patterns 5 669–678. [DOI] [PubMed] [Google Scholar]
- Salinas, L. S., E. Maldonado, M. Macias-Silva, T. K. Blackwell and R. E. Navarro, 2007. The DEAD box RNA helicase VBH-1 is required for germ cell function in C. elegans. Genesis 45 533–546. [DOI] [PubMed] [Google Scholar]
- Santos, A. C., and R. Lehmann, 2004. Germ cell specification and migration in Drosophila and beyond. Curr. Biol. 14 R578–R589. [DOI] [PubMed] [Google Scholar]
- Sengoku, T., O. Nureki, A. Nakamura, S. Kobayashi and S. Yokoyama, 2006. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 125 287–300. [DOI] [PubMed] [Google Scholar]
- Schisa, J. A., J. N. Pitt and J. R. Priess, 2001. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development 128 1287–1298. [DOI] [PubMed] [Google Scholar]
- Schmidt, D. J., D. J. Rose, W. M. Saxton and S. Strome, 2005. Functional analysis of cytoplasmic dynein heavy chain in Caenorhabditis elegans with fast-acting temperature-sensitive mutations. Mol. Biol. Cell 16 1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux, G., and R. E. Braun, 2006. Pathway to totipotency: lessons from germ cells. Cell 127 891–904. [DOI] [PubMed] [Google Scholar]
- Shim, Y. H., J. H. Chun, E. Y. Lee and Y. K. Paik, 2002. Role of cholesterol in germ-line development of Caenorhabditis elegans. Mol. Reprod. Dev. 61 358–366. [DOI] [PubMed] [Google Scholar]
- Smith, P., W. M. Leung-Chiu, R. Montgomery, A. Orsborn, K. Kuznicki et al., 2002. The GLH proteins, Caenorhabditis elegans P granule components, associate with CSN-5 and KGB-1, proteins necessary for fertility, and with ZYX-1, a predicted cytoskeletal protein. Dev. Biol. 251 333–347. [DOI] [PubMed] [Google Scholar]
- Spike, C. A., J. Bader, V. Reinke and S. Strome, 2008. DEPS-1 promotes P-granule assembly and RNA interference in C. elegans germ cells. Development 135 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, L. D., Z. Bao, D. Blasiar, T. Blumenthal, M. R. Brent et al., 2003. The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1 E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., 2005. Specification of the germ line, in WormBook, edited by The C. elegans Research Community. WormBook. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Strome, S., and R. Lehmann, 2007. Germ versus soma decisions: lessons from flies and worms. Science 316 392–393. [DOI] [PubMed] [Google Scholar]
- Strome, S., and W. B. Wood, 1982. Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 79 1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., and W. B. Wood, 1983. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35 15–25. [DOI] [PubMed] [Google Scholar]
- Strome, S., P. Martin, E. Schierenberg and J. Paulsen, 1995. Transformation of the germ line into muscle in mes-1 mutant embryos of C. elegans. Development 121 2961–2972. [DOI] [PubMed] [Google Scholar]
- Strome, S., J. Powers, M. Dunn, K. Reese, C. J. Malone et al., 2001. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell 12 1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styhler, S., A. Nakamura, A. Swan, B. Suter and P. Lasko, 1998. vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development 125 1569–1578. [DOI] [PubMed] [Google Scholar]
- Suntharalingam, M., and S. R. Wente, 2003. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell 4 775–789. [DOI] [PubMed] [Google Scholar]
- Timmons, L., and A. Fire, 1998. Specific interference by ingested dsRNA. Nature 395 854. [DOI] [PubMed] [Google Scholar]
- Walstrom, K. M., D. Schmidt, C. J. Bean and W. G. Kelly, 2005. RNA helicase A is important for germline transcriptional control, proliferation, and meiosis in C. elegans. Mech. Dev. 122 707–720. [DOI] [PubMed] [Google Scholar]
- Wickens, M., and A. Goldstrohm, 2003. Molecular biology. A place to die, a place to sleep. Science 300 753–755. [DOI] [PubMed] [Google Scholar]
- Wylie, C., 2000. Germ cells. Curr. Opin. Genet. Dev. 10 410–413. [DOI] [PubMed] [Google Scholar]
- Yigit, E., P. J. Batista, Y. Bei, K. M. Pang, C. C. Chen et al., 2006. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 127 747–757. [DOI] [PubMed] [Google Scholar]
- Zanotti, K. J., P. E. Lackey, G. L. Evans and M. R. Mihailescu, 2006. Thermodynamics of the fragile X mental retardation protein RGG box interactions with G quartet forming RNA. Biochemistry 45 8319–8330. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., and M. L. King, 2004. Sending RNAs into the future: RNA localization and germ cell fate. IUBMB Life 56 19–27. [DOI] [PubMed] [Google Scholar]