Abstract
Intranasal (i.n.) immunization is an effective regimen for the prophylaxis of respiratory diseases in early life. The aim of this study was to assess the need for nasal-associated lymphoid tissue (NALT) and cervical lymph nodes (CLN) in induction of protective immunity following mucosal vaccination of infant mice. We developed surgical techniques to eliminate NALT and CLN in young (8 days old) mice. I.n. vaccination of NALT- or CLN-deficient mice with pneumococcal polysaccharide conjugate vaccine plus interleukin-12 as a mucosal adjuvant (days 10 and 17) was followed by i.n. pneumococcal challenge (days 24–28). Mice were sacrificed on day 31 and nasal mucosal and systemic immune responses as well as pneumococcal colonization in the middle ear and nasopharynx were assessed. Elimination of NALT did not impair the ability of infant (3 week-old) mice to produce nasal or serum antibody responses following i.n. immunization. In contrast, surgical removal of CLN significantly impaired the ability to express IgA antibody in nasopharyngeal washes and total antibody in serum. Similarly, protection against pneumococcal colonization in the nasopharynx and middle ears of immunized mice was decreased in the absence of CLN but not in the absence of NALT. These findings suggest that surgical removal of NALT tissue, at least in a mouse model, does not affect the ability to respond to subsequent i.n. vaccination. In addition, in the young mice CLN play a more important role than NALT for induction of protective mucosal and systemic antibody responses following i.n. immunization.
Keywords: Intranasal, Immunization, NALT, CLN
1. Introduction
Infections of the upper respiratory tract, including middle ear (ME) infections, are preceded by colonization in the nasopharynx (NP). Thus, prevention of pathogen colonization at this site should effectively block both transmission and infection [1]. Secretory IgA antibodies (SIgA) are known to play a major role in protection against upper respiratory tract infections, and intranasal (i.n.) immunization is an effective regimen to induce such IgA antibodies in NP secretions, in addition to enhancing systemic immunity in humans [2]. Children are most susceptible to respiratory tract infections and recent studies have emphasized the importance of enhancing mucosal immunity in early life by i.n. immunization [3,4].
Antigen-specific SIgA is generated within mucosal tissues, which can be broadly classified as inductive and effector sites, and together, comprise the “common mucosal immune system” [5]. The components of the respiratory immune system include nasal-associated lymphoid tissue (NALT), cervical lymph nodes (CLN), and dispersed lymphoid cells in the epithelial layer and in the respiratory lamina propria [6]. Also, in a recent study, a NALT-like structure of lymphocyte aggregates that form follicles was identified in human nasal mucosa of children less than two years of age, indicating that an equivalent to mouse NALT can develop in humans [7]. NALT has been considered to be the major inductive site for initiation of SIgA immune responses in the respiratory tract [2,5,6]. In addition, CLN, which do not belong exclusively to the mucosal immune system, also share immune inductive properties with NALT and serve to amplify the specific responses generated within NALT [8]. Previous studies in adult mice showed that i.n. immunization can induce specific IgA response in both NALT and CLN [9,10]. However, NALT can only be identified in mice 7 days after birth, so its ability to function as a mucosal inductive site during early life is largely unknown [11].
In humans, NALT is absent but equivalent tissue is formed by the Waldeyer’s ring, which includes the nasopharyngeal (adenoids), palatine, tubal, and lingual tonsils [12]. Surgical removal of nasopharyngeal and palatine tonsils (i.e., adenoidectomy and tonsillectomy) are the most common major operations performed on children under general anesthesia in the United States but it is not clear how this might affect the ability to respond to i.n. vaccination.
Several studies evaluated the induction of mucosal IgA responses in the mice with defective organogenesis of NALT (lymphotoxin α/β−/− mice) [11] or in the mice lacking NALT as a result of surgery [13]. However, studies by using lymphotoxin α/β−/− mice eliminated the function of not only NALT but also CLN, and studies using surgical models did not include the young mice. In the young mice, functional evaluation of NALT has been challenging, largely due to its small size and difficulties in accessing its anatomic location. In the present study, we developed surgical techniques to eliminate NALT and CLN in infant (8 day old) mice. Using these surgical models in combination with an i.n. immunization protocol that employed pneumococcal polysaccharide conjugate vaccine and IL-12 as a mucosal adjuvant [14], we have now assessed the need for NALT and CLN in induction of protective immunity following mucosal vaccination of 10- and 17-days old mice, which correspond to the state of immune maturation of human infants [15].
2. Material and methods
2.1. Mice, NALT surgery and CLN surgery
BALB/cAnNCr mice, 4–6 weeks old, were purchased from Charles River Laboratories (Raleigh, NC) through a contract with the National Cancer Institute (Bethesda, MD). The mice were bred and maintained at Albany Medical College. All experimental procedures were in compliance with the guidelines of the institutional animal care and use committee.
NALT surgery was performed on day 8 after birth using a binocular microscope. The mice were laid in a supine position with their mouths held open to reveal access to the hard palate. NALT was clearly visualized as two parallel strips along the nasal septum. A 2- to 4-mm incision was made along the midline of the hard palate with care taken to cut only skin palate and to not cut underlying bone containing nasal mucosa. A surgical elevator was inserted through the incision and the skin palate was slightly separated from the hard palate alongside the incision (1 mm lateral to the incision). An 18-gauge needle from a bipolar coagulator (Codman & Shurtleff, Inc, Randolph, MA) was inserted through the incision and the structural integrity of the NALT was destroyed by cauterization. Another group of infant mice underwent sham surgery in which a 2- to 4-mm incision was made along the midline of the hard palate but no tissue was removed. After surgery, the wounds were sealed by tissue adhesive (3M Vetbond, Animal Care Products, St. Paul, MN) and the mice were weighed daily to ensure normal weight gain.
CLN were also excised on day 8 after birth using a binocular microscope. After a midline skin incision was made in the neck, the CLN were surgically removed, including the superficial CLN adjacent to the anterior edge of the submandibular salivary glands, the central (posterior) CLN deep within the musculature of the neck, and the facial CLN close to the ventral posterior edge of the parotid glands. Another group of infant mice underwent sham surgery by making a midline skin incision in the neck but leaving the CLN intact. The wounds were sealed by 3M Vetbond tissue adhesive.
2.2. Immunization
I.n. immunization was performed in untreated mice, in mice that received NALT or sham NALT surgery, and in mice that received CLN or sham CLN surgery. Immunization was performed beginning on day 10, i.e., 2 days after surgery. 2.5 μg of pneumococcal polysaccharide conjugate vaccine (Wyeth Vaccines, Pearl River, NY) was administered i.n. on day 10 together with 0.2 μg of IL-12 (Wyeth Vaccines) i.n. on days 10–13. Another group of mice received 2.5 μg of conjugate vaccine on day 10 without IL-12. The conjugate vaccine consisted of serotype 14 pneumococcal polysaccharide (PPS14) covalently linked at a ratio of 2:1 to CRM197 diphtheria toxoid. The initial preparations were given in a volume of 5 μl/mouse in PBS containing 1% normal mouse serum as vehicle. Mice were boosted i.n. on day 17 with 5 μg of PPS14 with or without IL-12 in a volume 10 μl, and sera and NP wash samples were collected for analysis one week later. Control mice received PBS vehicle only.
2.3. Isolation of mononuclear cells
CLN were removed and weighted on day 24 from untreated mice, and mice that received NALT-sham or NALT-surgery. CLN were gently passed through a steel mesh and mononuclear cells were further isolated according to a previously reported method [16].
2.4. Measurement of antibody levels by ELISA
Sera were obtained by orbital puncture. After the blood was collected, the mice were sacrificed and NP wash samples were collected from the NP cavity using 100 μl of cold PBS according to a previously reported method [17]. Leakage of serum proteins into the nasal mucosal compartment was monitored by measuring albumin levels in NP washes using Albustix (Bayer, Elkhart, IN). Samples were stored at −80°C prior to being assayed for PPS14-specific antibodies by ELISA. Briefly, 96-well or 386-well Nunc Immuno Polysorp plates (Nalge Nunc, Rochester, NY) were coated overnight at 4°C with 15μg/ml of PPS14 (American Type Culture Collection, Manassas, VA) (100 μl/well or 20μl/well, respectively) in PBS. The plates were washed in PBS containing 0.05% Tween 20 and blocked for 1 hr at 37°C with PBS containing 10% fetal calf serum (HyClone, Logan, UT). Two fold dilutions of mouse serum or NP wash fluid in PBS containing 10% fetal calf serum were added to the plates and incubated at 4°C overnight. After the plates were washed, alkaline phosphatase-conjugated goat anti-mouse Ig antibody (Southern Biothechnology, Birmingham, AL) was added and the plates were incubated at 4°C. Bound enzyme was detected by adding p-nitrophenyl substrate and measuring absorbance at 405 nm with a microplate reader (Bio-Tek Instruments, Winooski, VT). For NP samples, end-point titers were expressed as the reciprocal log2 of the last dilution that had an optical density at 405 nm (OD405) of >0.1 OD unit above the OD405 value of negative control samples obtained from unimmunized mice. Dilutions corresponding to 25% maximal binding were used as the titers for serum antibody.
2.5. Bacteria
S. pneumoniae strain TJO983, which expresses PPS14 (kindly provided by Dr. David E. Briles, University of Alabama at Birmingham, AL), was grown overnight on blood agar plates and cultured at 37°C in Todd-Hewitt broth supplemented with 0.5% yeast extract. The identity of the pneumococci was confirmed by colony morphology on blood agar plates and by sensitivity to optochin (Sigma, St. Louis, MO). Bacteria were harvested by centrifugation and washed twice in sterile PBS. The bacteria were resuspended in Todd-Hewitt broth containing 0.5% yeast extract and 15% glycerol, and stored in aliquots at −80ºC.
2.6. I. n. challenge with S. pneumoniae and induction of NP and ME colonization
Pneumococcal colonization studies were performed using a mouse model of NP and ME infection that was established after i.n. administration of pneumococci. In this model, the portal of pathogen entry into the ME would thus resemble the disease process in humans. One week after the booster immunization, mice were inoculated for five consecutive days (days 24–28) i.n. with 106 CFU of type 14 S. pneumoniae. For time course studies, the mice were sacrificed on days 25 to 36, and assayed for the presence of pneumococci in NP and ME wash fluids. For the rest of the studies, the mice were sacrificed on day 31. ME washes were collected as described previously [14]. NP and ME washes were diluted 10-fold and 50 μl of the diluted samples were plated onto blood agar to determine the concentrations of live bacteria. The mice were monitored by otomicroscopic observation to confirm tympanic membrane changes (vessel dilation, increased thickness, and reduced translucency).
2.7. Immunohistochemistry
For immunofluorescent tissue staining, groups of BALB/c mice were sacrificed 1 week after the day 7 booster immunization and then perfused transcardially with PBS, followed by perfusion with 10% formalin. The heads of the mice were immersed in 10% formalin, decalcified with 0.12 M EDTA, pH 7.0, for 3 weeks, and frozen in OCT compound in isopentane that was chilled in acetone/dry ice. The tissues were stored in the dark at −80°C until processing. Serial frozen sections of nasal mucosa (10 μm) were rehydrated in PBS and incubated with PBS/2% BSA (blocking reagent) for 10 min at room temperature. FITC goat anti-mouse-IgA (Southern Biotech, Birmingham, AL) was added and the sections were incubated for 1 h. The sections were washed and mounted in 10% PBS/90% glycerol, covered with a coverslip, and sealed with nail polish. Sections were kept in the dark at 4°C until analysis using a BX-FLA Olympus microscope (Tokyo, Japan) equipped with an Optronics Magnafire camera. Frozen sections were also stained with H&E. The immunostaining results shown are representative of one nasal mucosa per mouse and three mice per group.
3. Statistics
Specific antibody levels and bacterial concentrations were analyzed for statistical significance by the Mann-Whitney test. Bacterial numbers were expressed as medians and interquartile ranges because the data were not normally distributed. Differences of P<0.05 were considered to be significant.
4. Results
4.1. NALT and CLN removal in infant mice
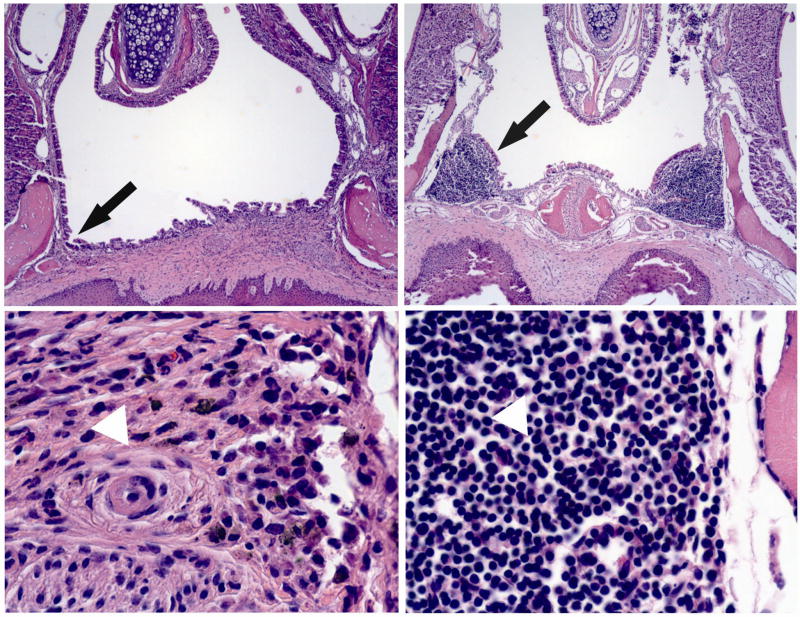
In the present study we developed a technique to destroy NALT in 8 days old mice. The use of cauterization resulted in significantly reduced bleeding during surgery. The surgery did not affect the feeding of mice as confirmed by normal weight gain. On day 17 after surgery, there was no presence of organized lymphoid tissue in the nasal cavity and only sparse numbers of lymphocytes in the area previously occupied by the NALT (Fig. 1).
Fig. 1.
Coronal sections from mice that were subjected to (upper and lower left) NALT surgery or (upper and lower right), sham surgery were stained with H&E (upper left and right = ×40 magnification; lower left and right = ×400 magnification). Upper left. The arrow indicates the absence of organized lymphoid tissue in a NALT-deficient mice 17 days after surgery. Upper right. The arrow indicates location of NALT in a sham-operated mouse 17 days after surgery. Lower left. The typical NALT location is occupied with scar tissue (arrowhead) in a NALT-deficient mouse. Lower right. The presence of lymphoid tissue (arrowhead) in a sham-operated mouse.
We also removed CLN in 8 days old mice. On day 17 after surgery, there was no evidence of lymphoid tissue in the location typical for CLN location.
4.2. I.n. infection to establish NP and ME pneumococcal colonization
In the present study we established a mouse model of ME infection after i.n. administration of pneumocci for five consecutive days (days 24–28) (Table 1). This model reproduces the natural route of pneumococcal infection and does not bypass the innate and specific immune mechanisms that are present at NP mucosa surfaces, in contrast to infections established by direct inoculation of bacteria into the ME [14,18]. All mice showed the presence of pneumococci in NP washes on days 25 to 36. No bacteria were cultured from NALT at any time point. The percentage of mice with ME infection gradually increased from day 25 until it peaked on day 31, and started to decrease thereafter, proportionate with the number of pneumococci present in the NP. Changes in tympanic membrane structure correlated with the presence of bacteria in the ME. Decreasing the inoculation dose (<106 CFU) or the number of inoculations (<5) significantly affected the probability of ME colonization. This is consistent with previous findings by others that a single intranasal challenge with S. pneumoniae type 14 induces ME colonization in less than 50% of mice [19].
Table 1.
Colonization of the NP and ME by pneumococci in BALB/c mice after i.n. inoculation with S. pneumoniae type 14
| Day after a birth | Number of mice | Inoculated dose | Mean No. of pneumococci cultured from NP wash
(CFU/ml) |
Percentage of mice exhibiting colonization | Percentage of mice exhibiting tympanic membrane changes | |
|---|---|---|---|---|---|---|
| NP | MEb | |||||
| 25 | 4 | 106 | 0.5 ×106 | 100 | 0 | 0 |
| 29 | 4 | 106 | 1.1 ×106 | 100 | 50 | 25 |
| 31 | 7 | 106 | 1.4 ×106 | 100 | 100 | 71 |
| 36 | 4 | 106 | 0.9 ×106 | 100 | 25 | 0 |
Mice were challenged with S. pneumoniae type 14 on days 24–28 after birth.
ME washes were pooled from the right and left ME.
4.3. Antigen-specific immune responses in infant mice
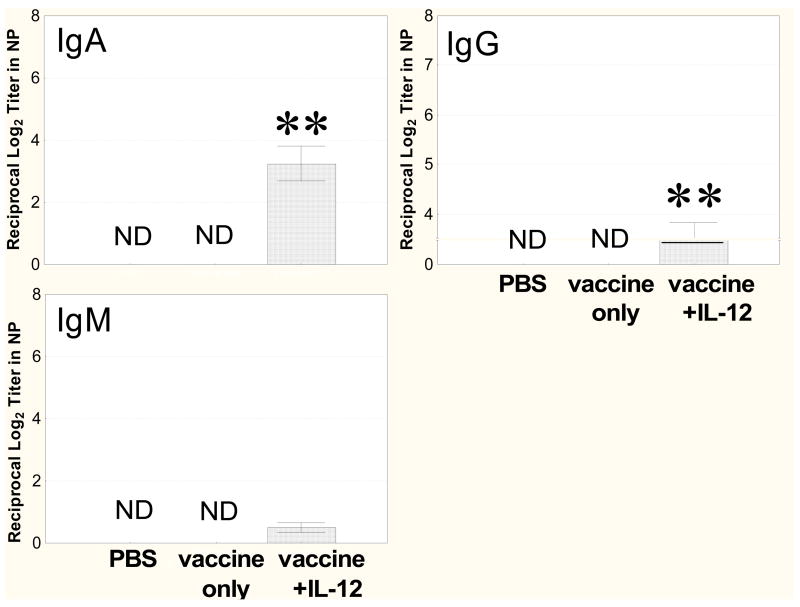
Anti-PPS14 antibody levels in NP washes after i.n. vaccination of infants were measured by ELISA. For i.n. vaccination, IL-12 was used as a mucosal adjuvant, a strategy that was previously shown by our laboratory to yield high levels of nasal IgA antibodies after vaccination of adult mice with PPS [20]. Indeed, we confirmed that specific antibodies in NP washes of 3 week-old mice were not induced by i.n. inoculation of vaccine alone (Fig. 2). However, after i.n. immunization in the presence of IL-12, levels of IgA antibodies were significantly elevated (P<0.01). Small amounts of IgG and IgM were detected in i.n. immunized mice treated with IL-12 but not in mice that received vaccine only.
Fig. 2.
Antibody levels in NP. Mice were immunized i.n. on days 10 and 17 with PPS14 conjugate vaccine plus IL-12 or primed with PBS vehicle or PBS only. I.n. immunized mice were sacrificed 1 week after the day 17 booster immunization, and NP washes were assayed by ELISA for total, IgA, IgG, and IgM anti-PPS14 antibody. Levels of total and nasal IgA antibody were significantly greater in the vaccinated group treated with IL-12 than in the mice treated with vaccine only (**, P < 0.01). ND - not detectable. Values are the mean ± SE for 8 mice/group.
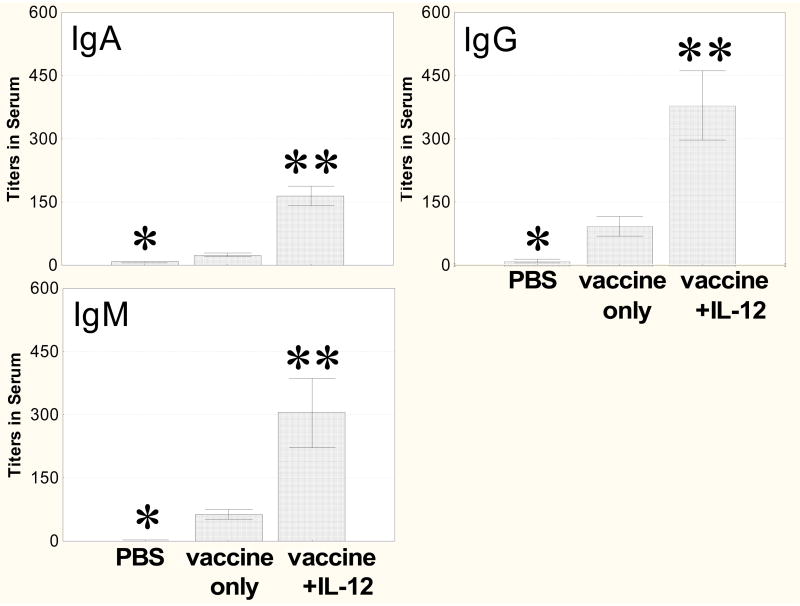
Levels of serum IgG, IgM, and IgA antibodies were also significantly augmented by i.n. vaccination in the presence of IL-12 compared to mice that received vaccine only (Fig. 3). Small amounts of specific antibodies were detected in i.n. immunized mice that received vaccine only. PPS-14-specific antibodies were not detected in the NP wash or sera of any mice treated with PBS-NMS alone or IL-12 alone (data not shown).
Fig. 3.
Antibody levels in sera. Mice were immunized i.n. as described in Fig. 2, sacrificed 1 week after the day 17 booster immunization, and serum was assayed by ELISA for total, IgA, IgG, and IgM anti-PPS14 antibody. Total, IgA, IgG and IgM titers were significantly greater in the vaccinated group treated with IL-12 than in mice treated with vaccine only (**, P < 0.01). Titers of total, IgA, IgG and IgM antibodies were significantly less in the PBS only group than in mice treated with vaccine only (*, P < 0.01). Values are the mean ± SE for 8 mice/group.
4.4. Colonization in NP and ME in infant mice
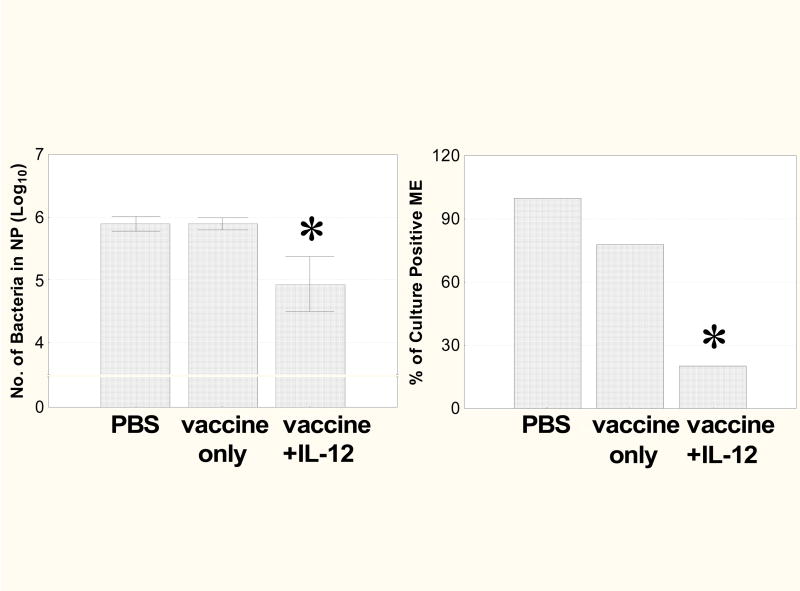
On days 24–28 after birth, vaccinated mice were inoculated i.n. with type 14 pneumococci. It was found that the number of pneumococci recovered in NP washes (Fig. 4, left) as well as the incidence of ME colonization (Fig. 4, right) was not reduced in mice that had received vaccine alone. In contrast, treatment with vaccine plus IL-12 caused markedly lower numbers of NP CFU (P<0.05) and a decreased incidence of ME colonization (P<0.05). There were no significant differences in numbers of bacteria recovered from animals treated with vehicle or IL-12 only.
Fig. 4.
Pneumococcus carriage in NP and ME. Mice were immunized i.n. as described in Fig. 2 and were inoculated i.n. with type 14 pneumococci on days 24–28. The mice were sacrificed on day 31, and NP and ME wash fluids were assayed for the presence of pneumococci. Protection against pneumococcal colonization in the NP (left) and ME (right) was significantly greater in the vaccinated group treated with IL-12 than in mice treated with vaccine only or PBS only (*, P < 0.05). Values are the mean ± SE of log10 CFU values (left) or percentage of culture-positive ears (right) for 8 mice/group.
4.5. Lymphocytes recovered from CLN in intact and NALT-deficient mice
The number of CLN cells and the weights of the CLN in various study groups are shown in Table 2. I.n. immunization in the presence of IL-12 significantly increased (P < 0.05) the number of lymphocytes in CLN compared to that in mice that received PBS-vehicle only. Interestingly, it was found that following i.n. immunization with PPS conjugate vaccine plus IL-12, both the sham surgery and NALT-deprived groups had significant elevations in numbers of lymphocytes in CLN (P < 0.05) that were not significantly different from each other. The increase in CLN cellularity was also accompanied by increase in the weights of the CLN.
Table 2.
Weight and cell count of CLN in intact, and in mice that received NALT-sham or NALT-surgery followed by i.n. administration of PBS, vaccine only or vaccine+IL-12
| Group
|
||||
|---|---|---|---|---|
| PBS | Vaccine only | Vaccine+IL-12 | ||
| CLN cell count | Intact | 6.17 ± 0.18 | 6.33 ± 0.27 | 7.72 ± 0.591 |
| (×105 cells /mouse) | Sham NALT-surgery | 6.17 ± 0.1 | 6.31 ± 0.37 | 7.71 ± 0.691 |
| NALT-surgery | 6.21 ± 0.19 | 6.2 ± 0.22 | 7.76 ± 0.651 | |
| CLN weight (mg) | Intact | 3.04 ± 0.13 | 3.26 ± 0.17 | 3.9 ± 0.081,2 |
| Sham NALT-surgery | 3.01 ± 0.16 | 3.28 ± 0.19 | 3.86 ± 0.151,2 | |
| NALT-surgery | 2.97 ± 0.26 | 3.17 ± 0.25 | 3.82 ± 0.181 | |
Data are presented as mean ± standard error (SE)
P < 0.05 as compared with PBS vehicle treated mice
P < 0.05 as compared with vaccine only treated mice
4.6. Mucosal and serum antibody responses after elimination of NALT or CLN
We next sought to determine whether the presence of NALT or CLN is a strict requirement for the induction of mucosal and serum antibody responses in infants. After surgical removal of NALT or CLN on day 8 after birth, mice were inoculated i.n. with PPS14 conjugate vaccine ± IL-12. Mice that underwent sham surgery were used as controls. Sham surgeries did not impair the ability of mice to produce specific mucosal or serum antibodies, when compared to mice without surgery. No antibody was detected in the nasal washes or serum of sham surgery mice that were treated with PBS vehicle alone.
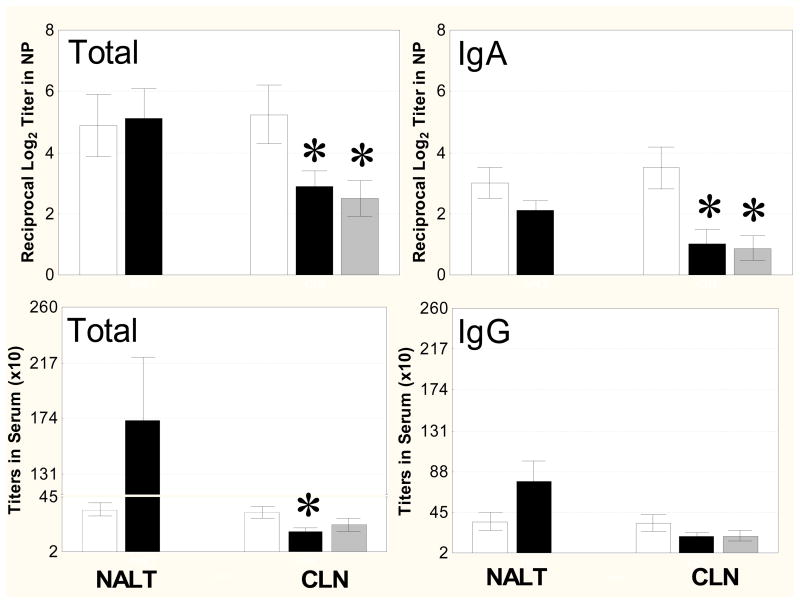
In vaccinated animals, it was found that both the sham surgery and NALT-deprived groups had significant elevations in nasal total (Fig. 5, upper left) and IgA antibody (Fig. 5, upper right) levels (P < 0.001) that were not significantly different from each other. However, only low levels of nasal total and IgA antibody responses were induced in mice after surgical removal of three different groups of CLN (superficial, central, and facial) (P<0.05 compared to the sham surgery group). Interestingly, a similar decrease in levels of nasal total and IgA antibody responses was induced in mice after surgical removal of only superficial CLN (P<0.05 compared to the sham surgery group). Immunofluorescent staining of the nasal mucosa reveled the presence of IgA+ cells in NALT-deficient (Fig. 6A) and intact (Fig. 6C) immunized mice treated with IL-12; however, there were fewer IgA+ cells in CLN-deficient mice (Fig. 6B). These cells are cytoplasmic IgA+ cells (likely plasmablasts or plasma cells), not merely surface IgA+ B cells. There were no detectable IgA+ cells in the nasal mucosa of unimmunized animals (data not shown).
Fig. 5.
Antibody levels in NP and sera after NALT or CLN surgery. NALT or CLN were removed on day 8 and the mice were immunized i.n. as described in Fig. 2. I.n. immunized mice were sacrificed 1 week after the day 17 booster immunization, and NP washes and sera were assayed by ELISA for anti-PPS14 antibody. Levels of total (upper left) and IgA (upper right) antibodies in NP washes and total antibody (lower left) in sera were significantly less in the absence of three different groups of CLN (superficial, central, and facial) (*, P < 0.05), but not in the absence of NALT compared with the sham surgery group. Levels of total (upper left) and IgA (upper right) antibodies in NP washes were significantly less in the absence of superficial CLN compared with the sham surgery group (*, P < 0.05 ). Values are the mean ± SE for 8 mice/group.▭-sham NALT or CLN surgery;▬-NALT or CLN (superficial, central, and facial) surgery;
 -CLN (superficial only) surgery.
-CLN (superficial only) surgery.
Fig. 6.
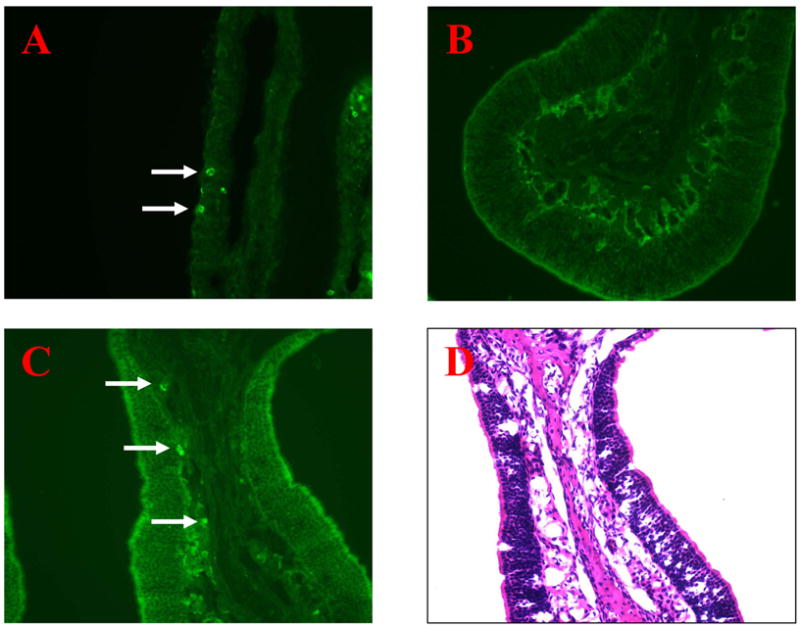
Fluorescent microscopy of nasal mucosa obtained from NALT-deficient (A), CLN-deficient (B), and intact mice (C) after i.n. immunization with PPS14 conjugate vaccine plus IL-12. Frozen sections were stained with anti-IgA antibody, and analyzed by fluorescent microscopy. The white arrows indicate IgA+ cells (A, C). NM sections of intact mice treated with IL-12 were also stained with H&E (D).Magnification is × 200.
Serum antibody responses were also assessed in NALT- and CLN-deficient mice. There were no significant differences in the levels of serum total (Fig. 5, lower left) and IgG antibodies (Fig. 5, lower right) between sham surgery and NALT-deprived groups. In contrast, the levels of serum total antibodies induced after surgical removal of three different groups of CLN (superficial, central, and facial) were lower than those after sham CLN surgery (Fig. 6, lower left) (P< 0.05). In fact, the levels of serum antibodies in CLN-deficient mice were comparable to those seen in intact mice that received i.n. vaccine alone. Surgical removal of only superficial CLN did not impair the ability of mice to produce serum total and IgG antibodies, when compared to mice after sham CLN surgery (P > 0.05).
4.7. Pneumococcal colonization after elimination of NALT or CLN
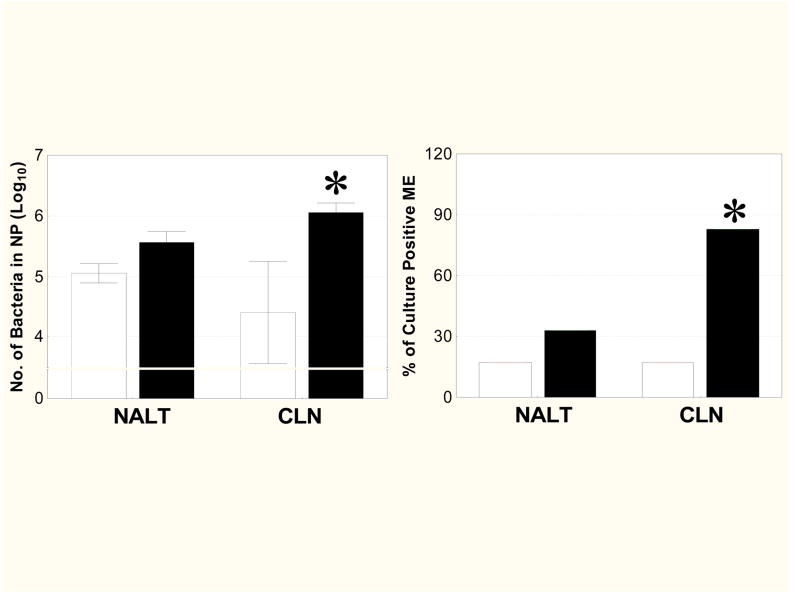
We next evaluated the roles of NALT and CLN in protection of the respiratory tract. I.n. vaccination of NALT- or CLN-deficient mice was followed by i.n. pneumococcal challenge. Mice that underwent sham surgery were used as controls. Sham surgeries did not impair the ability of mice to control NP and ME pneumococcal colonization following i.n. bacterial inoculation, when compared to mice without surgery.
NALT-deficient or sham NALT surgery mice demonstrated similar decreases in NP (Fig. 7, left) and ME (Fig. 7, right) colonization following i.n. pneumococcal challenge. In contrast, CLN-deficient mice demonstrated higher numbers of bacteria in NP washes as well as a higher incidence of ME colonization compared to sham CLN surgery mice (P<0.05). There were no decreases in NP or ME colonization in NALT- or CLN-deficient mice that were administered vaccine only or PBS (data not shown).
Fig. 7.
Pneumococcus carriage in NP and ME after NALT or CLN surgery. NALT or CLN were removed on day 8 and the mice were immunized i.n. as described in Fig. 2. I.n. immunized mice were inoculated i.n. with type 14 pneumococci on days 24–28. The mice were sacrificed on day 31, and NP and ME wash fluids were assayed for the presence of pneumococci. Protection against pneumococcal colonzation in the NP (left) or ME (right) following i.n. immunization with PPS14 plus IL-12 as a mucosal adjuvant was decreased in the absence of CLN (*, P < 0.05), but not in the absence of NALT compared with sham surgery mice. Values are the mean ± SE of log10 CFU values (left) or percentage of culture-positive ears (right) for 6 mice/group. ▭-sham NALT or CLN surgery; ▬-NALT or CLN (superficial, central, and facial) surgery.
5. Discussion
In the present study, we developed surgical technique to eliminate NALT in infant mice and assessed the importance of NALT in the induction of protective immunity in the upper respiratory tract. This study used an infant i.n. immunization protocol with PPS14 conjugate vaccine and IL-12 as adjuvant, and demonstrated the importance of CLN but not NALT for induction of protective mucosal antibody responses against pneumococcal infection in young animals.
There is substantial evidence that in the adult respiratory tract, NALT is a major inductive site for antigen-specific IgA antibody responses. In lymphotoxin α/β−/− mice, which show impaired organogenesis of NALT, i.n. immunization fails to induce mucosal IgA responses [11]. However, such mice also lack organized CLN. In contrast, i.n. administration of bacterial antigen in NALT-deficient mice (Id2−/−) induced the levels of antigen-specific antibodies which were comparable to those in wild type counterpart (Id2+/+) [21]. Furthermore, the results obtained in the current study showed that elimination of NALT in young mice by surgery did not impair their ability to produce nasal antibody responses following i.n. immunization with PPS conjugate vaccine. Another previous study has shown that surgical removal of NALT does not impair the ability of mice to generate specific mucosal IgA antibody following i.n. infection with influenza virus [13]. Similarly, in the gastrointestinal tract, it has been found that oral immunization of mice that lack Peyer’s patches induces intestinal IgA antibodies [22]. Taken together, it appears that specific SIgA responses can be induced in the absence of selected mucosal inductive sites, including NALT.
Based on the significant mucosal IgA antibody responses obtained in mice without NALT, it was important to determine the source of those responses. Thus, we addressed the possibility that CLN are required for the induction of antigen-specific nasal responses. Our data suggests that i.n. immunization induces cell proliferation or accumulation in CLN as evidenced by increase in cellularity and in the weights of the CLN. It is unlikely that NALT disseminated antigen-sensitized lymphocytes to the CLN, because the similar increases in the number of lymphocytes from CLN were observed both in sham surgery and NALT-deprived group of mice. Surgical removal of the CLN that drain both NALT (central CLN) and nasal mucosa (superficial CLN) was found to significantly impair the ability of the host to generate nasal IgA antibodies following i.n. immunization (4.3 fold decrease compared to sham-operated mice). A similar decrease in levels of nasal IgA antibodies (3.5 fold decrease) was observed when mice lacking only superficial CLN were i.n. immunized. Although the goal of the present study did not include studying induction of cellular responses in mucosal sites, it is possible that following i.n. immunization, lymphocytes in superficial CLN migrate into the respiratory lamina propria for subsequent IgA immune responses. Others have likewise reported that the absence of mesenteric lymph nodes that drain the intestinal mucosal areas, including Peyer’s patches, causes a significant impairment in the ability to generate mucosal IgA antibody responses following oral immunization [22]. These findings suggest that lymph nodes which drain mucosal tissues are a major source of immunocompetent cells that might be responsible for sustained antibody production in the upper respiratory tract, and can compensate for defects in immunological inductive functions if NALT is absent.
The present study demonstrated elevated levels of specific IgA antibodies in NP wash samples and the presence of IgA+ cells in the nasal mucosa of NALT-deficient mice treated with IL-12. These results suggest local production of specific IgA antibodies in NP washes of NALT-deficient mice rather than transudation from serum. However, we can not rule out the possibility that serum antibodies including IgG and IgM also reached NP mucosa. It is possible that the volume of PBS used for flushing the NP cavity (100 ul) diluted the NW wash samples and decreased the sensitivity of ELISA.
In addition to measuring antibody responses, the roles of NALT and CLN in protection of the respiratory tract from pneumococcal colonization was also evaluated. Elimination of NALT did not impair the ability of vaccinated hosts to control NP and ME pneumococcal colonization following i.n. bacterial inoculation. In contrast, protection against pneumococcal colonization was impaired in mice that underwent surgical removal of CLN prior to immunization. These findings indicate that in young mice CLN are essential to confer protection in the NP and ME after vaccination, whereas NALT plays only the minor role in that protection.
Several factors such as the distribution of specialized cells for antigen uptake, the use of adjuvant, and the maturity of the lymphoid tissues, could contribute to the present findings. Previously, it was though that particulate antigens are selectively sampled in NALT, whereas soluble antigens are taken up through the nasal mucosa and NALT [23,24]. Recent studies demonstrated the presence of a cluster of antigen-sampling cells in the turbinate portion of nasal cavity, which effectively took up both soluble and particle forms of inhaled antigens [21,25]. These findings provide evidence for a NALT-independent gateway for antigen sampling and subsequent induction of antigen-specific immune responses following i.n. administration of antigens. In the present study, the inclusion of IL-12 as a mucosal adjuvant enhanced the protective effects of the vaccine against pneumococcal infection. Although the exact mechanism of action of IL-12 as a mucosal adjuvant is largely unknown, it is possible that IL-12 enhances penetration of antigen through the nasal epithelia. In addition, IL-12 has been shown to have various stimulating effects on immunocompetent cells, including antigen-presenting cells, and B cells [26]. Another potentially relevant factor is the differential organogenesis of NALT and CLN in young mice. Initiation of NALT formation begins soon after birth [27]. Although NALT is present in 8 day old mice, its cellularity, T cell and B cell compartmentalization, lymphoid chemokine expression and high endothelial venules maturation is decreased compared to adult mice [11]. In contrast, CLN development begins during embryogenesis, and 8 day old mice have fully developed CLN [28]. Considering this, it is possible that in infants, NALT is less capable than CLN in functioning as an immunologic inductive tissue. Interestingly, in the human neonates, the total amount of lymphoid tissue in the form of lymph nodes, especially the CLN, is considerable and comparable to that in all tonsils, suggesting the importance of CLN in the protective immunity in upper respiratory tract in early age [29,30]. In the murine models, mucosal-independent IgA precursor cells have been demonstrated in the respiratory tract and intestinal mucosa and it has been suggested that B-1 cells can contribute to the induction of intestinal mucosal IgA antibody responses in the absence of Peyer’s patches [22,31,32]. Thus, it is possible that B-1 cells in the respiratory tract mucosa also contribute to induction of respiratory mucosal IgA antibody responses in the absence of NALT.
In the murine upper respiratory tract, the NALT is the only well-organized lymphatic tissue with fixed location in the NP. Therefore, this tissue can be regarded as the NP tonsil and as an equivalent of Waldeyer’s ring because structures similar to the palatine and lingual tonsils have not been observed in rodents [33]. It has been reported that after surgical removal of palatine tonsils and adenoids in children the local immune response to orally administered live polio vaccine is reduced, however, it is not known whether the surgical removal of adenoids may also impair the local immune response to i.n. administered vaccine [34]. The results presented in this study indicate that elimination of NALT tissue does not affect the induction of mucosal immune responses in the murine respiratory tract. Although human adenoids and murine NALT apparently play an important immune-inductive site role, extrapolation from our results on murine NALT to the situation in humans have to be regarded with caution. Adenoids and murine NALT show differences in terms of their cellular composition and isotype of antibody-producing cells [35]. In addition, adenoids act as a reservoir of S. pneumoniae and children with OM benefit from adenoidectomy because it eliminates the infectious source in the NP [36]. In contrast, pneumococci are unlikely to colonize in the murine NALT, and elimination of NALT, at least in our infant mice model, does not prevent subsequent NP colonization.
In summary, the results presented in this study indicate that elimination of NALT tissue does not affect the induction of mucosal immune responses in the murine respiratory tract. In young mice, NALT appear to play a minor role in the generation of mucosal IgA antibodies following i.n. immunization with PPS conjugate vaccine plus IL-12. In contrast, CLN appear to play an essential role in induction of respiratory IgA responses. In the absence of NALT, it is possible that CLN provide a compensatory immune response, and that response is sufficient to reduce NP and ME bacterial colonization.
Acknowledgments
This work was supported by NIH Grant AI41715 and by Philip Morris USA Inc. and Philip Morris International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu HY, Nahm MH, Guo Y, Russell MW, Briles DE. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–46. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen H, Bjamarson S, Del Giudice G, Moreau M, Siegrist CA, Jonsdottir I. Intranasal immunization with pneumococcal conjugate vaccines with LT-K63, a nontoxic mutant of heat-Labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice. Infect Immun. 2002;70:1443–52. doi: 10.1128/IAI.70.3.1443-1452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjarnarson SP, Jacobsen H, Del Giudice G, Trannoy E, Sigrist CA, Jonsdottir I. The advantage of mucosal immunization for polysaccharide-specific memory responses in early life. Eur J Immunol. 2005;35:1037–45. doi: 10.1002/eji.200425850. [DOI] [PubMed] [Google Scholar]
- 5.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis SS. Nasal vaccines. Adv Drug Deliv Rev. 2004;51:21–4. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 7.Debertin AS, Tschernig T, Tonjes H, Kleemann WJ, Troger HD, Pabst R. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol. 2003;134:503–7. doi: 10.1111/j.1365-2249.2003.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuercher AW, Coffin SE, Thurnheer MC, Fundova P, Cebra JJ. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J Immunol. 2002;168:1796–1803. doi: 10.4049/jimmunol.168.4.1796. [DOI] [PubMed] [Google Scholar]
- 9.Wu HY, Russell MW. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 10.Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J Immunol. 2003;170:1754–62. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- 11.Ying X, Chan K, Shenoy P, Hill M, Ruddle NH. Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin. Am J Pathol. 2005;166:135–46. doi: 10.1016/S0002-9440(10)62239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kempen MJ, Rijkers GT, van Cauwenberge PB. The immune response in adenoids and tonsils. Int Arch Allergy Immunol. 2000;122:8–19. doi: 10.1159/000024354. [DOI] [PubMed] [Google Scholar]
- 13.Wiley JA, Tighe MP, Harmsen AG. Upper respiratory tract resistance to influenza infection is not prevented by the absence of either nasal-associated lymphoid tissue or cervical lymph nodes. J Immunol. 2005;175:3186–96. doi: 10.4049/jimmunol.175.5.3186. [DOI] [PubMed] [Google Scholar]
- 14.Sabirov A, Metzger DW. Intranasal vaccination of neonatal mice with polysaccharide conjugate vaccine for protection against pneumococcal otitis media. Vaccine. 2006;24:5584–92. doi: 10.1016/j.vaccine.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 16.Kodama S, Suenaga S, Hirano T, Suzuki M, Mogi G. Induction of specific immunoglobulin A and Th2 immune responses to P6 outer membrane protein of nontypeable Haemophilus influenzae in middle ear mucosa by intranasal immunization. Infect Immun. 2000;68:2294–300. doi: 10.1128/iai.68.4.2294-2300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurono Y, Yamamoto M, Fujihashi K, et al. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1999;180:122–32. doi: 10.1086/314827. [DOI] [PubMed] [Google Scholar]
- 18.Sabirov A, Kodama S, Hirano T, Suzuki M, Mogi M. Intranasal immunization enhances clearance of nontypeable Haemophilus influenzae and reduces stimulation of tumor necrosis factor alpha production in the murine model of otitis media. Infect Immun. 2002;69:2964–71. doi: 10.1128/IAI.69.5.2964-2971.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malley R, Morse SC, Leite LC, Areas AP, Ho PL, Kubrusly FS, et al. Multiserotype protection of mice against pneumococcal colonization of the nasopharynx and middle ear by killed nonencapsulated cells given intranasally with a nontoxic adjuvant. Infect Immun. 2004;72:4290–92. doi: 10.1128/IAI.72.7.4290-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JM, Briles DE, Metzger DW. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect Immun. 2003;71:4780–88. doi: 10.1128/IAI.71.8.4780-4788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DY, Fukuyama S, Sagara H, Nagatake T, Takamura K, Nochi T, et al. Discovery of an alternative uptake system: the presence of respiratory M cells for inhaled antigens. Abstracts of the 13th International Congress of Mucosal Immunology; Tokyo, Japan. 2007. p. 162. [Google Scholar]
- 22.Yamamoto M, Rennert P, McGhee JR, Kweon MN, Yamamoto S, Dohi T, et al. Alternate mucosal immune system: organized Peyer’s patches are not required for IgA responses in the gastrointestinal tract. J Immunol. 2000;164:5184–91. doi: 10.4049/jimmunol.164.10.5184. [DOI] [PubMed] [Google Scholar]
- 23.Kraal G. Nasal-associated lymphoid tissue. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W, editors. Mucosal immunology. 3. New York: Academic Press; 2005. pp. 415–22. [Google Scholar]
- 24.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 25.Kiyono H. Airway immunity: uniqueness in organogenesis program and antigen-sampling system for mucosal vaccine development. Abstracts of the 13th International Congress of Mucosal Immunology; Tokyo, Japan. 2007. p. 50. [Google Scholar]
- 26.Arulanandam B, Van Cleave VH, Metzger DW. IL-12 is a potent neonatal vaccine adjuvant. Eur J Immunol. 1999;29:256–64. doi: 10.1002/(SICI)1521-4141(199901)29:01<256::AID-IMMU256>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Fukuyama S, Hiroi T, Yokota Y, Rennert PD, Yanagita M, Kinoshita N, et al. Initiation of NALT organogenesis is independent of the IL-7R, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(−)CD4(+)CD45(+) cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 28.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 29.Khlystova ZS, Abdumuratova DA, Savenko VA, Baryshev BB. Immunomorphologic characteristics of the tonsils and their lymph nodes in human fetuses. Vestn Otorinolaringol. 1989;2:55–8. [PubMed] [Google Scholar]
- 30.Collins P. Development of the cardiovascular and lymphatic systems. In: Berkowitz BKB, Borley NR, Crossman AR, et al., editors. Gray’s Anatomy. The Anatomical Basis for Clinical Practice. London: Elsevier; London: Elsevier; 2005. pp. 1029–56. [Google Scholar]
- 31.Hiroi T, Yanagita M, Iijima H, Iwatani K, Yoshida T, Takatsu K, Kiyono H. Deficiency of IL-5 receptor alpha-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cell in mucosa-associated tissues. J Immunol. 1999;162:821–8. [PubMed] [Google Scholar]
- 32.Tanaka N, Fukuyama S, Nagatake T, Takamura K, Murono Y, Kiyono H. CXCR5/CXCL13-independent nasal B1 cells for the induction of antigen-specific secretory IgA responses. Abstracts of the 13th International Congress of Mucosal Immunology; Tokyo, Japan. 2007. p. 193. [Google Scholar]
- 33.Sosa GA, Roux ME. Development of T lymphocytes in the nasal-associated lymphoid tissue (NALT) from growing Wistar rats. Clin Dev Immunol. 2004;11:29–34. doi: 10.1080/10446670410001670463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogra PL. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N Engl J Med. 1971;284:59–64. doi: 10.1056/NEJM197101142840201. [DOI] [PubMed] [Google Scholar]
- 35.Boyaka PN, Wright PF, Marinaro M, Kiyono H, Johnson JE, Gonzales RA, et al. Human nasopharyngeal-associated lymphoreticular tissues. Functional analysis of subepithelial and intraepithelial B and T cells from adenoids and tonsils. Am J Pathol. 2000;157:2023–35. doi: 10.1016/S0002-9440(10)64841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernstein JM. Waldeyer’s ring and otitis media: the nasopharyngeal tonsil and otitis media. Int J Pediatr Otorhinolaryngol. 1999;49:S127–32. doi: 10.1016/s0165-5876(99)00146-9. [DOI] [PubMed] [Google Scholar]