Abstract
Activation of neurons in the dorsomedial hypothalamus (DMH) appears to play an important role in signaling the excitation of brain regions responsible for experimental fever and for many of the physiological and behavioral changes seen in experimental stress or anxiety in rats. Here, we examined the effect of disinhibition of the DMH by unilateral microinjection of bicuculline methiodide (BMI) on Fos expression in selected regions of the brain that have been implicated in anxiety and responses to stress and fever in rats. Disinhibition of the DMH resulted in dramatic increases in local Fos expression and also increased the numbers of Fos-positive neurons in the lateral septal nucleus and in both the parvocellular and magnocellular subdivisions of the paraventricular nucleus, with greater increases ipsilateral to the injection site in the DMH. However, microinjection of BMI had no significant effect on Fos expression in the bed nucleus of the stria terminalis, another forebrain area implicated in stress and anxiety. In the brainstem, disinhibition of the DMH increased Fos expression in the nucleus tractus solitarius and the ventrolateral medulla bilaterally with greater increases again ipsilateral to the site of the microinjection, and also in the midline rostral raphe pallidus. Thus, disinhibition of neurons in the DMH in conscious rats results in increases in Fos expression in selected forebrain and brainstem regions that have been implicated in stress-induced physiological changes, anxiety, and experimental fever.
Keywords: bicuculline methiodide, microinjections, rats
1. INTRODUCTION
Recent evidence implicates neurons in the region of the dorsomedial hypothalamus (DMH) in the generation of a diverse array of physiologic and behavioral changes associated with the response to experimental stress and for thermoregulatory responses seen in exposure to cold and experimental fever in rats (for reviews, see DiMicco et al., 2002; DiMicco and Zaretsky, 2007). Microinjection of the GABAA receptor antagonist bicuculline methiodide (BMI) into the DMH evokes tachycardia, increased secretion of adrenocorticotropic hormone (ACTH), stimulation of intestinal motility, and intense escape behavior and “anxiety” (Shekhar and DiMicco, 1987; Shekhar et al., 1987; Shekhar, 1993; DeNovellis et al., 1995; Greenwood and DiMicco, 1995; Shekhar and Katner, 1995), a pattern of physiological and behavioral changes resembling those seen in response to neurogenic stressors, as well as increased core body temperature and sympathetically-mediated activation of interscapular brown adipose tissue (IBAT; Zaretskaia et al., 2002; Cao et al., 2004). Conversely, microinjection of the GABAA receptor agonist and neuronal inhibitor muscimol into the DMH suppresses the increases in heart rate, blood pressure, and plasma ACTH seen in experimental air stress (Stotz-Potter et al., 1996a, 1996b; McDougall et al., 2004), and produces an anxiolytic effect in behavioral paradigms (Shekhar et al., 1990; Shekhar, 1993; Shekhar and Katner, 1995). Microinjection of muscimol or kynurenate, a non-selective antagonist of ionotropic glutamate receptors, into the DMH also suppresses the increases in body temperature and sympathetic nerve activity to IBAT in anesthetized rats evoked by microinjection of prostaglandin E2 (PGE2) into the preoptic area (Zaretskaia et al., 2003; Madden and Morrison, 2004), an established model for fever. Based on these findings, activation of neurons in the DMH has been proposed to play a key role in activation of specific neural circuits that are ultimately responsible for many of the physiological changes seen in stress and in experimental fever.
The results of studies examining the expression of Fos, the protein product of the immediate early gene c-fos and a marker for functional cellular responses (Morgan and Curran, 1989; Martinez et al., 2002; for review see Konkle and Bielajew, 2004), support these roles for neurons in the DMH. Increased Fos expression has been noted in the DMH in various paradigms for “emotional” or neurogenic stress (Buijs et al., 1993; Cullinan et al., 1996; Krukoff and Khalili, 1997; Emmert and Herman, 1999; Palmer and Printz, 1999; Baffi and Palkovits, 2000; Briski and Gillen, 2001; Spitznagel et al., 2001) but not in hemorrhage (Thrivikraman et al., 2000), and Fos expression in the DMH is also increased in experimental models for fever and in cold exposure (Elmquist et al., 1996; Lacroix and Rivest, 1997; Baffi and Palkovits, 2000; McKitrick, 2000; Yoshida et al., 2002; Cano et al., 2003; Gautron et al., 2005). Microinjection of muscimol into the DMH markedly reduced the increase in Fos expression in the hypothalamic paraventricular nucleus (PVN) associated with experimental air jet stress but failed to influence that seen in hemorrhage (Morin et al, 2001). These results indicate that excitation of neurons in DMH activates specific effector circuits that are responsible for characteristic changes seen in response to exteroceptive stressors.
Thus, activation of neurons in the DMH may be responsible for excitation of downstream neural pathways relevant to many of the physiological changes seen in experimental stress and fever. Since disinhibition of neurons in the DMH by local microinjection of BMI appears to replicate most or all of these effects, we reasoned that disinhibition of neurons in the should increase Fos expression in these regions. Silveira and colleagues studied the effect of electrical stimulation as well as microinjection of the excitatory amino acid kainate and SR-95531, another GABAA receptor antagonist, at coordinates that approximated the location of the dorsomedial hypothalamic nucleus on fos expression in selected forebrain regions in Long-Evans rats (Silveira et al., 1995). However, the exact neural elements affected by electrical stimulation are not fully understood, and the doses of kainate (60 and 120 pmol) as well as volumes of all microinjections (200 nL) were much larger than those typically employed to evoke discrete activation of the DMH. Also, because the precise sites of microinjection or stimulation were not provided, the exact region of the hypothalamus that was activated in this study could not be determined. Therefore, we analyzed Fos expression in regions of the brain thought to play a role in anxiety, in cardiovascular and neuroendocrine effects of stress, and in experimental fever after microinjection of BMI or saline into identified sites in the DMH in conscious rats. We employed a reduced volume of injection (100 nL), and a dose of BMI (10 pmol) previously shown to produce relevant effects that are anatomically specific to the DMH (DeNovellis et al., 1995; Bailey and DiMicco, 2001; Zaretskaia et al., 2002). Areas selected for quantitative analysis included: the bed nucleus of the stria terminalis (BNST), also examined in the study of Silveira and colleagues (1995), and the ventral region of the lateral septal nucleus (LSV), two forebrain regions that (1) receive afferents from and send efferents to the DMH (ter Horst and Luiten, 1986; Thompson et al., 1996; Thompson and Swanson, 1998), (2) have been implicated in behavioral and neuroendocrine responses to stress and anxiety (Koolhaas et al., 1998; Ebner et al., 1999; Cecchi et al., 2002; Khoshbouei et al., 2002; Walker et al., 2003) and (3) where experimental stress has been reported to increase Fos expression (LSV: Sharp et al., 1991; Duncan et al., 1993; Beck and Fibiger, 1995; Imaki et al., 1995; BNST: Campeau and Watson, 2000; Ma and Morilak, 2004; Spencer and Day, 2004); the parvocellular and magnocellular regions of the PVN; the nucleus tractus solitarius (NTS) and the ventrolateral medulla (VLM), key brainstem regions involved in the central control of sympathetic activity regulating cardiovascular function (Ross et al., 1984; Sved et al., 1985; Willette et al., 1987; Agarwal et al., 1990); and the rostral raphe pallidus (rRP), a brain region implicated in the tachycardia evoked by chemical stimulation of the DMH (Samuels et al., 2002; Cao et al., 2004) and by experimental air jet stress (Zaretsky et al., 2003). In this study, microinjections of BMI into the DMH were unilateral. Given that projections of neurons in the DMH are predominantly ipsilateral (Thompson and Swanson, 1998), we anticipated that, as was reported previously (Silveira et al., 1995), significant lateralization of BMI-induced increases in Fos expression would be evident in areas where neuronal activation was directly dependent on neural circuitry, rather than secondary to the state of profound behavioral and “emotional” arousal so evoked. Although this dose of BMI has been employed extensively in microinjection studies targeting the DMH (Bailey and DiMicco, 2001; Samuels et al., 2002; Zaretskaia et al., 2002; Horiuchi et al., 2005; da Silva et al., 2006; de Menezes et al., 2006), the area of neuronal excitation evoked by such injections has never been delineated. Therefore, we also assessed the extent of the area of Fos expression in the vicinity of the injection site in the DMH. A preliminary report of this study was presented at the Annual Meeting of the Society for Neuroscience, San Diego, 2001.
2. RESULTS
Physiological response to microinjection of saline and BMI
Baseline parameters were not significantly different prior to injection of saline or BMI into the DMH (Table 1). Microinjection of saline 100nL into the DMH in control rats was followed by modest tachycardia and pressor responses of approximately 50 beats/min and 12 mmHg, respectively (fig. 1). These changes were transient, with both heart rate and blood pressure reaching their maxima at approximately 5 minutes after injection and returning to baseline by 10 minutes. A small increase in locomotor activity accompanied the cardiovascular changes, and this behavioral effect was modest and brief (i.e., less than 5 min) in duration.
Table 1.
Results of one-way ANOVA for blood pressure (BP), heart rate (HR), and locomotor activity (LA) in rats microinjected with saline versus BMI into the DMH.
| Baseline | Treatment | Time | Interaction | |
|---|---|---|---|---|
| BP | NS | 0.02 | <0.001 | <0.001 |
| F(1,10)=7.7 | F(34,340)=9.6 | F(34,340)=4.7 | ||
| HR | NS | <0.001 | <0.001 | <0.001 |
| F(1,10)=33.8 | F(34,340)=16.4 | F(34,340)=9.6 | ||
| LA | NS | <0.001 | <0.001 | <0.001 |
| F(1,10)=10.5 | F(34,340)=7.1 | F(34,340)=4.3 | ||
Baseline HR=386±5 beats/min (n=12), baseline BP=105±3 mmHg (n=12)
Fig. 1.
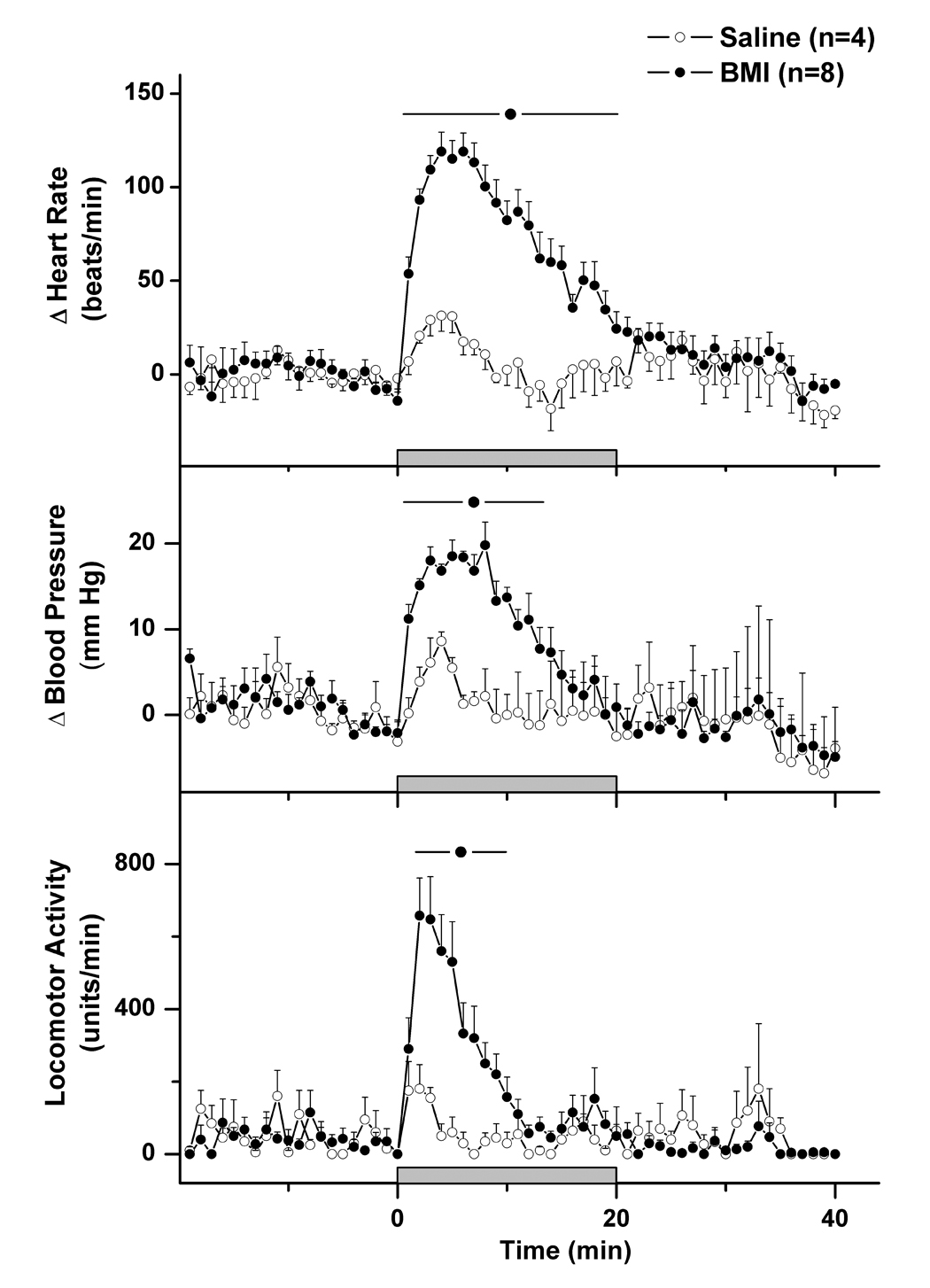
Mean (± SEM) heart rate (top), blood pressure (middle), and locomotor activity over time in rats receiving microinjection of saline (control rats - open circles) or BMI 10 pmol (filled circles) into the DMH. All rats were perfused at +90 min for subsequent analysis of Fos expression in selected brain regions.
In contrast to the effects of saline alone, unilateral microinjection of BMI 10 pmol/100 nL into the DMH elicited marked cardiovascular changes as have been described previously (DiMicco and Abshire, 1987; Wible et al., 1988; DeNovellis et al., 1995; fig. 1). The salient feature of the response was intense tachycardia, with increases in heart rate that averaged more than 120 beats/min at maximum and persisted for over 20 minutes. The BMI-induced tachycardia was paralleled by moderate elevations in blood pressure with peak increases averaging approximately 20 mmHg. These cardiovascular changes were accompanied by marked locomotor stimulation of shorter duration, a behavioral response that has been demonstrated previously to reflect an “anxiogenic” effect (Shekhar et al., 1990; Shekhar, 1993; Shekhar and Katner, 1995).
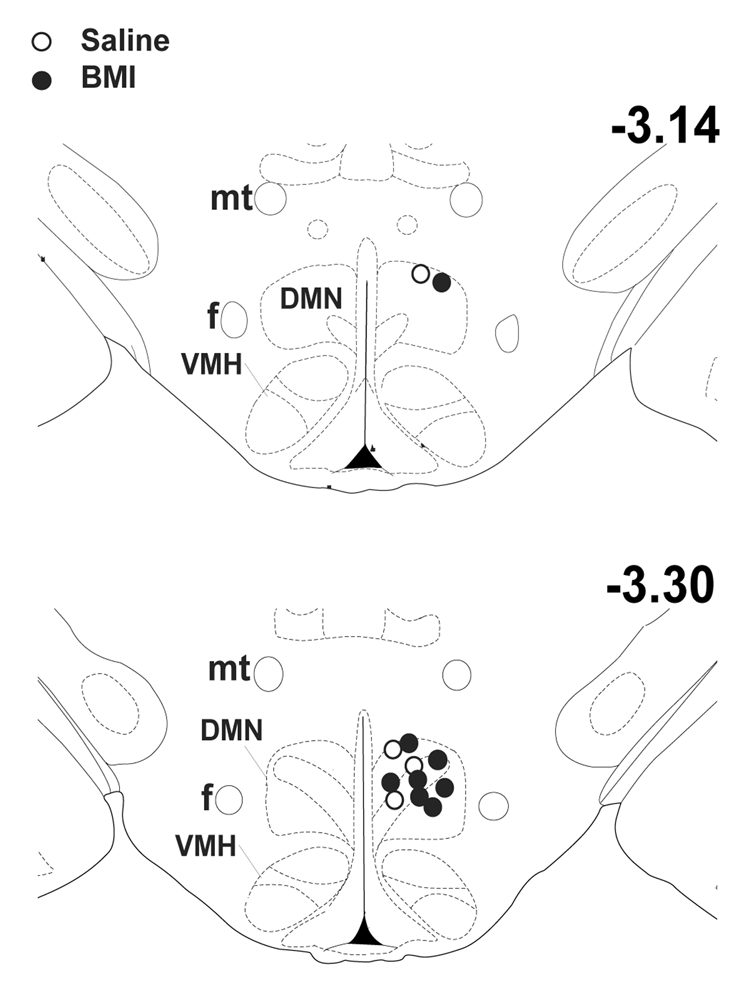
Injection sites were assessed from post-mortem histology to be highly localized to the region of the DMH. In every rat receiving BMI and all four saline-injected control rats, injection sites were estimated to be within the limits of the dorsomedial hypothalamic nucleus (DMN) itself (fig. 2) as defined by the atlas of Paxinos and Watson (1998).
Fig. 2.
Schematic coronal sections of rat brain at the level of the DMH adapted from the atlas of Paxinos and Watson (1998), depicting approximate sites of injection of BMI 10 pmol (filled circles) or saline (open circles) in the DMH in all 12 rats subjected to analysis. DMN – dorsomedial hypothalamic nucleus; f – fornix; mt – mammillothalamic tract; PH – posterior hypothalamus; VMH – ventromedial hypothalamic nucleus. Numbers indicate distance from bregma.
Fos expression in rats subjected to microinjection of saline or BMI into the DMH
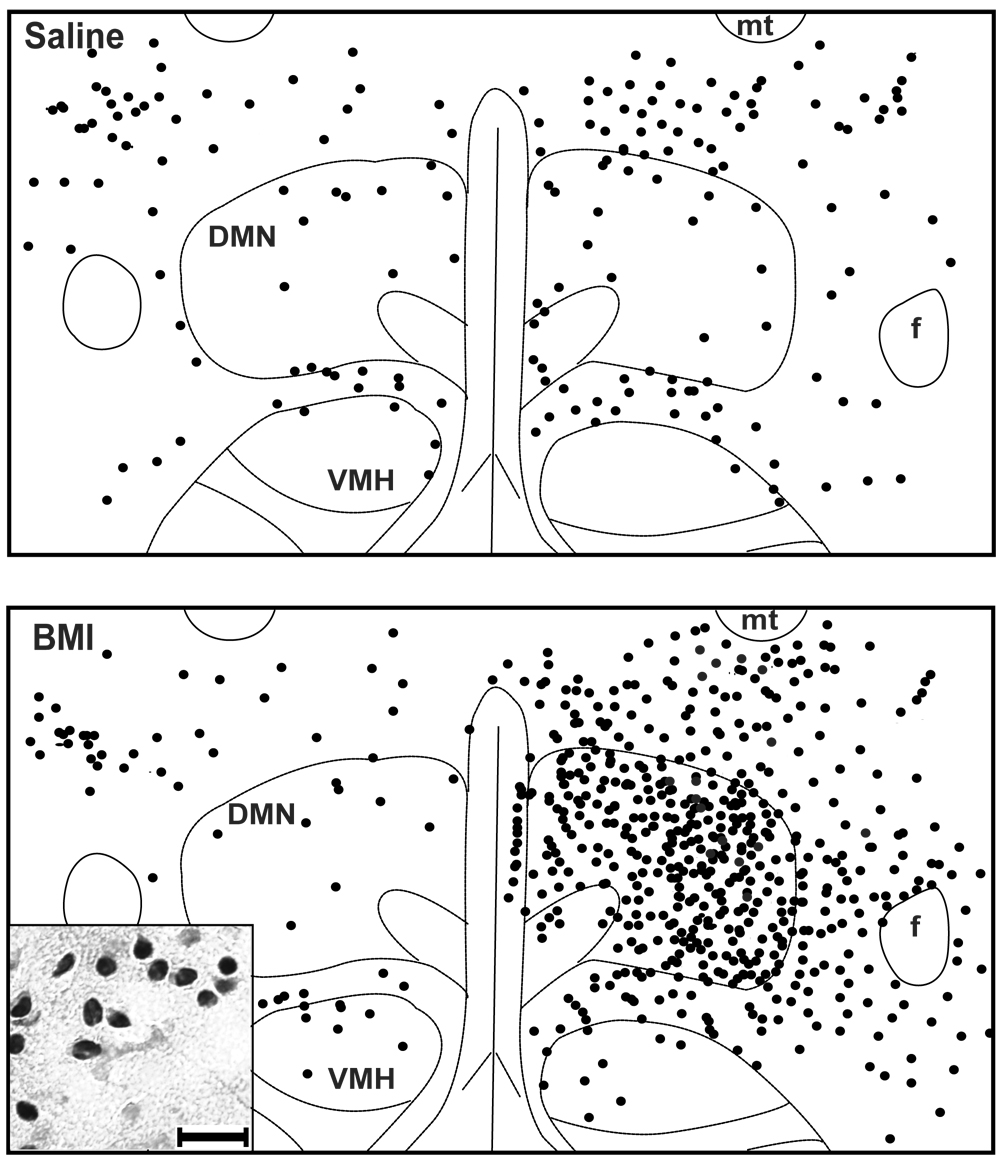
Rats subjected to unilateral microinjection of saline into the DMH exhibited modest but detectable levels of Fos expression in all regions examined (fig. 3–fig. 4). Diffuse but significant labeling was apparent in the DMN and surrounding region of the DMH with no difference in the number of Fos-positive neurons on injected versus uninjected sides (fig. 3–fig. 4), and modest labeling was also found in the other stress-related forebrain regions examined, including the PVN (fig. 4–fig. 5), LSV (fig. 4– fig. 5) and BNST (not shown).
Fig. 3.
Schematics depicting Fos expression in the DMH from two representative rats, treated with unilateral microinjections of either saline (ABOVE) or BMI 10 pmol (BELOW) into the DMH. Each dot represents one Fos-positive neuron. (For abbreviations, see legend of figure 2.) In both cases, right side of panel represents injected side. INSET – High (40x) magnification photomicrograph depicting cluster of Fos-positive neurons in the DMH. (calibration bar = 20 microns)
Fig. 4.
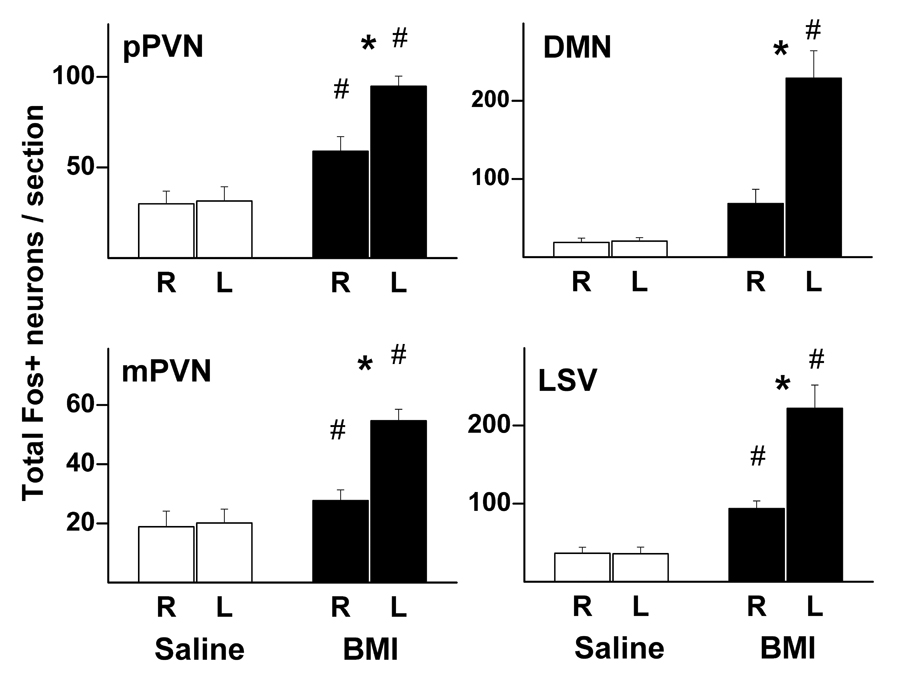
Graphic summary of mean number (± SEM) of Fos-positive neurons per rat on the right (R) and left (L) sides of the two major subdivisions of the PVN (pPVN – parvocellular; mPVN – magnocellular; saline – n=4; BMI – n=8), the DMN (saline – n=4; BMI – n=7), and the LSV (saline – n=4; BMI – n=6) in rats microinjected with saline 100 nL (open bars) or BMI 10 pmol (filled bars) into the left DMH. # - greater than corresponding value in saline-injected control rats; * - greater than uninjected side, p<0.05.
Fig. 5.
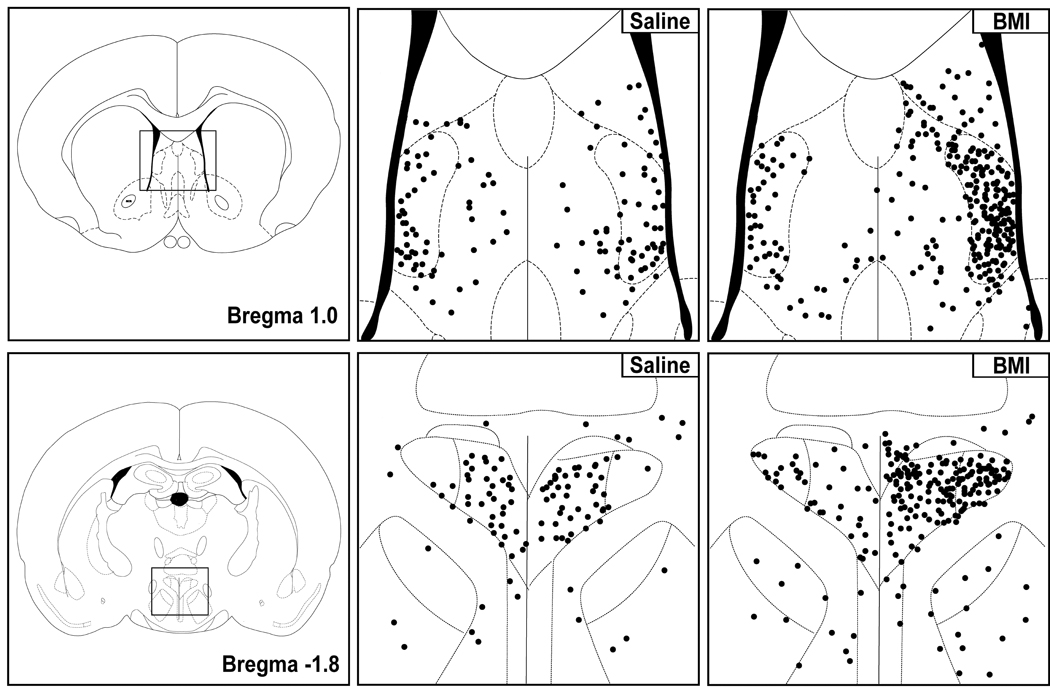
Schematics adapted from the atlas of Paxinos and Watson (1998) for orientation (FAR LEFT PANELS) and line drawings representing boxed areas in schematics (MIDDLE and RIGHT) depicting Fos expression in coronal sections through the LSV (ABOVE) and PVN (BELOW) from a representative saline-injected control rat (MIDDLE) and a rat after microinjection of BMI 10 pmol (RIGHT) into the left DMH. Each dot represents one Fos-positive neuron. In all cases, right side of panel is ipsilateral to injection site in the DMH.
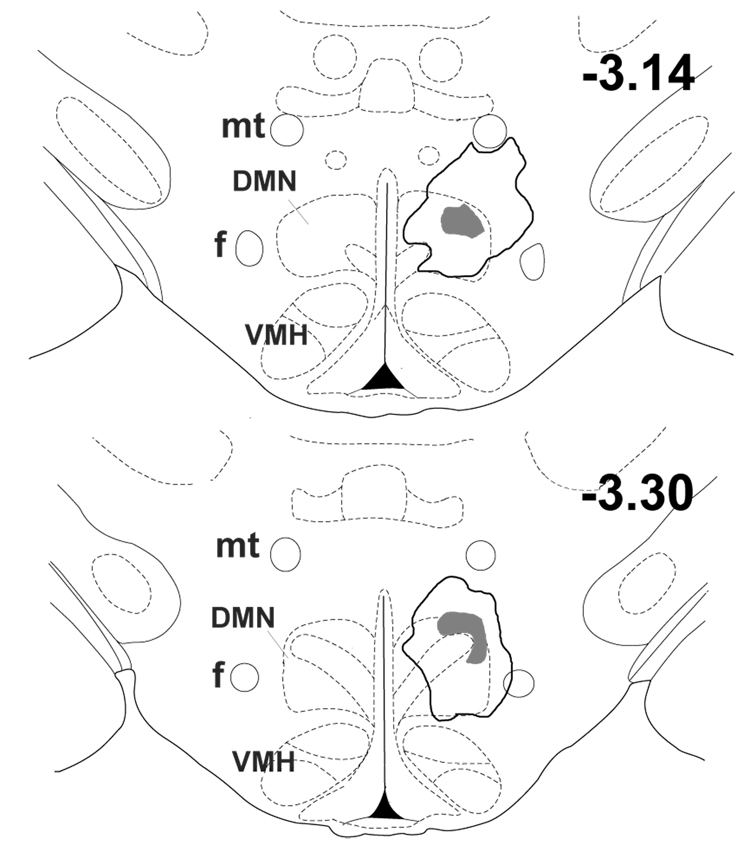
In rats subjected to unilateral microinjection of BMI into the left DMH, Fos expression was significantly elevated in almost every region examined, and these elevations were often highly lateralized to the injected side. The most intense Fos labeling observed anywhere in the brain in any group as seen in the DMH on the side ipsilateral to local microinjection of BMI (fig. 3 and fig. 4). The extent of the area emanating from the center of the injection site in the DMH where uniformly dense Fos expression was detectable varied modestly in different rats, but generally included most of the DMN and extended laterally into the perifornical region, dorsally into the dorsal hypothalamic area, and ventrally to the edge of the ventromedial hypothalamic nucleus (VMH; see fig. 6). The total extent of the area of dense and uniformly increased Fos expression evoked by BMI extended an average of 1.1 ± 0.1 mm in the anterior-posterior direction, and was estimated in each experiment by comparison with coronal sections from the atlas of Paxinos and Watson (1998). Thus, Fos expression extended anteriorly as far as −2.56 mm from Bregma in three of the eight rats and posteriorly as far as −3.80 mm in six animals, while in single individual rats, Fos expression extended as far anteriorly as −2.30 mm and as far posteriorly as −4.0 mm. Anteriorly, this region of increased Fos expression never reached the level of the PVN, while posteriorly it always extended somewhat into the anterior edge of the region designated as posterior hypothalamus (PH) by Paxinos and Watson (1998). A strong trend for increased Fos expression in the DMH on the side contralateral to the injection site failed to reach the level of statistical significance (p=0.11 versus saline-injected rats; fig. 3 and fig. 4).
Fig. 6.
Schematic coronal sections adapted from the atlas of Paxinos and Watson (1998) depicting the composite distribution of intense Fos expression extending continuously from the site of injection of BMI in the DMH. Numbers indicate distance from bregma. Intense Fos expression was apparent in all eight experiments at these two atlas levels only and is represented by shaded area. Bold line outlines area where uniformly intense fos expression was apparent in at least two experiments.
Microinjection of BMI into the DMH markedly elevated levels of Fos expression in the ipsilateral pPVN and, to a significantly lesser degree, the contralateral pPVN as well (fig. 4 and fig. 5). Disinhibition of the DMH also increased Fos expression in the mPVN, once again to a significantly greater degree on the side ipsilateral to microinjection of BMI. The most striking increase in Fos expression outside of the DMH was observed in the LSV where the number of Fos positive neurons ipsilateral to the site of microinjection of BMI was nearly six times that seen in saline treated rats (fig. 4 and fig. 5). Fos expression in the contralateral LSV was also significantly elevated. Injection of BMI into the DMH had no significant effect on Fos expression in the BNST, although a slight tendency for increased levels on the injected side was evident (data not shown).
In brainstem cardiovascular regions, microinjection of BMI generally increased Fos expression in all regions examined, and in bilateral structures these increases were greater on the side ipsilateral to the site of injection in the DMH (fig. 7). Fos expression was moderately elevated in the midline rRP in BMI-injected rats (fig. 8), with no evidence of increased expression in the adjacent parapyramidal region. Compared to vehicle controls, rats injected with BMI exhibited increased Fos expression in both pre- and post-commissural subregions of the NTS and in the C1 and A1 regions of the VLM, and in each case these increases were greater on the ipsilateral side (fig. 7).
Fig. 7.
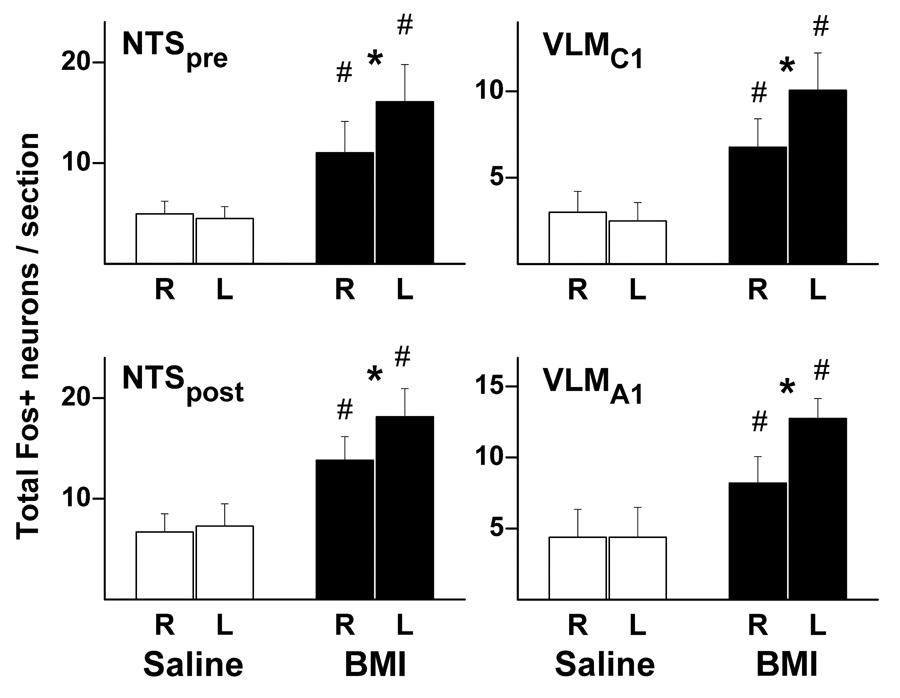
Graphic summary of mean number (± SEM) of Fos-positive neurons per rat in the pre-commissural (NTSpre; saline – n=4; BMI – n=6) and post-commissural (NTSpost; saline – n=4; BMI – n=7) subregions of the brainstem NTS (left) and in the VLM at levels corresponding to C1 (RVLMC1; saline – n=4; BMI – n= 6) and A1 (VLMA1; saline – n=4; BMI – n= 7) according to the atlas of Paxinos and Watson (1998). Rats subjected to unilateral microinjection of either saline 100 nL (open bars) or BMI 10 pmol (filled bars) into the left DMH. (R – right side; L – left side) # - greater than corresponding value in saline-injected control rats; * - greater than uninjected side, p<0.05.
Fig. 8.
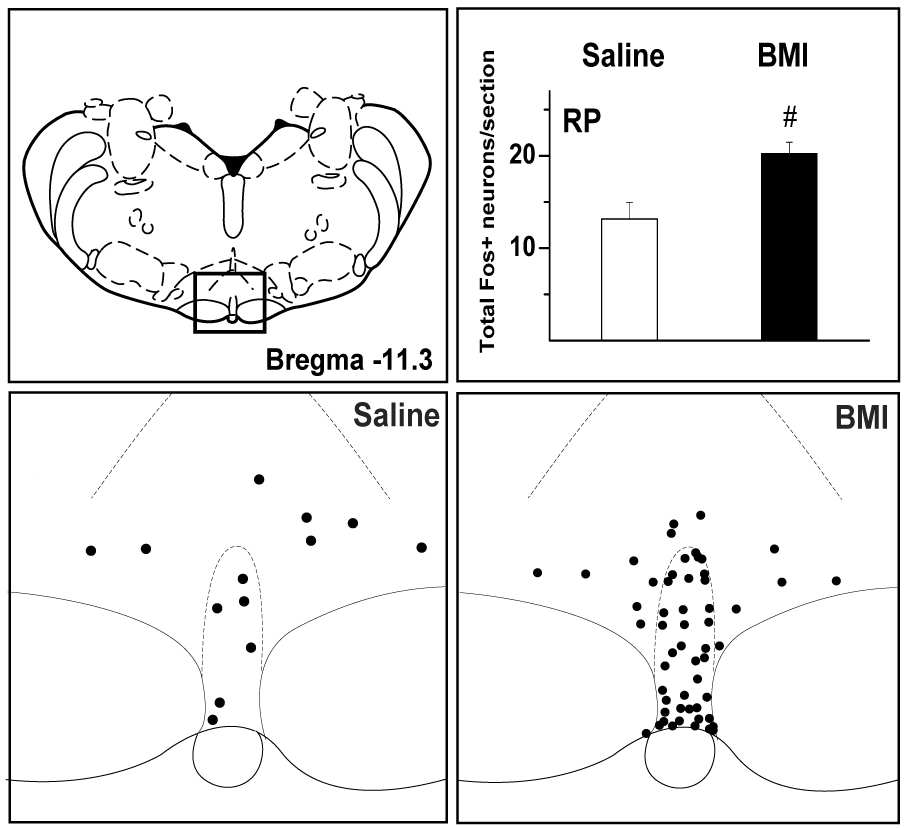
Fos expression in the rRP in saline- and BMI- treated rats. Schematic adapted from the atlas of Paxinos and Watson (1998) for orientation (TOP LEFT) and line drawings representing boxed area in schematic (BOTTOM) depicting Fos expression in the rRP in a representative saline-injected control rat (BOTTOM LEFT) and a rat after microinjection of BMI 10 pmol into the left DMH (BOTTOM RIGHT). Each dot represents one Fos-positive neuron. TOP RIGHT - Graphic summary of mean number (± SEM) of fos-positive neurons in the rRP. SALINE (left – open bar) – unilateral microinjection of saline 100 nL into the left DMH (n=4); BMI (right – filled bar) – unilateral microinjection of BMI 10 pmol into the left DMH (n=8). # - greater than corresponding value in saline-injected control rats, p<0.05.
3. DISCUSSION
The results of this study demonstrate the effect of disinhibition of the DMH in rats on Fos expression in brain regions selected for their involvement in anxiety, responses to stress, and fever, phenomena in which neurons in the DMH have been implicated. The finding that unilateral disinhibition of the DMH evoked increased Fos expression in most of these regions, and that these increases were greater ipsilateral to the site of injection in the DMH is consistent with previous data suggesting that neurons in the DMH play an important role in signaling excitation of central neural circuits responsible physiological and behavioral changes seen in these settings. Disinhibition of the DMH elicits autonomically-mediated increases in blood pressure and heart rate, as well as behavioral arousal characterized by intense locomotor activity (Shekhar and DiMicco, 1987) and “anxiety” (Shekhar, 1993). Increased blood pressure is known to elevate fos expression in the NTS (Chan and Sawchenko, 1998) and the intense behavioral arousal might similarly affect other brain regions. However, all of these changes should result in bilaterally symmetrical increases in Fos expression. Therefore, because projections emanating from the DMH are highly lateralized (ter Horst and Luiten, 1986; Thompson et al., 1996), increases that result from driving central neural circuitry from only one side of the DMH should produce greater increases in fos expression on the ipsilateral side. In fact, increases in Fos expression resulting from unilateral disinhibition of the DMH were always greater on the side ipsilateral to the injection, as was reported previously for stimulation of the medial hypothalamus (Silveira et al., 1995). Therefore, the presumptive increases in neuronal activity observed in these areas are most likely to represent the result of afferent synaptic excitation ultimately originating in disinhibited neurons in the region of the ipsilateral DMH.
In the region of the DMH itself, dramatic increases in Fos expression were apparent after local microinjection of BMI but not after similar injection of saline vehicle. Microinjection of BMI into the DMH has been employed in numerous studies in order to disinhibit neurons in the region (Horiuchi et al., 2004, 2005; Da Silva et al., 2006; McDowall et al., 2006; Nakamura and Morrison, 2007), but this study represents the first in which direct evidence for neuronal activation resulting from this disinhibition has been presented. In contrast to a previous report that employed electrical stimulation and relatively high doses of kainate for study (Silveira et al., 1995), we found that the extent of the area of uniformly increased Fos expression emanating from the site of microinjection in the DMH could be delineated with reasonable clarity. This region appeared to extend well beyond the dorsomedial nucleus to include the perifornical hypothalamus, the dorsal hypothalamic area, the medial zona incerta, and adjoining posterior hypothalamus (see figure 6). It is possible that the area directly affected by microinjected BMI may have been even more restricted, and that disinhibition of at the dendritic fields of distal neurons may have contributed to an extended area of increased fos expression. Nevertheless, given the extensive area over which neurons were affected in the present study where microinjection of 10 pmoles of BMI was employed, it seems likely that microinjection of much larger doses of this agent would have the potential to affect neurons relatively distant from the site of injection. Conversely, sympathetic or cardiovascular effects seen after microinjection of higher doses of BMI into nearby regions may well be a consequence of exciting neurons in the DMH (see DeNovellis et al., 1995). This would be particularly true in reports that have targeted the PVN with microinjections ranging from 50 pmol to as much as 2 nmol of BMI and assumed that sympathoexcitatory or cardiovascular effects noted were a consequence of disinhibiting neurons in the latter region (Martin et al., 1991; Reynolds et al., 1996; Zhang and Patel, 1998; Kenney et al., 2003; LaGrange et al., 2003; Reddy et al., 2005; Martin et al., 2006). The extent of the region activated by microinjection of 10 pmoles of BMI in this study make it highly plausible that at least some of the changes elicited by microinjection of these larger doses into the nearby PVN may be a consequence of spread or diffusion to the DMH.
A salient feature of the behavioral response to microinjection of BMI into the region of the DMH is behavioral arousal characterized by intense locomotor activity (Di Scala et al., 1984; Carrive et al., 1986; Shekhar and DiMicco, 1987) and “anxiety” (Shekhar, 1993), and we reasoned that Fos expression should be increased accordingly in those forebrain neurons whose activation might be relevant to these changes. The effect of BMI on Fos expression in the BNST and the LSV, two limbic structures closely associated with emotion and defensive reactions (for reviews, see Koolhaas et al., 1998; Garcia, 2002; Walker et al., 2003; Herman et al., 2005), provide an interesting contrast. A variety of stress paradigms have been reported to increase Fos expression in the BNST (Campeau and Watson, 2000; Greenwood et al., 2003; Ma and Morilak, 2004; Mantella et al., 2004; Bali et al., 2005, Kiyokawa et al., 2005; Spencer et al., 2005) and the LSV (Cullinan et al., 1995; Imaki et al., 1995; Lino-de-Oliveira et al., 2001). Accordingly, Fos expression was markedly enhanced in the ipsilateral LSV after microinjection of BMI in the DMH but not significantly changed in the BNST. Our negative finding in the BNST contrasts with that of Silveira and colleagues (1995) who employed electrical stimulation or microinjection of kainate 60 pmol or 120 pmol into the medial hypothalamus. Microinjection of as little as 0.5 pmoles of kainate into the DMH is sufficient to evoke marked cardiovascular and behavioral effects (DeNovellis et al., 1995), and electrical stimulation is known to stimulate fibers of passage as well as local neurons. Therefore, it seems likely that the increases in Fos expression in the BNST reported previously may not have been a consequence of activation of neurons specifically in the DMH. Alternatively, neurons in the DMH that may play a role in the recruitment of the BNST in stress, but, unlike those projecting to the LSV and other regions examined here, disinhibition alone may be insufficient for their activation.
Conversely, our observation that disinhibition of the DMH markedly enhances Fos expression in the LSV represents the first evidence that the activation of this region known to occur in stress could be signaled from the DMH. Projections from the DMH to the LSV have been described in the rat (Thompson et al., 1996). Activation of neurons in the LSV is thought to be a critical component of "flight" behavior (Roberts and Nagel, 1996), a response also associated with microinjection of GABAA receptor antagonists or kainate into the region of the DMH (Carrive et al., 1986; Shekhar et al., 1987; Silveira and Graeff, 1988; Siveira et al., 1995). Thus, our results suggests that, while neurons in both the BNST and LSV may be activated in stress, only in the latter region is signaling from the DMH likely to play a role in this excitation, and that activation of projections from the DMH to the LSV may mediate in part the behavioral changes evoked by stress in conscious rats.
In the brainstem, disinhibition of the DMH evoked increases in Fos expression in several autonomic areas of importance with regard to cardiovascular regulation. Experimental stress evokes increases in blood pressure and heart rate that are presumably mediated through increased sympathetic activity (Chiueh and Kopin, 1978; Kvetnansky et al., 1979). Accordingly, stress results in increased Fos expression in key brainstem regions thought to be relevant to these effects (Palmer and Printz, 1999; Dayas et al., 2001; Greenwood et al., 2003). In the present study, unilateral disinhibition of the DMH elevated arterial pressure and evoked significant increases in Fos expression that were restricted to the ipsilateral VLM, a region containing neurons primarily responsible for sympathetic regulation of blood pressure (for review see Madden and Sved, 2003) and where chemical stimulation is known to elicit marked pressor responses (Ross et al., 1984). Neurons in the DMH project directly to the VLM (Fontes et al., 2001), and disinhibition of the DMH excites presumptive pre-vasomotor neurons in the VLM (Horiuchi et al., 2004). Conversely, microinjection of muscimol into the VLM suppresses the increases in blood pressure and renal sympathetic nerve activity evoked from the DMH in anesthetized rats (Fontes et al., 2001; Horiuchi et al., 2004). Nevertheless, the present findings represent the first direct demonstration that disinhibition of the DMH results in excitation of neurons in this key brainstem region.
The present study also directly demonstrates for the first time that neurons in the rRP are activated by disinhibition of the DMH. The rRP is known to be the location of premotor cardiac sympathetic neurons and, as discussed above, disinhibition of neurons in the region of the RP results in sympathetically-mediated tachycardia closely resembling that seen after activation of neurons in the DMH (Morrison et al., 1999; Cao and Morrison, 2003). Because DMH-induced tachycardia can be markedly suppressed by microinjection of muscimol into the rRP (Samuels et al., 2002). the latter region has been proposed to represent the principal brainstem relay mediating DMH-induced tachycardia (Samuels et al., 2002, 2004; Cao and Morrison; 2003). The present findings thus support the conclusions of previous functional studies that pointed to a role for neuronal activity rRP in the increases in heart rate seen after disinhibition of the DMH.
Neurons in the NTS represent the first central synaptic relay for baroreceptor afferent activity (for review see Dampney et al., 2003) and increased arterial pressure is known to result in increased Fos expression in the region (Chan and Sawchenko, 1994, 1998; Dampney et al., 2003). Microinjection of BMI into the DMH evoked significant and bilateral increases in Fos expression in the same regions of the NTS, a finding consistent with the fact that this intervention elevated mean arterial pressure. However, DMH-induced Fos expression was significantly greater on the side ipsilateral to the side on which BMI was microinjected. This difference was most likely a consequence of synaptic excitation mediated through highly lateralized descending projections to the NTS.
The finding that disinhibition of the DMH increases Fos expression in the NTS may reflect one or both of two mechanisms that are consistent with current views of hypothalamic influences or stress on baroreflex function. Neurons in the DMH project to the NTS primarily ipsilaterally (ter Horst and Luiten, 1986; Thompson et al., 1996), and disinhibition of the DMH results in resetting of the baroreflex to a higher level (McDowall et al., 2006), an effect also seen in stress in rats (Hatton et al., 1997, Kanbar et al., 2007). Hypothalamic effects on baroreflex function seem to be exerted, at least in part, at the level of the NTS (Kunos and Vargas, 1995; Chen et al., 1996; Sevoz-Couche et al., 2003) and were historically described as inhibitory (see Nosaka, 1996). If so, then disinhibition of the DMH might have been expected to suppress the increase in Fos expression in the ipsilateral NTS associated with the increase in systemic arterial pressure. However, more recent analysis suggests that the sensitivity of baroreflex modulation of sympathetic activity is increased by stress in rats (Kanbar et al., 2007). Thus, the increased Fos expression in the NTS evoked by hypothalamic microinjection of BMI in the present study may represent DMH-induced enhancement of baroreceptor afferent input as is seen in stress. Alternatively, the increased Fos expression in the present study may represent activation of inhibitory GABAergic interneurons in the NTS that have been proposed to mediate stress- and DMH-induced suppression of the cardiac vagal component of the baroreceptor reflex (Kunos and Varga, 1995).
In summary, the findings of this study demonstrate that microinjection of BMI into the DMH evokes unilateral Fos expression in regions that have been implicated previously in the resulting physiological and behavioral effects and also thought to mediate stress-induced cardiovascular, neuroendocrine and behavioral changes. Furthermore, the overall pattern of Fos expression evoked from the DMH was similar to that previously reported for experimental stress in forebrain and brainstem structures. These results provide an anatomical correlate for previous functional data that implicate the DMH and projections to these regions in diverse components of the integrated physiological and behavioral responses to exteroceptive stress in rats.
4. EXPERIMENTAL PROCEDURES
Animals
Male Sprague Dawley rats (280–290 g) used in the study were housed individually under standard conditions with free access to food and water. The animals were kept in a temperature-controlled room (20–22°C) under a 12 hours light-dark cycle. All procedures involving animals in this study were adhered to National Institute of Health guidelines and were approved by Institutional Animal Care and Use Committee of the Indiana University School of Medicine.
Surgeries
Telemetric transmitters (DataSciences Inc., St. Paul, MN) were implanted under ketamine/xylazine anesthesia (80/11.5 mg/kg, i.p.) with catheter tips inserted into the femoral artery and secured in the abdominal aorta. Five days after telemetric probe implantation, rats were reanesthetized with pentobarbital (35 mg/kg, i.p., supplemented as needed) and mounted in a stereotaxic frame (Kopf Instruments) with the incisor bar at +5 mm above interaural line. A guide cannula for microinjection into the left DMH was implanted using target coordinates with respect to bregma: AP-1.2; RL+2.1; DV-9.1; holder fixed at an angle of 10° from sagittal. The implanted cannula was secured in place with three screws fixed to the skull and cranioplastic cement. The animal was then returned to his home cage and at least 7 days were allowed for recovery.
Experimental protocols
In order to verify placement of the guide cannulae, all rats were tested with microinjections of BMI (10 pmol in 100 nl) 3–4 days prior to experimentation described below. Heart rate, blood pressure and locomotor activity were recorded here and during all challenges described below by telemetry using the DataQuest system (DataSciences Inc., St.Paul, MN). Only those rats that responded to injection of BMI with maximal increases in heart rate over 100 beats per minute were included in the study.
Each experimental run was performed between 10:00 AM and 2:00 PM and involved groups of three rats: two rats received unilateral microinjections of BMI (10 pmol/100 nl), and one rat received an identical microinjection of saline vehicle (100 nl). All microinjections were performed in freely-moving animals without handling or restraint. Animals from a total of four experimental runs (eight rats receiving BMI and four rats receiving saline) were processed for analysis.
Perfusion and histology
Ninety minutes after microinjection of BMI or saline, rats were deeply anesthetized with pentobarbital and perfused transcardially with 100 ml of cold 0.9% saline followed by 100 ml of cold phosphate-buffered saline (PBS) containing 4% paraformaldehyde (pH 7.4). Brains were removed and post-fixed in the same fixative for 2 hours at 4°C and then submerged in PBS containing 25% sucrose for 48 hours at +4°C (with daily changes of the PBS–sucrose solution). Subsequently, frozen coronal sections were cut at a nominal thickness of 30 µm using a Leica cryostat, and stored at +4°C in PBS in culture plates. Sites of microinjection were approximated by a blinded observer from the cannula tracks and local tissue damage at the precise site of injection.
Immunohistochemical procedures and data analysis
After being washed and incubated in PBS containing blocking serum and 0.5% Triton X-100 for 1 hour, alternate sections of the brain were incubated for 48 hours at +4°C in anti-Fos rabbit polyclonal antibody (1:10,000; Ab-5,Oncogene Research Products, Cambridge, MA). The optimum dilution was determined to be 1:10,000 in preliminary experiments. Sections were then washed in PBS and incubated successively with (1) biotinylated anti-rabbit sheep antisera (1:200) for 1 hour; (2) avidin-biotin complex (dilution according to manufacturer’s protocol; Rabbit IgG Vectastain ABC Kit; Vector Laboratories, Burlingame, CA); (3) 0.02% 3,3’-diaminobenzidine tetrahydrochloride (DAB, Sigma) for 10 minutes; (4) 0.02 % DAB with 0.0005% H2O2 for 5–7 minutes with visual control of final precipitation of the color product. Sections were then washed, mounted on slides, air-dried, dehydrated and coverslipped with Entellan (EM Science, Gibbstown, NJ). Conventional digital microscopic images of studied areas of the brain were collected using Leica DM LB microscope (Germany), Spot CCD camera (Diagnostic Instruments, Sterling Heights, MI), and a PC Pentium III computer equipped with interface card.
Fos-positive cells were quantitated by an observer blinded to treatment groups in 6 (for LSV, BNST, PVN, VLM and NTS) or 10 (for RP) representative sections corresponding to the main body of the area or structure according to the atlas of Paxinos and Watson (1998). For LSV, sections analyzed were from approximately +1.0 through - 0.2 mm with respect to bregma. Only areas corresponding to the medial division and the medial part of the ventral division of the BNST were quantitated. In the PVN, the parvocellular and magnocellular subdivisions (pPVN and mPVN) were analyzed separately. Two separate regions of the VLM were approximated and analyzed, including: (1) all sections corresponding to C1, a region where epinephrine-containing neurons are found, located ventrally to nucleus ambiguus from −11.8 through −14.1 mm, including the region of a mixed population of epinephrine and norepinephrine-containing neurons; and (2) A1, a region of norepinephrine-containing neurons, located from approximately −14.1 through −14.3 mm from bregma (Dampney, 1994; Phillips et al., 2001). The RP was taken as the area of the medulla between the pyramids extending from the base of the brain dorsally about 500 microns and from approximately −10.3 through −12.3 mm from bregma. (The parapyramidal region, an area lateral to the RP extending over and adjacent to the pyramids, was also examined, but since no difference in fos expression was evident between saline- and BMI-injected rats, this data was not included in the analysis.) Two distinct regions of the NTS were quantitated: (1) the pre-commissural NTS, extending from approximately −11.8 through −13.4 with respect to bregma; and (2) the post commissural NTS extending from approximately −13.9 to −14.2 from bregma. The region of the DMH was also analyzed by counting Fos-positive neurons in the area approximating the DMN, the region two which all injection sites were localized (figure 2) and where Fos expression was clearly the most intense. (Nevertheless, increased fos expression in rats injected with BMI extended beyond the confines of the nucleus itself as described above.) Fos expression in a total of six sections, centered around those in which the zona compacta of the DMN was clearly evident, were quantitated.
Neurons were counted with ImageJ software (the Scion Corporation’s version of the NIH public domain) using the densitometry tool with the manual pre-set feature (counting criteria were defined specifically for each area and objective used for imaging). Data were summarized for 4 rats/region in saline-injected control rats, and for 6–8 rats/region for BMI-injected animals, and are expressed per section. Wherever sections were lost from a given region in a rat, the region for this animal was excluded from the analysis. These data were analyzed using two-way ANOVA with Newman-Keuls test was used for multiple post hoc comparisons (table 2). Data for baseline physiologic parameters were analyzed by one-way ANOVA (table 1).
Table 2.
Results of two-way ANOVA for Fos expression in rats microinjected with saline versus BMI into the DMH.
| Treatment | Side | Interaction | |
|---|---|---|---|
| LSV | 0.002 | 0.003 | 0.003 |
| F(1,8)=25 | F(1,8)=19.2 | F(1,8)=19.4 | |
| BNST | NS | NS | 0.005 |
| F(1,8)=5.3 | |||
| pPVN | 0.002 | <0.001 | 0.002 |
| F(1,10)=19.2 | F(1,10)=23.8 | F(1,10)=19.9 | |
| mPVN | 0.004 | <0.001 | <0.001 |
| F(1,10)=14.3 | F(1,10)=27.3 | F(1,10)=22.7 | |
| DMH | 0.002 | 0.008 | 0.009 |
| F(1,9)=18.8 | F(1,9)=11.6 | F(1,9)=11.1 | |
| VLMC1 | NS | NS | 0.021 |
| F(1,9)=7.8 | |||
| VLMA1 | NS | 0.011 | 0.011 |
| F(1,7)=11.7 | F(1,7)=11.7 | ||
| RP | 0.007 | - | - |
| F(1,10)=11.2 | |||
| NTSpre | 0.033 | 0.019 | NS |
| F(1,8)=6.6 | F(1,8)=8.6 | ||
| NTSpost | NS | 0.01 | 0.004 |
| F(1,9)=10.7 | F(1,9)=15.4 | ||
Acknowledgments
GRANTS This work was supported by NIH Grant NS 19883. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR015481-01 from the National Center for Research Resources, National Institutes of Health.
Abbreviations
- ACTH
adrenocorticotropic hormone
- BMI
bicuculline methiodide
- BNST
bed nucleus of the stria terminalis
- DMH
dorsomedial hypothalamus
- DMN
dorsomedial hypothalamic nucleus
- LSV
lateral septal nucleus, ventral part
- pPVN
paraventricular nucleus, parvocellular subdivision
- mPVN
paraventricular nucleus, magnocellular subdivision
- NTS
nucleus tractus solitarius
- VLM
ventrolateral medulla
- rRP
rostral raphe pallidus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal SK, Gelsema AJ, Calaresu FR. Inhibition of rostral VLM by baroreceptor activation is relayed through caudal VLM. Am. J. Physiol. 1990;258:R1271–R1278. doi: 10.1152/ajpregu.1990.258.5.R1271. [DOI] [PubMed] [Google Scholar]
- Baffi JS, Palkovits M. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinol. 2000;72:102–113. doi: 10.1159/000054577. [DOI] [PubMed] [Google Scholar]
- Bailey TW, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Bali B, Erdelyi F, Szabo G, Kovacs KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neurosci Lett. 2005;380:60–65. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene Fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski K, Gillen E. Differential distribution of Fos expression within the male rat preoptic area and hypothalamus in response to physical vs. psychological stress. Brain Res. Bull. 2001;55:401–408. doi: 10.1016/s0361-9230(01)00532-9. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J. Comp. Neurol. 1993;335:42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ., Jr Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo-pituitary-adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. J. Comp. Neurol. 2000;423:474–491. [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neurosci. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Carrive P, Schmitt P, Karli P. Flight induced by microinjection of D-tubocurarine or alpha-bungarotoxin into medial hypothalamus or periaqueductal gray matter: cholinergic or GABAergic mediation? Behavioural Brain Res. 1986;22:233–248. doi: 10.1016/0166-4328(86)90068-9. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neurosci. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chan RK, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J. Neurosci. 1998;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of Fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J. Comp. Neurol. 1994;348:433–460. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- Chen YL, Chan SH, Chan JY. Participation of galanin in baroreflex inhibition of heart rate by hypothalamic PVN in rat. Am. J. Physiol. 1996;271:H1823–H1828. doi: 10.1152/ajpheart.1996.271.5.H1823. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Kopin IJ. Hyperresponsivitiy of spontaneously hypertensive rat to indirect measurement of blood pressure. Am. J. Physiol. 1978;234:H690–H695. doi: 10.1152/ajpheart.1978.234.6.H690. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neurosci. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dampney RA. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog. Neurobiol. 1994;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell. Molec. Neurobiol. 2003;23:597–616. doi: 10.1023/A:1025080314925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva LG, Jr, Menezes RC, Villela DC, Fontes MA. Excitatory amino acid receptors in the periaqueductal gray mediate the cardiovascular response evoked by activation of dorsomedial hypothalamic neurons. Neurosci. 2006;139:1129–1139. doi: 10.1016/j.neuroscience.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Medullary neurones regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neurosci. 2001;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Fontes MA, DiMicco JA. Microinjection of muscimol into caudal periaqueductal gray lowers body temperature and attenuates increases in temperature and activity evoked from the dorsomedial hypothalamus. Brain Res. 2006;1092:129–137. doi: 10.1016/j.brainres.2006.03.080. [DOI] [PubMed] [Google Scholar]
- De Novellis V, Stotz-Potter EH, Morin SM, Rossi F, DiMicco JA. Hypothalamic sites mediating cardiovascular effects of microinjected bicuculline and EAAs in rats. Am. J. Physiol. 1995;269:R131–R140. doi: 10.1152/ajpregu.1995.269.1.R131. [DOI] [PubMed] [Google Scholar]
- De Micco JA, Abshire VM. Evidence for GABAergic inhibition of a hypothalamic sympathoexcitatory mechanism in anesthetized rats. Brain Res. 1987;402:1–10. doi: 10.1016/0006-8993(87)91041-9. [DOI] [PubMed] [Google Scholar]
- De Micco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Di Scala G, Schmitt P, Karli P. Flight induced by infusion of bicuculline methiodide into periventricular structures. Brain Res. 1984;309:199–208. [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GR. Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J. Neurosci. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Holsboer F, Landgraf R, Engelmann M. Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats. Eur. J. Neurosci. 1999;11:997–1002. doi: 10.1046/j.1460-9568.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J. Comp. Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain Fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am. J. Physiol. 2001;280:H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, synaptic plasticity, and psychopathology. Rev. Neurosci. 2002;13:195–208. doi: 10.1515/revneuro.2002.13.3.195. [DOI] [PubMed] [Google Scholar]
- Gautron L, Mingam R, Moranis A, Combe C, Laye S. Influence of feeding status on neuronal activity in the hypothalamus during lipopolysaccharide-induced anorexia in rats. Neurosci. 2005;134:933–946. doi: 10.1016/j.neuroscience.2005.03.063. [DOI] [PubMed] [Google Scholar]
- Greenwood B, DiMicco JA. Activation of the hypothalamic dorsomedial nucleus stimulates intestinal motility in rats. Am. J. Physiol. 1995;268:G514–G521. doi: 10.1152/ajpgi.1995.268.3.G514. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neurosci. 2003;120:269–281. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Hatton DC, Brooks V, Qi Y, McCarron DA. Cardiovascular response to stress: baroreflex resetting and hemodynamics. Am. J. Physiol. 1997;272:R1588–R1594. doi: 10.1152/ajpregu.1997.272.5.R1588. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MA, Dampney RA. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am. J. Physiol. 2004;287:R824–R832. doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Wakabayashi S, Dampney RA. Activation of 5-hydroxytryptamine 1A receptors suppresses the cardiovascular response evoked from the dorsomedial hypothalamic nucleus. Hypertens. 2005;46:173–179. doi: 10.1161/01.HYP.0000169970.68151.17. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Wang XQ, Demura H. Intracerebroventricular administration of corticotropin-releasing factor antagonist attenuates Fos mRNA expression in the paraventricular nucleus after stress. Neuroendocrinol. 1995;61:445–452. doi: 10.1159/000126867. [DOI] [PubMed] [Google Scholar]
- Kanbar R, Orea V, Barres C, Julien C. Baroreflex control of renal sympathetic nerve activity during air-jet stress in rats. Am. J. Physiol. 2007;292:R362–R367. doi: 10.1152/ajpregu.00413.2006. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Weiss ML, Mendes T, Wang Y, Fels RJ. Role of paraventricular nucleus in regulation of sympathetic nerve frequency components. Am. J. Physiol. 2003;284:H1710–H1720. doi: 10.1152/ajpheart.00673.2002. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Morilak DA. Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacol. 2002;27:25–34. doi: 10.1016/S0893-133X(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Mapping the neural circuit activated by alarm pheromone perception by Fos immunohistochemistry. Brain Res. 2005;1043:145–154. doi: 10.1016/j.brainres.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Bielajew C. Tracing the neuroanatomical profiles of reward pathways with markers of neuronal activation. Rev. Neurosci. 2004;15:383–414. doi: 10.1515/revneuro.2004.15.6.383. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Everts H, de Ruiter AJ, de Boer SF, Bohus B. Coping with stress in rats and mice: differential peptidergic modulation of the amygdala-lateral septum complex. Prog. Brain Res. 1998;119:437–448. doi: 10.1016/s0079-6123(08)61586-1. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Khalili P. Stress-induced activation of nitric oxide-producing neurons in the rat brain. J. Comp. Neurol. 1997;377:509–519. doi: 10.1002/(sici)1096-9861(19970127)377:4<509::aid-cne3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kunos G, Varga K. The tachycardia associated with the defense reaction involves activation of both GABAA and GABAB receptors in the nucleus tractus solitarii. Clin. Exp. Hypertens. 1995;17:91–100. doi: 10.3109/10641969509087057. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, McCarty R, Thoa NB, Lake CR, Kopin IJ. Sympatho-adrenal responses of spontaneously hypertensive rats to immobilization stress. Am. J. Physiol. 1979;236:H457–H462. doi: 10.1152/ajpheart.1979.236.3.H457. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Rivest S. Functional circuitry in the brain of immune-challenged rats: partial involvement of prostaglandins. J. Comp. Neurol. 1997;387:307–324. doi: 10.1002/(sici)1096-9861(19971020)387:2<307::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- LaGrange LP, Toney GM, Bishop VS. Effect of intravenous angiotensin II infusion on responses to hypothalamic PVN injection of bicuculline. Hypertens. 2003;42:1124–1129. doi: 10.1161/01.HYP.0000102181.83892.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino-de-Oliveira C, Sales AJ, Del Bel EA, Silveira MC, Guimaraes FS. Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Res. Bull. 2001;55:747–754. doi: 10.1016/s0361-9230(01)00566-4. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neurosci. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Sved AE. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell. Mol. Neurobiol. 2003;23:739–749. doi: 10.1023/A:1025000919468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am. J. Physiol. 2004;287:R1494–R1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- Martin DS, Egland MC, Barnes LU, Vogel EM. Adrenergic nerves mediate the venoconstrictor response to PVN stimulation. Brain Res. 2006;1076:93–100. doi: 10.1016/j.brainres.2005.12.116. [DOI] [PubMed] [Google Scholar]
- Martin DS, Segura T, Haywood JR. Cardiovascular responses to bicuculline in the paraventricular nucleus of the rat. Hypertens. 1991;18:48–55. doi: 10.1161/01.hyp.18.1.48. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with Fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Widdop RE, Lawrence AJ. Medial prefrontal cortical integration of psychological stress in rats. Eur. J. Neurosci. 2004;20:2430–2440. doi: 10.1111/j.1460-9568.2004.03707.x. [DOI] [PubMed] [Google Scholar]
- McDowall LM, Horiuchi J, Killinger S, Dampney RA. Modulation of the baroreceptor reflex by the dorsomedial hypothalamic nucleus and perifornical area. Am. J. Physiol. 2006;290:R1020–R1026. doi: 10.1152/ajpregu.00541.2005. [DOI] [PubMed] [Google Scholar]
- McKitrick DJ. Expression of fos in the hypothalamus of rats exposed to warm and cold temperatures. Brain Res. Bull. 2000;53:307–315. doi: 10.1016/s0361-9230(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Morin SM, Stotz-Potter EH, DiMicco JA. Injection of muscimol in dorsomedial hypothalamus and stress-induced Fos expression in paraventricular nucleus. Am. J. Physiol. 2001;280:R1276–R1284. doi: 10.1152/ajpregu.2001.280.5.R1276. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am. J. Physiol. 1999;276:R290–R297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am. J. Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka S. Modifications of arterial baroreflexes: obligatory roles in cardiovascular regulation in stress and poststress recovery. Jpn. J. Physiol. 1996;46:271–288. doi: 10.2170/jjphysiol.46.271. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Printz MP. Strain differences in Fos expression following airpuff startle in Spontaneously Hypertensive and Wistar Kyoto rats. Neurosci. 1999;89:965–978. doi: 10.1016/s0306-4522(98)00333-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain stereotaxic co-ordinates. 4th Edition. Burlington, MA: Academic Press; 1998. [Google Scholar]
- Phillips JK, Goodchild AK, Dubey R, Sesiashvili E, Takeda M, Chalmers J, Pilowsky PM, Lipski J. Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J. Comp. Neurol. 2001;432:20–34. doi: 10.1002/cne.1086. [DOI] [PubMed] [Google Scholar]
- Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am. J. Physiol. 2005;289:R789–R797. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- Reynolds AY, Zhang K, Patel KP. Renal sympathetic nerve discharge mediated by the paraventricular nucleus is altered in STZ induced diabetic rats. Nebr. Med. J. 1996;81:419–423. [PubMed] [Google Scholar]
- Roberts WW, Nagel J. First-order projections activated by stimulation of hypothalamic sites eliciting attack and flight in rats. Behav. Neurosci. 1996;110:509–527. doi: 10.1037//0735-7044.110.3.509. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J. Neurosci. 1984;4:474–494. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am. J. Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J. Physiol. 2002;538:941–946. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevoz-Couche C, Comet MA, Hamon M, Laguzzi R. Role of nucleus tractus solitarius 5-HT3 receptors in the defense reaction-induced inhibition of the aortic baroreflex in rats. J. Neurophysiol. 2003;90:2521–2530. doi: 10.1152/jn.00275.2003. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. Fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J. Neurosci. 1991;11:2321–2331. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. I. Behavioral measures. Brain Res. 1993;627:9–16. doi: 10.1016/0006-8993(93)90742-6. [DOI] [PubMed] [Google Scholar]
- Shekhar A, DiMicco JA. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacol. 1987;26:407–417. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Res. 1990;512:81–88. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Hingtgen JN, DiMicco JA. Selective enhancement of shock avoidance responding elicited by GABA blockade in the posterior hypothalamus of rats. Brain Res. 1987;420:118–128. doi: 10.1016/0006-8993(87)90246-0. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Katner JS. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacol. Biochem. Behav. 1995;50:253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- Silveira MC, Graeff FG. Defense reaction elicited by microinjection of kainic acid in the medial hypothalamus of the rat. Brazilian J. Med. & Biol. Res. 1988;21:569–571. [PubMed] [Google Scholar]
- Silveira MC, Sandner G, Di Scala G, Graeff FG. c-fos immunoreactivity in the brain following electrical or chemical stimulation of the medial hypothalamus of freely moving rats. Brain Res. 1995;674:265–274. doi: 10.1016/0006-8993(94)01451-m. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J. Comp. Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Day TA. Role of catecholaminergic inputs to the medial prefrontal cortex in local and subcortical expression of Fos after psychological stress. J. Neurosci. Res. 2004;78:279–288. doi: 10.1002/jnr.20242. [DOI] [PubMed] [Google Scholar]
- Spitznagel H, Baulmann J, Blume A, Unger T, Culman J. FOS expression in the rat brain in response to substance P and neurokinin B. Brain Res. 2001;916:11–21. doi: 10.1016/s0006-8993(01)02858-x. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stressinduced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996a;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J. Neurosci. 1996b;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF, Blessing WW, Reis DJ. Caudal ventrolateral medulla can alter vasopressin and arterial pressure. Brain Res. Bull. 1985;14:227–232. doi: 10.1016/0361-9230(85)90087-5. [DOI] [PubMed] [Google Scholar]
- ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res. Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J. Comp. Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res. - Brain Res. Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Nemeroff CB, Plotsky PM. Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic-pituitary-adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res. 2000;870:87–101. doi: 10.1016/s0006-8993(00)02405-7. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wible JH, Jr, Luft FC, DiMicco JA. Hypothalamic GABA suppresses sympathetic outflow to the cardiovascular system. Am. J. Physiol. 1988;254:R680–R687. doi: 10.1152/ajpregu.1988.254.4.R680. [DOI] [PubMed] [Google Scholar]
- Willette RN, Punnen-Grandy S, Krieger AJ, Sapru HN. Differential regulation of regional vascular resistance by the rostral and caudal ventrolateral medulla in the rat. J. Auton. Nerv. Syst. 1987;18:143–151. doi: 10.1016/0165-1838(87)90101-9. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Maruyama M, Hosono T, Nagashima K, Fukuda Y, Gerstberger R, Kanosue K. Fos expression induced by warming the preoptic area in rats. Brain Res. 2002;933:109–117. doi: 10.1016/s0006-8993(02)02287-4. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, Samuels BC, Cluxton LK, DiMicco JA. Microinjection of muscimol into raphe pallidus suppresses tachycardia associated with air stress in conscious rats. J. Physiol. 2003;546:243–250. doi: 10.1113/jphysiol.2002.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am. J. Physiol. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]