Abstract
Previous studies indicated that there is a separate hypothalamic control of follicle-stimulating hormone (FSH) release distinct from that of luteinizing hormone (LH). An FSH-releasing factor (FSHRF) was purified from rat and sheep hypothalami, but has not been isolated. We hypothesized that FSHRF might be an analogue of mammalian luteinizing hormone-releasing hormone (m-LHRH) and evaluated the activity of many analogues of m-LHRH and of the known LHRHs found in lower forms. Here we demonstrate that lamprey (l) LHRH-III has a potent, dose-related FSH- but not LH-releasing action on incubated hemipituitaries of male rats. l-LHRH-I on the other hand, had little activity to release either FSH or LH. m-LHRH was equipotent to l-LHRH-III to release FSH, but also had a high potency to release LH in contrast to l-LHRH-III that selectively released FSH. Chicken LHRH-II had considerable potency to release both LH and FSH, but no selectivity in its action. Salmon LHRH had much less potency than the others tested, except for l-LHRH-I, and no selectivity in its action. Because ovariectomized, estrogen, progesterone-treated rats are a sensitive in vivo assay for FSH- and LH-releasing activity, we evaluated l-LHRH-III in this assay and found that it had a completely selective stimulatory effect on FSH release at the two doses tested (10 and 100 pmols). Therefore, l-LHRH-III is a highly potent and specific FSH-releasing peptide that may enhance fertility in animals and humans. It may be the long sought after m-FSHRF.
Although the evidence from physiological studies for a separate hypothalamic control of follicle-stimulating hormone (FSH) release distinct from that of luteinizing hormone (LH) is compelling (1–5), proof of the separate hypothalamic control of FSH rests on the identification of an FSH-releasing factor (FSHRF). Crude extracts of the organum vasculosum lamina terminalis contained much more FSH-releasing activity than accounted for by the content of LH-releasing hormone (LHRH), and the slope of the dose-response curve in the in vitro bioassay for FSH release was much steeper than that obtained with LHRH (6). Extracts of the posterior median eminence similarly contained more FSH-releasing activity than could be accounted for by the content of LHRH (7).
FSHRF was purified originally from sheep hypothalami by gel filtration on Sephadex G-25 followed by carboxymethyl cellulose chromatography (2, 3). The release of FSH was measured by bioassay using either in vivo or in vitro assays of FSH-releasing activity (2, 3). Because LHRH has intrinsic FSH-releasing activity (8) and because Schally et al. could not separate FSH- from LH-releasing activity by fractionation on Sephadex G-25 using in vitro assays and measurement of FSH by immunoassay, they concluded that there was only one gonadotropin-releasing hormone (9).
Lumpkin et al. (10) separated the FSH- from the LH-releasing activity on the same column of Sephadex G-25 used originally (2) with bioassay of the activity in vivo in the ovariectomized (OVX), estrogen–progesterone-blocked rat, the original assay used to detect and separate the FSHRF from LHRH (2). The only difference was that plasma FSH and LH were measured by immunoassay rather than by bioassay. The LHRH in the various fractions was also measured by immunoassay (10). These results confirmed the earlier studies in which the hormones were measured by bioassay, and led us to attempt further purification of the FSHRF. Attempts to purify FSHRF by fractionation of lamb hypothalami were successful only at certain seasons of the year, and even then activity was lost on storage of samples at −20°C.
Because these were in vivo studies, it was necessary to confirm the activity by testing stalk-median eminence (SME) extracts on hemipituitaries from male rats incubated in vitro. Indeed, we confirmed the FSH-releasing activity of sheep and rat SME extracts in this assay and found that the FSH-releasing activity emerged from columns of Sephadex G-25, just prior to LHRH as with in vivo assay in the OVX, estrogen, progesterone-blocked rat (W.H.Y. and S.M.M., unpublished data).
Because the FSH-releasing activity of crude and purified extracts was relatively low compared with the LH-releasing activity, we anticipated difficulty in the isolation of FSHRF and hypothesized that it might be an analog of LHRH. Therefore, we screened the known LHRHs from lower forms for selective FSH-releasing activity and evaluated the activity of 25 analogs of LHRH in the in vivo assay (W.H.Y. and S.M.M., unpublished data). One of these analogs had only FSH-releasing activity, but the potency was very low and the slope of the dose-response curve was flat.
Of the known forms of LHRH from lower species (10), only chicken (c)-LHRH-II had slightly preferential FSH-releasing activity in vivo (W.H.Y. and S.M.M., unpublished data). Lamprey (l)-LHRH-I released FSH and LH but was of low potency (11). In 1993, Sower et al. (12) reported the structure of l-LHRH-III and found that it, like l-LHRH-I, stimulated estrogen and progesterone release from ovaries of lampreys. Because l-LHRH has been localized in axons in the arcuate-median eminence region of the human hypothalamus by immunocytochemistry that did not distinguish between l-LHRH-I and -III (13), we hypothesized that l-LHRH-III might be the long sought after FSHRF and evaluated its gonadotropin-releasing activity in vitro by incubating it with hemi-anterior pituitaries of adult, male rats.
METHODS
Adult male and female rats of the Sprague–Dawley strain (Holtzmann, Madison, WI; 200–250 g) were housed two per cage under controlled conditions of temperature (23–25°C) and lighting (on from 0500 to 1700 h). The animals had free access to a pellet diet and tap water.
In Vitro Studies.
After acclimatization for 5 or more days in the vivarium, male rats were killed by decapitation. Following removal of the posterior lobe, the anterior pituitary (AP) was bisected longitudinally, and each AP was incubated in a tube containing 0.5 ml of Krebs–Ringer bicarbonate (5 mM ascorbic acid; pH 7.4) buffer (KRB) in an atmosphere of 95% O2/5% CO2 in a Dubnoff shaker (50 cycles/min) for a period of 60 min. Following this preincubation, APs were incubated for 3 h in fresh KRB buffer alone or KRB containing graded concentrations of synthetic mammalian (m)-LHRH, l-LHRH-III, l-LHRH-I, c-LHRH-II, or salmon (s)-LHRH. The experiments were repeated twice. The medium was then aspirated and stored frozen at −20°C until radioimmunoassay.
In Vivo Experiments.
The rats were OVX while anesthetized with isoflurane and used for experiment 4 weeks later. Three days before blood sampling, each OVX rat was injected s.c. with 50 μg of estradiol benzoate (Sigma) dissolved in 0.1 ml sesame oil and 25 mg of progesterone (Lilly) dissolved in 0.5 ml sesame oil. One day before experiment, each animal was implanted with a jugular-atrial catheter as described (11). On the morning of the experiment, polyethylene (PE-50) tubing was connected to the distal end of the jugular-atrial catheter on the dorsum of the rat to facilitate blood sampling and intravenous injection. Animals were acclimatized for 1 h before the initial blood samples were withdrawn. Heparinized blood samples (0.5 ml) were collected just before and at 10, 30 and 60 min after injection of 0.5 ml of isotonic saline or l-LHRH-III (10 or 100 pmols). After removal of each blood sample, it was replaced with an equal volume of isotonic saline to help maintain blood volume.
Radioimmunoassays.
FSH and LH were measured by kits supplied by the National Institute of Arthritis Digestive Diabetes and Kidney Disease, and hormone values were expressed in terms of NIH-rFSH-RP-2 and NIH-rLH-RP-3 standards. The inter- and intraassay coefficients of variation for FSH assays were 7.5% and 5.2%, respectively, and 6.5% and 4.8% for LH, respectively.
Chemicals.
l-LHRH-III was synthesized by solid-phase methodology and purified to >97% purity by preparative reverse phase HPLC liquid chromatography (12). Other peptides were purchased from Peninsula Laboratories.
Statistics.
The significance of differences among multiple groups was determined by the analysis of variance (ANOVA) with subsequent Newman–Keuls multiple comparisons at each time point. Student’s t test was used to determine the significance of differences between two groups.
RESULTS
In Vitro Experiments.
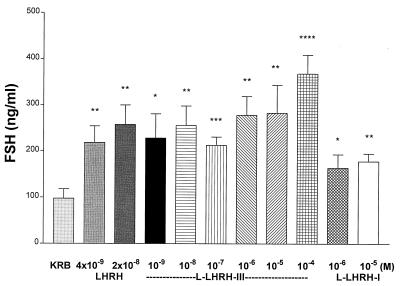
Effect of l-LHRH-I or -III and m-LHRH on FSH release. Lamprey LHRH-III released FSH in a dose-related fashion with a minimal effective concentration of 10−9 M, the lowest concentration tested, and a maximal effect at 10−6 M. Then, release plateaued to the highest concentration tested (10−4 M). l-LHRH-I produced a small release of FSH that was significant statistically at a concentration of 10−5 M and was significantly less than that produced by l-LHRH-III at the two concentrations tested (10−6 and 10−5 M). In contrast, m-LHRH induced FSH release at the two concentrations tested (4 × 10−9 and 2 × 10 −8 M), equivalent to that induced by l-LHRH-III (Fig. 1).
Figure 1.
The effect of m-LHRH, l-LHRH-III, and l-LHRH-I on FSH release from anterior hemipituitaries in vitro. In this and subsequent figures, values are mean +1 SEM. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; ∗∗∗∗, P < 0.0001 vs. KRB controls. Each concentration and control were tested in six tubes containing one hemipituitary per tube.
Effect of l-LHRH-I or -III and m-LHRH on LH release.
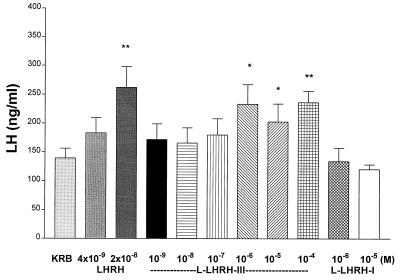
In contrast to its effects on FSH release, l-LHRH-III only released significant, and similar, concentrations of LH at the three highest concentrations tested (10−6–10−4 M), and l-LHRH-I was inactive at the concentrations tested (10−6 and 10−5 M). m-LHRH gave a dose-related stimulatory effect on LH release and was active at the lowest concentration tested (4 × 10−9 M) (Fig. 2).
Figure 2.
The effect of l-LHRH-III on LH release. l-LHRH-III stimulated LH release only at the high concentration of 10−6–10−4 M, whereas l-LHRH-I had no effect on LH release at doses of 10−6 and 10−5 M in vitro. ∗, P < 0.05, and ∗∗, P < 0.01 vs. KRB controls.
Effect of s- and c-LHRH-II on gonadotropin release.
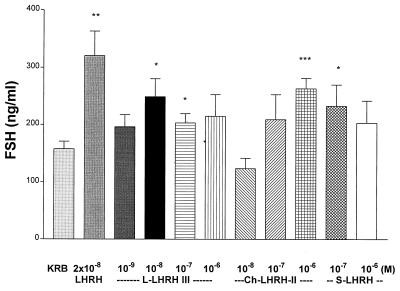
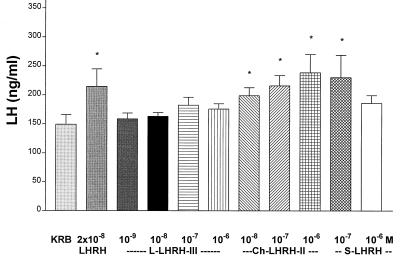
s-LHRH slightly stimulated both FSH (Fig. 3) and LH release (Fig. 4) at the two concentrations (10−7 and 10−6 M) tested. c-LHRH-II stimulated LH release at doses from 10−8–10−6 M, but the effect was not dose-related (Fig. 4). c-LHRH-II had equivalent LH-releasing activity to that of m-LHRH, but l-LHRH-III was devoid of LH-releasing activity in this experiment. c-LHRH-II only significantly increased FSH release at the highest concentration (10−6 M) tested. (Fig. 3). In this experiment, the minimal effective concentration for l-LHRH-III to release FSH significantly was 100-fold less than required for c-LHRH-II (Fig. 3).
Figure 3.
The effect of various LHRHs on FSH release. l-LHRH-III significantly stimulated FSH release from hemipituitaries in vitro at doses of 10−8 and 10−7 M. c-LHRH-II and s-LHRH stimulated FSH release at concentrations of 10−7 and 10−6 M, respectively. ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001 vs. KRB controls
Figure 4.
The effect of various LHRHs on LH release. l-LHRH-III (10−9–10−6 M) had no significant effect on LH release in vitro. c-LHRH-III stimulated LH release at doses of 10−8–10−6 M, and s-LHRH elevated LH release at the dose of 10−7 M (n = 6 in each group). ∗, P < 0.05 vs. KRB controls.
In Vivo Experiments.
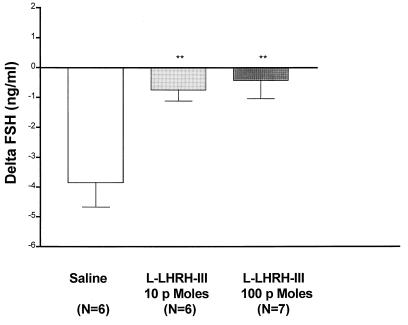
l-LHRH-III on FSH release. In the saline-injected control animals, there was a rapid and significant decline of plasma FSH within 10 min, which then returned toward control on the next measurement at 30 min, but had only reached a value that was not significantly less than control by 60 min (data not shown). This decline frequently occurs in this assay and is probably caused by the stress of injection and blood sampling. The lowest dose of l-LHRH-III injected (10 pmols) caused a highly significant increase (P < 0.01) in plasma FSH above that of saline-injected animals within 10 min (Fig. 5). Increasing the dose to 100 pmols caused a slightly larger effect at 10 min (P < 0.01 above controls) (Fig. 5), which was maintained at 30 min, whereas the effect of the 10-pmol dose had vanished by 30 min (data not shown). Thus, the 100-pmol dose had a more prolonged effect than the lower dose.
Figure 5.
The effect of l-LHRH-III (10 or 100 pmols) on FSH release in vivo. Values are expressed as changes of plasma FSH vs. initial values of each animal (delta FSH). At 10 min post-i.v. injection, l-LHRH-III produced a significant increase in plasma FSH above that of saline-injected animals. ∗∗, P < 0.01 vs. saline controls.
l-LHRH-III on LH release.
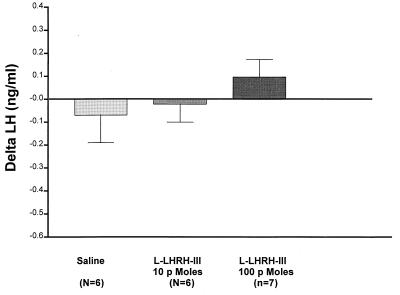
Injection of the saline diluent caused a slight decrease in LH concentrations in plasma at 10 min, which continued for the duration of the experiment but never reached statistical significance. The injection of the 10-pmol dose of l-LHRH-III produced no significant effect on plasma LH at 10 min (Fig. 6) or at any subsequent time point. The 100-pmol dose elevated LH slightly above the initial values at 10 min, but this change was not significant versus the saline-injected animals and by 60 min levels had returned to the preinjection values. Therefore, neither of the doses of l-LHRH-III employed had a significant effect on plasma LH in contrast to their highly significant elevation of FSH release.
Figure 6.
The effect of l-LHRH-III on LH release in vivo. l-LHRH-III had no significant effect on plasma LH release at doses of 10 or 100 pmols.
DISCUSSION
The results demonstrated that l-LHRH-III is a highly specific and potent FSH-releasing peptide. It behaves completely differently from l-LHRH-I, which has minimal effects on release of either FSH or LH. Also, s-LHRH and c-LHRH-II have low potency and lack specificity for FSH release. We have tested by in vivo assay the other known vertebrate LHRHs, and only one of them, c-LHRH-II, possessed slight selectivity for FSH release (W.H.Y. and S.M.M., unpublished data). This selectivity was not observed in the present in vitro experiments. Therefore, it appears highly likely that l-LHRH-III, a peptide that evolved 500 million years ago (12), has been preserved throughout evolution and is the m-FSHRF. Furthermore, axons of l-LHRH have been identified in human hypothalami by immunocytochemisty projecting from the arcuate region to the median eminence (13). The antibody used in that study did not distinguish between l-LHRH-I and -III. Therefore, we cannot be sure that l-LHRH-III is present in the human brain, but because l-LHRH-I has little FSH-releasing activity in comparison to that of l-LHRH-III, and because rat and sheep hypothalami contain highly specific FSH-releasing activity on bioassay in vivo and in vitro that elutes from columns of Sephadex G-25 at a position just prior to m-LHRH, it is highly likely that l-LHRH-III or a closely related molecule is m-FSHRF.
m-LHRH was approximately 1,000 times more potent to release LH than l-LHRH-III. Its decreased LH-releasing potency is probably accounted for by the fact that l-LHRH-III only has 60% homology with m-LHRH (Table 1). The entire difference is accounted for by the different amino acids in positions 5–8, which presumably cause a drastic decrease in LH and an increase in FSH-releasing capabilities. It is probable that the tetrapeptide, l-LHRH-III 5–8, reaches the active site of a putative FSHRF receptor. Presumably, the FSHRF receptor confers this specificity for FSH release, whereas the LHRH receptors stimulate the release of both hormones, albeit with a greater sensitivity for LH than FSH release, in vivo and in vitro (10, 11, 22). FSHRF receptors may reside on gonadotropes that contain only FSH. LHRH receptors (14, 15) may cause release of both hormones from gonadotropes that contain both FSH and LH. They may also be located on gonadotropes that only contain LH and be responsible for pulses of LH alone.
Table 1.
Structure of vertebrate LHRHs tested
| No. of amino acids
|
|
|---|---|
| 1 2 3 4 5 6 7 8 9 10 | |
| Mammal | pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2 |
| Lamprey III | pGlu-His-Trp-Ser-His-Asp-Trp-Lys-Pro-Gly-NH2 |
| Lamprey I | pGlu-His-Tyr-Ser-Leu-Glu-Trp-Lys-Pro-Gly-NH2 |
| Salmon | pGlu-His-Trp-Ser-Tyr-Gly-Trp-Leu-Pro-Gly-NH2 |
| Chicken II | pGlu-His-Trp-Ser-His-Gly-Trp-Tyr-Pro-Gly-NH2 |
In contrast to the roughly equal potency of m-LHRH to release FSH as that of l-LHRH-III in vitro, it has been apparent since the very beginning that m-LHRH has less potency in vivo to release FSH than the putative FSHRF. In early purification studies, we could not find any FSH release with doses of partially purified peptide that clearly released LH (S.M.M., unpublished data). Even the synthetic peptide, given by pulse injection, had little effect on FSH release, and it was only when it was given by infusion that it released FSH as well as LH (8, 23). Therefore, it was important to evaluate the activity of l-LHRH-III in vivo using the test animals that we have employed ever since the initial discovery of FSHRF. Indeed, l-LHRH-III released FSH and not LH at the two doses tested, 10 and 100 pmols. The release was not of very high magnitude, constituting about a 20% increase above the level in the saline-injected control animals. Consequently, we do not know if the dose-response curve of FSHRF is relatively flat in this particular assay. In vitro, we obtained a clear dose-response curve with l-LHRH-III. In any case, of the some 40 peptides that we have tested in this in vivo assay, l-LHRH-III is the only one with high potency to release FSH selectively and this characteristic was not shared by m-LHRH (10, 11). Because none of the peptides so far tested shares this pattern at low doses, this reinforces the possibility that l-LHRH-III or a closely related molecule could be m-FSHRF.
We discovered that GAP1–13 and [D-Trp9] GAP1–13 have selective FSH-releasing activity; however, only minimal such activity was observed in vivo at a dose of 1,000 pmols (11). Therefore, it is apparent that the l-LHRH-III is at least 100-fold more active than GAP peptides in vivo, which again suggests the l-LHRH-III may have physiologic significance.
We have fractionated rat hypothalami on Sephadex G-25 and have confirmed the pattern of elution of bio- and immunoreactive m-LHRH and FSH-releasing activity by the in vitro assay employed here. As in previous experiments with rat or sheep hypothalami, FSH-releasing activity emerged several tubes before LHRH and was completely separated from it. The tube containing bioactive FSHRF was in a peak of radioimmunoassayable l-LHRH-III (W.H.Y. et al., unpublished data). Therefore, we believe that l-LHRH-III or a very closely related molecule is m-FSHRF.
Pulsatile gonadotropin release in the rat is characterized by simultaneous pulses of FSH and LH and pulses of LH and FSH alone (1, 16). We hypothesize that the former two types of pulses may be accounted for by LHRH and the latter by FSHRF. Alternatively, FSHRF receptors may also exist on gonadotropes that secrete both gonadotropins and be responsible for the FSH released when both FSH and LH are simultaneously released. Several drugs, such as alcohol (1) and Δ-9-tetrahydrocannibanol (17), eliminate LH without affecting FSH pulses. This selective action to inhibit LHRH without affecting FSHRF release is shared by cytokines, such as interleukin 1 (16). Furthermore, the control of FSH-RF release is not mediated by nitric oxide that stimulates LHRH release (18).
The hypothalamic regions that control FSH and LH secretion are distinct. The perikarya of the LHRH neurons are located particularly in the medial preoptic area and their axons project to the lateral wings of the rostral and mid-median eminence (19). Stimulation of the preoptic region electrochemically (20) or by implants of prostaglandin E2 (21) stimulated LH but not FSH release, whereas lesions of this area blocked gonadal steroid-induced LH but not FSH release in the female rat (22).
On the contrary, electrochemical (20) or prostaglandin E2-induced stimulation (24) of the medial dorsal anterior hypothalamic area increased plasma FSH but not LH. Lesions of this region lowered basal FSH levels (5) and abolished pulsatile FSH release without altering basal or pulsatile LH release (25).
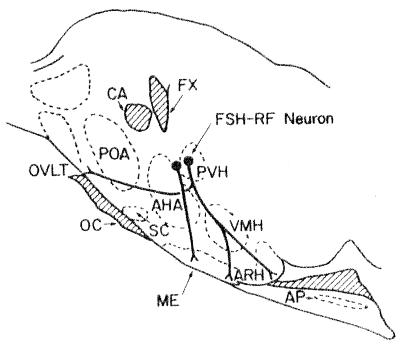
Stimulations caudally and ventrally from these regions resulted in concomitant release of FSH and LH (20, 21, 24). On the contrary, lesions of the rostral and mid-median eminence resulted in decreased basal (26) and pulsatile LH release§ with lesser suppression of FSH release, and lesions of the mid- and caudal median eminence occasionally blocked basal and pulsatile release of FSH without or with lesser blockade of LH release (26, §). Because extracts of the caudal median eminence had greater FSH-releasing activity than could be accounted for by their content of radioimmunoassayable LHRH (7), these findings taken together suggest that the perikarya of FSHRF-containing neurons are located in the region of the paraventricular nuclei with axons that project particularly to the mid and caudal median eminence, whereas the perikarya of the LHRH neurons in the medial preoptic area project particularly to the anterior and mid median eminence (Fig. 7). Because FSHRF activity was also found in the organum vasculosum lamina terminus (6), we hypothesize that axons of FSHRF neurons project to the organum vasculosum lamina terminus. They may release the peptide there or alternatively they may act as afferents providing information regarding the steroid milieu at this site lacking a blood brain barrier.
Figure 7.
Schematic diagram of the hypothalamic regions controlling FSHRF release. Possible location of the FSHRF neuronal system on a parasagittal section through the hypothalamic-pituitary region of the rat. (For further explanation see text.) OC, optic chiasm; CA, anterior commissure; SC, suprachiasmatic nucleus; FX, fornix; PVH, paraventricular nucleus; AHA, anterior hypothalamic area; DMH, dorsomedial nucleus; VMH, ventromedial nucleus; ARH, arcuate nucleus; PH, posterior hypothalamic nucleus; MM, medial mamillary nucleus; AP, anterior pituitary. [Reproduced from ref. 27 with permission (Elsevier, New York).]
The discovery of a FSHRF has important implications for veterinary and human medicine. For example, treatment of farm animals with FSHRF should lead to maturation of increased numbers of ovarian follicles and subsequent ovulations leading to production of litters of increased size. Conversely, inhibitory analogs of the peptide could be developed into potent antifertility drugs.
Acknowledgments
We thank Judy Scott and Jason P. Holland for their excellent secretarial assistance. This work was supported by National Institute of Health Grants DK43900 and MH51853.
ABBREVIATIONS
- FSH
follicle-stimulating hormone
- FSHRF
FSH-releasing factor
- LH
luteinizing hormone
- LHRH
luteinizing hormone-releasing hormone
- m
mammalian
- c
chicken
- l
lamprey
- s
salmon
- OVX
ovariectomized
- KRB
Krebs–Ringer bicarbonate buffer
Footnotes
Marubayashi, U., 73rd Annual Meeting of the Endocrine Society, June 10–15, 1991, Washington, DC, abstr. 460.
References
- 1.Dees W L, Rettori V, Kozlowski G, McCann S M. Alcohol. 1985;2:641–646. doi: 10.1016/0741-8329(85)90139-9. [DOI] [PubMed] [Google Scholar]
- 2.Dhariwal A P S, Nallar R, Batt M, McCann S M. Endocrinology. 1965;76:290–294. doi: 10.1210/endo-76-2-290. [DOI] [PubMed] [Google Scholar]
- 3.Dhariwal A P S, Watanabe S, Antunes-Rodrigues J, McCann S M. Neuroendocrinology. 1967;2:294–303. [Google Scholar]
- 4.Igarashi M, McCann S M. Endocrinology. 1964;74:446–452. doi: 10.1210/endo-74-3-446. [DOI] [PubMed] [Google Scholar]
- 5.Lumpkin M D, McCann S M. Endocrinology. 1984;115:2473–2480. doi: 10.1210/endo-115-6-2473. [DOI] [PubMed] [Google Scholar]
- 6.Samson W K, Snyder G, Fawcett C P, McCann S M. Peptides. 1980;1:97–102. doi: 10.1016/0196-9781(80)90041-8. [DOI] [PubMed] [Google Scholar]
- 7.Mizunuma H, Samson W K, Lumpkin M D, McCann S M. Life Sci. 1983;33:2003–2009. doi: 10.1016/0024-3205(83)90739-7. [DOI] [PubMed] [Google Scholar]
- 8.Schally A V, Redding R M, Nair G, Debeljuk L, White W F. Science. 1972;173:1036–1037. doi: 10.1126/science.173.4001.1036. [DOI] [PubMed] [Google Scholar]
- 9.Schally A V, Arimura A, Redding T W, Debeljuk L, Carter W, Dupont A, Vilchez-Martinez J A. Endocrinology. 1976;98:380–391. doi: 10.1210/endo-98-2-380. [DOI] [PubMed] [Google Scholar]
- 10.Lumpkin M D, Moltz J H, Yu W H, Samson W K, McCann S M. Brain Res Bull. 1987;18:175–178. doi: 10.1016/0361-9230(87)90188-2. [DOI] [PubMed] [Google Scholar]
- 11.Yu W H, Millar R P, Milton S C F, Del. Milton R C, McCann S M. Brain Res Bull. 1990;25:867–873. doi: 10.1016/0361-9230(90)90182-y. [DOI] [PubMed] [Google Scholar]
- 12.Sower S A, Chiang Y-C, Lovas S, Conlon J M. Endocrinology. 1993;132:1125–1131. doi: 10.1210/endo.132.3.8440174. [DOI] [PubMed] [Google Scholar]
- 13.Stopa E G, Sower S A, Svendsen C N, King J C. Peptides. 1988;9:419–423. doi: 10.1016/0196-9781(88)90278-1. [DOI] [PubMed] [Google Scholar]
- 14.Tsutsumi M, Zhou W, Millar R P, Mellon P L, Roberts J L, Flanagan C A, Dong K, Gillo B, Sealfon S C. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- 15.Reinhart J, Mertz L M, Catt K J. J Biol Chem. 1992;267:21281–21284. [PubMed] [Google Scholar]
- 16.Rettori V, Gimeno M F, Karara A, Gonzalez M C, McCann S M. Proc Natl Acad Sci USA. 1991;88:2763–2767. doi: 10.1073/pnas.88.7.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenger T, Rettori V, Snyder G D, Dalterio S, McCann S M. Neuroendocrinology. 1987;46:488–493. doi: 10.1159/000124870. [DOI] [PubMed] [Google Scholar]
- 18.Rettori V, Belova N, Dees W L, Nyberg C L, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCann S M. Annu Rev Pharmacol Toxicol. 1982;22:491–515. doi: 10.1146/annurev.pa.22.040182.002423. [DOI] [PubMed] [Google Scholar]
- 20.Kalra S P, Ajika K, Krulich L, Fawcett C P, Quidada M, McCann S M. Endocrinology. 1971;88:1150–1158. doi: 10.1210/endo-88-5-1150. [DOI] [PubMed] [Google Scholar]
- 21.Ojeda S R, Jameson H E, McCann S M. Endocrinology. 1977;100:1585–1594. doi: 10.1210/endo-100-6-1585. [DOI] [PubMed] [Google Scholar]
- 22.Bishop W, Kalra P S, Fawcett C P, Krulich L, McCann S M. Endocrinology. 1972;91:1404–1410. doi: 10.1210/endo-91-6-1404. [DOI] [PubMed] [Google Scholar]
- 23.Libertun C, Cooper K J, Fawcett C P, McCann S M. Endocrinology. 1974;94:518–525. doi: 10.1210/endo-94-2-518. [DOI] [PubMed] [Google Scholar]
- 24.Ojeda S R, Jameson H E, McCann S M. Endocrinology. 1977;100:1595–1603. doi: 10.1210/endo-100-6-1595. [DOI] [PubMed] [Google Scholar]
- 25.Lumpkin M D, McDonald J K, Samson W K, McCann S M. Neuroendocrinology. 1989;50:229–235. doi: 10.1159/000125227. [DOI] [PubMed] [Google Scholar]
- 26.Marubayashi U, McCann S M, Antunes-Rodrigues J. Brain Res Bull. 1989;23:193–200. doi: 10.1016/0361-9230(89)90147-0. [DOI] [PubMed] [Google Scholar]
- 27.McCann S M, Mizunuma H, Samson W K, Lumpkin M D. Psychoneuroendocrinology. 1983;8:299–308. doi: 10.1016/0306-4530(83)90004-5. [DOI] [PubMed] [Google Scholar]