Abstract
The relative contribution of alpha adrenergic receptor subtypes in the transduction of sympathetic nerve activity (SNA) during carotid baroreflex (CBR) engagement is not well understood. Therefore, we compared the hemodynamic consequence of CBR-mediated sympatho-excitation via neck pressure (NP) before and after alpha-2 adrenergic blockade with intra-arterial yohimbine. Leg blood flow was measured using 2D and Doppler ultrasound, and arterial blood pressure was determined directly. NP caused the expected vasoconstriction, and this response was significantly reduced (by 50-60%) when NP was repeated after yohimbine. These data indicate that alpha-2 adrenergic receptors contribute significantly to CBR-induced vasoconstriction in the human leg under resting conditions.
Keywords: Baroreflex, Yohimbine, Leg Blood Flow, ultrasound Doppler
In response to a hypotensive stimulus, the carotid baroreceptors respond with a reflex increase in sympathetic nerve activity (SNA). In the peripheral circulation, this results in vasoconstriction and subsequent decreased systemic vascular conductance via binding of norepinephrine (NE) to both alpha-1 and alpha-2 adrenergic receptors on vascular smooth muscle. However, the relative contribution of adrenergic receptor subtypes in the transduction of SNA during carotid baroreflex (CBR) engagement is not well understood in humans.
While the principle action of the CBR is towards arterial blood pressure homeostasis, we have demonstrated that acute CBR-mediated changes in sympathetic outflow and the subsequent impact on systemic vascular conductance also heavily influence beat-to-beat regulation of skeletal muscle blood flow (Keller et al., 2003; Wray et al., 2004a). From a clinical perspective, CBR sensitivity has been demonstrated to be diminished in heart failure (Eckberg et al., 1971), hypertension (Bristow et al., 1969), and chronic obstructive pulmonary disease (Patakas et al., 1982), conditions also characterized by dysfunction in skeletal muscle perfusion. In this context, characterizing the functional contribution of end-organ (i.e. alpha adrenergic) receptors to CBR regulation of muscle blood flow may indeed be of significant interest.
Therefore, we contrasted the hemodynamic consequence of acute, CBR-mediated increases in SNA before and after administration of intra-arterial yohimbine, a selective alpha-2 antagonist. This receptor subtype is of particular interest in the context of CBR regulation due to the established susceptibility of alpha-2 adrenergic receptors to metabolic inhibition, such as is seen during acute exercise (Thomas & Segal, 2004). We hypothesized that alpha-2 blockade would increase baseline vascular conductance, but that acute increases in CBR-mediated vasoconstriction would be unaffected due to compensatory action of the unblocked, alpha-1 adrenergic pathway.
Six healthy, young men (24 ±1 yrs) participated in the present study. Written informed consent was obtained from all participants, and experiments were approved by the local ethics committee of Copenhagen and Frederiksberg and conformed to the Declaration of Helsinki. All studies were performed in a thermoneutral environment, with subjects in a semi-recumbent position (approximately 30 deg reclined).
The femoral artery was cannulated (Arrow, 20 gauge) under local anesthesia (lidocaine, 5 ml, 20 mg/ml) and inserted in the proximal direction approximately 5 cm below the inguinal ligament. This catheter was used for drug infusion and arterial blood pressure measurements. An ultrasound Doppler machine (CFM 800, GE Medical) was utilized for determination of common femoral artery blood velocity and diameter, as described previously (Wray et al., 2004b; Brothers et al., 2006). Using femoral artery diameter (FAD) and mean blood velocity (Vmean), leg blood flow (LBF, ml/min) was calculated according to the equation: LBF = Vmean · Pi (FAD/2)2 · 60.
Carotid baroreflex (CBR) control of leg hemodynamics was assessed at rest by determining changes in HR, mean arterial blood pressure (MAP), and LBF before and during a single, 5-s pulse of neck pressure (NP) at +40 Torr, as described previously (Potts et al., 1993). NP was applied during a 10–15 s breath hold at end-expiration in order to minimize the respiratory modulation of HR and MAP. A minimum of 45 s was allowed to pass between each NP trial to allow physiological variables to return to pre-stimulus values. Peak responses for MAP were determined as the greatest change over a 4 s period of time in response to the application of NP, and changes from each of five trials were averaged to provide a mean response for each subject. Peak LBF was determined using the average of the 4 s time period at which the peak MAP response occurred, while pre LBF was determined using the average over 4 s immediately preceding each NP stimulus. From these values, leg vascular conductance (LVC, ml/min/mmHg) was calculated as: LVC = LBF / MAP.
Following the control NP trials, yohimbine (YOH) was prepared in sterile water (0.5 mg/ml) and infused at 5 μg/kg/min for 20 minutes. Post-yohimbine baseline measurements were recorded, and then NP was applied, as described above.
All data (apart from LBF) underwent analog-to-digital conversion and were sampled at 400 Hz, recorded on a PC, and analyzed offline with signal processing software (Windaq, Dataq Instruments, Akron, OH, USA). Within group differences in MAP and LBF were assessed by two-way analysis of variance, with Tukey's HSD procedure for post-hoc analysis. Statistical significance was set at P < 0.05.
Heart rate (59 ± 3 bpm, control; 62 ± 2 bpm, YOH) and common femoral artery diameter (0.95 ± 0.02, control; 0.96 ± 0.02, YOH) were not different between control and YOH conditions. Mean arterial blood pressure was significantly higher after YOH (99 ± 3 mmHg) compared to control (90 ± 2 mmHg). The time course for changes in HR, MAP, and LBF in response to NP was similar to previously reported values (Fadel et al., 2003), and did not differ between control and YOH trials.
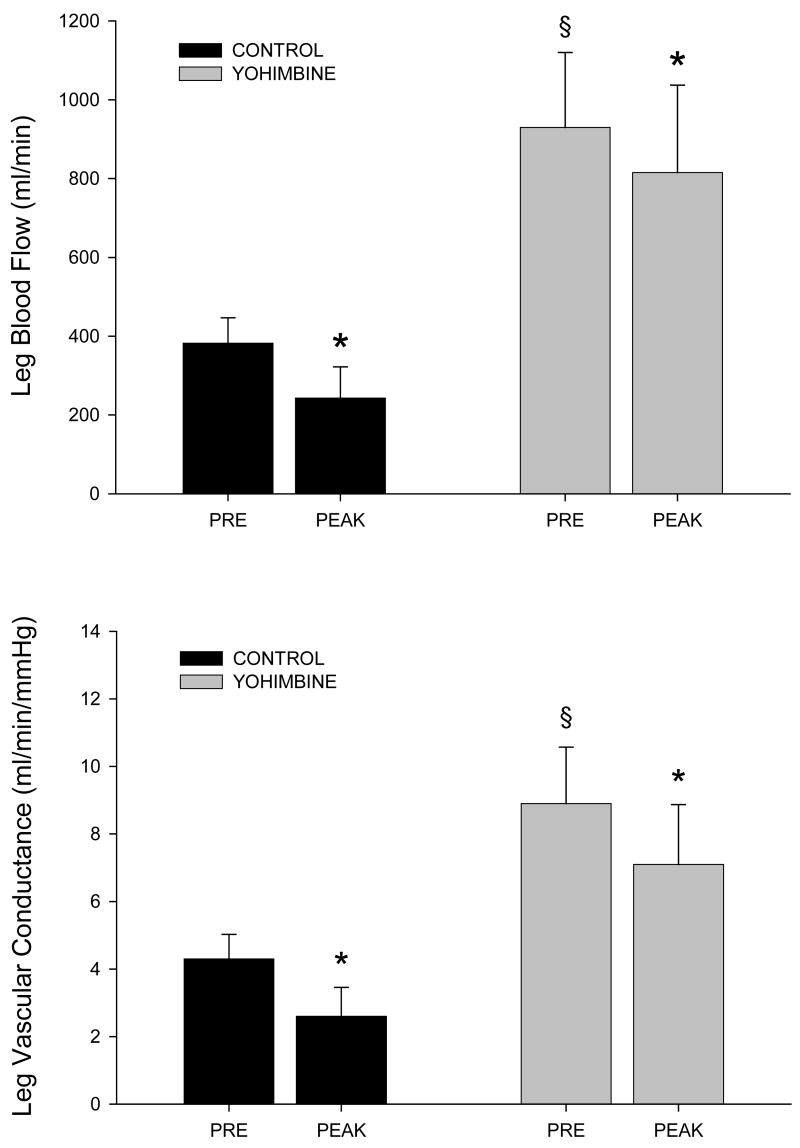
Figure 1 illustrates the hemodynamic responses to NP before and after YOH administration. Prior to alpha-2 adrenergic blockade, NP provoked a significant decrease in LBF (top panel) and calculated LVC (bottom panel). After alpha-2 adrenergic blockade, baseline LBF and LVC increased, and the vasoconstrictor response to NP was significantly reduced. NP increased mean arterial blood pressure slightly both before and after yohimbine (5 ± 1 mmHg, control; 8 ± 1 mmHg, YOH), though this pressor response was not significantly different (P = 0.18) between trials.
FIGURE 1.
Leg blood flow (top panel) and vascular conductance (bottom panel) in response to sympatho-excitation from 5-sec neck pressure (NP). PRE, prior to NP.
* Significantly different than PRE, P<0.05; § significantly different than control, P<0.05.
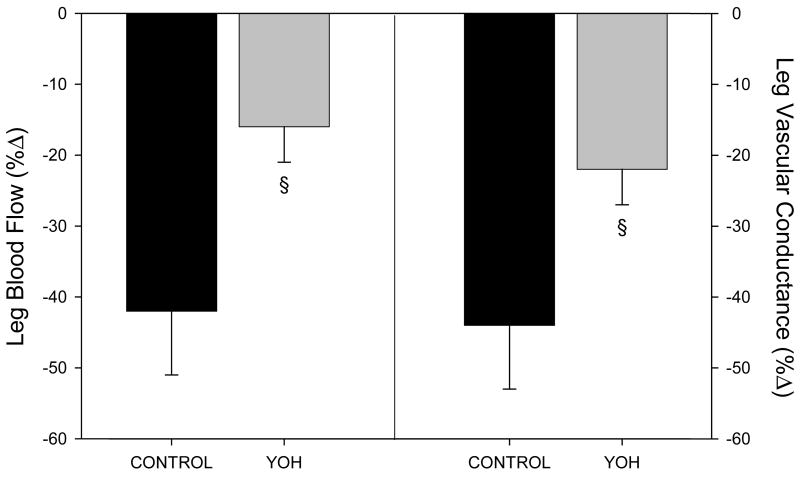
Figure 2 expresses the relative changes in LBF (left) and LVC (right) in response to NP before and after YOH administration. Together, these data identify a 50-60% reduction in vasoconstriction in response to NP after alpha-2 blockade. Thus, contrary to our hypothesis, functioning alpha-2 adrenergic receptors are essential for a normal vasoconstrictor response in the human leg during acute CBR-engagement under resting conditions.
FIGURE 2.
Relative changes in leg blood flow (left) and vascular conductance (right) in response to sympatho-excitation from 5-sec neck pressure (NP). YOH, yohimbine.
§ Significantly different than control, P<0.05.
The functional significance of alpha adrenergic subtypes in terms of cardiovascular control has been explored in animals and humans. A heterogeneous distribution of alpha receptors in the rat vasculature has been described, with the hypothesis that alpha-1 receptors are more involved in the maintenance of arterial blood pressure, while alpha-2 receptors are ideally situated to govern tissue perfusion (Anderson & Faber, 1991; McGillivray-Anderson & Faber, 1991). We have recently extended this work to humans, with findings that support a more distal location of alpha-2 adrenergic receptors and a widespread distribution of alpha-1 receptors, both proximal and distal in the leg vascular tree (Wray et al., 2004b). The current findings focus on only the distal, sympathetically-innervated adrenergic receptors, identifying a significant role of alpha-2 receptors in the transduction of CBR-induced sympathetic activity.
Prior studies in microvascular preparations have identified the vulnerability of alpha-2 adrenergic receptors to metabolic inhibition, an event that is also evident during acute exercise in animals and humans (Thomas & Segal, 2004). Thus, the current finding that alpha-2 adrenergic receptors contribute significantly to CBR-mediated vasoconstriction at rest may be of further interest during exercise, when alpha-2 receptor inhibition may result in a decline of CBR regulation in the exercising limb. However, further studies utilizing an acute exercise paradigm are required to fully address this issue.
Earlier work utilizing enteral (Farrow et al., 1990) and intra-arterial (Jie et al., 1985) YOH administration failed to identify drug-related differences in vasoconstriction during sympatho-excitation by way of lower body negative pressure, suggesting that cardiopulmonary baroreflex-mediated vasoconstriction is minimally affected by alpha-2 adrenergic blockade. Using a purely pharmacologic approach, Jie et al also demonstrated that alpha-2 antagonism reduces the vasoconstrictor effects of intra-arterial tyramine by 10-20% (1985). The current findings extend these previous studies through application of NP to evoke endogenous NE release through CBR-mediated sympathetic activation, thus representing a purely physiologic perturbation. This approach has provided characterization of post-junctional alpha-2 receptor responses induced exclusively by the CBR, thereby serving to further define the mechanisms by which this autonomic pathway regulates skeletal muscle blood flow.
All studies utilizing pharmacologic probes of receptor function must consider the potential interaction of the administered drug with non-targeted receptors. In the case of YOH, we acknowledge the risk of interaction with pre-junctional alpha-2 adrenergic receptors. However, evidence from sympathectomized animals suggest that this receptor population contributes minimally to alpha-2-mediated vasoconstriction (Buckwalter et al., 2001). In addition, recent work in humans has identified a role for beta-adrenergic receptors in CBR-mediated vasoconstriction (Pellinger & Halliwill, 2007). This receptor population was not antagonized in the present experimental paradigm, and thus it is unknown whether beta adrenergic receptor activation during NP contributed to the observed responses.
Acknowledgments
This study was supported in part grants from the Danish National Research Foundation (504-14), the National Institute of Health (HL045547) and NASA (NAG 9-1262). Dr. Sander's work was supported by grants and fellowships from the Danish Heart Foundation, the Danish Medical Research Council, the Novo Foundation, the Michaelsen Foundation, and the Kaj Hansen Foundation. Dr. Wray is currently supported by grants from the Tobacco-Related Disease Research Program (TRDRP 15RT-0100) and the Francis Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- Bristow JD, Gribbin B, Honour AJ, Pickering TG, Sleight P. Diminished baroreflex sensitivity in high blood pressure and ageing man. J Physiol. 1969;202:45P–46P. [PubMed] [Google Scholar]
- Brothers RM, Haslund ML, Wray DW, Raven PB, Sander M. Exercise-induced inhibition of angiotensin ii vasoconstriction in human thigh muscle. J Physiol. 2006;577:727–737. doi: 10.1113/jphysiol.2006.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates {alpha}-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol. 2003;88:671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- Farrow S, Mers A, Banta G, Steigerwalt S, Lockette W. Effect of the alpha 2-adrenergic antagonist yohimbine on orthostatic tolerance. Hypertension. 1990;15:877–880. doi: 10.1161/01.hyp.15.6.877. [DOI] [PubMed] [Google Scholar]
- Jie K, van Brummelen P, Vermey P, Timmermans PB, van Zwieten PA. Differences between exogenous and endogenous noradrenaline in the effects on vascular post-synaptic alpha 1- and alpha 2-adrenoceptors in man. J Hypertens Suppl. 1985;3:S145–147. [PubMed] [Google Scholar]
- Keller DM, Wasmund WL, Wray DW, Ogoh S, Fadel PJ, Smith ML, Raven PB. Carotid baroreflex control of leg vascular conductance at rest and during exercise. J Appl Physiol. 2003;94:542–548. doi: 10.1152/japplphysiol.00817.2002. [DOI] [PubMed] [Google Scholar]
- McGillivray-Anderson KM, Faber JE. Effect of reduced blood flow on alpha 1-and alpha 2-adrenoceptor constriction of rat skeletal muscle microvessels. Circ Res. 1991;69:165–173. doi: 10.1161/01.res.69.1.165. [DOI] [PubMed] [Google Scholar]
- Patakas D, Louridas G, Kakavelas E. Reduced baroreceptor sensitivity in patients with chronic obstructive pulmonary disease. Thorax. 1982;37:292–295. doi: 10.1136/thx.37.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinger TK, Halliwill JR. Effect of propranolol on sympathetically mediated leg vasoconstriction in humans. J Physiol. 2007;583:797–809. doi: 10.1113/jphysiol.2007.137422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol. 1993;265:H1928–1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Keller DM, Ogoh S, Sander M, Raven PB, Smith ML. Dynamic carotid baroreflex control of the peripheral circulation during exercise in humans. J Physiol. 2004a;559:675–684. doi: 10.1113/jphysiol.2004.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of alpha-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol. 2004b;555:545–563. doi: 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]