Abstract
Otitis media (OM) is one of the most common infectious diseases in humans. The pathogenesis of OM involves nasopharyngeal colonization (NP) and retrograde ascension of the pathogen up the Eustachian tube into the middle ear (ME). Due to increasing rates of antibiotic resistance, there is an urgent need for vaccines to prevent infections caused by the most common causes of bacterial OM, including nontypeable Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis. Current vaccine strategies aim to diminish bacterial NP carriage, thereby reducing the likelihood of developing acute OM. To be effective, vaccination should induce local mucosal mmunity both in the ME and in the NP. Studies in animal models have demonstrated that the intranasal route of vaccination is particularly effective at inducing immune responses in the nasal passage and ME for protection against OM. The mouse is increasingly used in these models, because of the availability of murine reagents and the existence of technology to manipulate murine models of disease immunologically and genetically. Previous studies confirmed the suitability of the mouse as a model for inflammatory processes in acute OM. Here, we discuss various murine models of OM and review the applicability of these models to assess the efficacy of mucosal vaccination and the mechanisms responsible for protection. In addition, we discuss various mucosal vaccine antigens, mucosal adjuvants and mucosal delivery systems.
1. Introduction
One of the most successful vaccines in human history is a mucosal vaccine, the oral poliovirus vaccine, and has been instrumental in eradicating this scourge from nearly the entire world. Another mucosal vaccine, FluMist, is similarly showing great promise against influenza. Thus, it is clear that improving the mucosal platform as a route for vaccination will be the next major advance in the field of vaccinology.
Otitis media (OM) is one of the most common infectious diseases in childhood and a frequent reason for prescribing antibacterials in infancy. Due to increasing rates of antibiotic resistance, there is an urgent need for vaccines to prevent bacterial OM infections. Mucosal administration of vaccines holds great promise as it allows induction of both mucosal and systemic immunity. However, for mucosal vaccination to be successful for OM, there are still major obstacles to be overcome. These include the diversity and substantial antigenic heterogeneity among the bacterial and viral pathogens that cause OM, and the low immunogenicity of mucosally administered antigens [1]. Possible solutions to these problems might involve the use of highly conserved protective antigens for bacterial and viral pathogens, and the use of appropriate adjuvants and antigen delivery systems aimed at enhancing the immunogenicity of experimental mucosal vaccines. The mouse provides an optimal model to approach these issues, because of the availability of murine reagents and the existence of technology to manipulate murine models immunologically and genetically. Previous studies confirmed the suitability of the mouse as a model to study inflammatory processes in acute OM and for evaluating putative vaccine antigens directed against OM using novel mucosal adjuvants and mucosal delivery systems [2–6]. Here, we discuss various murine models of OM and review the applicability and limitations of these models to assess the efficacy of mucosal vaccination. In addition, we discuss the mechanisms responsible for protection following mucosal vaccination and various approaches to improve the immunogenicity of mucosal vaccine antigens.
2. Rational for mucosal vaccination against OM
OM often presents as a polymicrobial disease involving complex coinfection with viral and bacterial pathogens. Acute OM is usually caused by bacteria, most commonly by Haemophilus influenzae (NTHi), Streptococcus pneumoniae (S. pneumoniae), and Moraxella catarrhalis (M. catarrhalis). Respiratory viruses, such as influenza, parainfluenza virus (PIV) and respiratory syncytial virus (RSV), are important co-pathogens and in most cases precede bacterial OM. The pathogenesis of OM involves nasopharyngeal (NP) bacterial colonization followed by retrograde ascension of the pathogen up the Eustachian tube into the middle ear (ME). Current vaccine strategies aim at diminishing bacterial NP carriage, thereby reducing the likelihood of developing acute OM. To be effective, vaccination should induce local mucosal immunity both in the NP and in the ME.
It is reasonable to believe that acute OM is a vaccine preventable disease. There is emerging evidence that immunological events are involved in the pathogenesis and resolution of acute OM. During OM, a large number of cells including lymphocytes and IgA-secreting cells are recruited to the middle ear [7,8]. In humans, immunization against influenza virus is associated with a reduction in viral disease as well as in acute OM [9,10]. Furthermore, the resolution of acute OM is correlated with the presence of specific ME mucosal and serum antibodies [11]. Taken together, these findings suggest that enhancement of specific immunity against respiratory pathogens can provide protection against OM.
Recent clinical studies have demonstrated that parenteral vaccine administration is effective at reducing OM caused by NTHi and S. pneumoniae [12–16]. The results from these studies suggest that serum antibody with bactericidal and opsonophagocytic activity can provide partial protection against OM. However, it is apparent that other immunization strategies need to be explored in order to enhance protection against OM. The human respiratory tract is an initial portal of entry for bacterial and viral pathogens that cause mucosal infections including OM, and, therefore, the mucosal immune response is likely to be important in protective immune responses against OM. At mucosal surfaces, secretory IgA (S-IgA) antibodies play a major role in protection. Local IgA responses in addition to serum antibody responses are induced through the use of mucosal vaccines whereas systemic immunization induces only limited or no S-IgA [17–19]. Other advantages offered by mucosal immunization compared to parenteral immunization include the ability to administer vaccines without a requirement for needles, thus improving patient compliance with vaccination schedules, and the capacity to induce immune responses capable of preventing infections at the site of exposure as well as at distant mucosal sites. Thus, optimal defense against major bacterial and viral pathogens of OM is likely to involve a mucosal route of vaccination.
3. Overview of mucosal immunity
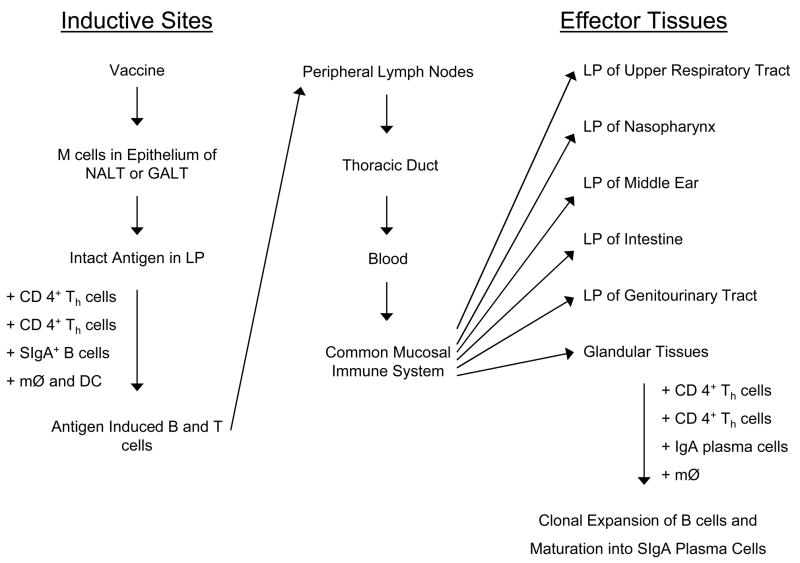
An overview of the mucosal immune system is depicted in Fig. 1. Mucosal immunity forms the first line of defense against most pathogens and consists of physicochemical barriers (mucous, epithelium), innate immune mechanisms, and adaptive host immunity, which at mucosal surfaces includes predominantly of S-IgA, CD4+ T cells, and antigen-specific cytotoxic T-lymphocytes (CTLs) [20]. The mechanisms responsible for protection by mucosal S-IgA antibodies are distinct from those of serum antibodies [21], and include antiadhesive activity, agglutination, neutralization of biologically active antigens, enhancement of innate antibacterial activity, and inhibition of complement-dependent IgM- or IgG-mediated reactions. The mucosal immune system can be divided into two functionally distinct compartments: (1) inductive sites, where antigen is encountered and initial stimulation of naïve T and B lymphocytes occurs, and (2) effector sites, where B cells differentiate into IgA plasma cells and produce S-IgA antibody to protect local and distal mucosal sites. Both mucosal inductive and effector sites are integrated into a common mucosal immune system. Inductive sites for mucosal immunity consist of organized mucosal-associated lymphoid tissue (MALT) and local draining lymph nodes. MALT is subdivided according to anatomical location and includes nasal-associated lymphoid tissue (NALT), bronchus-associated lymphoid tissue (BALT), and gut-associated lymphoid tissue (GALT). NALT and Peyer’s patches are though to be representative MALT in the respiratory and gastrointestinal tract, respectively. NALT is stimulated following intranasal (IN) administration of antigen, whereas Peyer’s patches are stimulated following oral antigen administration. In humans, NALT consists of the Waldeyer’s pharyngeal ring, which includes the adenoids and palatine tonsils. In addition, NALT-like structures consisting of lymphoid aggregates with follicle formation were identified in the human nasal mucosa of young children [22]. Rodents lack tonsils but have paired NALT on both sides of the nasopharyngeal (NP) duct, which are considered to be analogous to the Waldeyer’s ring in humans [23].
Fig. 1.
Induction of mucosal immunity following intranasal or oral administration of vaccine. The priming of antigen-specific, IgA-committed B cells occurs in the mucosal inductive sites and results in IgA antibody responses in effector tissues. NALT: nasal-associated lymphoid tissue; GALT: gut-associated lymphoid tissue; LP: lamina propria; SIgA: secretory IgA; MØ: macrophages; DC: dendritic cells.
Inductive sites contain all of the immunocompetent cells, including B cells, T cells, and antigen-presenting cells (macrophages, B cells and follicular dendritic cells) that are necessary for the development of effector and memory lymphocytes [19]. At mucosal surfaces, antigen is sampled by specialized microfold (M) cells in the lymphoid follicle-associated epithelium, which then delivers antigen to antigen-presenting cells. Antigen may also be sampled on mucosal surfaces by intra- and subepithelial dendritic cells, which migrate via draining lymph to local and regional lymph nodes where they present antigen to T cells. Naïve B and T lymphocytes enter MALT and lymph nodes via high endothelial venules (HEVs). After being primed to become memory B and T cells, they migrate from NALT and lymph nodes to the peripheral blood for subsequent extravasation into mucosal effector sites. The mechanism that guides lymphocytes back to the mucosa includes upregulation of expression of tissue-specific adhesion molecules and chemokine receptors on lymphocytes, which results in the attraction of IgA+ B cells to various mucosal tissues. This mechanism explains why mucosal immunization at one site can result in the secretion of specific IgA antibodies at other mucosal (or glandular) tissues, a finding that led to the term, the “common mucosal immune system.” [24].
The other important feature of the mucosal immune system is its “compartmentalization”, such that there is a preferential distribution of responding cells to effector sites that are anatomically or physiologically related to the inductive sites that received the original antigenic stimulation [25]. The underlying mechanism for this is thought to involve selective expression of adhesions (in tissues) and chemokine receptors (on lymphocytes), which are directly involved in mucosal homing of effector B and T cells following stimulation of certain inductive sites. For example, following IN immunization, IgA+ B cells and T cells that are generated in NALT enter the bloodstream, but they preferentially migrate back into the upper respiratory tract mucosa because they express L-selectin that interacts strongly with peripherial lymph node addressin, which is expressed by HEVs in the upper respiratory tract mucosa [26]. In contrast to NALT, α4β7 integrins and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) play predominant roles in the binding of primed lymphocytes to HEVs in the intestine following oral immunization [27]. This putative mechanism explains the induction of specific responses at mucosal sites where an antigen or pathogen was initially encountered, such as the upper respiratory tract following IN immunization [28,29] and the intestine following oral immunization [30]. Mucosal immunization also induces the production of serum IgA and IgG because mucosal dendritic cells can migrate and carry antigen to systemic inductive sites (i.e., lymph nodes, spleen) [31], and because a fraction of B cells activated in the mucosa or in the mucosal draining lymph nodes express peripheral homing receptors [32].
Protective immunity to pathogens involves the activation of two classes of T cells: CD4+ T helper (Th) cells and CD8+ CTLs [33]. Activation of CD4+ Th cells by foreign antigen leads to the secretion of appropriate cytokines for B-cell responses and immunoglobulin synthesis. Mature Th1 cells produce IL-2, IFN-γ, lymphotoxin (LT)-α, LT-β, and TNF- α, and mediate Th1 responses that are associated with IgG2a antibody responses in the mouse [34]. Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13, and provide effective help for IgA, IgG1, IgG2b, and IgE responses. In addition to TGF-β, Th1-derived IL-2 and Th2-derived IL-5, IL-6, and IL-10 are important IgA-enhancing cytokines for the activation and clonal expansion of S-IgA+ B cells and their terminal differentiation into IgA-producing plasma cells [34–36]. These plasma cells produce dimeric or polymeric forms of IgA which become S-IgA following covalent binding to secretory component that is synthesized by epithelial cells as part of the polymeric Ig receptor. The production of secretory component is up-regulated by both Th1 (IFN-γ) and Th2 (IL-4) cytokines [37–38]. Th1 type responses also up-regulate the function of CTLs that are believed to be major effectors for the elimination of infected cells [33]. Thus, induction of effective T cell immunity at mucosal sites involves both CD4+ T cells that produce cytokines for activation of antibody production and macrophages, and CTLs for more effective pathogen killing at mucosal surfaces [39].
Mucosal inductive and effector sites are distinct in terms of their cytokine profiles. CD4+ cells isolated from murine NALT demonstrate a Th0 cytokine profile, indicating that these T cells are capable of becoming Th1 or Th2 cells immediately after antigen exposure [40]. In contrast, the upper respiratory tract mucosa is considered to be a Th2 dominant site that supports the induction of IgA-producing cells. Of importance for vaccine development is the fact that various mucosal adjuvants influence the development of Th-1-type responses for protection against intracellular pathogens and Th-2-type responses required for protection against soluble antigens, allergens, and toxins [34].
3.1. Mucosal immune responses in the ME
Only a few immunocompetent cells are found in the normal ME mucosa, but acute or chronic inflammation results in significant recruitment of many immunocompetent cells including macrophages, T cells, B cells, dendritic cells and NK cells [8,41,42]. In an immune-mediated murine model of OM that was induced by challenge with keyhole limpet hemocyanin, many immunocytes were found to appear in the ME, including a dominant population of Mac-1+ cells, as well as Th cells and IgG+, IgA+, and IgM+ B cells. These findings suggest that immune responses can occur in the ME following appropriate antigenic or inflammatory stimulation. Kodama et al. [43] analyzed ME mucosa, NP mucosa and NALT from naïve mice for lymphocyte subset expression. In naïve mice, the composition of lymphocytes in ME mucosa was similar to that in NP mucosa, an example of an effector site, but was different from NALT, an inductive site. Both ME and NP mucosa contained large numbers of antibody-producing cells with a predominant IgA isotype. Another study by Kodama et al. [44] compared immunity in the ME, NP mucosa and NALT of mice after IN immunization with the P6 outer membrane protein of NTHi in the presence of cholera toxin (CT) as adjuvant. It was found that IN immunization induced P6-specific IgA antibody-producing cells in both the ME and NP. However, there were fewer numbers of specific antibody-producing cells in NALT. In addition, IN immunization induced in the ME mucosa CD4 T cells producing Th2 type cytokines (IL-5, IL-6, IL-10, TGF-β), which promote local IgA responses. We recently demonstrated that IN immunization with pneumococcal conjugate vaccine and IL-12 as a mucosal adjuvant induces a large number of IgA+ cells in ME mucosa [45]. Two important conclusions relevant to immunization against OM emerge from the above studies. First, these findings suggest that ME mucosa has characteristics of a mucosal effector site similar to the nasal passage mucosa. Secondly, the evidence suggests that NALT-targeted immunization is an effective regimen for induction of protective IgA responses in the ME. IN administration has become a popular route of mucosal immunization in mice in recent years, and is particularly effective for the generation of antibody responses in the upper respiratory tract. A primary role of NALT in the generation of immune responses in the ME is suggested by the relative proximity of the two tissues, which is in line with a concept of “compartmentalization” of the mucosal immune system. In support of this, comparative studies of IN and intragastric routes of immunization in mice have shown that IN immunization is superior to intragastric immunization for the induction of antigen-specific IgA antibody responses in nasal passages [46–48]. This may also hold true for the induction of mucosal immunity in the ME, because the latter represents an anatomical extension of the NP. Taken together, these findings suggest the feasibility of inducing vaccine-specific mucosal IgA responses in the ME by IN immunization.
4. Animal models of OM
Animal models are considered to be an essential element in studying (1) the common pathogens of acute OM, (2) the role of pathogen-specific immune responses in resolution of acute OM, and (3) tests of strategies for vaccination against this disease in humans. An advantage of using animal models includes control over the animals and the microenvironment during disease progression, which enables repeated observations of variables, such as immunologic status, histological changes, and the function of the ME. Several animal models have been used to study OM. A summary of currently available animal models of OM secondary to bacterial infection is provided in Table 1. It should be noted that various rodent species demonstrate a similarity in response to challenge. Doyle [49] established the criteria for an organism to be considered pathogenic in a particular model: (1) the organism induces pathologies similar to those observed in patients with the disease; (2) the pathologies can be objectively documented by otomicroscopy, tympanometry and histopathology; and (3) the organism is shown to reproduce in the ME space. Acute OM models of both S. pneumoniae and NTHi have been developed in several species, but it has been difficult to develop an animal model for M. catarrhalis. The animals can develop acute OM, but the bacteria are rapidly cleared from the ME. Most human bacterial pathogens are not natural colonizers or pathogens in rodents, therefore the immune responses of animals following infection in ME or NP could be different from those in humans.
Table 1.
Summary of animal models of OMa
| Animal species | Chinchilla | Gerbil | Guinea pig | Rat | Mouse |
|---|---|---|---|---|---|
| Pathogen recovery frequency (%):
NTHi SP MC |
> 90 > 90 0 |
> 90 > 09 0 |
NA [53] 100% [54] NA [55] |
> 90 > 90 < 60 |
100 [60] 100 [3] 0 [3] |
| Pathology frequency (%):
NTHi SP MC |
> 90 > 90 < 60 |
> 90 > 90 < 60 |
100 [53] 70 [56] >60 [55] |
> 90 > 90 < 60 |
100 [60] 100 [3,61] 0 [3] |
| Inbred strains | − | + | + | ++ | +++ |
| Genetic mutant models | − | − | − | + | +++ |
| Experimental reagents | − | + | ++ | +++ | +++ |
| Naturally occurring OM | − [50] | + [51,52] | +++ [57] | ++ [58,59] | + [62] |
| Cost | +++ | + | ++ | + | + |
The data for chinchilla, gerbil and guinea pig models were adapted from Doyle, 1989 [49].
OM, otitis media; NTHi, nontypeable H. influenza; SP, S. pneumoniae; MC, M. catarrhalis; NA, not available.
−, absent; + to +++, minimal to highly significant.
Various rodents, including mice, rats, gerbils, guinea pigs and chinchillas, have been used for induction of OM [63–65]. The structural features of the ME and Eustachian tubes in chinchillas and gerbils are similar, but both of these animals differ considerably from the rat [66]. Chinchillas and gerbils have large bulla, which is easily accessible for inoculation through the overlying skin and for serial sampling of ME fluid. The other advantages of chinchillas for an OM model include their general susceptibility to many human pathogens and the ability to induce OM after colonization of the NP [50]. However, in chinchillas, the tympanic membrane is not easily accessible for inspection with an ordinary microscope and for performing transtympanic challenge due to the elongated and S-shaped external auditory canal. In mice, rats, and guinea pigs, the structural features of the ME are similar but these animals have much smaller bulla compared to chinchillas and gerbils [63,66]. In these animals, pathogens can be inoculated through the tympanic membrane or through the bulla. The transbullar approach requires surgical exposure of the inferior bulla through an incision on the neck and blunt dissection of the soft tissue. One animal in particular that is now being increasingly studied in OM research is the mouse and this animal model is the focus of the present review. The mouse has several unique advantages over other species that are impossible to ignore. Experimental reagents for immunological studies are widely available, making it well suited for advanced research protocols. In addition, there is a broad range of transgenic and gene-deleted mouse strains to aid in delineation of the underlying genetic factors of host susceptibility to OM and specific pathophysiologic responses [67] (described in section 6). Furthermore, mice are inexpensive and easy to manipulate. Previous studies indicated the suitability of the mouse as a model for inflammatory processes in human acute OM, and thus, the results from the murine studies can be translated (with certain precautions) to humans [2–4;68].
4.1. Mouse models to assess protection against OM
Various models of ME infection and/or NP carriage, caused by common human pathogens, have been established in mice. These models have allowed an evaluation of candidate vaccine antigens and vaccine-specific immunity for prevention of bacterial and viral infections. In addition, individual mouse strains respond differently to infection, and this can result in unique patterns of susceptibility to infections in the NP and/or ME [69–70]. Melhus and Ryan [3] compared the propensity of BALB/c, Swiss-Webster and C57BL/6 strains of mice to develop acute OM and systemic disease after intrabullar inoculation of three OM pathogens. BALB/c mice were the most susceptible of the three strains, followed by C57BL/6 and Swiss-Webster mice. In addition, we recently evaluated development of OM-induced pneumococcal infection in BALB/c, BALB/c IFN-γ−/− and 129S1/SvImJ mice and found that 129S1/SvImJ mice were most susceptible to invasive disease following OM [45]. These findings indicate disparities in bacterial disease phenotype among various mouse strains. Such differences may help identify the gene(s) affecting host immune responses and host susceptibility to OM (described in Section 6). An approach to overcome strain variations in response to infection includes careful selection of specific mouse strains based on genetic background [67]. Furthermore, mice of the same genetic background may have unique susceptibilities to different bacterial pathogens. For example, Malley et al. [71] evaluated the susceptibility of C57BL/6 mice to development of bacterial carriage in the NP and the ME following IN inoculation of three capsular polysaccharide serotypes of pneumococci. In this study, the mice were more susceptible to pneumococcal serotypes 6B and 23F, and less susceptible to serotype 14. In various mouse models, the diagnosis of acute OM requires direct visualization of ME fluid behind the tympanic membrane and of tympanic membrane changes, such as dilated vessels, increased thickness, and reduced translucency by otomicroscopy. Both clinical symptoms (ME effusions, tympanic membrane appearance) and histopathology (Eustachian tube, ME) endpoints can be quantified. There are, however, certain limitations in using OM mouse models, described in Section 11.
4.1.1. IN inoculation model
In the IN inoculation model, initial bacterial colonization or viral replication occurs in the NP, which may be followed by invasion into the ME cavity. In this model, the portal of pathogen entry into the ME would thus resemble the disease process in humans. Anesthesia prior to IN inoculation and careful dosing of the inoculum (10 μl per adult mouse) allows minimal aspiration or swallowing of the inoculum, which in turn, makes the model highly reproducible. Whereas there are various established models of NP carriage that have been extensively used for the evaluation of the protective efficacy of IN vaccination, the actual spread of bacterial or viral infection into the Eustachian tube and ME has not been extensively studied. McCool and Weiser [72] evaluated the susceptibility of BALB/c, C57BL/6, and CBA/J mice to NP carriage after IN inoculation of S. pneumoniae strain P1121. Among these mice, BALB/c had the highest density and duration of colonization. Importantly, many aspects of this murine model resemble experimental P1121 pneumococcal carriage in humans, including the minimum colonizing dose (<104 CFU) and an average duration of colonization of several weeks [73]. Although development of OM is often sporadic following IN challenge with pathogen and infected ME show variability in bacterial numbers and inflammatory changes [74;our unpublished observations], Malley et al. [71] were able to induce pneumococcal infection in both NP and ME following IN inoculation of S. pneumoniae, as evidenced by positive NP and ME cultures. Similarly, after IN administration of a luciferase-expressing strain of S. pneumoniae that is known to efficiently colonize mucosal surfaces, McCullers et al. [75] confirmed the presence of pneumococci in NP and their subsequent expression in the ME by visualization of the bioluminescent bacteria. Imaging results showed that after pneumococcal IN inoculation, 100% of the mice developed NP colonization and 70% developed acute OM.
We have established a murine model of OM following IN infection of mice with type 14 S. pneumoniae for the evaluation of protective immune responses induced by mucosal vaccination [76]. We found progression of OM in normal mice following 5 daily IN inocula of pneumococci. Infection correlated with the presence of ME effusions and tympanic membrane changes. Time course studies showed that the optimal sampling time for measurement of bacterial carriage in NP and ME was day 8 after initial IN challenge. The possible mechanism responsible for ME infection could be induction of an inflammatory reaction at the Eustachian tube orifice in the NP following repeated exposure to S. pneumoniae.
Several studies confirmed the utility of this acute OM model for the evaluation of IN vaccine efficacy against NTHi [77] and pneumococcal disease [71,76]. IN vaccination prior to IN challenge was shown to confer protection against colonization in both NP and ME.
Several studies have utilized IN inoculation of viruses in order to induce OM. Meek et al. [78] developed a murine model of OM following IN inoculation of reovirus. The authors could detect reovirus antigen in the ME mucosa of infected animals, and histologic evidence of OM was found in half of all infected animals. Following infection, there were significant increases in B cell levels in the NP and Eustachian tubes. The number of infiltrating T cells, however, did not vary significantly from that in the uninfected control animals. Hirano et al. [79] demonstrated that IN inoculation of influenza virus induces inflammatory changes and increases in numbers of CD4+ and CD8+ T cells in the ME mucosa. McCullers et al. [75] infected pneumococcal-colonized mice with influenza virus and observed them for development of acute bacterial OM. These investigators found that 63% of virus-infected mice developed pneumococcal OM compared to 0% of mice mock-infected with PBS. Thus, virus infection allowed NP colonization to progress to OM. Hirano et al. [80] performed a study to further clarify the role of viral infection in development of OM. Mice were inoculated IN with influenza A virus and then examined for histological changes in the NP mucosa using a battery of lectins. Additionally, live NTHi or S. pneumoniae were inoculated into the NP after virus infection and the clearance of bacteria from the NP was examined. Influenza A virus infection changed the glycoconjugate composition of the NP mucosa. Moreover, similar to the findings of others [81,82], there was an increase in levels of NTHi and S. pneumoniae NP colonization in mice that had been infected with virus. Together, these findings could explain the enhanced inflammatory responses that were observed and the onset of bacterial OM following viral infection. Other mechanisms by which influenza virus could facilitate bacterial invasion include virus destruction of the respiratory epithelium, virus-induced immunosuppression, and up-regulated expression of molecules that bacteria utilize for epithelial attachment (i.e., platelet-activating factor receptor) [83]. Gitiban et al. [84] developed a mouse model of RSV infection of the NP and Eustachian tubes following IN challenge. Sections from the NP and Eustachian tubes were examined for RSV antigen expression and for inflammation. It was found that the respiratory and olfactory mucosae of the nasal cavity and the ciliated epithelium of the Eustachian tubes were infected with RSV, and this correlated with inflammatory changes in the NP and Eustachian tube mucosa [85]. Appel et al. [86] demonstrated that in the murine upper respiratory tract, progression of infection with Sendai virus (SeV), the murine counterpart of human PIV, correlates with mucosal inflammatory changes. It should be noted that mice are a natural host for SeV, and this virus may cause severe morbidity in this species [87]. Klements et al. [88] demonstrated that following IN challenge with SeV, all mice developed inflammatory changes in the NP and sinuses, which were associated with positive viral cultures from nasal washes and increased levels of tissue macrophages, neutrophils, and CD4+ and CD8+ T cells compared to uninfected animals.
4.1.2. Direct inoculation of bacteria into the ME
Either transbullar or transtympanic inoculation of bacteria into the ME can cause OM. The advantage of these techniques is the ability to precisely and reproducibly inoculate an exact number of microorganisms and thereby guarantee induction of disease with a low amount of variation between individual animals. Direct inoculation of infectious organisms by either route also allows induction of unilateral OM. A disadvantage of these techniques is the fact that direct ME inoculation is an artificial route of infection as it bypasses NP colonization. Bacterial inoculation using the transbullar approach requires surgical exposure of the inferior bulla through an incision on the neck and blunt dissection of the soft tissue. The pressure within the tympanic cavity is equilibrated by making two microholes in the bulla. The injection volume into the bulla can be as much as 10 μl of fluid in adult mice. This technique requires surgical skill to avoid damage to the adjacent major blood vessels. Meltus and Ryan [3] investigated the course of ME infection in three strains of mice following intrabullar inoculation of S. pneumoniae, NTHi, or M. catarrhalis. Otomicroscopic changes were measured as well as numbers of bacteria in the ME and blood. BALB/c mice were the most susceptible to S. pneumoniae and NTHi infection. By day 3, 75% of mice had developed OM and 50% had a positive ME bacterial culture. There was no evidence of ME infection following inoculation with M. catarrhalis. The authors concluded that the course of acute OM depends upon the mouse strain, the bacterial strain, and the bacterial dose. We applied intrabullar inoculation of H. influenzae or S. pneumoniae to induce OM as confirmed by ME inflammation and the presence of culturable bacteria 3 and 7 days after inoculation [60,76]. Direct ME challenge with either pathogen induced a severe local inflammatory response by day 3, which was reduced by day 7. We also confirmed the utility of this acute OM model for the evaluation of IN vaccine efficacy against NTHi and S. pneumoniae disease (Table 1). Hirano et al. [89] utilized intrabullar inoculation of NTHi into the ME of wild-type and toll-like receptor 4 (TLR4)-deficient mice to investigate the mechanism of protective innate immunity via TLR in the ME. The severity of acute OM was assessed by the presence of ME effusions, the degree of tympanic membrane changes and inflammatory responses in the ME. TLR4-deficient mice demonstrated an increased severity of OM and delayed resolution compared to their immunocompetent counterparts. The authors concluded that innate immune responses induced via TLR4 play an important role in eradicating NTHi infection in the ME.
Transtympanic inoculation of bacteria is relatively easy to perform and includes microscopic insertion of a needle through the tympanic membrane. However, injected fluid often drains from the ME via the hole in the tympanic membrane, resulting in an inoculation volume that is less precise compared to intrabullar inoculation. In addition, the resultant hole in the tympanic membrane can be a conduit for contamination and enhanced drainage of ME effusions via the Eustachian tube due to pressure equilibration [74]. McCoy et al. [90] inoculated heat-inactivated S. pneumoniae transtympanically and assessed ME inflammation on day 3 by measuring the amount of fluid accumulation in the ME, number of cells in the ME fluid, and the thickness of the tympanic membrane. MacArthur et al. [91] similarly transtympanically inoculated heat-killed S. pneumoniae strain 6A and established the parameters of ME inflammation in a mouse model of OM. The most significant ME measures of inflammation were the amounts of ME effusions, tympanic membrane thickness changes, and numbers of inflammatory cells.
Thus, the mouse is a useful animal model for induction of ME infection. The method of choice for OM induction, i.e., IN or direct ME administration of pathogens, depends on the experimental needs of the investigator. Induction of OM using an IN challenge model is preferred for studies that involve evaluating protective immunity against NP colonization and subsequent infection in the ME. Direct ME challenge, on the other hand, might be preferred for evaluating the role of protective immunity against ME challenge or resolution of OM as well as to evaluate the extent of the inflammatory response within the ME cavity.
5. Inflammatory responses in the ME and nasal cavity
Various pathogens have been administered into the nasal passages or ME of mice to induce specific immunity (influenza virus) or for actual challenge after immunization (bacterial/viral pathogens). This can result in various degrees of inflammatory changes, from transient influx of neutrophils into the mucosal surface that results from inoculation of S. pneumoniae [92] to severe epithelial damage induced by influenza virus [93].
Important differences have been noted in the inflammatory responses induced in the ME or nasal mucosa following intrabullar or IN challenge with the same pathogen. Thus, IN inoculation of S. pneumoniae resulted in nasal carriage without local inflammation [94] or was accompanied by mild inflammation which resolved within 1 day [92,95]. A study by Sun et al. [96] provided histological evidence that IN pneumococcal challenge is not necessarily accompanied by inflammation in nasal passages and bacterial challenge induced an increase in specific antibodies in wild type, but not polymeric IgR deficient (pIgR−/−) mice, i.e., there was no transudation of serum IgG into the respiratory tract. In contrast, direct ME inoculation of various strains of S. pneumoniae or NTHi results in extensive inflammation in the ME that includes the generation of effusion, infiltration of leukocytes into the mucosa and ME lumen, and mucosal hyperplasia [3,74]. The local ME inflammation lasts for 1–2 weeks [91]. The distinct inflammatory responses observed in these two anatomical sites could be explained, in part, by more effective clearance of bacterial pathogens from nasal passages than from the ME following challenge. The large surface area of nasal tissue and the wide opening of the upper airways into the NP likely facilitate bacterial clearance. In support of this, increasing the IN bacterial inoculation dose does not affect the probability of ascension of pathogen from the NP into the ME (our unpublished observations). In contrast, increasing of bacterial dose during direct ME challenge increases the frequency of OM and positive ME cultures [3].
Melhus and Ryan [3] examined the propensity of mice to develop bacteremia and succumb to systemic infection after intrabullar challenge with pathogen. Specifically, following intrabullar challenge with an invasive pneumococcal serotype 3 strain, all challenged animals succumbed within several days. Interestingly, in some mice, intrabullar challenge with strains of S. pneumoniae that are typically noninvasive as well as with encapsulated and even unencapsulated H. influenzae also resulted in systemic infection. In contrast, when 10-fold higher doses of S. pneumoniae type 3 were administered IN, all mice remained alive and exhibited nasal carriage without bacteremia or sepsis [94]. It should be noted that the ability of pneumococci to cause sepsis versus carriage after IN or ME challenge of mice is a function of both the mouse strain and the strain of pneumococci.
It should be noted that inclusion of mucosal adjuvants during IN vaccination can contribute to inflammatory responses in mucosal tissues. Previously, several studies showed the ability of CT and heat-labile enterotoxin (LT) [97,98] to induce inflammatory responses in nasal mucosa when administered IN. Van Ginkel et al. [98] demonstrated that CT induces a local inflammatory response in nasal passages, such as increased levels of IL-6 and IL-1β. This finding was supported by other observations that CT induces histological inflammation in nasal passages in a dose-dependent manner [97].
Thus, an inflammatory state can be induced in the ME and nasal passages either through live pathogen priming for immunization (e.g., sublethal doses of influenza virus), actual pathogen challenge, or through use of mucosal adjuvants such as CT and LT. The resulting inflammation may breach the mucosal barrier and increase transudation of serum antibody across mucosal surfaces. These findings might be related to the apparent influence of inflammation on the efficacy of vaccination as well as protection upon challenge, a topic that will be discussed in detail below.
6. Genetically modified animals to study OM
Mouse models provide an advantage over other animal models in that there is an extensive genetic toolkit available for manipulating the mouse genome, which is well-characterized and demonstrates homology with the human genome [99]. Genetically altered mice have provided an opportunity to assess the role of various factors in the enhanced protection observed following mucosal administration of vaccine candidates, including the importance of innate immunity [89], mucosal and systemic antibodies [96,100,101], T-cell immunity [102–104], and cytokines [45](described in section 8).
An initial step in exploring the genes that predispose to OM may include screening of inbred strains of mice. Recently, Zheng et al. [99] used tympanometry in an attempt to identify mouse strains showing differing ME functions. Of the 61 genetically diverse inbred strains tested, 15 strains exhibited abnormal tympanograms and these differences were thought to be the result of genetic factors which predispose to OM. Specifically, the LP/J mouse strain was shown to have an ME condition that results in a predisposition towards increased susceptibility to OM. Further mapping of the differences among inbred strains of mice in their susceptibility to OM will ultimately allow cloning and characterization of OM susceptibility genes [68]. However, analysis of the inbred strains will be complicated by the apparent complex genetic basis, and the low penetrance, of the disease phenotype.
Existing technologies allow development of genetically engineered mice with targeted gene mutations which result in increased susceptibility to OM. These mutant mice provide a great opportunity for unraveling the factors that predispose to OM in genetically susceptible hosts. Progress in this area was recently demonstrated by development of mutant Jeff [105,106] and Junbo mice [107]. These animals exhibit spontaneous, chronic OM which is initiated by murine pathogens. In the future, these mice might also be useful as a model to study OM induced by common human pathogens.
7. Effects of mucosal vaccines in a murine model
Our intent is to review the recent work on mucosally administered vaccines to prevent bacterial or viral infections that lead to OM. A literature search of the MEDLINE database (July 1986–June 2007) was conducted using the terms mouse, OM, colonization, pneumococcus, NTHi, M. catarrhalis, respiratory syncytial virus, parainfluenza and influenza. The search was restricted to studies conducted in mice and published in English. Product literature and references to pertinent review articles were also evaluated. The present review lists previous studies in mice relevant to mucosal administration of vaccines aimed at preventing ME infection (Table 2) or NP colonization (Tables 3–5). Also included are our own data regarding the protective efficacy of IN immunization against murine OM induced by intrabullar challenge with NTHi or S. pneumoniae (Table 6). We demonstrated protection against experimental OM as evidenced by enhanced clearance of bacterial pathogens and reduced inflammatory responses in the ME, such as the presence of ME effusions and tympanic membrane changes. The reader will see that the models reported in these tables and discussed in the text have been restricted to IN administration of vaccine, because this route was mostly used in the assessment of efficacy of mucosal vaccination against NP carriage and experimental OM. Importantly, initial studies with various experimental IN vaccines conducted in the mouse were shown to closely predict success in man [140].
Table 2.
Studies on intranasal vaccination to prevent ME carriage in mice
| System | Comments | Reference |
|---|---|---|
| Nontypeable H. influenzae | ||
| P6 (+ CT) | The protection was associated with enhanced ME mucosal and systemic responses as well as with reduced stimulation of TNF-α production in ME effusion. | [60] |
| P6 or rP6 (+ AdDP) | rP6 constitutes a promising vaccine candidate antigen. Vaccination with rP6 induced protection against OM and the mucosal response was stronger than to native P6. | [77] |
|
| ||
| S. pneumoniae | ||
| KNP (+ CTB) | •• Protection was demonstrated against both NP and ME colonization by three pneumococcal serotypes tested. The novel strategy of inexpensive vaccine for multiserotype protection. | [71] |
| PCV (+ IL-12) | • Neonatal immunization primed animals for enhanced ME mucosal and systemic antibody responses as infants, enhanced bacterial clearance from the ME, and increased survival against OM-induced invasive pneumococcal infection. | [45] |
| PCV (+ IL-12) | NALT-independent protection against NP carriage and ME invasion of challenged pneumococcal cells. | [76] |
Papers of particular interest, published within 1986–2007 period of review, have been highlighted as:
of special interest
of outstanding interest
ME, middle ear; CT, cholera toxin; r, recombinant; AdDP, adamantylamide dipeptide; OM, otitis media; KNP, killed nonencapsulated pneumococci; CTB, B subunit of cholera toxin; NP, nasopharyngeal; PCV, protein conjugate vaccine; IL-12, interleukin-12; NALT, nasal-associated lymphoid tissue;
Table 3.
Studies on intranasal vaccination to prevent nasal carriage by NTHi in mice
| System | Comments | Reference |
|---|---|---|
| Outer membrane protein-based vaccine | ||
| rP6 (+ CT) | Study indicates the good immunogenicity of rP6 to induce specific immune responses and protection against NTHi-induced infection. | [108] |
| P6 (+ CpG ODN) | •• The first study to use non-toxic adjuvant CpG for inducing immunity against NTHi along with the P6. | [109] |
| rLP4/rLP6/UspA2 (+ RC529-AF) | • IN administration of a mixture of the NTHi (P6+P4) and M. catarrhalis (UspA2) proteins resulted in systemic and mucosal antibody responses to each protein and reduced NP colonization by NTHi. | [110] |
| rP4 or rP4/rP6 (+CT) | •• Used combination of two surface protein antigens of NTHi for induction of mucosal and serum antibody responses. | [111] |
| Hap (+ mutant CT) | The induced protection was associated with induced specific mucosal IgA and systemic antibodies. | [112] |
|
| ||
| Lipooligosaccharide-based vaccine | ||
| dLOS-TT (+ CT) | The induced protection was associated with mucosal IgA and serum antibodies. | [113] |
NTHi, Nontypeable H. influenzae; CpG ODN, synthetic oligodeoxynucleotide; LP4/LP6, lipidated P4/6; UspA, ubiquitous cell surface protein A; IN, intranasal; RC529-AF, chemically synthesized adjuvant in an aqueous formulation; CT, cholera toxin; Hap, Haemophilus adhesin protein; dLOS-TT, detoxified lipooligosaccharide conjugated to tetanus toxoid.
Table 5.
Studies on intranasal vaccination to prevent intranasal viral challenge in mice
| System | Comments | Reference |
|---|---|---|
| Influenza | ||
|
| ||
| Live and inactivated vaccines | ||
| Live virus | IgA is primarily responsible for defense against virus. | [125] |
| Inactivated HA vaccine (+ CTB) | The degree of protecton against viral challenge correlated with levels of specific IgA and serum antibodies. Systemic immunization failed to induce nasal antiviral IgA antibodies. | [47] |
| Inactivated HA vaccine (+ CTB) | IN vaccination is more effective than parenteral immunization for providing cross-protection against nasal influenza infecton. | [126] |
| Inactivated trivalent vaccines (+ CTB) | Cross-protection against IN viral challenge associated with hemagglutinin-specific mucosal IgA | [127] |
| Inactivated vaccine (+ CTB) | • In the mice immunized primarily with later virus vaccine and boosted with another later virus vaccine, the cross-protection against challenge with the latest virus was provided more effectively than against challenge with the earliest virus. The strategy for inducing cross-protection against epidemic viral strain in humans. | [128] |
| Inactivated vaccine (+ CTB) | IN priming and IN boosting afforded the highest cross-protection, while combination of s.c. priming and IN or s.c. boosting afforded little cross-protection. | [129] |
| Inactivated virus (+ CTB) | • IN vaccination is superior to oral or systemic routes of vaccination in inducing specific antiviral IgA antibodies and protecting against IN viral challenge. | [130] |
| Inactivated vaccine (+ mutant CT) | Cross-protection against influenza correlated with nasal wash IgA. | [131] |
|
| ||
| Subunit and DNA vaccines | ||
| Subunit antigen (in liposomes) | Incorporation of IN vaccine into liposomes enhanced mucosal IgA and serum IgG responses. | [132] |
| Subunit antigen (+ LTB) | •• Local protection against influenza virus may be mediated not only by IgA but also by mucosal IgG antibodies. Both IN and i.m. routes were effective in conferring the protection. | [133] |
| Chitosan-DNA, expressing influenza HA and NP | •• I.m. priming and IN or i.m. boosting conferred the equal protection against influenza virus challenge. | [134] |
|
| ||
| RSV | ||
|
| ||
| Live and inactivated vaccines | ||
| Live and inactivated virus | Primary immunization with live, but not inactivated, RSV enhanced the production of mucosal RSV-specific IgA upon challenge. | [135] |
| Inactivated virus | • Simultaneous oral and IN administration of inactivated RSV induced specific mucosal immunity, which protected the upper respiratory tract from viral replication. | [110] |
|
| ||
| Subunit vaccines | ||
| F protein (+ CTB) | ••Combined IN plus parenteral immunization provided the better protection from viral replication in the upper respiratory tract than IN or parenteral immunization alone. Association of the enhanced protection with the induction of mucosal IgA, serum IgG and neutralizing antibody. | [136] |
| Chimeric FG (+ CTB) | IN vaccination was superior to parenteral immunization in inducing the protection in the upper respiratory tract against RSV. The enhanced protection correlated with the presence of mucosal IgA antibody. | [137] |
|
| ||
| Parainfluenza | ||
|
| ||
| Inactivated vaccine | ||
| IN killed SeV + oral | Protection against carriage was associated with the presence of IgA antiviral antibodies in nasal wash. Neither | [138] |
| killed SeV (+CT) | IN or oral immunization alone conferred the protection or induced mucosal IgA. | |
| Recombinant vaccine | ||
| RVVs carrying either the HN or the F gene of Sendai virus | • IN but not i.p. immunization induced SeV specific IgA antibodies in nasal wash, and protected from viral replication in the nose. The resistance to SeV in the nose could be abrogated by the IN instillation of anti-mouse IgA but not of anti-IgG antiserum. | [139] |
HA, hemagglutinin; s.c., subcutaneous; LTB, B subunit of heat-liable enterotoxin; i.m., intramuscular; NP, nucleoprotein; RSV, respiratory syncytial virus; F, fusion; protein; G, glycoprotein; SeV, Sendai virus; RVVs, recombinant vaccinia viruses; HN, hemagglutinin-neuraminidase.
Table 6.
Effect of intranasal immunization against otitis media (OM) induced by intrabullar challenge with nontypeable H. influenza (NTHi) or S. pneumoniae
| NTHi-induced OMa |
S. pneumoniae-induced OMb |
|||
|---|---|---|---|---|
| Day 3
|
Day 7
|
Day 3
|
Day 7
|
|
| PBS/P6+CT | PBS/P6+CT | PBS/PCV+IL-12 | PBS/PCV+IL-12 | |
| Mean concentration of bacteria in middle ear effusions (log10) | 5.93/4.41 c | 4.64/3.56d | 7.4/6.8d | 4/0d |
| Middle ear bacterial carriage (%) | 93/79 | 64/28 | 100/87 | 50/0d |
| Presence of middle ear effusions (%) | 100/100 | 79/28d | 100/100 | 50/37 |
| Tympanic membrane changes (%) | 93/87 | 21/0 | 87/75 | 25/0 |
Values obtained from the following publications: NTHi-induced OM (Sabirov et al., [60]); S. pneumonie-induced OM (Sabirov and Metzger, [45]).
Mice were inoculated IN with PBS or P6 and cholera toxin (P6+CT) prior to challenge with NTHi.
Mice were inoculated IN with PBS or pneumococcal conjugate vaccine and IL-12 (PCV+IL-12) prior to challenge with S. pneumoniae. Mice were killed on days 3 and 7 after the intrabullar challenge.
P< 0.001;
P< 0.05 (analyzed by Mann-Whitney test or Fisher’s exact test).
7.1. Candidate vaccine antigens
An effective vaccine formulation against OM must include bacterial antigens that are key targets for the immune system. Vaccine development is hampered by the extreme diversity of S. pneumoniae and NTHi capsular polysaccharides, and efforts are being made to identify protein surface antigens that are conserved among strains. Mucosally administered antiviral vaccines are also highly desirable since prevention of respiratory viral infection could be expected to substantially reduce the incidence of acute OM, which often develops as a complication of viral illness [141].
Several approaches have been used for induction of protective immunity in the ME and in nasal passages by mucosal vaccination. Studies in mice have demonstrated that IN vaccination is the most effective route for induction of protective immunity in the upper respiratory tract against OM. This approach is attractive because the nasal mucosa is the first portal of entry for most human pathogens and because the nasal mucosal environment does not induce degradation of antigens as opposed to the harsh environment in the gastrointestinal tract. One strategy employs whole killed or live attenuated pathogens as vaccines, in an attempt to induce broad protection. However, safety concerns with administration of whole microorganisms have prompted the use of capsular antigens and subunit outer membrane proteins (OMPs) with particular attention to virulence factors widely shared among strains.
In the mouse model, several targets have been identified as potential vaccine candidates against NTHi-mediated OM, including killed bacteria, OMPs, such as P4 and P6, core lipooligosaccharides (LOS), and the Hap adhesion protein [43,44,60,77,108–113,141–150]. Among these potential vaccine antigens, P6 is highly conserved among strains and is associated with crossreactive protection, whereas the other surface antigens demonstrate strain heterogeneity which limits their usefulness [151]. As a vaccine component, LOS is too toxic to be administered to humans, while detoxified LOS (dLOS or hapten) is not immunogenic. To overcome these problems, dLOS was covalently bound to antigenic proteins such as tetanus toxoid (TT) or a mutated form of diphtheria toxin. Such conjugate vaccines elicited long-lasting expression of antibodies with bactericidal activities against NTHi and M. catarhalis. Importantly, a dLOS-TT vaccine was the first investigational NTHi vaccine that has been tested and demonstrated to provide excellent immunogenicity in humans when administered systemically [12]. Moreover, IN immunization with the same conjugate significantly enhanced bacterial clearance in the mouse NP and thus holds promise for mucosal vaccination of humans [113]. The most effective vaccine might ultimately be one that includes several antigens of NTHi.
Capsular polysaccharide-based vaccines, including polysaccharide-protein conjugates have been shown to be effective in reducing S. pneumoniae infecton in humans and mouse models. However, the replacement by non-vaccine pneumococal serotypes causing OM after immunization has prompted the need for new pneumococcal vaccines. The possibility of using immunity to highly conserved and immunogenic pneumococcal surface proteins that may provide protection against all pneumococcal serotypes is currently being explored. Pneumococcal surface adhesion A (PsaA), pneumococcal histidine triad protein A (PhpA), pneumolysin, and pneumococcal protective protein A (PppA) are all conserved among S. pneumoniae strains [45,71,94,96,101–104,115,121,123,124,152,153]. In contrast, pneumococcal surface proteins A and C (PspA and PspC) demonstrate antigenic variability among different strains and immunization with these proteins elicits antibodies that cross-react with some but not all alleles. Alternatively, a combination of various proteins that have different protective functions may provide a broader protection. Finally, phosphorylcholine, a structural component of S. pneumoniae and NTHi, has also been evaluated for induction of cross-reactive protection against both pathogens[121].
Efforts to develop a M. catarrhalis vaccine have focused on killed bacteria [154], LOS [155], and OMPs, such as UspA2 [110]. A major obstacle in assessing potential vaccine antigens against M. catarrhalis includes the absence of a correlate of protection for this pathogen. A mouse model for the study of pulmonary clearance of M. catarrhalis is frequently used to study the effects of vaccination with M. catarrhalis antigens. In this model, mice are challenged by introducing bacteria into their lungs and the rate of clearance is followed as a measure of the immune response. IN administration of dLOS conjugated to mutated diphtheria toxin was found to induce mucosal and systemic antibodies and to enhance murine pulmonary clearance of M. catarrhalis [155].
Influenza vaccines tested in murine models include live topical vaccines, inactivated vaccines, and subunit vaccines consisting of antigens from pathogenic organisms, including the major surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA) [47,156–164]. In addition, a number of influenza DNA vaccine candidates are in various stages of development, with the hope that this vaccine approach induces a broad spectrum of immunity against multiple strains of influenza virus.
RSV vaccines include live [135] or attenuated virus [165–168], and a number of subunit vaccines based on the fusion (F) and major surface glycoprotein (G) proteins [136,137,169–174]. G is an integral membrane protein responsible for cell attachment, and F is responsible for fusion of the viral envelope with the cell membrane [175].
PIV vaccines include live murine SeV which is the closest known homologue of human PIV-1 [176], and subunit vaccines consisting of the H and N envelope glycoproteins, as well as F protein [139]. Studies in mice have demonstrated that immune responses induced against SeV cross-react with human PIV-1 [176]. This suggests the use of SeV as a candidate for a human PIV-1 vaccine.
8. Protective mechanisms against ME and IN challenge
8. 1. Role of humoral vs. T-cell immunity
Murine model systems have been invaluable for discerning the mechanism of mucosal vaccine-induced protection against OM. Considerable efforts have been made to assess the role of T- and B-cell dependent mechanisms in protection against respiratory pathogens following IN exposure to either live [102–104] or killed unencapsulated S. pneumoniae [103] (“whole-cell vaccine”). Traditionally, humoral immunity has been thought to mediate primary protection against pneumococci. However, recent studies demonstrated that IN immunization with wild type whole-cell pneumococci [102,103] conferred protection against NP carriage in immunocompromised μMT mice, which do not produce mature B cells or serum antibody, but did not protect T-cell deficient nude mice. Similar protection was demonstrated after IN administration of pneumococcal polysaccharides [124]. The results of these studies suggest that protection against respiratory pathogens can be induced in the absence of antibody and requires the presence of CD4+ T cells. Roche et al. [104] demonstrated that IN administration of a live nonencapsulated strain of S. pneumoniae to wild-type mice reduced NP colonization and conferred protection against systemic infection following IN challenge with a high dose of the parent strain. However, IN immunization failed to protect μMT mice or major histocompatibility complex class II-deficient mice which lack functional CD4+ T cells. These findings indicate that protection is both antibody- and CD4+ T cell-dependent. There are several potential explanations for these contrasting conclusions regarding the importance of antibody and T cells in protection of the respiratory tract from pneumococci. First, different experimental protocols were used - in some but not all cases, strong mucosal adjuvants such as CT or B subunit of CT (CTB) were used during vaccination [102,103,124]. In addition, it should be realized that μMT mice are known to produce mucosal (but not serum) IgA antibody [177], suggesting that use of μMT mice can not rule out a role for antibody-dependent mechanisms in vaccine protection. Thirdly, there are likely to be distinct protective immune responses to wild-type bacterial isolates [102,103] vs. attenuated mutant strains [104]. Encapsulation, for example, may obscure the immune response to underlying surface antigens. In support of this possibility, vaccination with attenuated mutant strains was shown to protect from subsequent IN challenge more efficiently than vaccination with wild-type isolates [104]. Similarly, differences may exist in the protective immune responses to killed [103] vs. live attenuated strains [104].
The precise mechanism by which CD4+ T cells mediate protection against colonization remains to be determined and several possibilities have been raised [103]. One possibility is that the CD4+ T cells responsible for protection are able to mediate optimal Th1 responses, similar to those shown to protect against intracellular bacteria. Another potential mechanism includes the presence in the whole-cell vaccines of TLR ligands, which can enhance Th1 responses. Finally, the potential contribution of the newly described Th17 T cell subset, which has been shown to mobilize neutrophils through granulopoesis and chemokine production [103], remains to be fully explored.
Thus, mature B cells are required for the protection against respiratory pathogens following IN immunization in at least some models. However, the findings also suggest an important role for T-dependent mechanisms even in the absence of B cells in protection against respiratory pathogens following IN immunization.
8.2. The role of mucosal and serum antibodies
One may speculate that the primary role of vaccines in limiting bacterial colonization at mucosal surfaces is to prevent initial infection. Secondarily, an increase in antibody concentrations caused by mucosal contact with pathogens in an immunized host could lead to a more rapid elimination of the pathogen from the mucosa. Mucosal immunization in various murine bacterial challenge models has been demonstrated to be effective at limiting acquisition of pathogens in the NP, ascension of pathogens from the NP to the ME, and pathogen replication in the ME. The analysis of protective immune responses in the ME fluids of infected mice is hampered by the difficulty in collecting ME fluids and their small volume. The concept of a compartmentalized mucosal immune system suggests that determination of specific antibodies in nasal fluids is the primary surrogate measure of immunity in the ME [25]. Likewise, immunization protocols that can induce protective immunity in the NP are expected to be effective in also activating protective immunity in the ME.
Studies using murine models have shown that IN and oral immunization is an effective regimen to induce expression of S-IgA and serum antibodies, as well as activation of mucosal Th cells, regulatory T cells and CTLs [43,178,179]. Co-administration of selected mucosal adjuvants favors the production of Th1- (IgG2a, IgG2b) and/or Th2-type (IgA, IgG1, IgE) antibodies, both in the mucosal and systemic compartments. Following IN immunization, the expression and function of antibodies in mediating bacterial clearance from mucosal surfaces as well as from the blood have been examined. In general, IN vaccination, which was effective in induction of nasal mucosal and serum antibodies, was also effective in the protection against carriage in ME or NP (Tables 2–6).
8.2.1. Protection against NTHi
Initial mucosal vaccination studies in mouse models were performed with whole killed NTHi or crude preparations of surface-exposed antigens (i.e., OMPs) as the immunogen, and the results from these studies established the parameters of effective immunity against NP infections as well as the potential of vaccination. For example, oral administration of formalin-killed NTHi was found to confer protection against nasal carriage by NTHi [142,143]. Similar protection was demonstrated after oral administration of OMP with GM-53, a chemically synthesized adjuvant [144]. Although this regimen was effective in inducing both mucosal and serum antibodies, the protective effect of oral vaccine was likely associated with mucosal IgA antibodies since oral immunization was more efficacious in a clearing nasal infection than subcutaneous immunization, which failed to induce mucosal antibodies [143]. Others have employed IN administration of OMP from NTHi [145,146], and two important conclusions resulted from those studies: (1) protection in the upper respiratory tract is correlated with the presence of specific IgA antibodies in nasal washes, and (2) nasal vaccine delivery was most efficacious in inducing mucosal IgA antibodies and in providing protection against nasal NTHi carriage compared to other routes of inoculation (oral, intra-tracheal, or systemic; [146]). IN administration of surface-expressed antigens from NTHi was used to elucidate the significance of immune responses to these antigens in clearing an infection. IN administration of the highly conserved NTHi P6 protein in the presence of adjuvant induced specific systemic as well as mucosal IgA responses in various effector sites, including nasal passages [44,148, 150], Eustachian tubes [43] and the ME [44]. Hirano et al. [147] demonstrated that IN administration of detoxified NTHi lipooligosaccharide (dLOS)-tetanus toxoid (TT) conjugates induced specific mucosal IgA and serum IgG antibodies with a predominance of IgG1 antibodies (Th2-type). The murine model used in this study provided useful information regarding the kinetics of antibody expression and cellular responses following multiple administration of dLOS-TT. Thus, studies with IN immunized mice demonstrated enhanced bacterial clearance following IN or ME bacterial challenge [45,60,113]. Moreover, NTHi numbers in nasal washes [113,144] or ME washes [60] were associated with vaccine-specific mucosal IgA and serum IgG antibody titers.
8.2.2. Protection against S. pneumoniae
Wu et al. [94] demonstrated that IN immunization with heat killed pneumococci or pneumococcal lysates in the presence of CTB as an adjuvant elicited protection against carriage. Further studies using murine models showed that protective immunity against pneumococcal infection could be conferred by antibodies against conserved pneumococal surface proteins or by serotype-specific anti-capsular antibodies. Antibodies neutralize the activity of virulence factors and function as an opsonin to enhance bacterial clearance. IN immunization of mice with pneumococcal serotype 1 polysaccharide conjugated to TT or type 3 polysaccharide conjugated to diphtheria toxoid together with a nontoxic mutant of Escherichia coli or a glyceride-polysorbate based adjuvant induced mucosal and systemic responses, and serum IgG antibody levels were significantly higher than after systemic immunization [180]. IN immunization with pneumococcal serotype 9V polysaccharide conjugated to pneumococcal proteins (pneumolysin or autolysin), and CT as adjuvant, elicited high 9V IgG and IgA antibody responses in serum and tissue homogenates [181]. Recently, we demonstrated protection against OM in unimmunized mice if the pneumococci were first opsonized with antibody from ME fluid or serum obtained from immunized mice before intrabullar challenge [45]. Several studies using mouse models have demonstrated the importance of serum antibodies for prevention of invasive pneumococcal disease following NP colonization or ME infection [45,94,116]. In IN immunized mice, the levels of serum antibodies correlated with rapid pneumococcal clearance from the blood [181] and lungs [180] following intraperitonial or IN challenge, respectively. It is expected that serum antibodies which are protective in the systemic compartment could also be protective at mucosal surfaces. Indeed, systemic immunization with PsaA synthetic peptides induced peptide-specific serum antibodies and provided cross-protection against nasal challenge with pneumococci [182]. Thus, protection at mucosal surfaces following IN immunization may be mediated by both mucosal IgA and serum antibodies. Specific IgA antibodies play a key role in clearing bacterial pathogens from mucosal sites by inhibiting the uptake and colonization of microorganisms [183]. The protective function of serum antibodies, especially IgG2a and IgG3 isotypes, has been shown to be associated with increased complement-mediated bactericidal and opsonophagocytic activity [101], as well as with increased binding avidity compared to IgA antibodies [181].
8.2.3. Protection against M. catarrhalis
Jiao et al. [155] inoculated mice IN with a M. catarrhalis dLOS-protein conjugate and the results revealed a negative correlation between bacterial counts in the lung, and IgA and IgG levels in nasal washes, lung lavage fluids, and serum. These data indicate that local and/or systemic LOS-specific antibodies are associated with enhanced bacterial clearance from the mouse lung. Importantly, IN immunization resulted in a higher level of bacterial clearance compared to subcutaneous immunization, supporting an important role for mucosal antibodies in the observed protection. Mason et al. [110] demonstrated that IN administration of a mixture of rLP4/rLP6/UspA2 proteins in the presence of the RC259-AF adjuvant induced specific IgA responses in the respiratory tract as well as specific serum IgG and IgA responses. The serum IgG subclass distribution was predominantly IgG2a, representing a Th1-type response. Importantly, serum antibodies from immunized mice showed complement-dependent bactericidal activity toward several strains of M. catarrhalis. Hou et al. [154] induced specific nasal and serum antibody responses following injection of whole killed M. catarrhalis into NALT tissue. Intra-NALT immunization was shown to be more effective at inducing specific mucosal and serum responses compared with those induced using the IN route, and this is likely due to more efficient priming of immunocompetent cells in a mucosal inductive site.
8.2.4. Protection against viruses
With respect to prevention of virally-induced acute OM, mucosal vaccines have been shown to provide effective protection against influenza virus, RSV and PIV in the respiratory tract (Table 5). Vaccine-induced protection against viral infections is likely to be associated with both humoral (S-IgA, serum IgG antibodies) and cell-mediated (CTLs) immunity [157,135,184]. Renegar et al. [185] demonstrated that S-IgA prevents influenza virus-induced pathology in the upper respiratory tract but serum IgG antibody failed to prevent viral infection of the nose although it did neutralize newly replicated virus after infection had been initiated. Studies of heterosubtypic immunity, i.e., crossreactive protection against different strains of influenza virus, also showed a correlation with S-IgA production (rather than cytotoxic T-cell reactivity) [186,187]. Others also demonstrated that passive transfer of IgA antibody, as well as IgG antibody, can confer protection against RSV infection in the upper respiratory tract [188]. The mechanism of IgA-mediated protection against viruses at mucosal surfaces may include both extracellular and intracellular neutralization [183,189]. IgG antibodies provide antiviral protection through direct neutralization as well as Fc receptor-mediated opsinophagocytosis [190]. IN immunization with recombinant SeV expressing the G glycoprotein of RSV induced SeV-specific and RSV-specific antibody-forming cells in lymph nodes, and a few antibody-forming cells in the bone marrow [191], and these increases were reflected by increased serum antibody levels [192]. Similarly, IN immunization of infant mice with human PIV-1 afforded protection against a subsequent challenge with SeV (mouse PIV-1) and this protection was associated with increased levels of specific antibodies in the serum [176]. Importantly, the response was completely cross-reactive between human PIV-1 and SeV. Several studies have compared the ability of mucosally and parenterally administered antiviral vaccines to induce mucosal vs. serum antibodies. Bastien et al. [170] demonstrated that both IN and intraperitoneal administration of an RSV G protein synthetic peptide mixed with CT induced specific IgG antibody in serum, whereas only IN administration induced specific IgA in nasal washes. Similarly, oral administration of an SeV-CT conjugate induced virus-specific IgA titers in nasal washes as well as specific antibodies in serum, and protected the upper respiratory tract against virus challenge with SeV [179]. In contrast, systemic immunization with the SeV-CT conjugate only induced virus-specific antibody responses in serum and failed to protect the upper respiratory tract against virus challenge. Thus, the enhanced protection against SeV conferred by the oral vaccine is likely to be due to specific S-IgA antibodies. Lovgren et al. [156] compared the induction of specific serum antibodies after IN, oral or subcutaneous administration of influenza glycoproteins in immunostimulating complexes (ISCOMs). Subcutaneous administration induced greater serum antibody titers compared to IN administration, whereas oral administration failed to induce serum antibody. The predominant IgG isotypes after IN or subcutaneous administration were IgG1 and IgG2a. Trudel et al. [166] demonstrated that IN administration of live RSV induced greater antibody titers in serum than those induced by intramuscular immunization, however, this difference could have been due to the fact that different adjuvants were used for IN and systemic vaccination. In addition to antibody-dependent protection, CD8+ cytotoxic T cells and CD4+ Th cells have been found to contribute to recovery from infection at mucosal surfaces by targeting internal viral proteins, but are not strong mediators of resistance to reinfection [166,193–196].
Considering the above, it is likely that mucosal specific IgA antibody protects against bacterial and viral pathogens at mucosal surfaces, whereas serum antibodies (as well as CD8+ and CD4+ T cells) mainly eradicate the pathogens after the infection has been initiated in the respiratory tract and/or ME [47,94,100,108,125].
8.3. Immunodeficiency models to understand the role of IgA in respiratory and ME immunity
The development of mice that are selectively deficient for expression of IgA (IgA−/− mice) or polymeric IgR (i.e., defective transport of IgA into the mucosal lumen) as a result of targeted genetic lesions has provided useful models to evaluate the role of IgA in upper respiratory tract and ME immunity [96,100,101,163,198].
Mbawuike et al. [163] demonstrated that in IgA−/− mice, IN immunization with a CTB-adjuvanted influenza vaccine provided protection against virus infection in the upper respiratory tract following IN challenge with a lethal dose of influenza virus. This finding challenged the notion that S-IgA is responsible for protection against virus infection in the respiratory tract. Similarly, IN immunization with live influenza virus conferred protection against heterosubtypic IN challenge in both IgA−/− and IgA+/+ mice, as evidenced by reduced virus titers in nasal washes [164,198]. In contrast, IN immunization of pIgR−/− mice with inactivated influenza virus failed to confer protection against virus infection in the upper respiratory tract, in parallel with a decrease in nasal wash IgA titer [100]. Moreover, the importance of S-IgA in host defense was shown by the finding that IN vaccination with pneumococcal polysaccharide conjugated to diphtheria toxoid in the presence of IL-12 as an adjuvant protected IgA+/+ but not IgA−/− mice against subsequent NP colonization with S. pneumoniae type 14 [96,101]. The same vaccination regimen similarly failed to protect pIgR−/− mice against nasal carriage, and this observation was associated with the absence of specific IgA antibody in nasal washes [96].
Recently, an effort has been made to present a unifying concept to explain apparently conflicting results obtained from various laboratories using IgA-deficient mice [199]. Differences in this regard may be related to the extent of inflammation induced during infection and the amount of transudated IgG antibody [199]. In general, it is the presence of inflammation that appears to determine whether IgA is necessary for any observed protection; that is, in the presence of inflammation, IgA-deficient mice are protected from infection, and in the absence of inflammation, IgA-deficient mice are not protected. This conclusion in supported by observations that S-IgA is not required for protection against IN challenge with S. pneumoniae type 3 or sublethal doses of influenza virus, or against intrabullar challenge with noninvasive pneumococci (unpublished observations), all of which result in significant inflammation and systemic infection. The latter effects facilitate passive transudation of serum IgG antibodies into mucosal secretions through damage to the epithelial layer and thus, compensate for the lack of IgA [60,185,199,200].
In essence, it is likely that IgA antibody provides an important first line of defense against infections of the respiratory tract. The presence of inflammation appears to determine whether IgA is necessary for any observed protection following IN or intrabullar challenge.
9. Mucosal adjuvants and delivery systems
Mucosal immunization suffers from two main problems: inefficient antigen retention and uptake at mucosal surfaces [201], and difficulties in eliciting immune responses to mucosally administered antigens [202]. With respect to oral immunization, the gastrointestinal tract prevents the absorption of many foreign molecules such as vaccine antigens [203]. Several strategies have been designed to improve the immunogenecity of vaccine antigens and to induce potent, long-lasting, and recallable mucosal immune responses manifested by expression of S-IgA antibodies in secretions, as well as specific antibody-secreting B cells and T effector cells in the relevant tissues [25]. These strategies include the use of purified synthetic adjuvants and delivery systems that can efficiently aid in uptake, processing, and presentation of vaccine antigens in mucosal tissues. Advantages include activation of only those elements of the local immune system that are required for protection, and limitation of the systemic distribution of the adjuvants. Novel adjuvants and delivery technologies are highly desirable for the successful development of mucosal vaccines against OM that have failed through the use of traditional approaches. Experiments in mice have revealed that balanced Th1- and Th2-type responses can be generated, offering the prospect of selectively inducing the most appropriate humoral or cell-mediated responses for protection against a particular infection.
The most widely experimentally used mucosal adjuvants in mice are CT and the closely related E. coli LT (Table 2), both of which can promote mucosal IgA responses, systemic IgG responses and CTL activation against co-administered antigens. Unfortunately, severe toxicity renders CT and LT unsuitable for clinical use in humans. To avoid toxicity, the isolated B subunits of CT [47,95,116,118,120,124] and LT [133] have been tested for their ability to augment immune responses against co-administered antigens. Both CTB and LTB are poor adjuvants when administered together with antigens by the oral route, although they display more significant activity when inoculated via the IN route [17]. In addition, genetically detoxified CT and LT mutants that have reduced toxicity, but retain adjuvanticity when given to animals by the IN route are now available [112,122,123,172]. It has been shown that CT primarily induces Th2 type responses characterized by CD4+ T cells that produce IL-4, IL-5, IL-6 and IL-10 and by the production of IgA, IgG1 and IgE antibodies [1]. Use of LT has been reported to produce a less polarized response, and to activate both Th1 and Th2 cells [204].
Several investigators have tested CpG oligodeoxynucleotides (ODN) as mucosal adjuvants [109,148]. IN administration of CpG ODN together with the NTHi P6 antigen resulted in both Th1 responses (CTL and IFN-γ) and Th2 responses (IgA) at mucosal sites and Th1 responses systemically [109,148]. Following IN administration, CpG ODN also enhanced serum antibody responses to type 9V polysaccharide conjugated to inactivated pneumolysin, and these responses included mixed Th1-type and Th2 type antibodies [205]. Kodama et al. [150] recently coadministered P6 protein with Flt3 ligand, which mobilizes and stimulates dendritic cells and myeloid and lymphoid progenitor cells, and demonstrated efficient induction of specific mucosal and serum antibodies. Co-inoculation of pneumococcal polysaccharide conjugate vaccine with IN administered IL-12, generally thought to be a Th1-inducing cytokine, was found to induce high levels of both Th1- and Th2-associated antibodies locally and in the bloodstream [45,96,101].
A variety of mucosal antigen delivery systems have been developed to increase uptake of antigen at mucosal surfaces, including biodegradable nanoparticles [160] and biopolymers such as chitosan [134], liposomes [158,159,132], and ISCOMs [156,157,166,169]. Studies with recombinant bacterial [115,206] and viral [191] vector systems have demonstrated the feasibility of creating live delivery systems that express recombinant DNA encoding foreign antigens but without the ability to allow reversion to a virulent phenotype [115,206]. ISCOMs have shown the potential to induce a full range of humoral and CTL responses, both of which appear to be important for antiviral protection [156,157,166,169]. Other strategies include the incorporation of antigens into various microparticles [134,160] or membrane-bound vesicles [158,133,159], procedures that promote antigen uptake and provide protection against proteolytic digestion.
10. Mucosal immunization in early life
In humans, OM has its onset in the first year of life, and therefore, a vaccine to prevent OM must target young infants and newborns. However, mucosal vaccination in this target population may be problematic because of the relatively poor immunogenicity of many vaccine candidates during early life due to immature antigen presenting and T cell function. In previous murine OM models, the effect of vaccination was studied in adult animals, however, as pointed out by Gu et al. [12], the immunogenicity of vaccines in adults might not always predict their activity at a young age. Thus, recent efforts have been made to evaluate the immunogenicity of vaccines in mouse models of early life. For example, we demonstrated that neonatal IN vaccination with pneumococcal conjugate vaccine in the presence of IL-12 can induce protective immunity against S. pneumoniae [45,76]. This immunization regimen was shown to enhance the ability of infant mice to clear pneumococcal infection from both the ME and NP [45,76]. Inclusion of IL-12 during neonatal IN vaccination may partially overcome defective antigen presenting cell function and thereby enhance priming for antibody responses [36].
Another innovative approach has been explored to prevent NTHi-induced disease in young mice via maternal IN immunization [149]. Such a vaccination approach holds promise in stimulating antibody responses to the P6 protein and potentially preventing OM due to NTHi carriage in the mother, as well as OM in the infant through passive transfer of maternal antibodies across the placenta. Similarly, maternal IN immunization of mice with PspA and CTB was reported to significantly reduce nasal carriage of pneumococci in the offspring following IN challenge with pneumococci [152].
11. Limitation of the mouse model and mucosal vaccination strategy
How closely does the mouse immune response and the pathogenesis of ME disease resemble that in humans? Murine antibody responses and the stages of immune maturation are similar to those in humans [207]. Nevertheless, while it appears that induction of OM is reproducible in mice, the major bacterial (NTHi, S. pneumoniae and M. catarrhalis) and viral (influenza virus, PIV, RSV) pathogens for humans are not natural murine pathogens [64,85,208,209]. Since these microbes are exclusively human pathogens under natural conditions, the colonization patterns and the immune responses observed following infection of mouse ME and NP might be different from those of humans. This is a significant limitation to the usefulness of the rodent model. In order to overcome this limitation, multiple IN inoculations or direct inoculation into the ME space must be performed to induce disease (as described in section 4.1). Alternative approaches include using mutant bacterial strains that adhere to, and invade, murine mucosa more efficiently than wild type bacteria [210] and the use of genetically modified mice which demonstrate increased susceptibility to human pathogens. Even so, the observed inflammatory responses are often less prominent than those in humans. Therefore, transudation of serum antibody is also less efficient in the mouse compared to humans, where transudation of serum antibody appears to be very important. Nevertheless, both antibody and cellular systemic immune responses in the mouse mimic the human immune response sufficiently to be informative. Another limitation is that the mouse ME is not easily accessible for inoculation and for removal of fluid, and the small size precludes sampling of large quantities of ME effusion. An alternative is to use larger species such as chinchillas, guinea pigs, rats, or gerbils for induction of OM. However, despite the above disadvantages, the mouse model is still the most useful for testing hypotheses regarding immune responses and pathogenensis, although certain precautions should be taken in extrapolating results from murine studies to humans. Experience with oral vaccines has shown that vaccines which work perfectly in mice can fail to perform well in other animal species including humans [211]. It has to be noted that most of the vaccines in current use are parenteral vaccines and only very few mucosal vaccines have been approved for human use, primarily because the low immunogenicity of soluble antigens and the lack of safe and efficacious mucosal adjuvants [212]. In addition, it is uncertain whether a fully effective dose of vaccine can be administered mucosally. It has been difficult to gain approval for mucosal vaccines in humans with the notable recent exception of the IN FluMist influenza vaccine. However, progress in this field may allow increased development of needle-free vaccines against mucosally acquired pathogens, including those that lead to OM.
12. Conclusions and future directions
In our opinion, improving the mucosal platform as a route for vaccination will be the next major advance in the field of vaccinology. Studies in murine models have been instrumental in the development of many successful mucosal vaccines to prevent bacterial and viral infections that often lead to OM. Mucosal immunization studies, particularly those utilizing IN inoculation, have identified several vaccine candidates for key bacterial and viral targets against OM, and the types of immune responses associated with protection from infection in the ME. The progress in this field was demonstrated by the development of various nonreplicating subunit vaccines, DNA vaccines, recombinant replicating vaccines, and, microbial vector-based mucosal vaccines. Mouse models of NP colonization and ME infection have been used by several groups to show that IN vaccine administration with the use of appropriate adjuvants and antigen delivery systems can be effective for induction of protection against NP carriage and experimental OM. In addition, transgenic and knockout mice have been invaluable for discerning the mechanisms of mucosal vaccine-induced protection against OM and elucidating the roles of various components of a protective immune response. Over the next years, emphasis should be placed in several areas to advance the field of vaccine development:
Priorities for future mucosal vaccine development against OM should focus on multipathogen vaccines which include combinations of protective protein antigens from all three causative bacteria. The emphasis should be placed on development of strategies using antigens which are highly conserved among similar bacterial species as well as those shared among different bacterial species, such as phosphorylcholine. The induction of protective responses following mucosal administration of more than one antigen from similar pathogens should continue to be tested. The evaluation of combined bacterial-viral vaccine formulations should also continue to be an important area of investigation. Although IN vaccination appears to be an attractive route for prevention of OM, future studies should also focus on immunization by mixed, mucosal routes (nasal-oral) or a combination of mucosal and parenteral routes.
Novel mucosal adjuvants and mucosal delivery systems should continue to be developed as a means to enhance the efficacy of mucosal and systemic immune responses.
The immunogenicity of mucosal vaccines in infants needs to be further evaluated in the mouse models of early life and maternal immunization.
There is a need to develop a mouse model of OM caused by combinations of bacterial pathogens (NTHi and S. pneumoniae) and viruses. Such polymicrobial models will allow testing of multipathogen vaccines against OM. The efficacy of promising vaccine candidates against M. catarrhalis must also be assessed in a mouse pulmonary clearance model.
One of the priorities for research that can readily exploit mouse models includes further unraveling of the genetic factors that are involved in susceptibility to OM and that influence host immune responses to pathogens/mucosal vaccines. Current and future progress in developing mice with targeted gene mutations that result in increased susceptibility or spontaneous development of OM will provide significant new insights into the mechanisms responsible for disease progression and the requirements for protection.
If there is sufficient effort and resources devoted to resolving these issues, we predict that in the next decade or two, it will be possible to routinely utilize mucosal vaccines for prevention of OM so that this disease will ultimately represent a historical footnote and will no longer be a significant cause of human morbidity and mortality.
Table 4.
Studies on intranasal vaccination to prevent nasal carriage by S. pneumoniae in mice
| System | Reference | |
|---|---|---|
| Pathogenic and non-pathogenic bacteria | ||
| KNP (+ CT or CTB) | •• The protection was demonstrated against both colonization in the NP and ME. | [71,114] |
| Live-attenuated bacteria | • Live S. pneumoniae attenuated through deletion of genes encoding virulence factors was effective in induction of cross-serotype protection. Protection did not depend on a response to the virulence factors such as C-Ps, pneumolysin and PspA. | [104] |
| rLactobacilli expressing PsaA | •• Recombinant lactobacilli expressing PsaA induced systemic and mucosal immune responses which where protective against pneumococcal nasal carriage. | [115] |
|
| ||
| Protein subunit-based vaccine | ||
| PspA or 6B PS-TT (+ CTB) | IN but not systemic immunization is effective in inducing specific mucosal and serum IgA antibodies, and in providing the cross-protecton. | [116] |
| rPspA, rPsaA, rPneumolysin (+ CTB) | PsaA is better than PspA or pneumolysin in protection | [117] |
| PspA, PspC, PsaA, pneumolysin | •• Demonstrated that the best protein vaccines for prevention of infection may be those that include more than one protection-eliciting pneumococcal protein. Immunization with a mixture of PsaA and PspA elicited better protection against carriage by two pneumococcal strains than either protein alone. | [118] |
| PspA (+ IL-12) | IN administration of IL-12, known activator of Th1 and NK cells, augmented the production of both Th1- and Th2- associated antibody isotype both in mucosal and systemic compartments. | [119] |
| PspC (+ CTB) | Reduced the nasal carriage of a pneumolysin-minus mutant, but not wild-type strain. | [120] |
| Phosphorylcholine-KLH (+ CT) | •• Elicited cross-protection against nasal carriage by S.pneumoniae and NTHi. Provides a potential as a broad spectrum IN vaccine | [121] |
| PppA (+ MPL or CT mutant) | Elicited a high titer of serum antibody and reduced the recovery of challenged pneumococcal cells from nasal wash. | [122] |
| RPhpA (+ mutant CT) | Protection against NP colonization as well as against death and bacteremia following IN challenge with a heterologous pneumococcal strain | [123] |
|
| ||
| Polysaccharide -based vaccine | ||
| C-Ps (+ CT or CTB) | • Cross-serotype protection against nasal carriage. Protection is independent of antibody but dependent on CD4+ | [124] |
KNP, killed nonencapsulated pneumococci; CTB, B subunit of cholera toxin; C-Ps, pneumococcal cell wall polysaccharide; PspA, pneumococcal surface protein A; PsaA, pneumococcal surface adhesion; PS-TT, polysaccharide, conjugated to tetanus toxoid; PspC, pneumococcal surface protein C; IL-12, interleukin 12; NK, natural killer cells; KLH, keyhole limpet hemocyanin; PppA, pneumococcal protective protein; MPL, monophosphoryl lipid A; rPhpA, pneumococcal histidine protein A
Acknowledgments
We thank Professor Michael E. Pichichero and Dr. Toru Takimoto (Department of Microbiology and Immunology, University of Rochester Medical Center, Rochester, New York) for their help in carefully reviewing this manuscript.
Footnotes
This paper is dedicated to the memory of Professor Goro Mogi for his lasting contribution to the field of Mucosal Immunology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moyle PM, McGeary RP, Blanchfield JT, Toth I. Mucosal immunisation: adjuvants and delivery systems. Curr Drug Deliv. 2004;1(4):385–96. doi: 10.2174/1567201043334588. [DOI] [PubMed] [Google Scholar]
- 2.Krekorian TD, Keithley EM, Fierer J, Harris JP. Type B Haemophilus influenzae-induced otitis media in the mouse. Laryngoscope. 1991;101(6 Pt 1):648–56. doi: 10.1288/00005537-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Melhus A, Ryan AF. A mouse model for acute otitis media. APMIS. 2003;111(10):989–94. doi: 10.1034/j.1600-0463.2003.1111012.x. [DOI] [PubMed] [Google Scholar]
- 4.Bikhazi P, Ryan AF. Expression of immunoregulatory cytokines during acute and chronic middle ear immune response. Laryngoscope. 1995;105(6):629–34. doi: 10.1288/00005537-199506000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M, Leonard G, Kreutzer DL. Murine model of interleukin-8-induced otitis media. Laryngoscope. 1997;107(10):1405–8. doi: 10.1097/00005537-199710000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14(2):430–45. doi: 10.1128/CMR.14.2.430-445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim DJ. Functional morphology of the lining membranes of the middle ear and Eustachian tube. An overview Ann Otol Rhinol Laryngol. 1974;83(Suppl 11):5–22. doi: 10.1177/0003489474083s1102. [DOI] [PubMed] [Google Scholar]
- 8.Ichimiya I, Kawauchi H, Mogi G. Analysis of immunocompetent cells in the middle ear mucosa. Arch Otolaryngol Head Neck Surg. 1990;116(3):324–30. doi: 10.1001/archotol.1990.01870030088015. [DOI] [PubMed] [Google Scholar]
- 9.Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child. 1991;145(4):445–8. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- 10.Russell F, Mulholland K. Prevention of otitis media by vaccination. Drugs. 2002;62(10):1441–5. doi: 10.2165/00003495-200262100-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kurono Y, Lim DJ, Mogi G. Middle ear and eustachian tube. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W, editors. Mucosal immunology. 3. New York: Academic Press; 2005. pp. 1509–16. [Google Scholar]
- 12.Gu XX, Rudy SF, Chu C, McCullagh L, Kim HN, Chen J, et al. Phase I study of a lipooligosaccharide-based conjugate vaccine against nontypeable Haemophilus influenzae. Vaccine. 2003;21(17–18):2107–14. doi: 10.1016/s0264-410x(02)00768-5. [DOI] [PubMed] [Google Scholar]
- 13.Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–8. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 14.Pelton SI. Vaccination for the prevention of acute otitis media: proof of concept and current challenges. Pediatr Ann. 2002;31(12):804–9. doi: 10.3928/0090-4481-20021201-09. [DOI] [PubMed] [Google Scholar]
- 15.Harrison CJ. Changes in treatment strategies for acute otitis media after full implementation of the pneumococcal seven valent conjugate vaccine. Pediatr Infect J. 2003;22(8 Suppl):S120–30. doi: 10.1097/00006454-200308001-00003. [DOI] [PubMed] [Google Scholar]
- 16.Shinefield HR, Black S. Efficacy of pneumococcal conjugate vaccines in large scale field trials. Pediatr Infect Dis J. 2000;19(4):394–7. doi: 10.1097/00006454-200004000-00036. [DOI] [PubMed] [Google Scholar]
- 17.Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunization and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21(Suppl 2):S89–95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 18.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171(6):1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 19.Zuercher AW. Upper respiratory tract immunity. Viral Immunol. 2003;16(3):279–89. doi: 10.1089/088282403322396091. [DOI] [PubMed] [Google Scholar]
- 20.van Ginkel FW, Nguyen HH, McGhee JR. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000;6(2):123–32. doi: 10.3201/eid0602.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell MW, Kilian M. Biological activities of IgA. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal immunology. 3. London: Academic Press; 2005. pp. 267–90. [Google Scholar]
- 22.Debertin AS, Tschernig T, Tönjes H, Kleemann WJ, Tröger HD, Pabst R. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol. 2003;134(3):503–7. doi: 10.1111/j.1365-2249.2003.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, et al. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13(6):219–24. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 24.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 25.Russell MW, Martin MH, Wu HY, Hollingshead SK, Moldoveanu Z, Mestecky J. Strategies of immunization against mucosal infections. Vaccine. 2000;19(Suppl 1):S122–7. doi: 10.1016/s0264-410x(00)00290-5. [DOI] [PubMed] [Google Scholar]
- 26.Csencsits KL, Walters N, Pascual DW. Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: alpha(E) versus L-selectin. J Immunol. 2001;167(5):2441–5. doi: 10.4049/jimmunol.167.5.2441. [DOI] [PubMed] [Google Scholar]
- 27.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 28.VanCott TC, Kaminski RW, Mascola JR, Kalyanarman VS, Wassef NM, Alving CR, et al. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J Immunol. 1998;160:2000. [PubMed] [Google Scholar]
- 29.Bergquist C, Johansson EL, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2672. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudin A, Johansson E-L, Berquist C, Holmgren J. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect Immun. 1998;66:3390. doi: 10.1128/iai.66.7.3390-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacPherson GG, Liu LM. Dendritic cells and Langerhans cells in the uptake of mucosal antigens. Curr Top Microbiol Immunol. 1999;236:33–5. doi: 10.1007/978-3-642-59951-4_3. [DOI] [PubMed] [Google Scholar]
- 32.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–9. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 33.Nugent J, Po AL, Scott EM. Design and delivery of non-parenteral vaccines. J Clin Pharm Ther. 1998;23(4):257–85. [PubMed] [Google Scholar]
- 34.Boyaka PN, McGhee JR, Czerkinsky C, Mestecky J. Mucosal vaccines: an overview. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal immunology. 3. London: Academic Press; 2005. pp. 855–74. [Google Scholar]
- 35.Boyaka PN, Marinaro M, Jackson RJ, Menon S, Kiyono H, Jirillo E, et al. IL-12 is an effective adjuvant for induction of mucosal immunity. J Immunol. 1999;162(1):122–8. [PubMed] [Google Scholar]
- 36.Arulanandam BP, Van Cleave VH, Metzger DW. IL-12 is a potent neonatal vaccine adjuvant. Eur J Immunol. 1999;29(1):256–64. doi: 10.1002/(SICI)1521-4141(199901)29:01<256::AID-IMMU256>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Coffman RL, Seymour BW, Lebman DA, Hiraki DD, Christiansen JA, Shrader B, et al. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 38.Yanagita M, Hiroi T, Kitagaki N, Hamada S, Ito HO, Shimauchi H, et al. Nasopharyngeal-associated lymphoreticular tissue (NALT) immunity: fimbriae-specific Th1 and Th2 cell-regulated IgA responses for the inhibition of bacterial attachment to epithelial cells and subsequent inflammatory cytokine production. J Immunol. 1999;162(6):3559–65. [PubMed] [Google Scholar]
- 39.McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10(2):75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 40.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998;28(10):3346–53. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Jecker P, Pabst R, Westermann J. Proliferating macrophages, dendritic cells, natural killer cells, T and B lymphocytes in the middle ear and Eustachian tube mucosa during experimental acute otitis media in the rat. Clin Exp Immunol. 2001;126(3):421–5. doi: 10.1046/j.1365-2249.2001.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi M, Kanai N, Watanabe A, Oshima O, Ryan AF. Lymphocyte subsets in immune-mediated otitis media with effusion. Eur Arch Otorhinolaryngol. 1992;249(1):24–7. doi: 10.1007/BF00175666. [DOI] [PubMed] [Google Scholar]
- 43.Kodama S, Hirano T, Suenaga S, Abe N, Suzuki M. Eustachian tube possesses immunological characteristics as a mucosal effector site and responds to P6 outer membrane protein of nontypeable Haemophilus influenzae. Vaccine. 2006;24(7):1016–27. doi: 10.1016/j.vaccine.2005.07.110. [DOI] [PubMed] [Google Scholar]
- 44.Kodama S, Suenaga S, Hirano T, Suzuki M, Mogi G. Induction of specific immunoglobulin A and Th2 immune responses to P6 outer membrane protein of nontypeable Haemophilus influenzae in middle ear mucosa by intranasal immunization. Infect Immun. 2000;68(4):2294–300. doi: 10.1128/iai.68.4.2294-2300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabirov A, Metzger DW. Intranasal vaccination of neonatal mice with polysaccharide conjugate vaccine for protection against pneumococcal otitis media. Vaccine. 2006;24(27–28):5584–92. doi: 10.1016/j.vaccine.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 46.Kurono Y, Mogi G, Kodama S, Yamamoto M, McGhee JR, Kiyono H. Mucosal immune responses in mice immunized intranasally with outer membrane proteins of Haemophilus influenzae. Abstracts of the 9th International Congress of Mucosal Immunology; Sydney, Australia. 1997. p. 28. [Google Scholar]
- 47.Tamura S, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6(5):409–13. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 48.McCluskie MJ, Davis HL. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine. 2000;4–519:413–22. doi: 10.1016/s0264-410x(00)00208-5. [DOI] [PubMed] [Google Scholar]
- 49.Doyle WJ. Animal models of otitis media: other pathogens. Pediatr Infect Dis J. 1989;8(1 Suppl):S45–7. [PubMed] [Google Scholar]
- 50.Giebink GS. Otitis media: the chinchilla model. Microb Drug Resist. 1999;5(1):57–72. doi: 10.1089/mdr.1999.5.57. [DOI] [PubMed] [Google Scholar]
- 51.Means LW, Daniel HJ, Jordan LH, Loesche PJ. Nonsusceptibility to otitis media of the laboratory gerbil. Meriones unguiculatus. Physiol Psychol. 1975;3:229–30. [Google Scholar]
- 52.Thompson TA, Gardner D, Fulghum RS, Daniel HJ, Allen WE, Worthington JM, et al. Indigenous nasopharyngeal, auditory canal, and middle ear bacterial flora of gerbils: animal model for otitis media. Infect Immun. 1981;32(3):1113–8. doi: 10.1128/iai.32.3.1113-1118.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takoudes TG, Haddad J., Jr Hydrogen peroxide in acute otitis media in guinea pigs. Laryngoscope. 1997;107(2):206–10. doi: 10.1097/00005537-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Cook RD, Postma DS, Brinson GM, Prazma J, Pillsbury HC. Cytotoxic changes in hair cells secondary to pneumococcal middle-ear infection. J Otolaryngol. 1999;28(6):325–31. [PubMed] [Google Scholar]
- 55.Sato K. Experimental otitis media induced by nonviable Moraxella catarrhalis in the guinea pig model. Auris Nasus Larynx. 1997;24(3):233–8. doi: 10.1016/s0385-8146(96)00022-3. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura H, Watanabe N, Bundo J, Shinoda M, Mogi G. Oral vaccine therapy for pneumococcal otitis media in an animal model. Arch Otolaryngol Head Neck Surg. 1991;117(8):889–94. doi: 10.1001/archotol.1991.01870200083014. [DOI] [PubMed] [Google Scholar]
- 57.Wells JR, Gernon WH, Ward G, Davis RK, Hays LL. Otosurgical model in the guinea pig (Cavia porcellus) Otolaryngol Head Neck Surg. 1986;95(4):450–7. doi: 10.1177/019459988609500406. [DOI] [PubMed] [Google Scholar]
- 58.Daniel HJ, Carmine FH, Cook RA. Otitis media in two strains of laboratory rats. J Aud Res. 1971;11:276–8. [Google Scholar]
- 59.Daniel HJ, Means LW, Dressel ME, Loesche PJ. Otitis media in laboratory rats. Physiol Psycol. 1973;1:7–8. [Google Scholar]
- 60.Sabirov A, Kodama S, Hirano T, Suzuki M, Mogi G. Intranasal immunization enhances clearance of nontypeable Haemophilus influenzae and reduces stimulation of tumor necrosis factor alpha production in the murine model of otitis media. Infect Immun. 2001;69(5):2964–71. doi: 10.1128/IAI.69.5.2964-2971.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ichimiya I, Suzuki M, Hirano T, Mogi G. The influence of pneumococcal otitis media on the cochlear lateral wall. Hear Res. 1999;131(1–2):128–34. doi: 10.1016/s0378-5955(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 62.Committee on Infectious Diseases of Mice and Rats, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Infectious Diseases of Mice and Rats. Washington, D.C: The National Academic Press; 1991. [Google Scholar]
- 63.Hellmstrom S, Salen B, Stenfors LE. Anatomy of the rat middle ear. A study under the dissection microscope. Acta Anat (Basel) 1982;112:346–52. doi: 10.1159/000145527. [DOI] [PubMed] [Google Scholar]
- 64.Foxwell AR, Kyd JM, Cripps AW. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol Mol Biol Rev. 1998;62(2):294–308. doi: 10.1128/mmbr.62.2.294-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westman E. Thesis. Umea University; Sweeden: 2003. Animal models for studies of middle ear pathology. In: Experimental acute otitis media. Aspects on treatment, protection and structural changes; pp. 19–20. Sundsvall, Kaltes Grafiska AB. [Google Scholar]
- 66.Daniel HJ, 3rd, Fulghum RS, Brinn JE, Barrett KA. Comparative anatomy of eustachian tube and middle ear cavity in animal models for otitis media. Ann Otol Rhinol Laryngol. 1982;91(1 Pt 1):82–9. doi: 10.1177/000348948209100118. [DOI] [PubMed] [Google Scholar]
- 67.Zheng QY, Hardisty-Hughes R, Brown SD. Mouse models as a tool to unravel the genetic basis for human otitis media. Brain Res. 2006;1091(1):9–15. doi: 10.1016/j.brainres.2006.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim DJ, Hermansson A, Hellström SO, Hussl B, Alper CM, Iino Y, et al. Recent advances in otitis media. 3. Animal models; anatomy and pathology; pathogenesis; cell biology and genetics. Ann Otol Rhinol Laryngol Suppl. 2005 Jan;194:31–41. [PubMed] [Google Scholar]
- 69.Trune DR. Mouse models for immunologic diseases of the auditory system. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. Boca Raton: CRC Press; 2001. pp. 505–31. [Google Scholar]
- 70.McGinn MD, Bean-Knudsen D, Ermel RW. Incidence of otitis media in CBA/J and CBA/CaJ mice. Hear Res. 1992;59(1):1–6. doi: 10.1016/0378-5955(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 71.Malley R, Morse SC, Leite LC, Areas AP, Ho PL, Kubrusly FS, et al. Multiserotype protection of mice against pneumococcal colonization of the nasopharynx and middle ear by killed nonencapsulated cells given intranasally with a nontoxic adjuvant. Infect Immun. 2004;72(7):4290–2. doi: 10.1128/IAI.72.7.4290-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCool TL, Weiser JN. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun. 2004;72(10):5807–13. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCool TL, Cate TR, Tuomanen EI, Adrian P, Mitchell TJ, Weiser JN. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect Immun. 2003;71(10):5724–32. doi: 10.1128/IAI.71.10.5724-5732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryan AF, Ebmeyer J, Furukawa M, Pak K, Melhus A, Wasserman SI, et al. Mouse models of induced otitis media. Brain Res. 2006;1091(1):3–8. doi: 10.1016/j.brainres.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 75.McCullers JA, Karlstrom A, Iverson AR, Loeffler JM, Fischetti VA. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3(3):28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabirov A, Metzger DW. Intranasal vaccination of infant mice induces protective I mmunity in the absence of nasal-associated lymphoid tissue. Vaccine. doi: 10.1016/j.vaccine.2008.01.027. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertot GM, Becker PD, Guzman CA, Grinstein S. Intranasal vaccination with recombinant P6 protein and adamantylamide dipeptide as mucosal adjuvant confers efficient protection against otitis media and lung infection by nontypeable Haemophilus influenzae. J Infect Dis. 2004;189(7):1304–12. doi: 10.1086/382508. [DOI] [PubMed] [Google Scholar]
- 78.Meek RB, 3rd, McGrew BM, Cuff CF, Berrebi AS, Spirou GA, Wetmore SJ. Immunologic and histologic observations in reovirus-induced otitis media in the mouse. Ann Otol Rhinol Laryngol. 1999;108(1):31–8. doi: 10.1177/000348949910800105. [DOI] [PubMed] [Google Scholar]
- 79.Hirano T, Tomiguchi M, Mabuchi H, Kodama S, Suzuki M. Analysis of the cellular immune responses to influenza a virus infection in the middle ear. Abstracts of the 9th International Symposium on Recent Advances in Otitis Media; St. Pete Beach, FA, USA. 2007. p. 104. [Google Scholar]
- 80.Hirano T, Kurono Y, Ichimiya I, Suzuki M, Mogi G. Effects of influenza A virus on lectin-binding patterns in murine nasopharyngeal mucosa and on bacterial colonization. Otolaryngol Head Neck Surg. 1999;121(5):616–21. doi: 10.1016/S0194-5998(99)70068-9. [DOI] [PubMed] [Google Scholar]
- 81.Francis TE, de Torregrosa MV. Combined infection of mice with H. influenzae and influenza virus by the intranasal route. J Infect Dis. 1945;76:70–7. [Google Scholar]
- 82.McCullers JA, Regh JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186(3):341–50. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 83.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19(3):571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gitiban N, Jurcisek JA, Harris RH, Mertz SE, Durbin RK, Bakaletz LO, et al. Chinchilla and murine models of upper respiratory tract infections with respiratory syncytial virus. J Virol. 2005;79(10):6035–42. doi: 10.1128/JVI.79.10.6035-6042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Domachowske JB, Bonville CA, Rosenberg HF. Animal models for studying respiratory syncytial virus infection and its long term effects on lung function. Pediatr Infect Dis J. 2004;23(11 Suppl):S228–34. doi: 10.1097/01.inf.0000144672.81955.a4. [DOI] [PubMed] [Google Scholar]
- 86.Appell LH, Kovatch RM, Reddecliff JM, Gerone PJ. Pathogenesis of Sendai virus infection in mice. Am J Vet Res. 1971;32(11):1835–41. [PubMed] [Google Scholar]
- 87.Faisca P, Desmecht D. Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res Vet Sci. 2007;82(1):115–25. doi: 10.1016/j.rvsc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Klemens JJ, Thompson K, Langerman A, Naclerio RM. Persistent inflammation and hyperresponsiveness following viral rhinosinusitis. Laryngoscope. 2006;116(7):1236–40. doi: 10.1097/01.mlg.0000224526.43698.52. [DOI] [PubMed] [Google Scholar]
- 89.Hirano T, Kodama S, Fujita K, Maeda K, Suzuki M. Role of Toll-like receptor 4 in innate immune responses in a mouse model of acute otitis media. FEMS Immunol Med Microbiol. 2007;49(1):75–83. doi: 10.1111/j.1574-695X.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 90.McCoy SL, Kurtz SE, Macarthur CJ, Trune DR, Hefeneider SH. Identification of a peptide derived from vaccinia virus A52R protein that inhibits cytokine secretion in response to TLR-dependent signaling and reduces in vivo bacterial-induced inflammation. J Immunol. 2005;174(5):3006–14. doi: 10.4049/jimmunol.174.5.3006. [DOI] [PubMed] [Google Scholar]
- 91.MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear Res. 2006;219(1–2):12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 92.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73(11):7718–26. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harford CG, Leidler V, Hara M. Effect of the lesion due to influenza virus on the resistance of mice to inhaled pneumococci. J Exp Med. 1949;89:53–67. doi: 10.1084/jem.89.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu HY, Virolainen A, Mathews B, King J, Russell MW, Briles DE. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog. 1997;23(3):127–37. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 95.Briles DE, Novak L, Hotomi M, van Ginkel FW, King J. Nasal colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect Immun. 2005;73(10):6945–51. doi: 10.1128/IAI.73.10.6945-6951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun K, Johansen FE, Eckmann L, Metzger DW. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J Immunol. 2004;173(7):4576–81. doi: 10.4049/jimmunol.173.7.4576. [DOI] [PubMed] [Google Scholar]
- 97.Hodge LM, Marinaro M, Jones HP, McGhee JR, Kiyono H, Simecka JW. Immunoglobulin A (IgA) responses and IgE-associated inflammation along the respiratory tract after mucosal but not systemic immunization. Infect Immun. 2001;69(4):2328–38. doi: 10.1128/IAI.69.4.2328-2338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Ginkel FW, Jackson RJ, Yoshino N, Hagiwara Y, Metzger DJ, Connell TD, et al. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect Immun. 2005;73(10):6892–902. doi: 10.1128/IAI.73.10.6892-6902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng QY, Tong YC, Alagramam KN, Yu H. Tympanometry assessment of 61 inbred strains of mice. Hear Res. 2007;231(1–2):23–31. doi: 10.1016/j.heares.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asahi Y, Yoshikawa T, Watanabe I, Iwasaki T, Hasegawa H, Sato Y, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168(6):2930–8. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 101.Lynch JM, Briles DE, Metzger DW. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect Immun. 2003;71(8):4780–8. doi: 10.1128/IAI.71.8.4780-4788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trzcinski K, Thompson C, Malley R, Lipsitch M. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect Immun. 2005;73(10):7043–6. doi: 10.1128/IAI.73.10.7043-7046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci USA. 2005;102:4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roche AM, King SJ, Weiser JN. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect Immun. 2007;75(5):2469–75. doi: 10.1128/IAI.01972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hardistry RE, Erven A, Logan K, Morse S, Guionaud S, Sancho-Oliver S, et al. The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media. J Assoc Res Otolaryngol. 2003;4:130–8. doi: 10.1007/s10162-002-3015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hardisty-Hughes RE, Tateossian H, Morse SA, Romero MR, Middleton A, Tymowska-Lalanne Z, et al. A mutation in the F-box gene, Fbxo11, causes otitis media in the Jeff mouse. Hum Mol Genet. 2006;15(22):3273–9. doi: 10.1093/hmg/ddl403. [DOI] [PubMed] [Google Scholar]
- 107.Parkinson N, Hardisty-Hughes RE, Tateossian H, Tsai HT, Brooker D, Morse S, et al. Mutation at the Evi1 locus in Junbo mice causes susceptibility to otitis media. PLoS Genet. 2006;2(10):e149. doi: 10.1371/journal.pgen.0020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hotomi M, Yamanaka N, Shimada J, Suzumoto M, Ikeda Y, Sakai A, et al. Intranasal immunization with recombinant outer membrane protein P6 induces specific immune responses against nontypeable Haemophilus influenzae. Int J Pediatr Otorhinolaryngol. 2002;65(2):109–16. doi: 10.1016/s0165-5876(02)00076-9. [DOI] [PubMed] [Google Scholar]
- 109.Abe N, Kodama S, Hirano T, Eto M, Suzuki M. Nasal vaccination with CpG oligodeoxynucleotide induces protective immunity against non-typeable Haemophilus influenzae in the nasopharynx. Laryngoscope. 2006;116(3):407–12. doi: 10.1097/01.mlg.0000199740.04730.d4. [DOI] [PubMed] [Google Scholar]
- 110.Mason KW, Zhu D, Scheuer CA, McMichael JC, Zlotnick GW, Green BA. Reduction of nasal colonization of nontypeable Haemophilus influenzae following intranasal immunization with rLP4/rLP6/UspA2 proteins combined with aqueous formulation of RC529. Vaccine. 2004;22(25–26):3449–56. doi: 10.1016/j.vaccine.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 111.Hotomi M, Ikeda Y, Suzumoto M, Yamauchi K, Green BA, Zlotnick G, et al. A recombinant P4 protein of Haemophilus influenzae induces specific immune responses biologically active against nasopharyngeal colonization in mice after intranasal immunization. Vaccine. 2005;23(10):1294–300. doi: 10.1016/j.vaccine.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 112.Cutter D, Mason KW, Howell AP, Fink DL, Green BA, St Geme JW., 3rd Immunization with Haemophilus influenzae Hap adhesin protects against nasopharyngeal colonization in experimental mice. J Infect Dis. 2002;186(2):1115–21. doi: 10.1086/344233. [DOI] [PubMed] [Google Scholar]
- 113.Hirano T, Hou Y, Jiao X, Gu XX. Intranasal immunization with a lipooligosaccharide-based conjugate vaccine from nontypeable Haemophilus influenzae enhances bacterial clearance in mouse nasopharynx. FEMS Immunol Med Microbiol. 2003;35(1):1–10. doi: 10.1111/j.1574-695X.2003.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 114.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001;69(8):4870–3. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oliveira ML, Arêas AP, Campos IB, Monedero V, Perez-Martínez G, Miyaji EN, et al. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 2006;8(4):1016–24. doi: 10.1016/j.micinf.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu HY, Nahm MH, Guo Y, Russell MW, Briles DE. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175(4):839–46. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 117.Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, Schilling M, et al. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine. 2000;18(16):1707–11. doi: 10.1016/s0264-410x(99)00511-3. [DOI] [PubMed] [Google Scholar]
- 118.Briles DE, Ades E, Paton JC, Sampson JS, Carlone GM, Huebner RC, et al. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68(2):796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arulanandam BP, Lynch JM, Briles DE, Hollingshead S, Metzger DW. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect Immun. 2001;69(11):6718–24. doi: 10.1128/IAI.69.11.6718-6724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balachandran P, Brooks-Walter A, Virolainen-Julkunen A, Hollingshead SK, Briles DE. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect Immun. 2002;70(5):2526–34. doi: 10.1128/IAI.70.5.2526-2534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tanaka N, Fukuyama S, Fukuiwa T, Kawabata M, Sagara Y, Ito HO, et al. Intranasal immunization with phosphorylcholine induces antigen specific mucosal and systemic immune responses in mice. Vaccine. 2007;25(14):2680–7. doi: 10.1016/j.vaccine.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 122.Green BA, Zhang Y, Masi AW, Barniak V, Wetherell M, Smith RP, et al. PppA, a surface-exposed protein of Streptococcus pneumoniae, elicits cross-reactive antibodies that reduce colonization in a murine intranasal immunization and challenge model. Infect Immun. 2005;73(2):981–9. doi: 10.1128/IAI.73.2.981-989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y, Masi AW, Barniak V, Mountzouros K, Hostetter MK, Green BA. Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect Immun. 2001;69(6):3827–36. doi: 10.1128/IAI.69.6.3827-3836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74(4):2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Renegar KB, Small PA., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65(4):2146–8. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tamura SI, Asanuma H, Ito Y, Hirabayashi Y, Suzuki Y, Nagamine T, et al. Superior cross-protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur J Immunol. 1992;22(2):477–81. doi: 10.1002/eji.1830220228. [DOI] [PubMed] [Google Scholar]
- 127.Tamura S, Ito Y, Asanuma H, Hirabayashi Y, Suzuki Y, Nagamine T, et al. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992;149(3):981–8. [PubMed] [Google Scholar]
- 128.Tamura S, Asanuma H, Ito Y, Yoshizawa K, Nagamine T, Aizawa C, et al. Formulation of inactivated influenza vaccines for providing effective cross-protection by intranasal vaccination in mice. Vaccine. 1994;12(4):310–6. doi: 10.1016/0264-410x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 129.Asanuma H, Koide F, Suzuki Y, Nagamine T, Aizawa C, Kurata T, et al. Cross-protection against influenza virus infection in mice vaccinated by combined nasal/subcutaneous administration. Vaccine. 1995;13(1):3–5. doi: 10.1016/0264-410x(95)80002-u. [DOI] [PubMed] [Google Scholar]
- 130.Hirabayashi Y, Kurata H, Funato H, Nagamine T, Aizawa C, Tamura S, et al. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990;8(3):243–8. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- 131.Watanabe I, Hagiwara Y, Kadowaki SE, Yoshikawa T, Komase K, Aizawa C, et al. Characterization of protective immune responses induced by nasal influenza vaccine containing mutant cholera toxin as a safe adjuvant (CT112K) Vaccine. 2002;20(29–30):3443–55. doi: 10.1016/s0264-410x(02)00351-1. [DOI] [PubMed] [Google Scholar]
- 132.de Haan A, Geerligs HJ, Huchshorn JP, van Scharrenburg GJ, Palache AM, Wilschut J. Mucosal immunoadjuvant activity of liposomes: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine. 1995;13(2):155–62. doi: 10.1016/0264-410x(95)93129-w. [DOI] [PubMed] [Google Scholar]
- 133.de Haan L, Verweij WR, Holtrop M, Brands R, van Scharrenburg GJ, Palache AM, et al. Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine. 2001;19(20–22):2898–907. doi: 10.1016/s0264-410x(00)00556-9. [DOI] [PubMed] [Google Scholar]
- 134.Davis SS. The use of soluble polymers and polymer microparticles to provide improved vaccine responses after parenteral and mucosal delivery. Vaccine. 2006;24(Suppl 2):S2/7–S2/10. doi: 10.1016/j.vaccine.2005.01.102. [DOI] [PubMed] [Google Scholar]
- 135.Valosky J, Hishiki H, Zaoutis TE, Coffin SE. Induction of mucosal B-cell memory by intranasal immunization of mice with respiratory syncytial virus. Clin Diagn Lab Immunol. 2005;12(1):171–9. doi: 10.1128/CDLI.12.1.171-179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walsh EE. Humoral, mucosal, and cellular immune response to topical immunization with a subunit respiratory syncytial virus vaccine. J Infect Dis. 1994;170(2):345–50. doi: 10.1093/infdis/170.2.345. [DOI] [PubMed] [Google Scholar]
- 137.Oien NL, Brideau RJ, Walsh EE, Wathen MW. Induction of local and systemic immunity against human respiratory syncytial virus using a chimeric FG glycoprotein and cholera toxin B subunit. Vaccine. 1994;12(8):731–5. doi: 10.1016/0264-410x(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 138.Nedrud JG, Liang XP, Hague N, Lamm ME. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987;139(10):3484–92. [PubMed] [Google Scholar]
- 139.Takao SI, Kiyotani K, Sakaguchi T, Fujii Y, Seno M, Yoshida T. Protection of mice from respiratory Sendai virus infections by recombinant vaccinia viruses. J Virol. 1997;71(1):832–8. doi: 10.1128/jvi.71.1.832-838.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davis SS. Nasal vaccines. Adv Drug Deliv Rev. 2001;51(1–3):21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 141.Giebink GS, Kurono Y, Bakaletz LO, Kyd JM, Barenkamp SJ, Murphy TF, et al. Recent advances in otitis media. 6. Vaccine. Ann Otol Rhinol Laryngol Suppl. 2005;194:86–103. [PubMed] [Google Scholar]
- 142.Kurono Y, Shimamura K, Mogi G. Inhibition of nasopharyngeal colonization of Hemophilus influenzae by oral immunization. Ann Otol Rhinol Laryngol. 1992;157:11–5. doi: 10.1177/0003489492101s1004. [DOI] [PubMed] [Google Scholar]
- 143.Kurono Y, Shigemi H, Kodama S, Mogi G. Effects of oral and systemic immunization on nasopharyngeal clearance of nontypeable Haemophilus influenzae in BALB/c mice. Laryngoscope. 1996;106(5 Pt 1):614–8. doi: 10.1097/00005537-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 144.Suzuki M, Kurono Y, Kodama S, Shigemi H, Mogi G. Enhancement of nasal clearance of nontypeable Haemophilus influenzae by oral immunization with outer membrane proteins. Acta Otolaryngol. 1998;118(6):864–9. doi: 10.1080/00016489850182594. [DOI] [PubMed] [Google Scholar]
- 145.Hotomi M, Saito T, Yamanaka N. Specific mucosal immunity and enhanced nasopharyngeal clearance of nontypeable Haemophilus influenzae after intranasal immunization with outer membrane protein P6 and cholera toxin. Vaccine. 1998;16(20):1950–6. doi: 10.1016/s0264-410x(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 146.Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, Mogi G, et al. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1999;180(1):122–32. doi: 10.1086/314827. [DOI] [PubMed] [Google Scholar]
- 147.Hirano T, Jiao X, Chen Z, Van Waes C, Gu XX. Kinetics of mouse antibody and lymphocyte responses during intranasal vaccination with a lipooligosaccharide-based conjugate vaccine. Immunol Lett. 2006;107(2):131–9. doi: 10.1016/j.imlet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 148.Kodama S, Abe N, Hirano T, Suzuki M. Safety and efficacy of nasal application of CpG oligodeoxynucleotide as a mucosal adjuvant. Laryngoscope. 2006;116(2):331–5. doi: 10.1097/01.mlg.0000194222.93067.f7. [DOI] [PubMed] [Google Scholar]
- 149.Yamauchi K, Hotomi M, Billal DS, Suzumoto M, Yamanaka N. Maternal intranasal immunization with outer membrane protein P6 maintains specific antibody level of derived offspring. Vaccine. 2006;24(25):5294–9. doi: 10.1016/j.vaccine.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 150.Kodama S, Hirano T, Abe N, Suzuki M. A single nasal dose of Flt3 ligand enhances mucosal immune responses in the nasopharynx. Abstracts of the 9th International Symposium on Recent Advances in Otitis Media; St. Pete Beach, FA. 2007. p. 71. [Google Scholar]
- 151.Barenkamp SJ. Rationale and prospects for a nontypable Haemophilus influenzae vaccine. Pediatr Infect Dis J. 2004;23(5):461–2. doi: 10.1097/01.inf.0000125892.66941.a9. [DOI] [PubMed] [Google Scholar]
- 152.Katsurahara T, Hotomi M, Yamauchi K, Suzumoto M, Billal D, Briles D, et al. Protection of pneumococcal infection by maternal immunization with pneumococcal surface protein a (PspA). Abstracts of the 9th International Symposium on Recent Advances in Otitis Media; St. Pete Beach, FA. 2007. p. 112. [Google Scholar]
- 153.Tai SS. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit Rev Microbiol. 2006;32(3):139–53. doi: 10.1080/10408410600822942. [DOI] [PubMed] [Google Scholar]
- 154.Hou Y, Hu WG, Hirano T, Gu XX. A new intra-NALT route elicits mucosal and systemic immunity against Moraxella catarrhalis in a mouse challenge model. Vaccine. 2002;20(17–18):2375–81. doi: 10.1016/s0264-410x(02)00097-x. [DOI] [PubMed] [Google Scholar]
- 155.Jiao X, Hirano T, Hou Y, Gu XX. Specific immune responses and enhancement of murine pulmonary clearance of Moraxella catarrhalis by intranasal immunization with a detoxified lipooligosaccharide conjugate vaccine. Infect Immun. 2002;70(11):5982–9. doi: 10.1128/IAI.70.11.5982-5989.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lövgren K. The serum antibody response distributed in subclasses and isotypes after intranasal and subcutaneous immunization with influenza virus immunostimulating complexes. Scand J Immunol. 1988;27(2):241–5. doi: 10.1111/j.1365-3083.1988.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 157.Lövgren K, Kåberg H, Morein B. An experimental influenza subunit vaccine (iscom): induction of protective immunity to challenge infection in mice after intranasal or subcutaneous administration. Clin Exp Immunol. 1990;82(3):435–9. doi: 10.1111/j.1365-2249.1990.tb05467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bergmann KC, Waldman RH. Enhanced murine respiratory tract IgA antibody response to oral influenza vaccine when combined with a lipoidal amine (avridine) Int Arch Allergy Appl Immunol. 1988;87(3):334–5. doi: 10.1159/000234695. [DOI] [PubMed] [Google Scholar]
- 159.el Guink N, Kris RM, Goodman-Snitkoff G, Small PA, Mannino RJ. Intranasal immunization with proteoliposomes protects against influenza. Vaccine. 1989;7(2):147–51. doi: 10.1016/0264-410x(89)90055-8. [DOI] [PubMed] [Google Scholar]
- 160.Moldoveanu Z, Novak M, Huang WQ, Gilley RM, Staas JK, Schafer D, et al. Oral immunization with influenza virus in biodegradable microspheres. J Infect Dis. 1993;167(1):84–90. doi: 10.1093/infdis/167.1.84. [DOI] [PubMed] [Google Scholar]
- 161.Ben Ahmeida ET, Gregoriadis G, Potter CW, Jennings R. Immunopotentiation of local and systemic humoral immune responses by ISCOMs, liposomes and FCA: role in protection against influenza A in mice. Vaccine. 1993;11(13):1302–9. doi: 10.1016/0264-410x(93)90099-j. [DOI] [PubMed] [Google Scholar]
- 162.Tamura S, Yamanaka A, Shimohara M, Tomita T, Komase K, Tsuda Y, et al. Synergistic action of cholera toxin B subunit (and Escherichia coli heat-labile toxin B subunit) and a trace amount of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994;12(5):419–26. doi: 10.1016/0264-410x(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 163.Mbawuike IN, Pacheco S, Acuna CL, Switzer KC, Zhang Y, Harriman GR. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J Immunol. 1999;1;162(5):2530–7. [PubMed] [Google Scholar]
- 164.Zhang Y, Pacheco S, Acuna CL, Switzer KC, Wang Y, Gilmore X, et al. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology. 2002;105(3):286–94. doi: 10.1046/j.0019-2805.2001.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reuman PD, Keely SP, Schiff GM. Rapid recovery in mice after combined nasal/oral immunization with killed respiratory syncytial virus. J Med Virol. 1990;32(1):67–72. doi: 10.1002/jmv.1890320112. [DOI] [PubMed] [Google Scholar]
- 166.Trudel M, Nadon F, Séguin C, Brault S, Lusignan Y, Lemieux S. Initiation of cytotoxic T-cell response and protection of Balb/c mice by vaccination with an experimental ISCOMs respiratory syncytial virus subunit vaccine. Vaccine. 1992;10(2):107–12. doi: 10.1016/0264-410x(92)90026-g. [DOI] [PubMed] [Google Scholar]
- 167.Hishiki H, Zuercher AW, Valosky J, Coffin SE. Regional differences in the early mucosal immune response induced by primary inoculation of mice with respiratory syncytial virus. Microb Pathog. 2004;36(3):141–6. doi: 10.1016/j.micpath.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 168.Etchart N, Baaten B, Andersen SR, Hyland L, Wong SY, Hou S. Intranasal immunisation with inactivated RSV and bacterial adjuvants induces mucosal protection and abrogates eosinophilia upon challenge. Eur J Immunol. 2006;36(5):1136–44. [Google Scholar]
- 169.Hu KF, Elvander M, Merza M, Akerblom L, Brandenburg A, Morein B. The immunostimulating complex (ISCOM) is an efficient mucosal delivery system for respiratory syncytial virus (RSV) envelope antigens inducing high local and systemic antibody responses. Clin Exp Immunol. 1998;113(2):235–43. doi: 10.1046/j.1365-2249.1998.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bastien N, Trudel M, Simard C. Complete protection of mice from respiratory syncytial virus infection following mucosal delivery of synthetic peptide vaccines. Vaccine. 1999;17(7–8):832–6. doi: 10.1016/s0264-410x(98)00267-9. [DOI] [PubMed] [Google Scholar]
- 171.Tebbey PW, Unczur CA, LaPierre NA, Hancock GE. A novel and effective intranasal immunization strategy for respiratory syncytial virus. Viral Immunol. 1999;12:41–5. doi: 10.1089/vim.1999.12.41. [DOI] [PubMed] [Google Scholar]
- 172.Tebbey PW, Scheuer CA, Peek JA, Zhu D, LaPierre NA, Green BA, et al. Effective mucosal immunization against respiratory syncytial virus using purified F protein and a genetically detoxified cholera holotoxin, CT-E29H. Vaccine. 2000;18(24):2723–34. doi: 10.1016/s0264-410x(00)00058-x. [DOI] [PubMed] [Google Scholar]
- 173.Kim HJ, Kim JK, Seo SB, Lee HJ, Kim HJ. Intranasal vaccination with peptides and cholera toxin subunit B as adjuvant to enhance mucosal and systemic immunity to respiratory syncytial virus. Arch Pharm Res. 2007;30(3):366–71. doi: 10.1007/BF02977620. [DOI] [PubMed] [Google Scholar]
- 174.Cyr SL, Jones T, Stoica-Popescu I, Brewer A, Chabot S, Lussier M, et al. Intranasal proteosome-based respiratory syncytial virus (RSV) vaccines protect BALB/c mice against challenge without eosinophilia or enhanced pathology. Vaccine. 2007;25(29):5378–89. doi: 10.1016/j.vaccine.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 175.Smith FS, Portner A, Leggiadro RJ, Turner EV, Hurwitz JL. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology. 1994;205(2):453–61. doi: 10.1006/viro.1994.1665. [DOI] [PubMed] [Google Scholar]
- 176.Sangster M, Smith FS, Coleclough C, Hurwitz JL. Human parainfluenza virus type 1 immunization of infant mice protects from subsequent Sendai virus infection. Virology. 1995;212(1):13–9. doi: 10.1006/viro.1995.1448. [DOI] [PubMed] [Google Scholar]
- 177.Macpherson AJ, Lamarre A, Mccoy K, Harriman GR, Odermatt B, Dougan G, et al. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2(7):625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 178.Boyaka PN, McGhee JR, Czerkinsky C, Mestecky J. Mucosal vaccines: an overview. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W, editors. Mucosal immunology. 3. New York: Academic Press; 2005. pp. 855–874. [Google Scholar]
- 179.Liang XP, Lamm ME, Nedrud JG. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988;141(5):1495–501. [PubMed] [Google Scholar]
- 180.Jakobsen H, Schulz D, Pizza M, Rappuoli R, Jónsdóttir I. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines with nontoxic mutants of Escherichia coli heat-labile enterotoxins as adjuvants protects mice against invasive pneumococcal infections. Infect Immun. 1999;67(11):5892–7. doi: 10.1128/iai.67.11.5892-5897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee CJ, Lee LH, Frasch CE. Protective immunity of pneumococcal glycoconjugates. Crit Rev Microbiol. 2003;29(4):333–49. doi: 10.1080/713608018. [DOI] [PubMed] [Google Scholar]
- 182.Johnson SE, Dykes JK, Jue DL, Sampson JS, Carlone GM, Ades EW. Inhibition of pneumococcal carriage in mice by subcutaneous immunization with peptides from the common surface protein pneumococcal surface adhesin a. J Infect Dis. 2002;185(4):489–96. doi: 10.1086/338928. [DOI] [PubMed] [Google Scholar]
- 183.McGhee JR, Lamm ME, Strober W. Mucosal immune responses: an overview. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal immunology. 2. London: Academic Press; 1999. pp. 485–506. [Google Scholar]
- 184.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis. 2005;58(4):195–207. [PubMed] [Google Scholar]
- 185.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–86. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 186.Takase H, Murakami Y, Endo A, Ikeuchi T. Antibody responses and protection in mice immunized orally against influenza virus. Vaccine. 1996;14(17–18):1651–6. doi: 10.1016/s0264-410x(96)00128-4. [DOI] [PubMed] [Google Scholar]
- 187.Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, et al. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21(6):1337–44. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 188.Weltzin R, Hsu SA, Mittler ES, Georgakopoulos K, Monath TP. Intranasal monoclonal immunoglobulin A against respiratory syncytial virus protects against upper and lower respiratory tract infections in mice. Antimicrob Agents Chemother. 1994;38(12):2785–91. doi: 10.1128/aac.38.12.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Freihorst J, Ogra PL. Mucosal immunity and viral infections. Ann Med. 2001;33(3):172–7. doi: 10.3109/07853890109002074. [DOI] [PubMed] [Google Scholar]
- 190.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166(12):7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 191.Takimoto T, Hurwitz JL, Zhan X, Krishnamurthy S, Prouser C, Brown B, et al. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol. 2005;18(2):255–66. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- 192.Takimoto T, Hurwitz JL, Coleclough C, Prouser C, Krishnamurthy S, Zhan X, et al. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78(11):6043–7. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–17. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 194.Girard MP, Cherian T, Pervikov Y, Kieny MP. A review of vaccine research and development: human acute respiratory infections. Vaccine. 2005;23(50):5708–24. doi: 10.1016/j.vaccine.2005.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mackenzie CD, Taylor PM, Askonas BA. Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology. 1989;67(3):375–81. [PMC free article] [PubMed] [Google Scholar]
- 196.Lightman S, Cobbold S, Waldmann H, Askonas BA. Do L3T4+ T cells act as effector cells in protection against influenza virus infection. Immunology. 1987;62(1):139–44. [PMC free article] [PubMed] [Google Scholar]
- 197.Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160(1):322–7. [PubMed] [Google Scholar]
- 198.Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, Epstein SL. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol. 2001;166(12):7437–45. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- 199.Metzger DW. IgA and respiratory immunity. In: Kaetzel CS, editor. Mucosal Immune Defense: Immunoglobulin A. Plenum Publishers; New York: 2007. pp. 269–90. [Google Scholar]
- 200.Koskela M. Antibody response of young children to parenteral vaccination with pneumococcal capsular polysaccharides: a comparison between antibody levels in serum and middle ear effusion. Pediatr Infect Dis. 1986;5(4):431–4. doi: 10.1097/00006454-198607000-00011. [DOI] [PubMed] [Google Scholar]
- 201.Russell-Jones GJ. Oral vaccine delivery. J Control Release. 2000;65(1–2):49–54. doi: 10.1016/s0168-3659(99)00231-x. [DOI] [PubMed] [Google Scholar]
- 202.Kaiserlian D, Etchart N. Epicutaneous and transcutaneous immunization using DNA or proteins. Eur J Dermatol. 1999;9(3):169–76. [PubMed] [Google Scholar]
- 203.Foss DL, Murtaugh MP. Mechanisms of vaccine adjuvanticity at mucosal surfaces. Anim Health Res Rev. 2000;1(1):3–24. doi: 10.1017/s1466252300000025. [DOI] [PubMed] [Google Scholar]
- 204.Pizza M, Giuliani MM, Fontana MR, Monaci E, Douce G, Dougan G, et al. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19(17–19):2534–41. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- 205.Lee CJ, Lee LH, Gu XX. Mucosal immunity induced by pneumococcal glycoconjugate. Crit Rev Microbiol. 2005;31(3):137–44. doi: 10.1080/10408410591005093. [DOI] [PubMed] [Google Scholar]
- 206.Mercenier A, Müller-Alouf H, Grangette C. Lactic acid bacteria as live vaccines. Curr Issues Mol Biol. 2000;2(1):17–25. [PubMed] [Google Scholar]
- 207.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19(25–26):3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 208.Murphy TF. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Rev Vaccines. 2005;4(6):843–53. doi: 10.1586/14760584.4.6.843. [DOI] [PubMed] [Google Scholar]
- 209.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7(5):449–55. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 210.Tu le N, Jeong HY, Kwon HY, Ogunniyi AD, Paton JC, Pyo SN, et al. Modulation of adherence, invasion, and tumor necrosis factor alpha secretion during the early stages of infection by Streptococcus pneumoniae ClpL. Infect Immun. 2007;75(6):2996–3005. doi: 10.1128/IAI.01716-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Silin DS, Lyubomska OV, Jirathitikal V, Bourinbaiar AS. Oral vaccination: where we are? Expert Opin Drug Deliv. 2007;4(4):323–40. doi: 10.1517/17425247.4.4.323. [DOI] [PubMed] [Google Scholar]
- 212.De Magistris MT. Mucosal delivery of vaccine antigens and its advantages in pediatrics. Adv Drug Deliv Rev. 2006;58(1):52–67. doi: 10.1016/j.addr.2006.01.002. [DOI] [PubMed] [Google Scholar]