Abstract
It is widely conjectured that muscle shortens because portions of myosin molecules (the “cross-bridges”) impel the actin filament to which they transiently attach and that the impulses result from rotation of the cross-bridges. Crystallography indicates that a cross-bridge is articulated–consisting of a globular catalytic/actin-binding domain and a long lever arm that may rotate. Conveniently, a rhodamine probe with detectable attitude can be attached between the globular domain and the lever arm, enabling the observer to tell whether the anchoring region rotates. Well-established signature effects observed in shortening are tension changes resulting from the sudden release or quick stretch of active muscle fibers. In this investigation we found that closely correlated with such tension changes are changes in the attitude of the rhodamine probes. This correlation strongly supports the conjecture about how shortening is achieved.
Keywords: muscle contraction, energy transduction, myosin conformation, highly reactive thiol, rhodamine
The molecular mechanism of muscle contraction involves the cyclical interaction of the myosin cross-bridge and the actin filament while myosin hydrolyzes ATP. Elucidation of this mechanism is sought at two levels, one local to the cross-bridge—explaining how myosin transduces the free energy in ATP into the potential to do work (1)—and the other is at larger scale—explaining how the force to move myosin and actin filaments is generated (2–4). Experimentally, we need to detect and correlate local and global structural changes accompanying energy transduction and force generation in actomyosin. The spectroscopic probe techniques can give the required comprehensive “two view” description of the muscle contraction mechanism.
An extrinsic fluorescent probe linked specifically to a side chain on the myosin cross-bridge or subfragment 1 (S1) emits a signal that can be detected and interpreted in terms of local and/or global movement of the S1 (5, 6). The dual sensitivity of a probe can accurately reflect the local and global nature of contraction when both capabilities are exploited (7–10). For instance, by using a fluorescent probe, we recently investigated changes in the local conformation of the probe binding cleft of S1, a deep cleft containing the highly reactive cysteine (Cys-707 or highly reactive thiol, SH1) and a nucleotide-sensitive tryptophan 510 (Trp-510) (11), in response to ATP hydrolysis (12).† Alternatively, signals from several spectroscopic probes on SH1 in cross-bridges from fibers in steady-state conditions reported the global orientation of the cross-bridge in various physiological states including isometric contraction (6, 14–16). These global-orientation-detecting signals, when appropriately manipulated, combine to constrain fully a model for the cross-bridge angular trajectory during contraction (7, 8).
Time-resolved fluorescence experiments have similarly reported local and/or global properties of the cross-bridge. Relevant to our work are how the closely related methods of polarized fluorescence correlation spectroscopy (FCS) (17, 18) and polarized fluorescence photobleaching recovery (PFPR) (19, 20) were used to detect transient cross-bridge orientation (21, 22). The polarized FCS from probe-modified cross-bridges in active isometric fibers detected for the first time the rotational movement of active cross-bridges, established their rotational relaxation time in isometric conditions, and confirmed the stochastic nature of their repetitive rotational movements (21). PFPR from similarly labeled cross-bridges in active isometric fibers detected submillisecond cross-bridge relaxation rates and large probe angular displacement (22).
Another time-resolved technique observes the polarized emission from fluorescent-probe-modified cross-bridges during cross-bridge movement synchronized by rapid release/stretch of an actively contracting fiber. This technique detected a correlation between the rotation of probes bound to a myosin light chain, located in the lever arm domain of the cross-bridge, and tension transients accompanying the rapid release/stretch of the probe-modified muscle fiber (23) but detected no like correlation with probes bound elsewhere, namely, to SH1 (24). We report herein a reinvestigation of the latter observation. Our data show that the SH1 region of the cross-bridge undergoes significant rotational movement upon rapid length changes in active fibers that correlate with the fiber tension transients.
METHODS
Chemicals.
The fluorescent labels 5′-iodoacetamido tetramethylrhodamine (5′IATR), 5′-iodoacetamidofluorescein (5′IAF), and 5-[2-(iodoacetyl)aminoethyl]aminonaphthalene-1-sulfonic acid (1,5-IAEDANS) are from Molecular Probes. ADP, ATP, dithiothreitol, phenylmethylsulfonyl fluoride, and actin are from Sigma. All chemicals are analytical grade.
Solutions.
Rigor solution contains 80 mM KCl, 5 mM MgCl2, 2 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, and 5 mM potassium phosphate buffered at pH 7.0. Relaxing solution is rigor solution plus 4 mM ATP. MgADP solution is rigor solution plus 4 mM ADP. Activating solution is relaxing solution with 0.1 mM CaCl2 replacing the EGTA.
Preparation of 5′IAF-Labeled Actin.
Rabbit skeletal actin was specifically modified with 5′IAF at Cys-374 as described (25, 26). The dye/actin monomer molar ratio was 0.4.
Preparation and Modification of Muscle Fibers.
Rabbit psoas muscle fibers were obtained as described (21) and kept in a relaxing solution containing 50% (vol/vol) glycerol at −15°C for up to several weeks. We prepared 5′IATR-labeled glycerinated muscle fibers also as described (27). We reinvestigated the location of 5′IATR within the components of modified fibers by extracting the proteins from modified fiber bundles and separating them by molecular weight by using SDS/PAGE (28). For comparison, two other iodoacetamido-based probes, 5′IAF and 1,5-IAEDANS, were also located in the modified fibers by the same method. Fiber labeling with 5′IAF and 1,5-IAEDANS was done according to Ajtai and Burghardt (29) and Borejdo and Putnam (14), respectively.
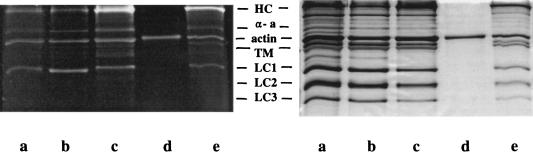
Fig. 1 shows the distribution of the probes among the protein components of the fiber by comparing images of the same gel using the fluorescence from the attached label and from Commassie blue staining. 5′IAF-labeled actin was also run on the gel as a fluorescent molecular weight marker. The distribution of probes in Fig. 1 agrees with earlier work indicating that 77–86% of the total probe intensity is from the myosin heavy chain, 8–14% from actin, 4–7% from myosin light chain 1, and 2–5% from α-actinin (14, 27, 29). Fig. 1 shows no significant labeling of tropomyosin by these probes in contrast to a recent finding (24).
Figure 1.
Comparison of a SDS/PAGE gel containing purified 5′IAF-labeled actin and proteins extracted from modified fibers under UV illumination with detection of the fluorescence emission (Left) and the same gel after Commassie staining and visualization by transmitted visible light (Right). Identified proteins include myosin heavy chain (HC), α-actinin (α-a), tropomyosin (TM), and myosin light chains (LC1, LC2, and LC3). Lanes contain extracts from 5′IAF (lane a), 1,5-IAEDANS (lane b), and 5′IATR (lane c) labeled fibers, 5′IAF-labeled actin (lane d), and the extract from an overlabeled nonspecifically 5′IATR-modified fiber (lane e). Only the nonspecifically labeled fiber, lane e, shows any measurable intensity from tropomyosin. Actin is the second most heavily labeled protein, after the myosin heavy chain, in all of the fiber extracts.
The SH1 labeling specificity was evaluated by comparison of the Ca2+- and K+-EDTA ATPase activities of myosin extracted from labeled fibers (27, 28, 30, 31). All of the 5′IATR on the heavy chain resides on SH1 (28). The myosin K+-EDTA ATPase activity indicates a 5′IATR/S1 molar ratio of 0.32 in agreement with earlier work (28). Fibers labeled to this degree exhibited a peak isometric tension of 180 ± 40 kN/m2 (mean ± SD). Identically handled but unmodified fibers exhibited a peak isometric tension of 172 ± 50 kN/m2. This result suggests no measurable impairment of isometric tension due to the modification of SH1 with 5′IATR.
Optics.
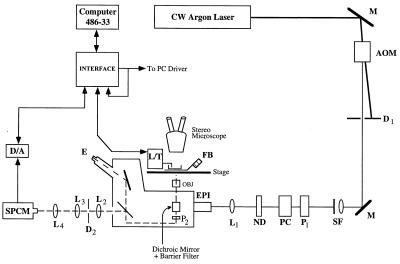
Fig. 2 shows the optical setup used in the experiments. An acousto-optical modulator (AOM) (IntraAction, Bellwood, IL) attenuates the 514.5-nm line from a CW argon ion laser. The first-order diffraction beam from the AOM is selected at the diaphragm (D1) for illuminating the fiber sample. A spatial filter (SF) and a Glan–Thompson polarizer (P1) restores the Gaussian profile and linear polarization of the attenuated beam distorted by the AOM. The Pockels cell (PC; Lasermetrics, Englewood, NJ) alternates the electric field polarization of the attenuated beam between parallel and perpendicular to the fiber axis. The neutral density filter (ND) further attenuates the illumination beam. The lens (L1) at the epiillumination port of the inverted microscope (Zeiss Axiovert 135) focuses the laser beam on the field diaphragm and subsequently on the sample after reflection at the dichroic mirror and transmission through the ×10 0.3 numerical aperature air-immersion objective (OBJ). The objective also collects emitted light. The microscope stage holds the temperature-controlled fiber bath (FB) that has a quartz bottom to allow transmission of the excitation and emitted light. The fiber length/tension controller (L/T; model 303, Cambridge Technology, Watertown, MA) is also located on the microscope stage. A long working distance, variable magnification stereo microscope (Zeiss, SV8) gives a magnified view of the moving and fixed arms of the length/tension controller where the fiber is mounted. Fluorescence collected by the objective at wavelengths of ≥590 nm transmits the dichroic mirror, barrier filter, manually operated emission polarizer (P2), image plane diaphragm (D2), and lenses (L2–L4). Lenses L2 and L3 with diaphragm D2 prevent stray light from interring the detector. Lens L4 focuses the fluorescence signal from the sample onto the active area of an avalanche photodiode detector/discriminator single-photon-counting module, SPCM-200-PQ (EG & G Optoelectronics, Quebec, Canada). The fluorescence emission or transmitted light image of the sample can also be sent to the eyepiece (E) for viewing.
Figure 2.
Time-resolved fluorescence polarization instrument for detecting cross-bridge rotation in muscle fibers undergoing length transients. The vertically polarized laser beam reflects from mirrors (M), attenuates at the acousto-optical modulator (AOM), and transmits a diaphragm (D) spatial filter (SF), polarizer (P), Pockels cell (PC), neutral-density filter (ND), and lens (L) before entering the epiillumination port of an inverted microscope. The microscope stage holds the temperature-controlled fiber bath (FB) and the fiber length/tension controller (L/T). Fluorescence emitted by the fiber sample is collected by the objective (OBJ) and analyzed by a polarizer before detection at the single photon-counting module (SPCM) or viewing at the eyepiece (E). Digital signals from the SPCM are analog converted (D/A) and monitored by the interface.
Data Acquisition.
A 33-MHz “486” personal computer, with two interface cards (Keithly Metrabyte, Taunton, MA) for counter/timer functions (CTM05) and digital-to-analog (D/A) or A/D conversion in real time (DAS1601) and custom-written software, control the combined optical and mechanical experiment. The CTM05 generates, the clock for timing the experiment, a square-tooth voltage defining the laser polarization state (using the Pockels Cell, PC) that alternates with each clock pulse, and a voltage step defining the fiber length (using the length/tension controller). Four A/D input channels, triggered and clocked by the CTM05, simultaneously monitor PC state, fiber length, tension, and fluorescence intensity. The SPCM detector/discriminator produces a logic pulse for each photon detected. These pulses are counted for a sample observation time, and the sum was latched and converted to a voltage by an 8-bit D/A converter (AD558, Analog Devices, Norwood, MA). The counter is then cleared and ready to count pulses for the next sample time. The analog voltage representing fluorescence intensity from the AD558 is monitored by the A/D input on the DAS1601. The SPCM has a 200-ns dead time accounted for by using the relation described by Yuan and Axelrod (32). Counting rates did not exceed 2 × 105 pulses per s. We verified that the system functions correctly for sample observation times of ≥5 μs.
Mechanics.
Single glycerinated muscle fibers at resting length were draped between stainless steel hooks rigidly connected to the moving and fixed arms of the length/tension controller. The hooks were precoated with acetone-diluted Duco cement. Additional diluted Duco cement, applied to the fiber at the outer side of each hook, firmly attached the fiber, without glue on any part of the sample from which mechanical or optical measurements were made. This procedure prevents excessive sarcomere elongation at the fiber ends during force generation. The fiber sample between the hooks was 2 mm long. The focused laser beam, ≈10 μm in diameter, was located within 200 μm of the fixed arm of the length/tension controller to minimize fiber translation inside the illuminated region during the length transient. Fiber length change rates of 0.3% (3 nm per half-sarcomere) per 1.5 ms were used. All experiments were conducted at 4°C.
Fluorescence Polarization.
Change in cross-bridge orientation was detected by using the polarized fluorescence from the cross-bridge-bound probe. Polarized fluorescence was quantified in terms of ratios, Q∥ and Q⊥, defined as,
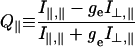 |
and
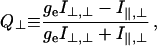 |
1 |
where I is the fluorescence intensity and ge corrects for the polarization-dependent transmission efficiency of excitation intensity through the instrument optics. The subscripts (∥ and ⊥) refer to the direction of light polarization relative to the fiber symmetry axis, the first to excitation and the second to emission. For any probe distribution, the absolute values of Q∥ and Q⊥ cannot exceed one. In the limit of probes orienting with dipoles parallel to the fiber axis Q∥ → 1 and Q⊥ → −1. Probes orienting with dipoles perpendicular to the fiber axis give Q∥ → −1 and Q⊥ → 1. The microscope optical axis is parallel to the direction of propagation of the excitation and emission beams making Q∥ = Q⊥ for a random distribution of probes.
Two sequential emitted-light-intensity samples provide one polarization ratio because the excitation light polarization alternates between ∥ and ⊥. The emission polarizer was rotated manually so Q∥ and Q⊥ were measured separately. No attempt was made to combine I∥,∥ and I∥,⊥ into a third independent polarization ratio because these quantities were measured at quite different times so that their combination could be sensitive to slow systematic changes in fiber order. In all experiments, light intensity was sampled every 500 μs so that the polarization ratios have a time resolution of 1 ms.
The A/D channels sample the excitation light polarization, fiber length, tension, and fluorescence emission for a data collection run. A time window, defined relative to the release/stretch event, designates the time blocks for signal averaging of the data stream in one run. In this case one data collection run consisted of 19 release/stretch cycles of a quick release followed by a quick restretch to the original length. An active fiber was subjected to a set of five runs. Between each run was a pause of 10 s for data handling. Between each set the active fiber was relaxed for 3 min and then reactivated for another set of data collection runs. A single active fiber was subjected to less than or equal to seven sets of data collection runs. Measurements on fibers in relaxation and rigor and in the presence of MgADP were conducted similarly except that there was no delay between sets. We detected no significant change in isometric tension or steady-state fiber polarization ratios during the course of these experiments.
Steady-state fluorescence polarization from the labeled fibers was also measured by using the time-resolving apparatus but with zero amplitude length jumps. The data from a single run was time-averaged. In addition to the quantities in Eq. 1, we observed,
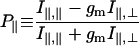 |
and
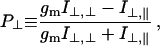 |
2 |
in the steady-state. In this equation, gm corrects for the polarization-dependent transmission efficiency of emission intensity through the instrument optics. Limiting cases of probes orienting parallel or perpendicular to the fiber axis, or randomly oriented, produce polarization ratios P∥ and P⊥ identical to Q∥ and Q⊥, respectively. Only three of the quantities P∥, P⊥, Q∥, and Q⊥ are independent, but all are listed in our results to facilitate comparison with steady-state values reported earlier and with time-resolved values reported herein.
RESULTS
Steady-State Fluorescence Polarization from Muscle Fibers.
Table 1 shows the steady-state fluorescence polarization ratios from Eqs. 1 and 2 for 5′IATR-labeled muscle fibers in rigor, in relaxation, in the presence of MgADP, and in isometric contraction. The values agree with those reported previously (8).
Table 1.
Steady-state fluorescence polarization ratios from 5′IATR-labeled fibers
| Ratio
|
||||
|---|---|---|---|---|
| Rigor | MgADP | Relax | Active | |
| P∥ | 0.28 | 0.60 | 0.42 | 0.48 |
| P⊥ | 0.52 | 0.00 | 0.29 | 0.20 |
| Q∥ | 0.27 | 0.50 | 0.37 | 0.40 |
| Q⊥ | 0.52 | 0.14 | 0.34 | 0.28 |
Effect of Release/Stretch Transients on Active Muscle Fibers.
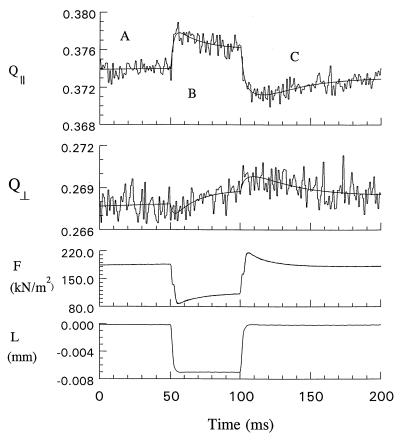
Fig. 3 shows the time-dependent polarization ratios Q∥(t) and Q⊥(t), tension per unit fiber cross-sectional area F(t), and length L(t) from contracting 5′IATR-modified fibers. The polarization ratio, Q∥(t), changes in response to changes in L(t), in a manner closely correlated with F(t). These changes in Q∥(t) went undetected by other workers (24), so it is necessary to emphasize that the changes reported herein are statistically significant. The experimental trace of Q∥(t) represents measurements on 50 different single fibers. Because the standard deviation of points in the trace is equal to or less than the differences between the trace and the smooth curve that represents it,‡ we can say that in response to the experimentally imposed L(t), the smooth Q∥(t) changes by about 3 SDs. If the usual Gaussian assumption is made, the probability of such a large change occurring by chance would be about 1 in 1000. Also persuasive that we observe real events is the high correlation (ρ = −0.87) between Q∥(t) and F(t). [The correlation is much less (ρ = +0.21), but not zero, between Q⊥(t) and F(t)].
Figure 3.
Fluorescence polarization ratios Q∥ and Q⊥, fiber force per cross-sectional area F, and fiber length L, as functions of time. Active fibers were subjected to a 0.3% quick release followed by a quick stretch back to the original length while monitoring the polarization ratio and F. The smooth solid lines in the plots of Q∥, Q⊥, and F are the best fits to the data with piecewise continuous functions on the domains A, B, and C. The fitted curves are sums of exponentials given in Eqs. 3 and 4 with parameters given in Tables 2 and 3. The fitted curve is indistinguishable from the data in the plot of F.
In our protocol, Q∥(t) and F(t) from a single release/stretch cycle do not relax fully to their steady-state values before a new cycle begins. Consequently, the average of these nonstationary signals drifts as shown most clearly in the prerelease time domain where both Q∥(t) and F(t) rise slightly toward their steady-state values. This drift causes the apparent steady-state value of Q∥(t) from Fig. 3 to be lower than that observed from unperturbed active isometric fibers in the steady state (see Table 1).
Fig. 3 also shows the time-dependent polarization ratio Q⊥(t) from contracting 5′IATR-modified fibers. The average of results from 20 active single fibers formed this trace. The standard deviation of points in Q⊥(t) is about equal to the deviations of the data from the smooth fitted lines that are also shown. Unlike Q∥(t), the changes in Q⊥(t) from the length transients are not statistically significant; however, the curve indicates trends consistent with the results for Q∥(t). As in Q∥(t), the averaging of nonstationary signals causes the apparent steady-state value of Q⊥(t) from Fig. 3 to be lower than that observed from unperturbed active isometric fibers in the steady state (see Table 1).
The ≈1.5-ms step response time of the length/tension controller implies that relaxation times τi and φi of ≈1 ms in Tables 2 and 3 are likely instrument (not sample)-limited. In time domain A, Q∥(t), Q⊥(t), and F(t) are rising slowly with a relaxation time of ≈384 ms. This time constant is similar to that observed from cycling isometric cross-bridges (21) and, as mentioned above, appears because we averaged slightly nonstationary signals. In time domain B, the 3 nm per half-sarcomere quick release of an active fiber produced an instantaneous increase in Q∥ and decrease in Q⊥ and F. The magnitude of change in Q∥ and Q⊥ is ≈0.005 and ≈0.001, respectively. After release Q∥, Q⊥, and F relax toward their steady-state values with an apparent relaxation time of ≈17 ms. In time domain C, rapidly restretching the active fiber produces a decrease (increase) in Q∥ (Q⊥) that contains both an instantaneous and a finite relaxation. The stretch produced an instantaneous decrease (increase) in Q∥ (Q⊥) of ≈0.004 (≈0.001) followed by another decrease (increase) of ≈0.006 (≈0.002) with a ≈10-ms relaxation time and then an increase (decrease) of ≈0.006 (≈0.003) with a ≈23-ms relaxation time. The total downward (upward) displacement of Q∥ (Q⊥) after rapidly restretching the active fiber is ≈0.009 (≈0.003), somewhat larger than that after a quick release. In time domain C, rapidly restretching the active fiber produces an instantaneous increase in F and then a decrease that relaxes toward its steady-state value with a relaxation time between 7 and 17 ms. The fit of F in time domain C was significantly improved by using three exponentials terms in Eq. 4; however, the fit appeared qualitatively very similar if the two terms containing the 7- and 17-ms relaxations were replaced by one with an intermediate relaxation time.
Table 2.
Amplitudes and relaxation times for fluorescence polarization ratios in time domains A, B, and C
| Parameter | Domain A | Domain B | Domain C |
|---|---|---|---|
| Δtq | t | t − 50.5 | t − 101.0 |
| Q∥,⊥(Δtq = 0) | 0.3739, 0.2676 | 0.3740, 0.2678 | 0.3763, 0.2688 |
| p∥,⊥1 | 0.0010, 0.0014 | 0.0049, −0.0014 | −0.0036, 0.0008 |
| p∥,⊥2 | 0, 0 | −0.0028, 0.0023 | −0.0055, 0.0019 |
| p∥,⊥3 | 0, 0 | 0, 0 | 0.0057, −0.0030 |
| τ1 | 384 | 1 | 1 |
| τ2 | — | 17 | 10 |
| τ3 | — | — | 23 |
Table 3.
Amplitudes and relaxation times for tension/area in time domains A, B, and C
| Parameter | Domain A | Domain B | Domain C |
|---|---|---|---|
| Δtf | t | t − 53 | t − 103 |
| F (Δtf = 0) | 184 | 131 | 169 |
| f1 | 22 | −50 | 62 |
| f2 | 0 | 34 | −18 |
| f3 | 0 | 0 | −31 |
| φ1 | 384 | 1 | 1 |
| φ2 | — | 17 | 7 |
| φ3 | — | — | 17 |
Effect of Release/Stretch Transients on Fibers in Rigor, in MgADP, or in Relaxation.
We performed length-jump experiments identical to those on active fibers (above) on passive labeled muscle fibers in rigor, in the presence of MgADP, and in relaxation. As observed previously in the steady state (31, 33–35), the cross-bridge orientation did not appear to change upon releasing or when restretching the fiber in these states. We observed Q∥ from fibers in rigor or in the presence of MgADP occasionally changing slightly (up or down) with a release and then returning to the prerelease value upon restretching. Experiments from several fibers showed that when the infrequent changes in Q∥ occurred upon release, an increase or decrease in Q∥ was equally likely. In all cases, we could trace the change in Q∥ upon release to probe rotation when the stiff fiber in rigor or in the presence of MgADP bends. This artifact in Q∥ could then be removed by prestretching the fiber before resuming the length transient experiments.
DISCUSSION
Active fiber tension recovery after a quick release is widely taken as a signature phenomenon of active muscle. It has been characterized as having four phases (3, 36). Phases 1 and 2 relate to elastic and active elements of the cross-bridge response that take up the slack in an active fiber quickly without cross-bridge detachment from actin. Phases 3 and 4 are slower and involve cross-bridge detachment from actin. The active element of the cross-bridge response to a quick release has a relaxation time of ≈1 ms and probably corresponds to rotation of the S1 while attached to actin. We detect this rotation in time domain B of Fig. 3 as an instantaneous change in polarization. In this case, “instantaneous” is defined by as fast as or faster than we can release an active fiber. Phase 3 of the tension recovery is an order of magnitude slower; we think that we detect it as a polarization change occurring with an ≈17-ms relaxation (see Eq. 3 and Table 2). In this phase of tension recovery, the lower tension-generating cross-bridges are detaching from actin and are being replaced by higher tension-generating cross-bridges. The polarization changes because cross-bridges that are unbinding and the cross-bridges that are binding to actin have different orientation distributions. Our data suggests that the cross-bridges attaching are more orientationally disordered than those detaching.
Like active tension recovery after a quick release, active tension recovery after a quick stretch is characterized as having four phases of recovery; however, the time constants associated with these phases are different from those after a release (3, 36). In phase 2 and phase 3, the recoveries after a stretch are 5–10 times slower than those observed after a release (37). The phase 2 response to a quick stretch has a relaxation time of the order of 10 ms. We detect this phase of cross-bridge response in time domain C of Fig. 3 as both an instantaneous and a slower (≈10 ms) relaxation (see Eq. 3 and Table 2). Recent mechanical measurements suggest that the slower phase 2 recovery after a stretch does not reflect the reversal of active cross-bridge rotation in a release but comes from cross-bridge detachment and reattachment on this time scale (37). Our polarization data are consistent with this interpretation if we attribute the reversal of the active cross-bridge rotation to the instantaneous change in the polarization and the slower polarization relaxation to cross-bridge detachment and reattachment. Again, our data suggest that the cross-bridges entering attachment are more orientationally disordered than those leaving attachment.
It is not always appreciated that structural changes inferred from observations of attached probes are determined not just by the extent of the structural change but on how propitiously the probe is positioned to report the change. The size of the change in Q∥ after quick release/stretch length perturbations is small when compared with polarization changes from 5′IATR at SH1 in response to a physiological state change, for instance when MgADP binds to the rigor cross-bridge (16, 38). In the present case, we expected that the polarization changes would be small because of the orientation of the 5′IATR probe on S1. We had observed and correlated the signals from several different spectroscopic probes attached to SH1 in cross-bridges, including the 5′IATR probe, using a model-independent and multiprobe analysis method (7, 8). The measurements were made on fibers in steady-state conditions. We found the orientation of each probe relative to the principal hydrodynamic axis of S1 and used this information to deduce the rotational trajectory of the S1 during contraction. Designating a polar or torsional rotation as one in which the long or short dimension of the S1 rotates, we found that force generation involves predominantly polar rotations of S1 with amplitudes of 35–45°. State transitions in the cross-bridge cycle related to the ATPase activity, such as binding or releasing of nucleotides involve predominantly torsional rotations with amplitudes spanning 5–10°. The particular orientation that 5′IATR has on S1 makes the probe very sensitive to torsional rotation of S1 and significantly less sensitive to the polar rotation of S1 accompanying force generation. The release/stretch length perturbation of active fibers should affect the polar degree of freedom of S1 associated with force production and minimally alter 5′IATR orientation.
We have reported above [as have others before us (6, 21, 39)] that the peak isometric tension developed by a modified fiber is the same as that developed by a native fiber. Additional similarities between the two types of fiber are seen in Fig. 3. For example, after sudden changes in L(t) the Q∥(t) from labeled fibers relaxes toward a steady-state just as does F(t), and the F(t) values of labeled and native fibers are the same. All these similarities suggest that at least in organized fibers, the various processes of contraction are not seriously impaired by the chemical modification entailed in labeling. Various, sometimes opposing (40), conclusions have been reached from experiments with reconstituted systems (41). In attempting to extend observations on reconstituted fibers to organized fibers, it may be thought that the apparent unperturbability of organized fibers arises because labeled cross-bridges, although fatally compromised, are passively moved in synchrony with active neighboring cross-bridges. In addition to colliding with the maximum tension similarity of labeled and native fibers, this argument is countered by certain fluctuation experiments (21). In these the polarized fluorescence fluctuations from probes modifying SH1s in steady-state isometric contraction detect stochastic cross-bridge rotations at frequencies similar to the corresponding tension fluctuations (42). The theory that active cross-bridges move passive ones under these circumstances seems difficult to reconcile with stochastic behavior, and also one has to wonder why these rare events so often happen next to a labeled cross-bridge in a sparsely labeled fiber.
Thus, the foregoing experiments, conservatively interpreted, establish the main point of this work; by using a widely accepted criterion for contraction-related activity (characteristic responses of active muscle to sudden length changes), we find that such activity is manifest in fluorescence polarization signals from probes attached to Cys-707 and that the pattern of these signals is that expected to result from rotational movement.
It is of interest to consider briefly the implications of our findings for the many molecular models proposed to account for contraction. Although all models have postulated ATPase-linked conformational changes in actomyosin (1), the “first-generation” models envisioned thrust motion to be a global “rigid-body” rotation of an actin-attached S1 moiety of myosin (2–4). Early experimental evidence (15, 43–45) seemed consistent with this simple view. However, more refined evidence (46–49) and new high-resolution structures of S1 (50–52) have stimulated a second generation of models whose central feature is bending within the myosin molecule (not changes in the actomyosin interface). Just what region of myosin bends is controversial. A suggestion that thrust is accompanied by the closing (50, 53) of the substrate (ATP) cleft was withdrawn after it was found that the opening–closing amplitude was very small (51, 54, 55). Reasoning from experiments distinct from the present ones, we proposed (12) that thrust (bending) is associated with the closing of a different cleft, one including Cys-707. The present experiments reinforce that suggestion, since they show that contraction-related events are reported by a probe bound to this cysteine. Others (23) have reported that probes at a site considerably tailward from Cys-707 (at Cys-108 of the regulatory light chain) respond to sudden length changes when the fiber as a whole is in the rigor state. If Cys-707 and Cys-108 were connected by rigid structures, one would expect that the region around Cys-707 would similarly respond, but, as we have reported herein, it does not. An interesting explanation might be that there is an elastic element between the two sites. [Such an element, possibly in the cross-bridge, has always been invoked to explain the phase 1 recovery of tension after imposing a sudden length change on an active fiber (3)].
In summary, our data indicates that 5′IATR specifically bound to SH1 in S1 rotates during active tension relaxation in response to a rapid change in fiber length. The fluorescence polarization of the probe and the fiber tension follow similar time courses, indicating that the two are correlated and suggesting that the cross-bridge rotates to cause muscle shortening. That the SH1-bound probe participates in cross-bridge rotation to produce force defines a minimal volume of S1 (containing the light chain binding region and the probe binding cleft) that actively rotates during contraction. The difference in the responses of these two regions of S1 to length changes of fibers in rigor suggests that an elastic element, possibly the one giving a phase 1 response in tension relaxation experiments after length transients in active fibers, resides between SH1 and the light chain binding region.
Acknowledgments
We gratefully acknowledge Prof. M. F. Morales for helpful suggestions and for critically reading the manuscript, Dr. E. H. Hellen and Mr. J. E. Lyke for their contributions to the development of the time-resolved fluorescence polarization instrument, Mr. G. C. Harrer for fabricating several of the metal parts for the instrument, and Prof. J. W. Shriver for lending us the length/tension controller. This work was supported by grants from the National Institutes of Health (R01AR39288), the American Heart Association (930 06610), and the Mayo Foundation.
ABBREVIATIONS
- S1
myosin subfragment 1
- SH1
highly reactive thiol
- 5′IATR
5′-iodoacetamidotetramethylrhodamine
- 5′IAF
5′-iodoacetamidofluorescein
- 1
5-AEDANS, 5-[2-(iodoacetyl)aminoethyl]aminonaphthalene-1-sulfonic acid
Footnotes
The sequence numeration of Maita et al. (13) will be used throughout this paper.
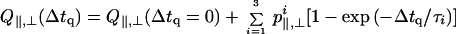 |
3 |
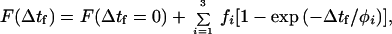 |
4 |
References
- 1.Morales M F, Botts J. Proc Natl Acad Sci USA. 1979;76:3857–3859. doi: 10.1073/pnas.76.8.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley H E. Science. 1969;164:1356–1366. [PubMed] [Google Scholar]
- 3.Huxley A F, Simmons R M. Nature (London) 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- 4.Lymn R W, Taylor E W. Biochemistry. 1971;10:4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- 5.Botts J, Takashi R, Torgerson P, Hozumi T, Muhlrad A, Mornet D, Morales M F. Proc Natl Acad Sci USA. 1984;81:2060–2064. doi: 10.1073/pnas.81.7.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nihei T, Mendelson R A, Botts J. Biophys J. 1974;14:236–242. doi: 10.1016/S0006-3495(74)85911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burghardt T P, Ajtai K. Biochemistry. 1994;33:5376–5381. doi: 10.1021/bi00184a004. [DOI] [PubMed] [Google Scholar]
- 8.Ajtai K, Toft D J, Burghardt T P. Biochemistry. 1994;33:5382–5391. doi: 10.1021/bi00184a005. [DOI] [PubMed] [Google Scholar]
- 9.Burghardt T P, Ajtai K. Biophys Chem. 1996;60:119–133. doi: 10.1016/0301-4622(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 10.Ajtai K, Burghardt T P. Biochemistry. 1995;34:15943–15952. doi: 10.1021/bi00049a009. [DOI] [PubMed] [Google Scholar]
- 11.Rayment I, Holden H M. Trends Biochem Sci. 1994;19:129–134. doi: 10.1016/0968-0004(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Ajtai K, Burghardt T P. Biochim Biophys Acta. 1996;1296:1–4. doi: 10.1016/0167-4838(96)00086-6. [DOI] [PubMed] [Google Scholar]
- 13.Maita T, Yajima E, Nagata S, Miyanishi T, Nakayama S, Matsuda G. J Biochem. 1991;110:75–87. doi: 10.1093/oxfordjournals.jbchem.a123546. [DOI] [PubMed] [Google Scholar]
- 14.Borejdo J, Putnam S. Biochim Biophys Acta. 1977;459:578–595. doi: 10.1016/0005-2728(77)90056-1. [DOI] [PubMed] [Google Scholar]
- 15.Nihei T, Mendelson R A, Botts J. Proc Natl Acad Sci USA. 1974;71:274–277. doi: 10.1073/pnas.71.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borejdo J, Assulin O, Ando T, Putnam S. J Mol Biol. 1982;158:391–414. doi: 10.1016/0022-2836(82)90205-4. [DOI] [PubMed] [Google Scholar]
- 17.Koppel D E, Axelrod D, Schlessinger J, Elson E L, Webb W W. Biophys J. 1976;16:1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrenberg M, Rigler R. Chem Phys. 1974;4:390–401. [Google Scholar]
- 19.Axelrod D, Koppel D E, Schlessinger J, Elson B, Webb W W. Biophys J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith L M, Weis R M, McConnell H M. Biophys J. 1981;36:73–91. doi: 10.1016/S0006-3495(81)84717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borejdo J, Putnam S, Morales M F. Proc Natl Acad Sci USA. 1979;76:6346–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellen E H, Ajtai K, Burghardt T P. J Fluoresc. 1995;5:355–367. doi: 10.1007/BF01152562. [DOI] [PubMed] [Google Scholar]
- 23.Irving M, Allen T S C, Sabido-David C, Cralk J S, Brandmeler B, Kendrick-Jones J, Corrie J E T, Trentham D R, Goldman Y E. Nature (London) 1995;375:688–690. doi: 10.1038/375688a0. [DOI] [PubMed] [Google Scholar]
- 24.Berger C L, Craik J S, Trentham D R, Corrie J E T, Goldman Y E. Biophys J. 1996;71:3330–3343. doi: 10.1016/S0006-3495(96)79526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawyer W H, Woodhouse A G, Czarnecki J J, Blatt E. Biochemistry. 1988;27:7733–7740. doi: 10.1021/bi00420a023. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y-L, Taylor D L. J Histochem Cytochem. 1980;28:1198–1206. doi: 10.1177/28.11.6107318. [DOI] [PubMed] [Google Scholar]
- 27.Ajtai K, Ilich P J K, Ringler A, Sedarous S S, Toft D J, Burghardt T P. Biochemistry. 1992;31:12431–12440. doi: 10.1021/bi00164a019. [DOI] [PubMed] [Google Scholar]
- 28.Ajtai K, Burghardt T P. Biochemistry. 1989;28:2204–2210. doi: 10.1021/bi00431a035. [DOI] [PubMed] [Google Scholar]
- 29.Ajtai K, Burghardt T P. Biochemistry. 1992;31:4275–4288. doi: 10.1021/bi00132a018. [DOI] [PubMed] [Google Scholar]
- 30.Ajtai K, Poto L, Burghardt T P. Biochemistry. 1990;29:7733–7741. doi: 10.1021/bi00485a023. [DOI] [PubMed] [Google Scholar]
- 31.Burghardt T P, Ajtai K. Proc Natl Acad Sci USA. 1989;86:5366–5370. doi: 10.1073/pnas.86.14.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan Y, Axelrod D. Biophys J. 1995;69:690–700. doi: 10.1016/S0006-3495(95)79944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.dos Remedios C G, Millikan R G C, Morales M F. J Gen Physiol. 1972;59:103–120. doi: 10.1085/jgp.59.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowder M S, Cooke R. Biophys J. 1987;51:323–333. doi: 10.1016/S0006-3495(87)83338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naylor G R S, Podolsky R J. Proc Natl Acad Sci USA. 1981;78:5559–5563. doi: 10.1073/pnas.78.9.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford L E, Huxley A F, Simmons R M. J Physiol (London) 1977;269:441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V. J Physiol (London) 1997;498.1:3–15. doi: 10.1113/jphysiol.1997.sp021837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajtai K, Burghardt T P. Biochemistry. 1986;25:6203–6207. doi: 10.1021/bi00368a055. [DOI] [PubMed] [Google Scholar]
- 39.Crowder M S, Cooke R. J Muscle Res Cell Motil. 1984;5:131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- 40.Root D D, Reisler E. Biophys J. 1992;63:730–740. doi: 10.1016/S0006-3495(92)81646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobkov A A, Bobkova E A, Homsher E, Reisler E. Biophys J. 1997;72:A220. [Google Scholar]
- 42.Borejdo J. Biophys J. 1980;29:49–64. doi: 10.1016/S0006-3495(80)85117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendelson R, Putnam S, Morales M. J Supramol Struct. 1975;3:162–168. doi: 10.1002/jss.400030209. [DOI] [PubMed] [Google Scholar]
- 44.Stone D B, Mendelson R A. Biophys J. 1986;49:444a. [Google Scholar]
- 45.Curmi P M G, Stone D B, Schneider D K, Spudich J A, Mendelson R A. J Mol Biol. 1988;203:781–798. doi: 10.1016/0022-2836(88)90209-4. [DOI] [PubMed] [Google Scholar]
- 46.Highsmith S, Eden D. Biochemistry. 1993;32:2455–2458. doi: 10.1021/bi00061a001. [DOI] [PubMed] [Google Scholar]
- 47.Wakabayashi K, Tokunaga M, Kohno I, Sugimoto Y, Hamanaka T, Takezawa Y, Wakabayashi T, Amemiya Y. Science. 1992;258:443–447. doi: 10.1126/science.1411537. [DOI] [PubMed] [Google Scholar]
- 48.Cheung H C, Gryczynski I, Malak H, Wiczk W, Johnson M L, Lakowicz J R. Biophys Chem. 1991;40:1–17. doi: 10.1016/0301-4622(91)85025-l. [DOI] [PubMed] [Google Scholar]
- 49.Mendelson R A, Schneider D K, Stone D B. J Mol Biol. 1996;256:1–7. doi: 10.1006/jmbi.1996.0063. [DOI] [PubMed] [Google Scholar]
- 50.Rayment I, Rypniewski W R, Schmidt-Base K, Smith R, Tomchick D R, Benning M M, Winkelmann D A, Wesenberg G, Holden H M. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 51.Fisher A J, Smith C A, Thoden J B, Smith R, Sutoh K, Holden H M, Rayment I. Biochemistry. 1995;34:8960–8972. doi: 10.1021/bi00028a004. [DOI] [PubMed] [Google Scholar]
- 52.Smith C A, Rayment I. Biochemistry. 1996;35:5404–5417. doi: 10.1021/bi952633+. [DOI] [PubMed] [Google Scholar]
- 53.Rayment I, Holden H M, Whittaker M, Yohn C B, Lorenz M, Holmes K C, Milligan R A. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 54.Luo Y, Wang D, Cremo C R, Pate E, Cooke R, Yount R G. Biochemistry. 1995;34:1978–1987. doi: 10.1021/bi00006a019. [DOI] [PubMed] [Google Scholar]
- 55.Franks-Skiba K, Hwang T, Cooke R. Biochemistry. 1994;33:12720–12728. doi: 10.1021/bi00208a025. [DOI] [PubMed] [Google Scholar]