Abstract
The effect of illumination on alertness can be assessed by comparing the efficacy of an anesthetic under light vs. dark conditions. Results from such tests on wild-type flies and visual mutants demonstrate that, surprisingly, light has both positive and negative influences on arousal. These dual effects may explain aspects of the fly's daily activity and have potential clinical implications.
VISIBLE light is generally regarded as an arousing stimulus. Given the inverse relationship between arousal and anesthesia (Pfaff 2006), one expects that illumination would oppose the ability of general anesthetics to induce sedation and/or hypnosis. In the clinic, noxious stimuli are known to have such an influence (Röpcke et al. 2001) but, to our knowledge, the effect of light on anesthesia has not been previously reported. We examined this question in the model organism Drosophila melanogaster, which not only has strong behavioral responses to light (Wheeler et al. 1993) but also is affected by general anesthetics in much the same way as are higher organisms (Morgan and Sedensky 2003).
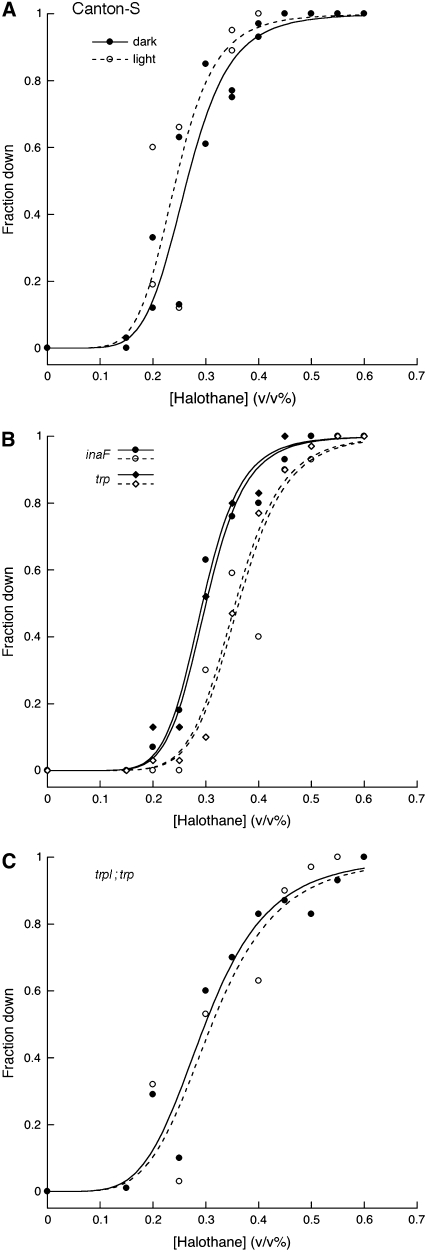
We tested the wild-type Canton-S strain for sensitivity to the volatile anesthetic halothane in either dim red light, which fruit flies perceive poorly if at all, or ambient room light. Contrary to our expectation, the half-effective concentration (EC50) of halothane that prevents flies from righting themselves and climbing after brief shaking showed very little change upon shift from dim red light to ambient light (Figure 1A). Moreover, this barely significant effect (Table 1, line 1) was in the direction opposite to that expected if light is an arousing stimulus. The failure to obtain a rightward shift in the concentration-response curve could merely mean that a modest light intensity is insufficiently powerful to overcome the effects of anesthetics on arousal. But it is also possible that light exerts both positive and negative influences, and these are roughly balanced under the conditions of our experiment. Although this alternative is less parsimonious, support for it comes from the effect of light on anesthetic sensitivity in flies with genetically altered vision. To ensure that visually impaired and wild-type animals developed under equivalent circumstances, all flies in this study were dark-reared. We first examined flies with perturbations that affect transient receptor potential (TRP), a subunit of the channels that carry a major component of the light-induced current in fly eyes (Oberwinkler 2002; Montell 2005). Flies bearing a mutation in inaF, a gene known to be required for normal trp function (Li et al. 1999) and to encode a polypeptide that interacts with TRP (Cheng and Nash 2007), were tested for halothane sensitivity. In the dark, the mutant flies had nearly normal sensitivity but, when subsequently exposed to ambient light, they became significantly less sensitive to halothane (Figure 1B; Table 1, line 2). A severe mutation in the trp gene itself conferred virtually identical behavior (Figure 1B; Table 1, line 3), as did an inaF;;trp double mutant (not shown). It appears that removal of one element of visual signaling reveals the arousing properties of light.
Figure 1.—
Effect of light on anesthetic sensitivity in wild type and flies with altered TRP signaling. The fraction of flies unable to climb out of the conical base of a tube is plotted against the halothane concentration to which they were exposed. Briefly, flies that were raised in total darkness on cornmeal/molasses agar at 25° were anesthetized with carbon dioxide, sorted in groups of ∼10, and returned to the dark incubator. The next day, 3–6 groups of each genotype were loaded without further recourse to anesthesia into perforated Falcon tubes, which were then placed in a glove box under dim red light illumination and equilibrated with a fixed concentration of halothane. After 40 min, the flies in each tube were tapped to the bottom and tested for their climbing ability as described (Guan et al. 2000; Campbell and Nash 2001). While the anesthetic concentration was maintained, fluorescent room lights were then turned on (yielding ∼230 lx within the glove box) and the flies in each tube were retested 40 min later. These flies were discarded and the dark/light tests with fresh flies were performed at a different halothane concentration. Thus, as opposed to earlier work with this assay, each data point reflects the behavior of 30–60 flies that were exposed to only a single concentration of halothane. Data are presented for the Canton-S control strain (A), for strains with a mutation in trp or inaF (B), and a strain with mutations in both trp and trpl (C). Solid and open symbols denote data points collected in dim red light and ambient room light, respectively; duplicate data points at a given concentration represent independent measurements done at least 6 months apart. For each strain and each condition the data points were fit to a sigmoidal function as described (Guan et al. 2000); the two resulting parameters that characterize each curve (EC50 and steepness) are given in Table 1. Our stock of Canton-S flies was originally obtained from J. S. de Belle (de Belle and Heisenberg 1996). The mutant alleles, each of which is either a molecular null or a severe hypomorph (Niemeyer et al. 1996; Cheng and Nash 2007) were as follows: inaFP106x, trp301, and trpl302. Each mutation was introduced into the Canton-S background by multiple (typically six) rounds of backcrossing; in each round, the desired flies were identified by PCR detection (primer sequences available on request) of a polymorphism created by or tightly linked to the mutation.
TABLE 1.
Parameters of concentration-response curves
| % EC50 (95% C.I.)
|
|||
|---|---|---|---|
| Strain | Dark | Light | Slope constant (± SEM) |
| Canton-S | 0.27 (0.26–0.28) | 0.25 (0.24–0.26) | 6.45 ± 0.34 |
| inaF[P106×] | 0.30 (0.28–0.31) | 0.36 (0.34–0.38) | 7.51 ± 0.62 |
| trp[301] | 0.30 (0.28–0.31) | 0.35 (0.33–0.37) | 8.31 ± 0.69 |
| trpl [302];trp[301] | 0.30 (0.28–0.32) | 0.30 (0.28–0.32) | 4.86 ± 0.38 |
| w[1118] | 0.35 (0.33–0.37) | 0.21 (0.20–0.23) | 4.47 ± 0.30 |
| w[hd] | 0.26 (0.25–0.28) | 0.18 (0.17–0.19) | 5.91 ± 0.40 |
| w[1118];;trp[301] | 0.27 (0.25–0.29) | 0.24 (0.22–0.26) | 4.15 ± 0.34 |
Curves were fit to a sigmoid function as before (Guan et al. 2000) with the aid of a commercial statistics package (SPSS, Chicago). To best assess the influence of illumination on a given strain, curves generated in dark and light were analyzed together to generate a single slope constant (± SEM) that defines their steepness. The analysis also yields EC50 values [each with its own 95% confidence interval (C.I.)] that define anesthetic potency in tests of each strain in the dark and in the light. A significant effect of illumination is ascertained when the 95% C.I. for the curve generated in ambient light fails to overlap the 95% C.I. for the curve generated in dim red light.
Light can influence behavior by effects on either visual or nonvisual pathways (Giebultowicz 2001). Thus, the effects of light in a trp mutant could reflect nonvisual photoreception (Stanewsky 2002), whose impact might be revealed only when vision is reduced. To explore this possibility, we tested the halothane sensitivity of a trpl;trp double mutant, which displays almost no light-induced current (Niemeyer et al. 1996). The ineffectiveness of illumination on halothane sensitivity in this strain (Figure 1C; Table 1, line 4) implies that light-dependent arousal must depend on the vision that remains in a trp/inaF mutant. The simplest possibility for the interplay of light and halothane in such mutants would be for the anesthetic to affect transient receptor potential-like (TRPL) channels, whose role in retinal signaling is overshadowed by TRP channels (Bähner et al. 2002). However, at a concentration of 0.5%, halothane had no obvious effect on the shape of the electroretinogram (ERG) elicited from trp mutant flies by light pulses of 3–20 sec duration (R. L. Scott and H. A. Nash, unpublished observations). This argues against a dramatic effect of the anesthetic on the functioning of TRPL channels. Although we cannot rule out the possibility that subtle effects of halothane on retinal physiology (Rajaram and Nash 2004) might have different consequences for behavior of the mutant vs. that of wild-type flies, we thus favor the idea that the effects of light impinge on drug action at a deeper level of the nervous system.
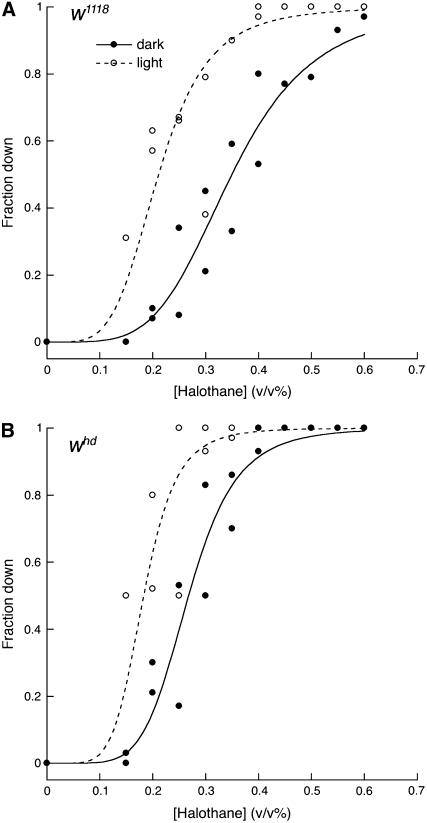
The data shown in Figure 1 are consistent with the hypothesis that illumination has opposing effects on arousal in wild-type flies, but another possibility must be considered. According to this alternative, light has only positive effects on arousal but it is less effective in wild-type flies than in trp/inaF mutants. Although this hypothesis must invoke a paradoxical effect of illumination on mutants with poor vision, it is hard to rule out a priori. However, if light were only an arousing stimulus, illumination should never raise the anesthetic sensitivity of flies above the level seen in the dark. In disagreement with this prediction, we find that mutations in the white gene confer light-dependent hypersensitivity. Our initial observations were made with flies bearing the w1118 mutation. As reported before (Campbell and Nash 2001), these are more resistant than congenic controls when tested in dim red light. But when dark-reared w1118 flies were exposed to ambient light, they increased rather than decreased their sensitivity (Figure 2A). This light-dependent hypersensitivity is not merely a consequence of the initial resistance conferred by the w1118 mutation. Flies carrying the whd80k17 mutation, although they display normal sensitivity when tested in the dark, are also hypersensitive under ambient light conditions (Figure 2B); the same is true for flies bearing the w1 mutation (not shown). It thus appears that, in the absence of a functional White gene product, illumination has an antiarousing effect (Table 1, lines 5 and 6). Although some behavioral effects of white mutations are known to be manifest in the dark (Diegelmann et al. 2006), it might be fruitful to examine other reported effects of white (Nichols et al. 2002; Hoyer et al. 2008) to see the extent to which they reflect the calmative effect of illumination on the mutants.
Figure 2.—
Illumination effects on anesthetic depth in white mutants. Halothane sensitivity was determined in dim red light or ambient light as in Figure 1 for Cantonized strains bearing either the w1118 mutation (A) or the whd80k17 (abbreviated as whd) mutation (B). EC50 values and slope constants for each curve are given in Table 1.
We do not know the basis for the opposite effects of white and trp/inaF mutations. We can only presume that the reduced acuity in the former and the residual vision in the latter are responsible for converting the same optical input into nonidentical outputs, each of which differentially activates the optic lobe. These two outputs appear to operate in parallel since, when a double w1118;;trp301 mutant was tested for the influence of light on halothane sensitivity (Table 1, line 7), neither mutation was strongly epistasitic over the other.
In summary, our data show that genetic manipulation of vision in Drosophila can change light-dependent effects on anesthetic depth in either direction. Given our rudimentary understanding of the molecular and cellular basis of anesthesia in flies, little can be said about the mechanism that underlies these effects. Indeed, the mutant phenotypes described in this article may serve as exemplars of the ability of mutations to influence drug sensitivity in ways that are distant from drug action (Nash 2002). However, by focusing attention on those regions of the nervous system that receive input from the visual system, our observations should assist mechanistic studies that will use localized manipulation of excitability (Luan and White 2007) to define the functional anatomy of anesthetic responsiveness.
Many lines of evidence point to an inverse relationship between anesthesia and arousal in vertebrates (Pfaff 2006). The same is likely to be true in Drosophila. This assertion is not only based on a priori reasoning but also supported by the recent measurement of anesthetic effects on local field potentials recorded from fly brains. In that work (van Swinderen 2006), volatile anesthetics were shown to depress the power of 20–30 Hz brain activity, a feature that correlates well with selective attention in flies (van Swinderen and Greenspan 2003). Our observations on illumination and anesthesia thus imply that the visual stream provides both positive and negative influences on arousal. There are several potential implications of this conclusion. One possibility is that the capacity of light to both activate and depress locomotor behavior underlies aspects of the fly's daily pattern of movement. For example, flies (even those whose circadian clock is disarmed) respond to lights-on with a burst of activity but subsequently reduce their locomotion (Wheeler et al. 1993). More generally, the dual effects of light on alertness may rationalize the occasional finding of “paradoxical” effects of light that are opposite of its typically positive masking effect on behavior (Nash et al. 2002; Rieger et al. 2003). Our demonstration that relatively subtle changes in vision can significantly influence arousal in flies may also have clinical relevance. Because light has a powerful influence on behavior in virtually all animals and because genetic polymorphisms as well as nongenetic factors should yield a broad spectrum of visual faculties among the patient population, our studies raise the issue of illumination as a source of variability in the depth of clinical anesthesia.
Acknowledgments
We acknowledge Joseph Campbell for the initial observations of halothane resistance in an inaF mutant. We thank Joy Gu for technical assistance, Robert Scott for electroretinography, and Ben White for helpful discussions and insightful suggestions. This research was supported by the Intramural Program of the National Institutes of Mental Health.
References
- Bähner, M., S. Frechter, N. Da Silva, B. Minke, R. Paulsen et al., 2002. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron 34 83–93. [DOI] [PubMed] [Google Scholar]
- Campbell, J. L., and H. A. Nash, 2001. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J. Neurobiol. 49 339–349. [DOI] [PubMed] [Google Scholar]
- Cheng, Y., and H. A. Nash, 2007. Drosophila TRP channels require a protein with a distinctive motif encoded by the inaF locus. Proc. Natl. Acad. Sci. USA 104 17730–17734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle, J. S., and M. Heisenberg, 1996. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: a case study of the mushroom body miniature gene (mbm). Proc. Natl. Acad. Sci. USA 93 9875–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann, S., M. Zars and T. Zars, 2006. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn. Mem. 13 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebultowicz, J. M., 2001. Peripheral clocks and their role in circadian timing: insights from insects. Philos. Trans. R. Soc. Lond. Ser. B 356 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, Z., R. L. Scott and H. A. Nash, 2000. A new assay for the genetic study of general anesthesia in Drosophila melanogaster: use in analysis of mutations in the 12E region. J. Neurogenet. 14 25–42. [DOI] [PubMed] [Google Scholar]
- Hoyer, S. C., A. Eckart, A. Herrel, T. Zars, S. A. Fischer et al., 2008. Octopamine in male aggression of Drosophila. Curr. Biol. 18 159–167. [DOI] [PubMed] [Google Scholar]
- Li, C., C. Geng, H. T. Leung, Y. S. Hong, L. L. Strong et al., 1999. INAF, a protein required for transient receptor potential Ca(2+) channel function. Proc. Natl. Acad. Sci. USA 96 13474–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, H., and B. H. White, 2007. Combinatorial methods for refined neuronal gene targeting. Curr. Opinion. Neurobiol. 17 572–580. [DOI] [PubMed] [Google Scholar]
- Montell, C., 2005. Drosophila TRP channels. Pflugers Arch. 451 19–28. [DOI] [PubMed] [Google Scholar]
- Morgan, P. G., and M. M. Sedensky, 2003. Simple genetic models for anesthetic action, pp. 231–248 in Neural Mechanisms of Anaesthesia, edited by J. F. Antognini, E. E. Carstens and D. E. Raines. Humana Press, Totowa, NY.
- Nash, H. A., 2002. In vivo genetics of anesthetic action. Brit. J. Anesth. 89 143–155. [DOI] [PubMed] [Google Scholar]
- Nash, H. A., R. L. Scott, B. C. Lear and R. Allada, 2002. An unusual cation channel mediates photic control of locomotion in Drosophila. Curr. Biol. 12 2152–2158. [DOI] [PubMed] [Google Scholar]
- Nichols, C. D., J. Ronesi, W. Pratt and E. Sanders-Bush, 2002. Hallucinogens and Drosophila: linking serotonin receptor activation to behavior. Neuroscience 115 979–984. [DOI] [PubMed] [Google Scholar]
- Niemeyer, B. A., E. Suzuki, K. Scott, K. Jalink and C. S. Zuker, 1996. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell 85 651–659. [DOI] [PubMed] [Google Scholar]
- Oberwinkler, J., 2002. Calcium homeostasis in fly photoreceptor cells. Adv. Exp. Med. Biol. 514 539–583. [DOI] [PubMed] [Google Scholar]
- Pfaff, D., 2006. Brain Arousal and Information Theory. Harvard University Press, Cambridge, MA.
- Rajaram, S., and H. A. Nash, 2004. A specific alteration in the electroretinogram of Drosophila melanogaster is induced by halothane and other volatile general anesthetics. Anesth. Analg. 98 1705–1711. [DOI] [PubMed] [Google Scholar]
- Rieger, D., R. Stanewsky and C. Helfrich-Forster, 2003. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J. Biol. Rhythms 18 377–391. [DOI] [PubMed] [Google Scholar]
- Röpcke, H., B. Rehberg, M. Koenen-Bergmann, T. Bouillon, J. Bruhn et al., 2001. Surgical stimulation shifts EEG concentration-response relationship of desflurane. Anesthesiology 94 390–399. [DOI] [PubMed] [Google Scholar]
- Stanewsky, R., 2002. Clock mechanisms in Drosophila. Cell Tissue Res. 309 11–26. [DOI] [PubMed] [Google Scholar]
- van Swinderen, B., 2006. A succession of anesthetic endpoints in the Drosophila brain. J. Neurobiol. 66 1195–1211. [DOI] [PubMed] [Google Scholar]
- van Swinderen, B., and R. J. Greenspan, 2003. Salience modulates 20–30 Hz brain activity in Drosophila. Nat. Neurosci. 6 579–586. [DOI] [PubMed] [Google Scholar]
- Wheeler, D. A., M. J. Hamblen-Coyle, M. S. Dushay and J. C. Hall, 1993. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J. Biol. Rhythms 8 67–94. [DOI] [PubMed] [Google Scholar]