Abstract
BUCCI, D.J., M.E. HOPKINS, A.A. NUNEZ, S.M. BREEDLOVE, C.L. SISK, J.T. NIGG. Effects of sex hormones on associative learning in the spontaneously hypertensive rat.
Pavlovian conditioning of a visual stimulus paired with food was examined in spontaneously hypertensive rats (SHR), which are a commonly used model for Attention-Deficit/Hyperactivity Disorder (ADHD), and in Wistar rats (normoactive control). In gonadally intact rats of both strains, males spent more time in the food cup following onset of the light than did females, indicating a stronger association of the conditioned stimulus (CS) with reward. Gonadectomy carried out in adulthood affected conditioning differently in the two strains. In Wistar rats, gonadectomy had no effect on conditioned responding in females, but reduced conditioned responding in males, effectively eliminating the sex difference in behavior. This result suggests that circulating androgens in male Wistar rats normally aid conditioning in this task. In contrast, gonadectomy enhanced conditioning in both sexes in the SHR rats, indicating that androgens and/or estrogens impair conditioned associations in this strain. These data indicate that gonadal steroids can influence conditioning in rats and that the valence of steroid action on this behavior is strain-dependent. To the extent that SHR serves as a model of ADHD in humans, the influence of steroids on associative learning may play a role in the expression of ADHD-like behaviors.
Keywords: Attention-Deficit/Hyperactivity Disorder, gonadectomy, sex difference, gonadal steroids, SHR
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is among the most common childhood psychological disorders, affecting approximately 3–5% of children and persisting into adolescence and adulthood for an estimated 60–80% of people who are affected [1]. It is now well established that ADHD is more common in boys than girls, with sex ratio estimates ranging from 2:1 to 5:1 [2,3,4] across clinic and population samples. Nonetheless, given the high prevalence rates for ADHD, a significant number of girls have the disorder, although historically they have been under-researched and under-identified. Moreover, there is evidence that in adulthood the prevalence rate in females and males is equivalent [5].
Recent studies suggest that the neural and cognitive mechanisms involved in ADHD may differ by sex, in parallel to sex differences in the behavioral and cognitive manifestation of the disorder. Only a few studies have compared boys and girls with ADHD on cognitive or neural measures, but at least some of these studies suggest that girls exhibit more extreme cognitive symptoms when matched to boys on symptom severity [6,7,8]. Twin studies suggest potential sex differences in genetic influences on ADHD [9,10]. These types of findings underscore the need to answer longstanding calls in the field for examination of sex hormone effects on ADHD, as articulated in an NIH consensus statement over a decade ago [11]. These sex differences suggest that ADHD could reflect permanent effects of perinatal gonadal hormonal exposures (e.g., androgens) and/or ongoing effects of reproductive or adrenal stress axis hormones. Although hormones are a likely route to sex differences, causal effects on behavior and cognition are difficult to study without animal models.
The present study thus examined the influence of sex hormones on behavior in Spontaneously Hypertensive rats (SHR), a commonly used animal model of ADHD [12]. Although no animal model of ADHD is fully satisfactory, SHR rats exhibit deficits in sustained attention, increased motor activity, shortened delay-of-reinforcement gradients, and altered learning and extinction reminiscent of impairments associated with ADHD [13,14,15,16,17]. Hypofunctional dopaminergic and noradrenergic systems are present in SHR rats and have been argued to be similar to the neurochemical abnormalities observed in groups of children with ADHD [12]. Yet to date, only a few studies have examined sex differences in SHR rats (or other animal models of ADHD), and although these are not conclusive, they raise the possibility of sex differences in the strain with regard to learning and behavior [18]. Moreover, only two studies have investigated the effects of perinatal hormone manipulations on behavior in SHR rats, and no studies have examined the influence of sex hormones on behavior in adulthood in this strain or any other animal model of ADHD.
Male and female SHR rats were trained in a Pavlovian conditioning task in which a visual stimulus was paired with food reward. The ability of SHR rats to learn a simple stimulus-reward association was compared to male and female Wistar rats (a common, normo-active strain). The activational role of sex hormones in mediating behavioral differences was examined by including additional groups of male and female SHR and Wistar rats that had undergone gonadectomy as adults. After the conditioning procedure was finished, all rats were tested in an open field apparatus to assess locomotor activity. It was hypothesized that if circulating sex hormones contribute to sex differences in SHR rats, then we would expect to observe sex-different effects of gonadectomy in this strain.
Materials and methods
Subjects
Intact and gonadectomized male and female SHR and Wistar rats were obtained from Harlan Laboratories, Indianapolis, IN, USA. Gonadectomy was performed by the supplier 1 week before the rats were shipped to the laboratory. Upon arrival, all rats were 8 weeks of age. Sample sizes for the Wistar strain were 8 intact males, 9 castrated males, 8 intact emales, and 10 ovariectomized females. For the SHR strain there were 9 intact males, 11 castrated male, 9 intact females, and 11 ovariectomized females. Rats had free access to water and food (Purina standard rat chow; Nestlé Purina, St. Louis, MO, USA) during a seven-day acclimation period and maintained on a 14/10-hr light-dark cycle. Rats were subsequently handled and weighed daily for three days to establish baseline body weights and then body weights were gradually reduced to 85% of baseline over a 10 day period. Rats were housed individually during the experiment because they had undergone surgery and it was necessary to avoid the possibily that grooming by cagemates would irritate the wound, and because the behavioral procedures required food restriction, requiring that each rat was fed a specific amount of food each day depending on his/her weight. Animals were monitored and cared for throughout the experiment in compliance with the principles of laboratory animal care, including the Association for Assessment and Accreditation of Laboratory Animal Care guidelines, the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, and the Dartmouth College Institutional Animal Care and Use Committee.
Apparatus
The conditioning procedures took place in standard operant conditioning chambers (24 cm × 30.5 cm × 29 cm; Med Associates, St. Albans, VT, USA) constructed of aluminum front and back walls, clear acrylic sides and top, and grid floors. The chambers were enclosed in sound-attenuating cabinets (62 cm × 56 cm × 56 cm) equipped with exhaust fans for air flow and background noise (~68dB). A dimly illuminated food cup was recessed in the center of one end wall and a 6-W jeweled panel light, which served as the visual conditioned stimulus (CS), was located 5 cm above the opening. An infrared photobeam was located across the entry of the food cup to monitor placement of the snout into the food cup to retrieve food pellets.
Behavioral Procedures
Rats were first trained to eat from the food cup during a single 30-min session in which two 45-mg food pellets (Noyes, New Brunswick, NJ, USA) were randomly delivered into the food cup 8 times. The subsequent ten daily conditioning sessions each lasted for approximately 30 min and included six trials consisting of a 10-sec presentation of the panel light co-terminating with delivery of two food pellets. The inter-trial intervals averaged 5 min and varied from day to day.
Analysis of Conditioning Data
Breaks in the infrared photobeam located across the entry of the food cup were monitored by the computer during presentation of the light as well as during the 5-sec period before the start of a trial (pre-CS responding) and the 5-sec period immediately after food was delivered (post-CS responding). Two measures of conditioned responding were analyzed: the amount of time spent with the snout inside the food cup, and the number of entries into the food cup. The time spent with the snout inside the food cup was particularly important because measures of response rate or the number of responses can be affected by the hyperactive phenotype of SHR rats [17] and potentially confound an accurate assessment of learning ability. Group differences in conditioned responding were assessed using a repeated measures analysis of variance (ANOVA) with Strain (Wistar or SHR), Sex, and Treatment (intact or gonadectomized) as the between-subjects variables and Session as the within-subjects variables. All analyses were followed up with pair-wise comparisons (Fishers PLSD) and were conducted using an alpha level of 0.05.
Open Field Exploration
One day after the final conditioning session locomotor activity was assessed in an open field apparatus. The open field chamber (43.2 × 43.2 cm) was composed of plexiglas walls and was connected to a computer running Open Field Activity Software (Med Associates). The chamber was equipped with 16 photobeams mounted on the sides to monitor locomotor activity. On the test day, rats were placed individually in the open field chamber and were allowed to explore the chamber for 15 min, during which time the total distance traveled was monitored by the computer. The chamber was cleaned with Quatricide disinfectant between testing each rat.
Analysis of Activity Data
The total distance traveled was averaged into five 3-min blocks. Locomotor activity was analyzed using a repeated measures ANOVA with Block as the within-subjects variable and Strain (Wistar or SHR), Sex, and Treatment (intact or gonadectomized) as the between-subjects variables. In addition, correlations between locomotor activity in the open field and conditioned responding during the light were assessed by calculating Pearson correlation coefficients. An alpha level of 0.05 was used for all analyses.
Results
Conditioning Task
The amount of time spent with the snout inside the food cup during presentation of the light served as the primary measure of conditioned responding and is presented in Figures 1A (Wistar rats) and 1B (SHR rats). As expected, responding increased over the trials in all groups as training progressed, indicating that rats learned to associate the light with food reward. This was confirmed by a repeated measures ANOVA that revealed a significant main effect of Session [F(9,603)=78.5, p<0.0001]. In addition, there was a significant main effect of Sex [F(1,67)=5.8, p<0.02] but no Sex X Strain (p>0.3), Sex X Treatment (p>0.4), or Sex X Strain X Treatment interaction (p>0.3), indicating that male rats spent more time with their snouts in the food cup than female rats regardless of strain or treatment. Lastly, there was a significant Strain X Treatment interaction [F(1,67)=4.8, p<0.03] and a significant Strain X Treatment X Session interaction [F(9,603)=3.3, p<0.001]. Post-hoc analyses revealed that gonadectomy improved conditioning in male and female SHR rats (p<0.05) but not in Wistar rats (p>0.2).
Figure 1.
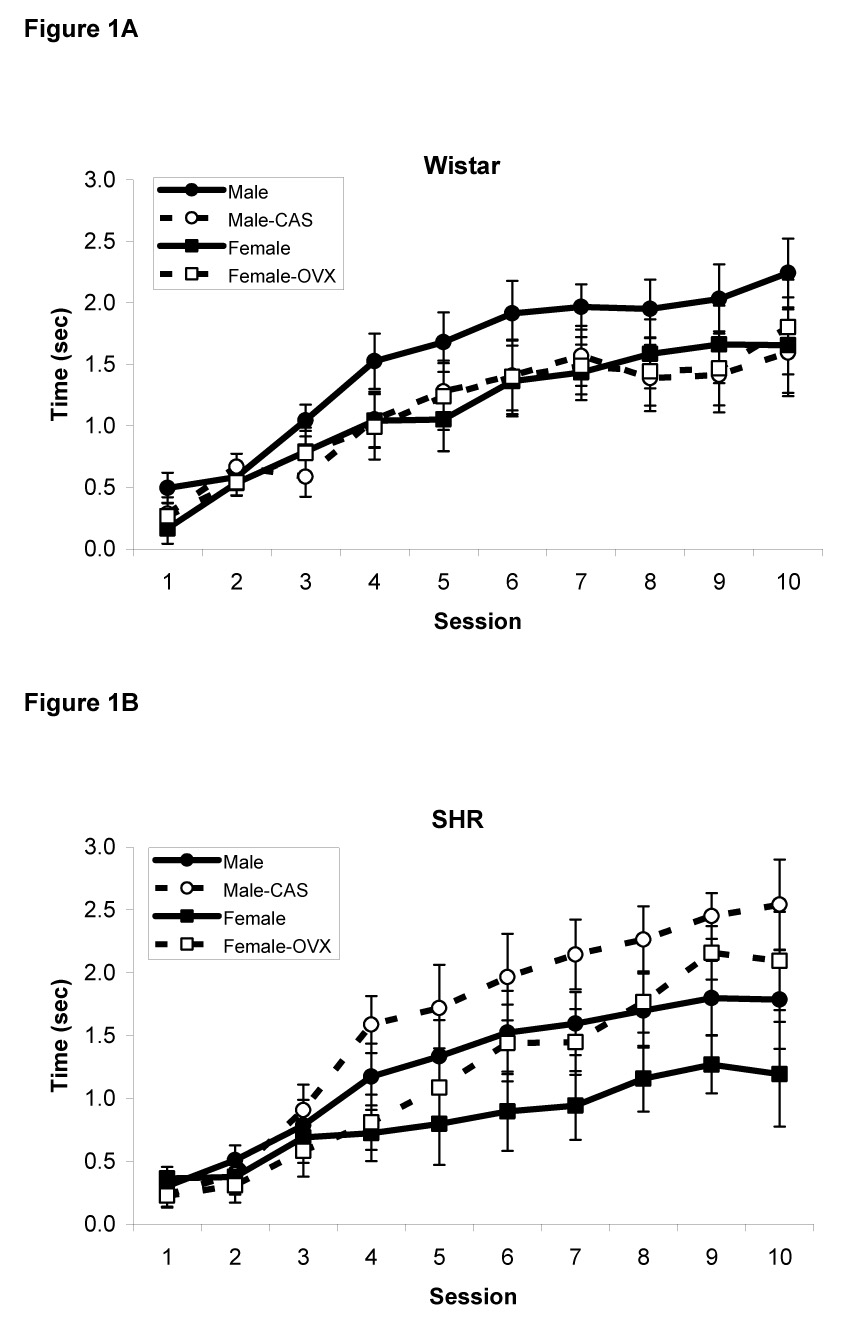
Amount of time spent inside the food cup during presentation of the visual CS in male and female intact and gonadectomized Wistar (A) and SHR rats (B). Gonadectomy enhanced conditioning in SHR rats but not in Wistar rats. Data are mean ± S.E.M.
Time spent in the food cup during the 5 sec prior to the start of a trial (pre-CS responding) was very low in all groups of rats, indicating that placing the head into the food cup was specific to presentation of the CS (Figure 2A). A repeated measures ANOVA revealed only a significant effect of Strain [F(1,67)=17, p<0.0001]. The mean amount of time spent in the food cup prior to onset of the light was slightly higher for Wistar rats (0.32 ± 0.03 sec) than for SHR rats (0.18 ± 0.02 sec). Responding during the 5 sec period after the visual stimulus was turned off and food was delivered (post-CS responding) is illustrated in Figure 2B. A repeated measures ANOVA only revealed that post-CS responding was slightly was higher in SHR rats (4.1 ± 0.1 sec) than Wistar rats (3.8 ± 0.1 sec), i.e., a main effect of strain [F(1,67)=6.9, p<0.01], consistent with the model of greater reward sensitivity in the SHR strain.
Figure 2.
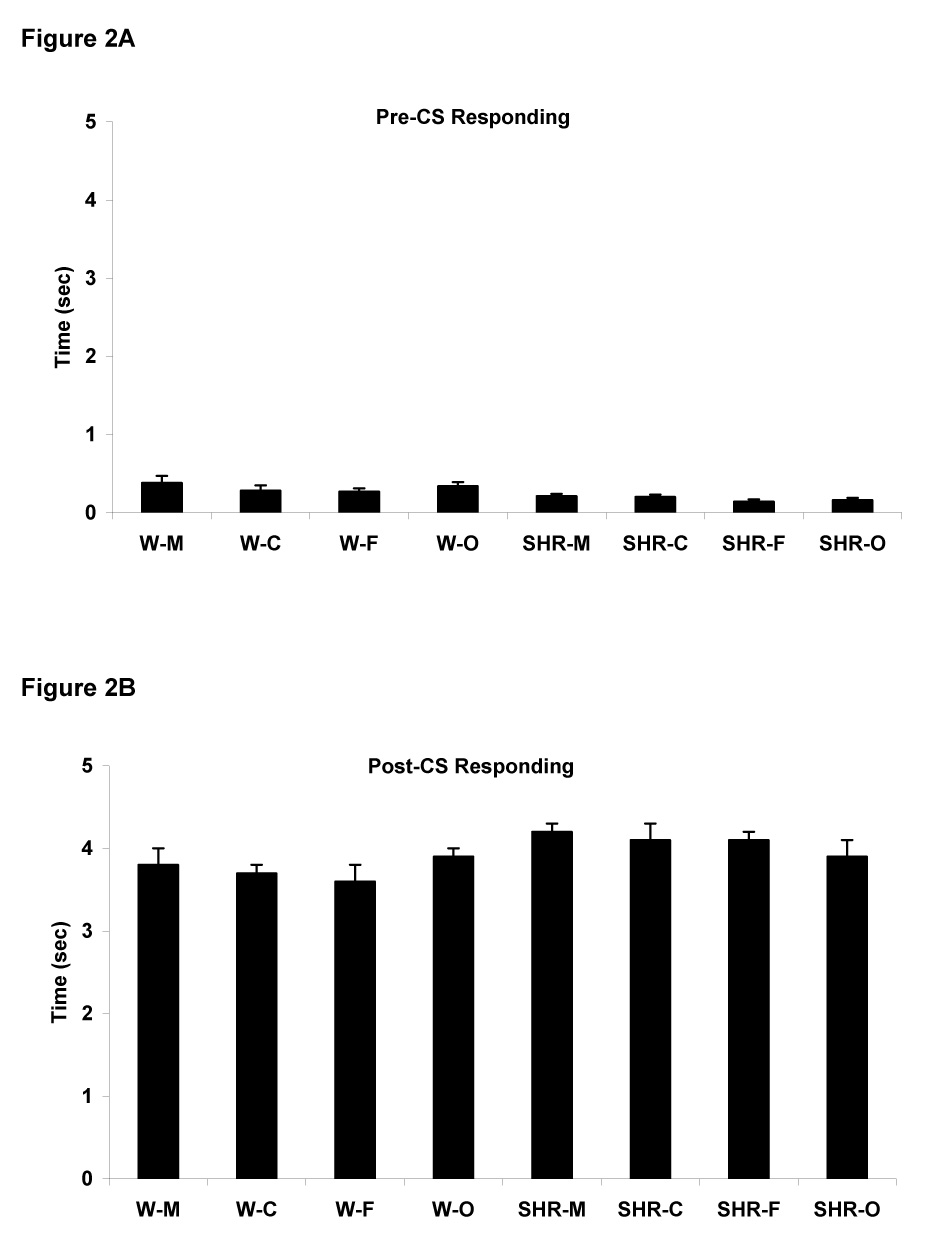
Amount of time spent inside the food cup prior to CS onset (A, pre-CS behavior) and after the CS was turned off and food was delivered (B, post-CS responding). Data are means across all 10 session ± S.E.M.
In addition to measuring the amount of time spent with the snout in the food cup, we also analyzed the number of entries into the food cup during presentation of the light. There were no significant differences detected (Ps>0.2), indicating that any differences in the amount of time spent in the food cup were not simply due to one or more treatment groups committing more food cup entries.
Locomotor Activity
Data were not available for one of the intact male SHR rats, so the analysis of locomotor activity was carried out using 74 rats. The distance traveled by Wistar and SHR rats in the open field is illustrated in Figure 3A and 3B, respectively. A repeated measures ANOVA revealed a main effect of Block [F(4,56)=43.2, p<0.0001] indicating that locomotor activity habituated over the course of the 15 min test session, as is typical in this type of design. In addition, there was a significant main effect of Sex [F(1,66)=17.5, p<0.0001] indicating that overall, female rats traversed a greater distance than male rats. The main effect of Strain was also significant [F(1,66)=35, p<0.0001] in that Wistar rats exhibited greater locomotor activity than SHR rats. Lastly, the Sex X Treatment interaction was significant [F(1,66)=7.4, p<0.01); post-hoc comparisons revealed that gonadectomy increased locomotor activity in male rats but decreased locomotor activity in female rats. Notably, there were no significant correlations between locomotor activity measured in the open field test and the time spent in the food cup during presentation of the light (r<0.15, p>0.2).
Figure 3.
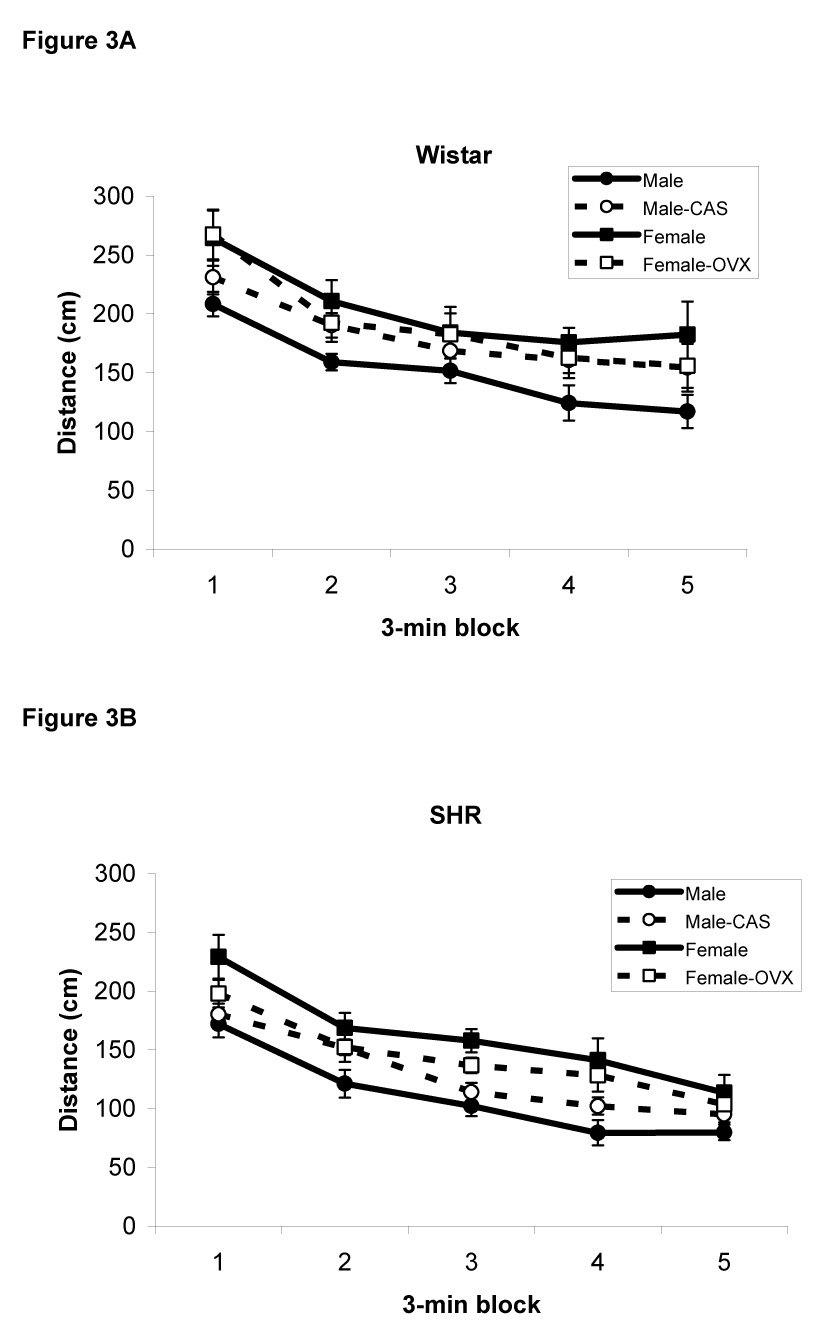
Distance traveled in the open field apparatus by male and female intact and gonadectomized Wistar (A) and SHR rats (B). Female rats exhibited more locomotor activity than males rats. In addition, Wistar rats traveled a longer distance than SHR rats. Data are mean ± S.E.M.
Discussion
ADHD is a disorder that cannot be understood without grasping the reasons for sex differences in its prevalence and possibly, in its expression [6,7,8]. Research on sex hormones is among the most important routes of investigation for solving this question, yet basic animal studies of hormonal effects on learning and behavior relevant to ADHD have been lacking. The present study thus examined the activational effects of sex hormones on behavior in a common albino strain of rats (Wistar) and in SHR rats, a widely-studied animal model of ADHD [12]. In a task designed to assess the ability of rats to learn a simple stimulus-reward relationship, we found that male rats exhibited more conditioned responding than female rats regardless of strain or gonadal intactness. However, gonadectomy differentially affected conditioning in the two strains in that the loss of circulating hormones improved conditioning in SHR rats but not in Wistar rats. These findings have several implications for understanding the activational affects of hormones on simple cognitive function in males and females as well as the interaction of sex hormones and genotype in ADHD.
The finding of a sex difference in conditioning is not surprising in light of previous research on sex differences in learning in humans as well as laboratory animals. For example, many studies of spatial learning ability in rats indicate that males out-perform females [19,20,21,22], although some studies suggest otherwise [23]. Likewise, studies in humans provide evidence of a sex difference in spatial learning and memory in favor of males [24] and recent studies in mice indicate that androgen receptor stimulation usually has beneficial effects on learning [25]. On the other hand, adult female rats outperform males in acquisition of a conditioned eyeblink response [26]. In the current study, while the direction of the sex difference was the same in both strains of rats, the developmental origin of the sex difference in conditioning may differ in the SHR and Wistar strains. In the Wistar rats, the sex difference in conditioning appears to be abolished by gonadectomy, primarily because castration of males reduces their response to the level of females. Ovariectomy of Wistar females is without effect. These findings suggest that the sex difference in response of intact individuals in this strain is “activational” in males, accounted for by the normal sex difference in circulating androgens. Removing circulating androgens in males abolishes the sex difference. Thus future studies could pursue the question of which brain regions are being affected by androgens to increase responding in males of this strain. It would also be interesting to know whether providing female Wistar rats with male-typical levels of testosterone would increase their responses.
In contrast, the sex difference in conditioning in SHR rats appears to be independent of adult gonadal hormones, as it persists following gonadectomy. This sex difference may therefore be “organizational”, caused by androgen-induced masculinization of the brain of males perinatally [27]. Although the sex difference in responsiveness in SHR rats is not dependent on circulating gonadal hormones, gonadectomy nevertheless has a dramatic effect on conditioning in both sexes. This finding strongly indicates that circulating gonadal hormones inhibit this behavior in both sexes of this strain. This is consistent with the recent finding that castration reversed deficits in stroke-prone SHR rats in a spontaneous alternation task and also normalized aberrant levels of androgen receptors [28,29]. As ovaries secrete relatively little androgen, these findings suggest that estrogens may normally inhibit conditioning in this strain. Presumably estrogenic stimulation of the gonadally intact males’ brains is via aromatization of testosterone to estrogens [27]. If so, then the decreased responsiveness of Wistar males following castration may reflect the loss of estrogen stimulation of the brain. As mentioned above, one could then ask why estrogen stimulation of the brains of Wistar males alters conditioning in the opposite direction from SHR males.
The differential effect of gonadectomy in the two strains suggests that gonadal hormones indeed affect conditioning, but that the nature of the effect depends on the genetic background. While this finding complicates a global understanding of the role of gonadal hormones in cognitive function, it suggests that the genetic background that makes SHR rats appear behaviorally distinctive from other strains may achieve this effect by altering response to steroid hormones. Thus one question that could be pursued in future studies is whether the SHR or Wistar strains are most representative of rats in general. If future studies find that gonadectomy consistently reduces conditioning in other rat strains, then the SHR response to castration, being in the opposite direction, may represent a critical difference responsible for the other behavioral distinctions in this strain. In other words, the distinctive response to gonadal hormones seen in SHR rats may be responsible for the ADHD-reminiscent behavior of these animals.
It is informative to compare the results of our study to those obtained in two previous studies in which sex hormones were manipulated perinatally. In one study, treating male SHR rats with testosterone early in life (postnatal day 10) impaired spatial memory when rats were later tested at postnatal day 45 [30]. A second study reported that androgen treatment on postnatal day 7 resulted in increased distance traveled in an open field apparatus [31]. Both of these findings are consistent with the present data in that removal of testosterone in adult SHR rats improved conditioning.
The pattern of results obtained in the conditioning task does not appear to be confounded by systematic differences in performance between strains or sex. For example, increased locomotor activity was observed in females in the open field test, consistent with previous findings [32]. Although it is possible this may have contributed to the sex difference in conditioned responding, analysis of pre-CS food cup behavior did not reveal any sex differences in baseline food cup responding. In addition, there was no correlation between locomotor activity and any measure of conditioned behavior. Moreover, the effects of gonadectomy on locomotor activity and associative learning were not in the same direction. We also do not believe that the results can be explained by the effects of gonadectomy on the hypertensive phenotype of SHR rats. First, hypertension is usually not present in this strain until later in adulthood [33,34]. Secondly, the effects of gonadetomy on hypertension are in opposite directions in males and females [35,36], whereas the effects on behavior were in the same direction in this study. Finally, we point out that although gonadectomy has been shown to differentially affect eating behavior in males and females [37], we did not observe any sex differences in post-CS responding; that is, males and females spent similar amounts of time in the food cup when food was delivered. Thus the sex differences in learning are not likely attributable to differences in motivation.
One potential issue with the intact females in this study is that it was not determined which day of the estrous cycle they were in when locomotor activity was assessed. To the extent that diestrus or proestrus or estrus is over-represented on that particular day, this could have skewed the group mean (e.g., females usually more active on the day of estrus). However, previous studies indicate that there are no differences in open field activity across different stages of estrus in female SHR rats [38]. With regard to the conditioning procedure, each female would be expected to have gone through at least 2 full cycles over the course of the conditioned sessions and so cyclic variation in hormone levels might be expected to “even out” over the 10 days of trials.
The observation that locomotor activity in the open field was greater in Wistar rats compared to SHR rats in our study is consistent with others that have used Wistar rats as a comparison strain. Indeed, SHR rats do not exhibit hyperactivity in an open field when compared to common albino strains such as Wistar or Sprague-Dawley rats [39]. Instead, locomotor activity appears increased in SHR rats only when they are compared to WKY rats (but see [40]) which were derived from the same albino Wistar-Kyoto stock [38]. Although some have argued that this makes WKY the most suitable comparison strain in studies involving SHR rats, mounting data indicates that the behavioral profile of WKY rats is unlike any other albino strain and that these rats may not serve as an appropriate “normal” comparison strain [13,42]. There is evidence that WKY rats are abnormally hypoactive [43,44,45] and they are currently being investigated as a model of depression [46,47,48]. There is also evidence that they do not share as much genetic background with SHR rats as originally suggested [49,50]. Thus, many current studies involving SHR rats use Wistar or Sprague-Dawley rats as a comparison strain. Although the lack of hyperactivity in the open field in SHR rats could be used to argue against the use of SHR rats as a model of ADHD, it is important to consider that assessing activity via distance traveled in an open field may not be the best measure of hyperactivity. More relevant measures of activity might be obtained by assessing task-relevant responding in operant reinforcement schedules, for example. In that setting, SHR rats exhibit hyperactivity and impulsive motor behavior compared to both WKY as well as more common albino strains [51], but see [52].
In summary, the findings presented here provide evidence for sex differences in simple learning in SHR rats. These data are consistent with the few other studies that have investigated sex differences in behavior in this strain as well as studies in humans that indicate that females with ADHD may have greater cognitive impairment [6,7,8]. The performance of male and female SHR rats has been shown to differ in an operant discrimination paradigm [18,53] as well as a Pavlovian model of conditioned inhibition [54]. In the latter study, female SHR rats were slower to learn a serial feature negative discrimination, consistent with a greater deficit in inhibitory behavior compared to males. However, gonadectomy affected males and female SHR rats similarly, indicating that circulating sex hormones in adulthood are not sufficient to explain the observed sex difference in adulthood. These data suggest that pre-existing differences early in development may be responsible for the finding that girls exhibit more severe cognitive dysfunction than boys diagnosed with ADHD. In addition, another major finding of the present study was that the organizational versus activational effects of sex hormones may affect SHR rats differently than other strains of albino rats. If this result turns out to be consistent across other behavioral domains related to ADHD (e.g., inhibition, working memory, sustained attention), it would provide evidence that the distinctive response to gonadal hormones in SHR rats may also contribute to sex differences in ADHD-like behavior. Further studies are also needed to more fully characterize the effects of sex hormones on behavior in SHR rats by comparing the effects of gonadectomy and possibly hormone replacement at other times during development, e.g., perinatally.
Acknowledgements
The authors thank Silas Cole and Matthew Mackey for assistance in collecting the behavioral data. This research was supported by NIMH Grant MH070542.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. U.S. Government Printing Office. 4th ed. U.S. Government Printing Office; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Lahey BB, Miller TI, Gordon RA, Riley AW. Developmental epidemiology of the disruptive behavior disorders. In: H.C. Quay HC, Hogan AE, editors. Handbook of Disruptive Behavior Disorders. New York: Kluwer/Plenum; 1999. pp. 23–48. [Google Scholar]
- 4.Wolraich ML, Hannah JN, Pinnock TY, Baumgaertel A, Brown J. Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:319–324. doi: 10.1097/00004583-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Feifel D. Attention-deficit hyperactivity disorder in adults. Postgrad. Med. 1991;100:207–218. doi: 10.3810/pgm.1996.09.78. [DOI] [PubMed] [Google Scholar]
- 6.Ernst M, Liebenauer LL, King AC, Fitzgerald GA, Cohen RM, Zametkin AJ. Reduced brain metabolism in hyperactive girls. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33:858–868. doi: 10.1097/00004583-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Gershon J. A meta-analytic review of gender differences in ADHD. J. Atten. Disord. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- 8.Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Faraone SV, Biederman J, Keenan K, Tsuang MT. A family-genetic study of girls with DSM-III attention deficit disorder. Am. J. Psychiatry. 1991;148:112–117. doi: 10.1176/ajp.148.1.112. [DOI] [PubMed] [Google Scholar]
- 10.Rhee SR, Waldman ID, Hay DA, Levy F. Aetiology of sex differences in the prevalence of DSM-III-R ADHD: A comparison of two models. In: Levy F, Hay DA, editors. Attention, Genes, and ADHD. Philadelphia: Brunner-Routledge; 2001. pp. 139–156. [Google Scholar]
- 11.Arnold LE. Sex differences in ADHD: Conference summary. J. Abnorm. Child Psychol. 1996;24:555–569. doi: 10.1007/BF01670100. [DOI] [PubMed] [Google Scholar]
- 12.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Clements KM, Wainwright PE. Spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rats differ in performance on a win-shift task in the water radial arm maze. Behav. Brain Res. 2006;167:295–304. doi: 10.1016/j.bbr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 15.Johansen EB, Sagvolden T. Response disinhibition may be explained as an extinction deficit in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav. Brain Res. 2004;149:183–196. doi: 10.1016/s0166-4328(03)00229-8. [DOI] [PubMed] [Google Scholar]
- 16.Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci. Biobehav. Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 17.Sagvolden T, Hendley ED, Knardahl S. Behavior of Hypertensive and Hyperactive Rat Strains: Hyperactivity Is Not Unitarily Determined. Physiol. Behav. 1992;52:49–57. doi: 10.1016/0031-9384(92)90432-2. [DOI] [PubMed] [Google Scholar]
- 18.Berger DF, Sagvolden T. Sex differences in operant discrimination behaviour in an animal model of attention-deficit hyperactivity disorder. Behav. Brain Res. 1998;94:73–82. doi: 10.1016/s0166-4328(97)00171-x. [DOI] [PubMed] [Google Scholar]
- 19.Barrett RJ, Ray OS. Behavior in the open field, Lashley III maze, shuttle-box, and Sidman avoidance as a function of strain, sex, and age. Dev. Psychol. 1970;3:73–77. [Google Scholar]
- 20.Dawson JL, Cheung YM, Lau RT. Developmental effects of neonatal sex hormones on spatial and activity skills in the white rat. Biol. Psychol. 1975;3:213–229. doi: 10.1016/0301-0511(75)90036-8. [DOI] [PubMed] [Google Scholar]
- 21.Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behav. Brain Res. 1993;53:1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- 22.Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav. Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Bucci DJ, Chiba AA, Gallagher M. Spatial learning in male and female Long-Evans rats. Behav. Neurosci. 1995;109:180–183. doi: 10.1037//0735-7044.109.1.180. [DOI] [PubMed] [Google Scholar]
- 24.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav. Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 25.Rizk A, Robertson J, Raber J. Behavioral performance of tfm mice supports the beneficial role of androgen receptors in spatial learning and memory. Brain Res. 2005;1034:132–138. doi: 10.1016/j.brainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm. Behav. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puts DA, Jordan CL, Breedlove SM. Defending the brain from estrogen. Nat. Neurosci. 2006;9:155–156. doi: 10.1038/nn0206-155. [DOI] [PubMed] [Google Scholar]
- 28.Jesmin S, Togashi H, Sakuma I, Mowa CN, Ueno K, Yamaguchi T, Yoshioka M, Kitabatake A. Gonadal hormones and frontocortical expression of vascular endothelial growth factor in male stroke-prone, spontaneously hypertensive rats, a model for attention-deficit/hyperactivity disorder. Endocrinology. 2004;145:4330–4343. doi: 10.1210/en.2004-0487. [DOI] [PubMed] [Google Scholar]
- 29.Togashi H, Ueno K, Jesmin K, Sakuma I, Matsumoto M, Inoue Y, Yoshioka M. Proc. 33rd Annual Meeting of the Soc. for Neurosci. New Orleans, LA: 2003. Effects of gonadectomy and gonadal hormone replacement on short-term memory impairment in an animal model of attention-deficit/hyperactivity disorder. [Google Scholar]
- 30.King JA, Barkley RA, Delville Y, Ferris CF. Early androgen treatment decreases cognitive function and catecholamine innervation in an animal model of ADHD. Behav. Brain Res. 2000;107(1–2):35–43. doi: 10.1016/s0166-4328(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 31.Li JS, Huang YC. Early androgen treatment influences the pattern and amount of locomotion activity differently and sexually differentially in an animal model of ADHD. Behav. Brain Res. 2006;175(1):176–182. doi: 10.1016/j.bbr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Hendley ED, Wessel DJ, Atwater DG, Gellis J, Whitehorn D, Low WC. Age, sex and strain differences in activity and habituation in SHR and WKY rats. Physiol. Behav. 1985;34:379–383. doi: 10.1016/0031-9384(85)90199-4. [DOI] [PubMed] [Google Scholar]
- 33.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OHL. Myocardial Fibrosis and Stiffness With Hypertrophy and Heart Failure in the Spontaneously Hypertensive Rat. Circulation. 1995;91:161–170. doi: 10.1161/01.cir.91.1.161. [DOI] [PubMed] [Google Scholar]
- 34.Boluyt MO, Bing OH, Lakatta EG. The ageing spontaneously hypertensive rat as a model of the transition from stable compensated hypertrophy to heart failure. Eur Heart J. 1995;16 Suppl N:19–30. doi: 10.1093/eurheartj/16.suppl_n.19. [DOI] [PubMed] [Google Scholar]
- 35.Martin DS, Biltoft S, Redetzke R, Vogel E. Castration reduces blood pressure and autonomic venous tone in male spontaneously hypertensive rats. J Hypertens. 2005;23:2229–2236. doi: 10.1097/01.hjh.0000191903.19230.79. [DOI] [PubMed] [Google Scholar]
- 36.Packer CS, Pelaez NJ, Kramer JA. Estrogen protects against hypertension in the spontaneously hypertensive rat, but its protective mechanism is unrelated to impaired arterial muscle relaxation. J Gend Specif Med. 2001;4:20–27. [PubMed] [Google Scholar]
- 37.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eikelis N, Van Den Buuse M. Cardiovascular responses to open-field stress in rats: sex differences and effects of gonadal hormones. Stress. 2000;3:319–334. doi: 10.3109/10253890009001137. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson SA, Cada AM. A longitudinal study of short- and long-term activity levels in male and female spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rats. Behav Neurosci. 2003;117:271–282. doi: 10.1037/0735-7044.117.2.271. [DOI] [PubMed] [Google Scholar]
- 40.Sagvolden T, Pettersen MB, Larsen MC. Spontaneously Hypertensive Rats (SHR) as a putative model of childhood hyperkinesis; SHR behavior compared to four other strains. Physiol. Behav. 1993;54:1047–1055. doi: 10.1016/0031-9384(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 42.Pare WP. Investigatory behavior of a novel conspecific by Wistar Kyoto, Wistar and Sprague-Dawley rats. Brain Res. Bull. 2000;53:759–765. doi: 10.1016/s0361-9230(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 43.Chess AC, Keene CS, Wyzik EC, Bucci DJ. Stimulus processing and associative learning in Wistar and WKHA rats. Behav. Neurosci. 2005;119:772–780. doi: 10.1037/0735-7044.119.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drolet G, Proulx K, Pearson D, Rochford J, Deschepper CF. Comparisons of behavioral and neurochemical characteristics between WKY, WKHA, and Wistar rat strains. Neuropsychopharmacology. 2002;27:400–409. doi: 10.1016/S0893-133X(02)00303-2. [DOI] [PubMed] [Google Scholar]
- 45.Pare WP. Stress ulcer and open-field behavior of spontaneously hypertensive, normotensive, and Wistar rats. Pavlov J. Biol. Sci. 1989;24:54–57. doi: 10.1007/BF02964537. [DOI] [PubMed] [Google Scholar]
- 46.Baum AE, Solberg LC, Churchill GA, Ahmadiyeh N, Takahashi JS, Redei EE. Test- and behavior-specific genetic factors affect WKY hypoactivity in tests of emotionality. Behav. Brain Res. 2006;169:220–230. doi: 10.1016/j.bbr.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braw Y, Malkesman O, Dagan M, Bercovich A, Lavi-Avnon Y, Schroeder M, Overstreet DH, Weller A. Anxiety-like behaviors in pre-pubertal rats of the Flinders Sensitive Line (FSL) and Wistar-Kyoto (WKY) animal models of depression. Behav. Brain Res. 2006;167:261–269. doi: 10.1016/j.bbr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8:925–932. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- 49.Johnson ML, Ely DL, Turner ME. Steroid sulfatase and the Y chromosome hypertensive locus of the spontaneously hypertensive rat. Steroids. 1995;60:681–685. doi: 10.1016/0039-128x(95)00091-4. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto K. Spontaneous hypertension in rats. Int. Rev. Exp. Pathol. 1969;7:227–270. [PubMed] [Google Scholar]
- 51.Sagvolden T. The spontaneously hypertensive rats as model of ADHD. In: Solanto M, Castellanos FX, editors. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001. pp. 221–237. [Google Scholar]
- 52.van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Sagvolden T, Berger DF. An animal model of attention deficit disorder: The female shows more behavioral problems and is more impulsive than the male. Eur. Psychologist. 1996;1:113–122. [Google Scholar]
- 54.Bucci DJ, Hopkins ME, Keene CS, Sharma M, Orr LE. Sex differences in learning and inhibition in spontaneously hypertensive rats. Behavioural Brain Research. doi: 10.1016/j.bbr.2007.08.022. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


