Abstract
Citric acid cycle intermediates are absorbed from the gastrointestinal tract through carrier-mediated mechanisms, although the transport pathways have not been clearly identified. This study examines the transport of citric acid cycle intermediates in the Caco-2 human colon carcinoma cell line, often used as a model of small intestine. Inulin was used as an extracellular volume marker instead of mannitol since the apparent volume measured with mannitol changed with time. The results show that Caco-2 cells contain at least three distinct transporters, including the Na+-dependent di- and tricarboxylate transporters, NaDC1 and NaCT, and one or more sodium-independent pathways, possibly involving organic anion transporters. Succinate transport is mediated mostly by Na+-dependent pathways, predominantly by NaDC1, but with some contribution by NaCT. RT-PCR and functional characteristics verified the expression of these transporters in Caco-2 cells. In contrast, citrate transport in Caco-2 cells occurs by a combination of Na+-independent pathways, possibly mediated by an organic anion transporter, and Na+-dependent mechanisms. The non-metabolizable dicarboxylate, methylsuccinate, is also transported by a combination of Na+-dependent and -independent pathways. In conclusion, we find that multiple pathways are involved in the transport of di-and tricarboxylates by Caco-2 cells. Since many of these pathways are not found in human intestine, this model may be best suited for studying Na+-dependent transport of succinate by NaDC1.
Keywords: Caco-2 cells, transporter, sodium, organic anion, succinate, citrate, small intestine
1. Introduction
The Na+/dicarboxylate cotransporters of the SLC13 family transport citric acid cycle intermediates together with Na+ ions [1]. The low affinity transporter, NaDC1, is found on the luminal membranes of intestinal and renal epithelial cells. Although the function of NaDC1 has been studied mostly in the kidney, where it reabsorbs filtered citric acid cycle intermediates, it is also important in the intestine. The normal function of the intestinal NaDC1 is to absorb dicarboxylates from the diet and from gastrointestinal secretions [2]. In recent years, a new role for NaDC1 in metabolism and aging has been suggested; mutations in NaDC1 homologs in Drosophila (Indy protein) and Caenorhabditis elegans result in lifespan extension [3,4]. Both Indy and the C. elegans protein are found in the midgut, analogous to small intestine, suggesting that the mechanism of lifespan extension may be similar to that produced by caloric restriction [5].
There is very little information about intestinal transport of citric acid cycle intermediates in mammals. There is evidence for intestinal transepithelial transport of citrate in humans [6] and of citrate, succinate and α-ketoglutarate in hamsters [7]. Studies with isolated brush border membrane vesicles suggest that transport across the apical membrane is entirely sodium-dependent, with properties very similar to those of NaDC1 [8–11]. The intestinal basolateral membrane appears to contain sodium-independent pathways for transport of citrate and tricarballylate, a non-metabolizable tricarboxylate. The basolateral transporter may be an anion exchanger, since it is trans-stimulated by other di- or tricarboxylates [10]. Nevertheless, detailed information is lacking on the transport systems for citric acid cycle intermediates in the small intestine and there are no model systems currently available for the study of regulation of these transporters. The human colon adenocarcinoma cell line, Caco-2, has often been used as a model of enterocytes, because Caco-2 cells differentiate into polarized cells with many of the functional properties of enterocytes [12,13]. Therefore, we investigated the Caco-2 cells as a potential model system for studying transport of citric acid cycle intermediates.
The results of this study show that the apical membrane of differentiated Caco-2 cells contains at least three transport systems for di- and tricarboxylates. The Na+-dependent transport of succinate and citrate in Caco-2 cells is mediated by a combination of NaDC1 and the Na+/citrate transporter, NaCT, although NaDC1 is the predominant transport pathway. NaCT is not normally expressed in the small intestine [14]. The Na+-independent transport of citrate in Caco-2 cells is likely mediated by one or more organic anion transporters, possibly OAT2 and/or OAT4. There is also transepithelial transport of methylsuccinate across Caco-2 cell monolayers, indicating the presence of an additional Na+-independent transporter on the basolateral membrane. RT-PCR experiments show that Caco-2 cells express different dicarboxylate and organic anion transporters than human small intestine. The current studies establish that di- and tricarboxylate uptakes by Caco-2 cells occur via multiple transport pathways, predominantly by NaDC1 as well as by NaCT and organic anion transporters that are not found in normal small intestine. We conclude that the Caco-2 cell line is probably useful for studying regulation of NaDC1, but is not a good model of the transport pathways for citric acid cycle intermediates in small intestine.
2. Methods
2.1. Caco-2 cell culture
The human colon adenocarcinoma cell line, Caco-2, was obtained from the American Type Culture Collection (Rockville, MD) and cultured in DMEM with 4.5 g/l glucose (GIBCO-Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (Hyclone), 100 units/ml penicillin G, 100 μg/ml of streptomycin, 1% (v/v) sodium pyruvate, and 1% (v/v) nonessential amino acids (Sigma). Cells were maintained at 37°C in an atmosphere of 5% CO2 and cultures were passaged at about 90% confluence. For transport experiments with cells attached to plastic, cells were plated at a density of 2 × 105 cells/well onto 24 well plates coated with 5 μg/cm2 rat-tail collagen I (BD Bioscience-Clontech). For transepithelial transport measurements, cells were seeded at high density (1 × 106 cells/24 mm insert or 2 × 105 cells/6.5 mm insert) on Transwell™ polycarbonate filter tissue culture inserts (Costar). Cell monolayers were maintained with daily medium changes for 20 or 21 days after seeding. Cells were incubated with serum-free medium for 24 hours prior to transport assays. The differentiated phenotype of the cells was observed by microscopy. The integrity of cell monolayers grown on Transwells was evaluated by measuring the transepithelial electrical resistance (TEER) with an epithelial voltmeter (EVOM; World Precision Instrument) and the magnitude of paracellular flux of [3H] or [14C]-labeled mannitol.
2.2. Transport studies in attached cell monolayers
The transport of [14C]succinate (40 mCi/mmol, Moravek), [14C]citrate (55 mCi/mmol, American Radiolabeled Chemical Inc.), or [3H]methylsuccinate, a gift from Dr. Chari Smith (GlaxoSmithKline), in attached cells was conducted using a modification of our previous protocol [15]. Briefly, cells were washed twice with 1 ml choline buffer (120 mM choline chloride, 5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 5 mM D-glucose, 25 mM HEPES, adjusted to pH 7.4 with 1M Tris) to remove medium. The uptake experiments were performed at 37°C on a platform rotator. The transport of succinate and citrate was measured in sodium buffer (same as choline buffer, except that choline is replaced by NaCl) or choline buffer. The transport buffer also contained [3H] or [14C]-labeled extracellular marker to determine the residual extracellular volume. After the incubation period, typically 60 minutes, the uptake measurements were stopped with four washes of ice-cold choline buffer (1 ml each) and the buffer was removed. The cells were then solubilized in 0.25 ml of 1% sodium dodecyl sulfate (SDS). The radioactivity was determined using dual-label liquid scintillation counting. Uptakes were normalized for the residual extracellular volume determined from the extracellular marker counts.
2.3. Transepithelial transport and intracellular accumulation
Cells plated on Transwell inserts were used for studies of transepithelial transport. Transport studies were conducted 21–23 days after seeding when TEER values exceeded 600 ω·cm2 (after correction for resistance values of inserts alone). Prior to initiating the transport studies, culture inserts were washed twice by dipping in 250 ml of choline buffer, and placed in fresh 6 or 24 well plates. Cell monolayers were preincubated from the apical and basolateral sides with 1.5 ml and 2.6 ml of prewarmed choline buffer per well, respectively (in 6 wells), or with 0.1 ml and 0.6 ml per well, respectively (in 24 wells) and allowed to equilibrate for 30 min at 37°C. Transport was initiated by adding sodium or choline buffer containing [3H]methylsuccinate at a final concentration of 100 μM. [14C]mannitol was also added to determine the magnitude of paracellular fluxes across the epithelium and confirm maintenance of monolayer integrity during the flux measurements. Data were used only if paracellular fluxes of [14C]mannitol were less than 0.2%. Epithelial layers were incubated for 60 min at 37°C. To measure transepithelial transport, 500 μl samples were taken from the receiver side, either the apical or basolateral chamber. Fluxes in the absorptive (apical to basolateral) and secretory (basolateral to apical) direction were measured and expressed as fmol/hr-cm2. To determine the intracellular accumulation, at the end of the incubation period the intact epithelial monolayer on the tissue culture insert was sequentially transferred through 4 beakers each containing 500 ml of cold choline buffer, then the membrane containing the cell monolayer was carefully excised from the insert. Cell-associated radioactivity was determined by dual-label liquid scintillation counting.
2.4. Construction of human NaCT expression plasmids
The full-length coding sequence of human NaCT (GenBank AY151833) was amplified from human liver cDNA (Clontech) using the following primers: sense, 5′-TTG AAT TCC CGC CAT GGC CTC GGC GC-3′ and antisense, 5′-CCC GCT CGA GCT AAG TCT CAA TAT GTG TC-3′, including EcoRI and XhoI restriction sites (underlined) to facilitate subcloning. The PCR product was subcloned into pCR 2.1 vector using the TopoTA cloning kit (Invitrogen), followed by subcloning into the mammalian expression plasmid, pcDNA3.1 (Invitrogen). The cDNA sequence was verified by the Protein Chemistry Laboratory of the University of Texas Medical Branch.
2.5. Functional expression in HRPE cells
Human retinal pigment epithelial (HRPE) cells (Coriell Institute) were maintained in Modified Eagle’s medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin G, 100 μg/ml of streptomycin at 37°C with 5% CO2. For transient transfections, cells were seeded onto 24 well culture plates at a density of 1.2 × 105 cells/well. Transient transfections with plasmids containing hNaDC1 [15] or hNaCT with FuGENE 6 (Roche) were conducted 24 hours later according to the manufacturer’s instructions. Each well was transfected with 1.8 μg FuGENE6 and 0.6 μg DNA. Transport assays were performed 48 hours after transfection as described for Caco-2 cells except that the extracellular marker correction was not done. Uptakes in transfected HRPE cells were corrected for background counts by subtracting counts in control cells transfected with the pcDNA3.1 vector plasmid.
2.6. RT-PCR
Total RNA was extracted from Caco-2 cells 21 days after confluence using RNeasy mini kits (Qiagen) according to manufacturer’s instructions. First-strand cDNA was synthesized from 5 μg of RNA using the SuperScript II RNaseH-Reverse Transcriptase kit (Invitrogen) with random hexamer primers. Human small intestine cDNA was purchased from Origene. The quality of cDNA was assessed by amplification of β-actin using primers from Clontech. The PCR reaction was done using the FailSafe™ PCR system (Epicentre Biotechnologies, Madison, WI) with 2X Buffer F and sequence-specific primers (Table I). The PCR protocol was 94°C for 3 min, followed by 28 cycles of 94°C for 45 sec, 60°C for 45 sec, 72°C for 1 min, and 72°C for 15 min final elongation. For hOAT3, the PCR was done using 2X Buffer J and the cycles were shortened to 30 sec each. PCR products were visualized on agarose gels stained with ethidium bromide. The identity of PCR products obtained from Caco-2 cells was verified by sequencing after subcloning into the pCR2.1 plasmid (TA cloning kit).
Table I.
Oligonucleotide primers used for PCR amplification of human Na+/dicarboxylate cotransporters (NaDC and NaCT), and organic anion transporters (OAT).
| Protein(Genbank No.) | Primer sequences (5′ to 3′) | Predicted size (bp) |
|---|---|---|
| hNaDC1 (U26209) | CCCTTAATCCTGTTCCCTATGA TGGGGGAAGAGCGAGTTGA | 581 |
| hNaDC3 (AF154121) | CACCGCCTCCACTGCCATGAT GACGGGAAGAAGAACAAGATGG | 749 |
| HNaCT (AY151833) | GGAGCTGCCAGGGAGTCAAGTG GGAGGGGGATAAAATGGAGTTTTC | 647 |
| hOAT1 (AF124373) | GGTTCTTCATTGAGTCGGCCC GCCACAGGCAAAGCACCGTA | 690 |
| hOAT2 (AF210455) | CCAGGAGGCTGTGAGCAAAGTG CTGTGCCTGCCTCGTCTCTG | 612 |
| hOAT3 (NM_004254) | CACCGCAAGTGACCTGTTCC CAGGAAGAGGGCAGCACTG | 540 |
| hOAT4 (NM_018484) | ATGCTGGACAATGGCTCTGC GTCAGGTTCTTGGCCTCCTTG | 778 |
2.7. Data analysis
Data are presented as means ± S.E.M. Statistical analysis was done using Student’s t-test or One-way analysis of variance followed by Dunnett’s test (SigmaStat program, Jandel Scientific). In all analyses, differences were considered statistically significant when p < 0.05.
3. Results
3.1. Extracellular volume measurement in Caco-2 cells
Mannitol is often used for estimating the extracellular volume of attached cell monolayers, including Caco-2 [16]. However, we found that the apparent extracellular volume measured with both [3H] and [14C] mannitol appeared to increase over time (Fig. 1), suggesting that mannitol is not a good measure of extracellular volume in Caco-2 cells grown on plastic. In a previous study using isolated sympathetic ganglia, mannitol was found to be metabolized, with different metabolites depending on the isotope (3H or 14C), which produced differences in apparent extracellular volume [17]. Another possible explanation could be that the radiolabeled mannitol is accumulating through paracellular pathways in aqueous domes under the monolayer [18]. We tested [14C]inulin as an alternative extracellular marker and there was no change in apparent volume over time (Fig. 1). Therefore, [14C]inulin counts were used to estimate the extracellular volume of the Caco-2 monolayers, approximately 0.35 μl per well, which was then used in correcting uptake calculations.
Fig. 1.
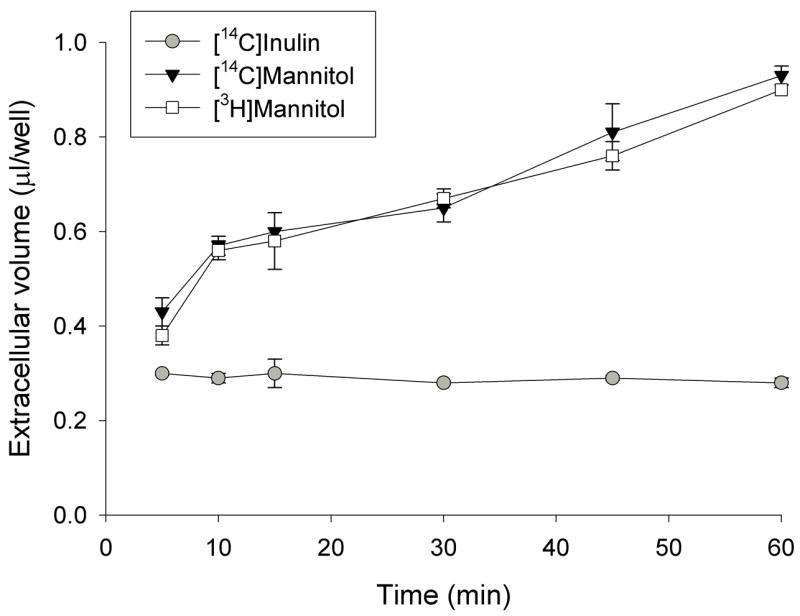
The apparent extracellular volume of Caco-2 cell monolayers determined using [3H] and [14C]mannitol and [14C]inulin in a time course up to 1 h. Caco-2 cells grown on plastic were incubated in sodium buffer containing [3H] and [14C]mannitol or [14C]inulin for the time indicated.
3.2. Succinate and citrate transport in Caco-2 cells grown on plastic
The time course of succinate and citrate uptake in Caco-2 cells was measured in the presence and absence of sodium over a 60 minute time course (Fig. 2). The sodium-dependent transport of succinate was essentially linear over 60 min (Fig. 2A). In order to maximize signal above the counts associated with the extracellular fluid, 60 minute uptakes were used for the experimental time points. For example, in the experiment shown in Fig. 2A, the [14C]succinate counts associated with extracellular fluid were approximately 4.2 pmol/well, approximately one third of the intracellular accumulation. The citrate time course is very different from that of succinate (Fig. 2B). The sodium-dependent component of citrate transport was not evident until after 30 minutes, and there was a substantial sodium-independent component. Again, for accurate measurement above the extracellular [14C]citrate counts, we used 60 minute uptake measurements.
Fig. 2.
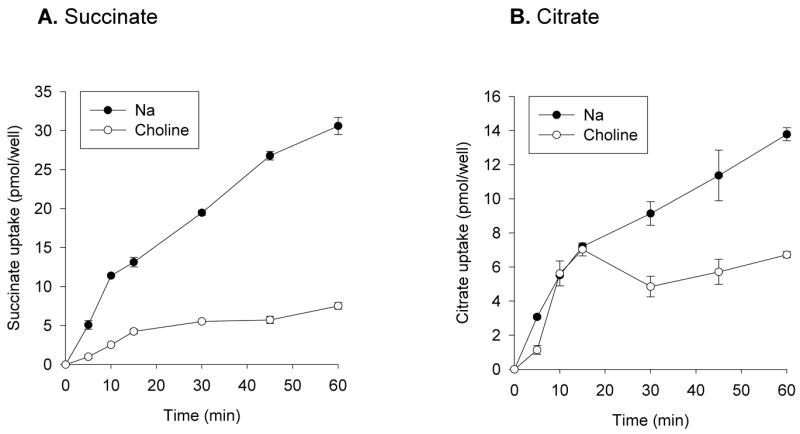
Time course of uptake of succinate and citrate by Caco-2 cells. Monolayers grown on plastic were incubated with 10μM [14C]succinate (A) or [14C]citrate (B) in sodium or choline buffer for time points up to 1 hour. Each data point represents the mean ± S.E.M, n = 4 determinations.
Consistent with the time course experiments (Fig. 2), measurements of single transport points showed that most of the succinate transport was sodium-dependent, whereas approximately half the citrate transport was sodium-dependent. The succinate transport activity (60 minute time point) was 329 ± 17 fmol/min-well (n = 19 experiments) in sodium and 92 ± 17 fmol/min-well (n = 9) in choline, results not shown. The citrate transport activity was 257 ± 18 fmol/min-well (n = 24) in sodium and 149 ± 12 fmol/min-well (n = 29) in choline. The proportion of sodium-independent citrate transport varied considerably between experiments, and in some experiments there was no detectable sodium-independent transport of citrate, suggesting variability in expression of the sodium-independent citrate transporters. At present we have no explanation for this variability. The sodium-independent citrate transport pathway was not influenced by cell passage number, differentiation state, glutamine supplementation or by lot or source of serum (results not shown).
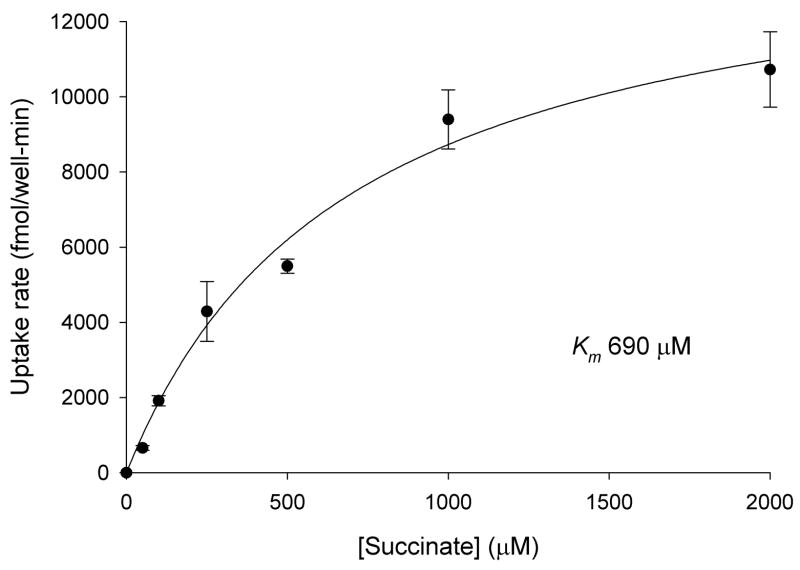
3.3 Kinetics of succinate transport in Caco-2 cells
The kinetics of Na+-dependent succinate transport were measured in Caco-2 cells. In the experiment shown in Fig. 3, the Km for succinate was 0.69 mM and in a second experiment, the Km was 0.77 mM. This result indicates that succinate transport in Caco-2 cells is handled primarily by a low-affinity transporter, most likely NaDC1 because the human NaDC1 has a Km for succinate of ~0.8 mM [19]. The human NaCT has a Km for succinate (estimated from inhibition experiments) of approximately 1.9 mM [14] and the high affinity transporter, hNaDC3, has a Km for succinate of 20 μM [20].
Fig. 3.
Kinetics of Na+-dependent succinate transport in Caco-2 cells. [14C]succinate transport was measured in sodium and choline buffers for 30 min. Na+-dependent transport was calculated from the difference between uptakes in sodium and choline. Each data point shows the mean ± range (n = 2 wells) from a single experiment.
3.4. RT-PCR analysis of Na+-dependent dicarboxylate transporters
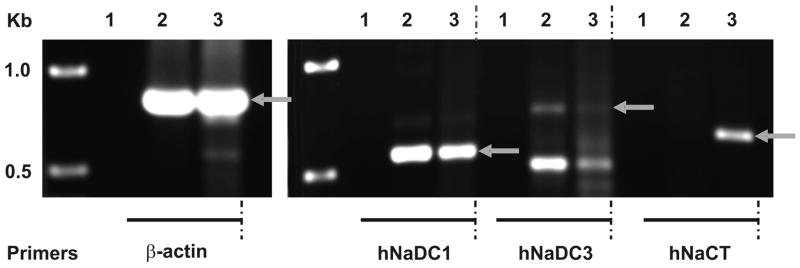
We next used RT-PCR to examine whether message for the three known Na+-coupled succinate and citrate transporters, NaDC1, NaDC3 and NaCT, is found in Caco-2 cells and in normal human intestine. Normal human intestine contained mRNA for NaDC1 but not NaCT (Fig. 4), consistent with the tissue distribution pattern of NaDCs [21] and NaCT [14]. There was a faint signal in the NaDC3 RT-PCR reaction, indicating that NaDC3 may be present in human intestine. Northern blots of rat intestinal mRNA do not contain NaDC3 message [22], but the sensitivity of RT-PCR is greater than Northern blotting and there may be species differences. Caco-2 cells resembled normal intestine because they expressed NaDC1 (Fig. 4). However, the RT-PCR reaction with NaCT primers was also positive in Caco-2 cells, unlike normal human intestine. There was a very faint signal with NaDC3 primers but we were unable to verify the identity by sequencing. Therefore, potential candidates for Na+-dependent transport of succinate and citrate in Caco-2 cells are NaDC1 and NaCT.
Fig. 4.
RT-PCR analysis of Na+/dicarboxylate cotransporter mRNA in human intestine (lane 2) and Caco-2 cells (lane 3), water controls (lane 1) using specific primers for hNaDC1, hNaDC3 and hNaCT. Amplification with β-actin primers to test the quality and relative quantity of cDNA was performed in parallel. Size standards are shown in leftmost lane. Arrows show the expected size of products. The identity of hNaDC1 and hNaCT products was verified by sequencing.
We attempted to identify NaDC1 protein in Caco-2 cells by Western blotting but were unable to obtain consistent results. In two experiments there was a single protein band at 75 kDa, similar to the protein found in intact human brush border membrane vesicles (results not shown). However, in five experiments the blots contained multiple protein bands with different relative abundance, and some of the blots did not contain the 75 kDa band. Although the antibodies recognize the human NaDC1 [23], the abundance in Caco-2 cells may be too low for accurate detection particularly if our antibodies cross-react with other more abundant proteins.
3.5. Succinate and citrate transport by HRPE cells transfected with hNaDC1 or hNaCT
Fig. 5 shows the Na+-dependent succinate and citrate transport activity by HRPE cells transiently transfected with hNaDC1 or hNaCT. For both NaDC1 and NaCT, there was no sodium-independent transport of succinate or citrate. HRPE cells expressing hNaDC1 had about 10-fold higher rate of succinate transport compared with citrate (Fig. 5, left). HRPE cells transiently transfected with hNaCT cDNA (Fig. 5, right) had lower transport of both succinate and citrate compared with hNaDC1-expressing cells, and the rate of succinate transport was about half the rate of citrate transport. This result is consistent with previous findings that NaDC1 prefers succinate whereas NaCT transports citrate most efficiently [1,14,24]. By comparison, the rate of Na+-dependent citrate transport relative to succinate transport in Caco-2 cells (about 46%) is greater than would be expected if Caco-2 cells expressed only NaDC1. Therefore, Na+-dependent transport of succinate and citrate in Caco-2 cells appears to be mediated predominantly by NaDC1, with some contribution by another sodium-dependent transporter, possibly NaCT.
Fig. 5.
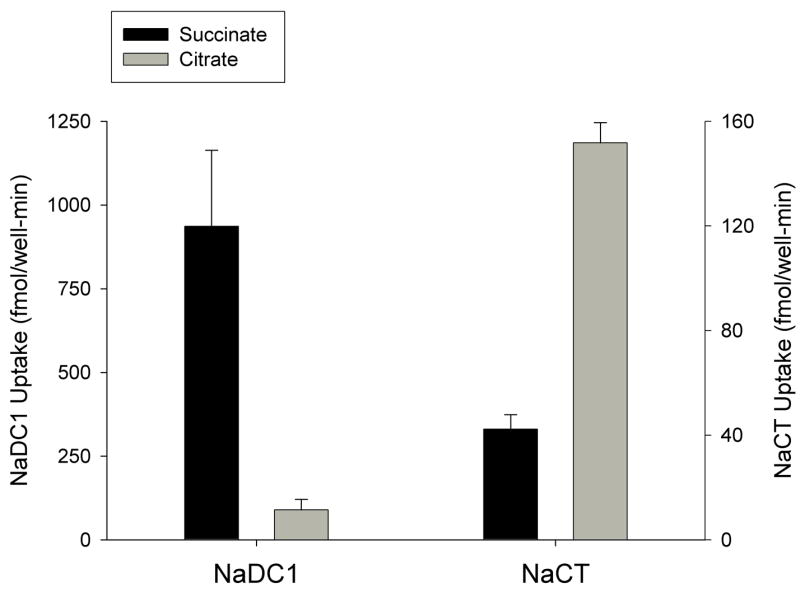
Transport of succinate and citrate in by HRPE cells transfected with hNaDC1 or hNaCT in pcDNA3.1 vector. Uptakes of 10 μM [14C]succinate or [14C]citrate in HRPE cells expressing hNaDC1 (A), or hNaCT (B) were measured for 30 min in sodium- or choline-containing transport buffer. Na+-dependent transport was calculated from the difference between uptakes in sodium and choline. Data shown are the means ± range or S.E.M. (n = 2–8 separate transfection experiments).
3.5. Na+-independent citrate transport in Caco2 cells
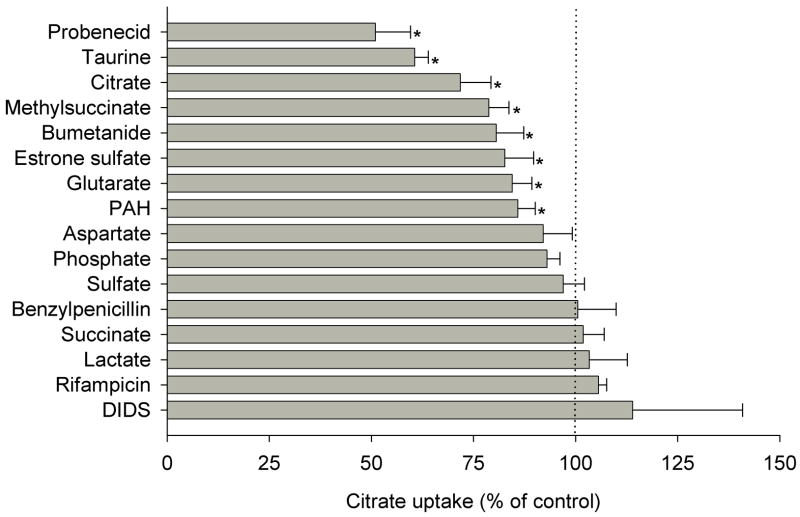
There have been no reports in the literature of sodium-independent transporters for citrate. Because other dicarboxylates, such as α-ketoglutarate and glutarate, are carried by the organic anion transporters or OATs [25,26], we examined the substrate specificity of the sodium-independent citrate transport pathway using test inhibitors, particularly inhibitors of the OAT family. Na+-independent citrate transport in Caco-2 cells was inhibited by other di- or tricarboxylates including citrate, methylsuccinate and glutarate, but not by succinate (Fig. 6). Because 1 mM citrate only produced ~30% inhibition of [14C] citrate, the Km for the combined transport pathways is likely to be greater than 1 mM. Citrate transport was also inhibited by probenecid, bumetanide and p-amino-hippurate (PAH) which are substrates of the known OATs [27–30]. Moreover, citrate transport was inhibited by substrates of OAT4 including taurine, and estrone sulfate [31–33]. However, citrate transport was not inhibited by rifampicin, which rules out other organic anion transporters, including OATPs, as potential transporters [34]. Furthermore, citrate transport was insensitive to phosphate, aspartate, or sulfate. These results are consistent with the functional characteristics of OAT transporters, possibly OAT2 and OAT4, mediating sodium-independent transport of citrate in Caco-2 cells.
Fig. 6.
Substrate specificity of Na+-independent citrate transport in Caco-2 cells. Transport of 10 μM [14C]citrate in Caco-2 cells was measured for 60 min in choline buffer in the absence (control) or presence of the indicated test inhibitors or substrates. Inhibitor concentrations were 1 mM, except for 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS, 0.1mM) and p-aminohippuric acid, (PAH, 5mM). The data are expressed as a percentage of control in the absence of test inhibitor. Data shown are means ± range or S.E.M of 2–8 experiments. * p < 0.05, significantly different from the uptakes in control group.
3.6. RT-PCR analysis of organic anion transporters
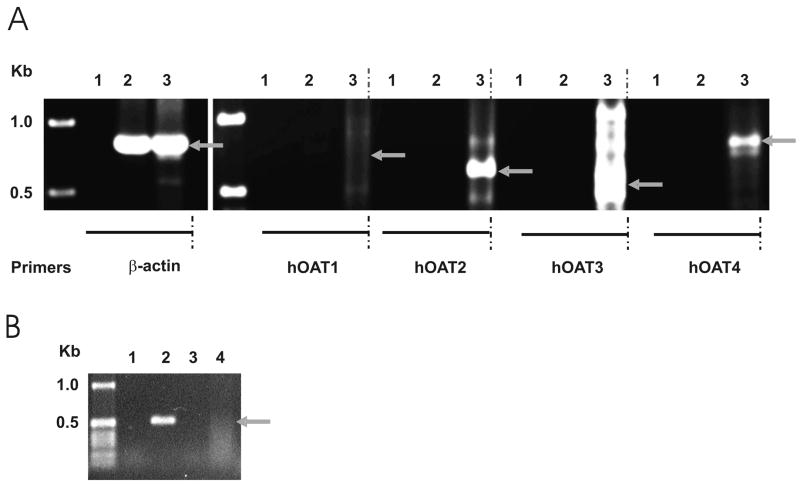
The inhibitor experiment suggested that the Na+-independent transport of citrate might be mediated by OAT2 and OAT4, therefore we tested whether these transporters are expressed in Caco-2 cells using RT-PCR with sequence-specific primers. Previous studies indicate that OAT transporters are not expressed in small intestine [28,31,35,36], and these findings were verified here (Fig. 7A). In contrast, RT-PCR reactions with Caco-2 cells produced positive signals with OAT2 and OAT4 primers, consistent with the mRNA expression profile of OATs in Caco-2 cells [37]. There were multiple products with the OAT3 primers under the conditions used for the other PCR reactions, but changes to the reaction buffer and a decrease in the cycle time produced a positive signal in human kidney, which is known to express OAT3, and no signals in human intestine or Caco2 cells (Fig. 7B). Therefore, OAT2 and OAT4 may be the transporters responsible for Na+-independent transport of citrate in Caco-2 cells.
Fig. 7.
A. RT-PCR analysis of human organic anion transporters (hOATs) in human intestine (lane 2) and Caco-2 cells (lane 3). Controls containing water in place of primers are in lane 1. Control amplification with β-actin primers was performed in parallel. Size standards are shown in leftmost lane. Arrows show the expected size of PCR products. The identity of hOAT2 and hOAT4 products was verified by sequencing. B. RT-PCR using hOAT3 primers under optimized conditions (Buffer J, 30 sec cycles, as described in Methods). Lane 1, water control; lane 2, human kidney; lane 3, human intestine; lane 4, Caco-2 cells.
3.7. Transepithelial transport and intracellular accumulation of methylsuccinate by Caco-2 cells
In initial experiments, the transepithelial fluxes of succinate and citrate were too low to measure accurately (data not shown). Therefore, we tested the transport of the non-metabolizable substrate, methylsuccinate, in Caco-2 cells grown on permeable supports. Methylsuccinate is a known substrate of NaDC1 [38] and OAT4 [33]. In preliminary studies, we found that NaCT also transports methylsuccinate, although at a much slower rate than citrate (Na+-dependent transport of 100 μM substrate: citrate 3.5 pmol/well-min vs methylsuccinate 0.6 pmol/well-min). In the apical to basolateral direction, methylsuccinate accumulation in the cell across the apical membrane exhibited both sodium-dependent and -independent components, whereas transepithelial methylsuccinate fluxes across the epithelium were only sodium-independent (Fig. 8A). In the basolateral to apical direction, both the intracellular accumulation and transepithelial flux were only sodium-independent (Fig. 8B). The rate of transepithelial transport from the basolateral to apical side of the membrane was approximately double that in the apical to basolateral direction. In a single experiment, cellular accumulation of methylsuccinate across the basolateral membrane was not affected by the organic anion transport inhibitors, DIDS (0.1 mM) or bumetanide (1 mM) (results not shown).
Fig. 8.
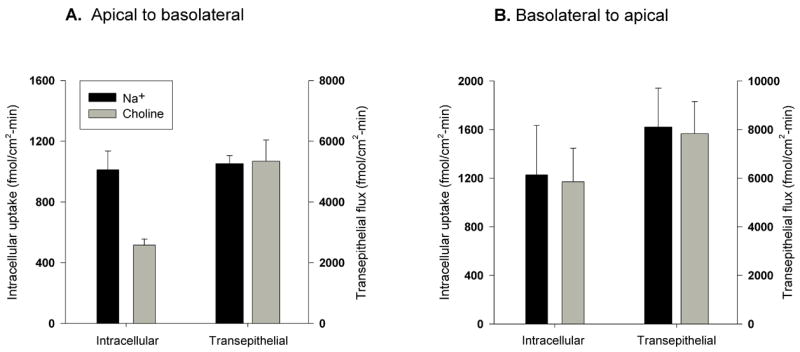
Transepithelial transport of [3H]methylsuccinate by Caco-2 cells. Intracellular accumulation and transepithelial transport of [3H]methylsuccinate (100 μM) were determined in the apical to basolateral (A) or basolateral to apical direction (B). Uptakes were measured for 60 min in sodium and choline buffers. Data are the means ± S.E.M, n = 3 independent experiments.
Discussion
In the current study, we have examined the transport pathways for di- and tricarboxylates in the Caco-2 cell line, originally derived from human colon carcinoma. Succinate transport in Caco-2 cells appears to be almost entirely sodium-dependent, with properties consistent with the low affinity Na+/dicarboxylate cotransporter, NaDC1. Although NaCT is present in the cells, it probably does not contribute much to succinate transport. Citrate transport is mediated by a combination of sodium-dependent and sodium-independent pathways, including NaDC1, NaCT and possibly OAT2 and OAT4. Transepithelial transport across Caco-2 monolayers was studied using the non-metabolizable dicarboxylate, methylsuccinate. Methylsuccinate is a substrate of NaDC1, but it is also carried by several sodium-independent transporters found on the apical and basolateral membranes.
The Na+-dependent transport of succinate and citrate, and probably also methylsuccinate, across the apical membrane of Caco-2 cells was determined to be mediated by a combination of the Na+/dicarboxylate cotransporter, NaDC1, and the Na+/citrate cotransporter, NaCT. Normal human intestine contains mRNA for NaDC1 but Caco-2 cells contain mRNA encoding both NaDC1 and NaCT. The human NaDC1 transports both succinate and citrate, with Km values of 0.8 mM (succinate) and 7 mM (citrate) and similar Vmax values [19]. In contrast, the human NaCT prefers citrate to succinate; the Km value for citrate is 0.6 mM whereas the Km for succinate (estimated from inhibition studies) is around 2 mM [14]. Functional assays indicate that both NaDC1 and NaCT are active in Caco-2 cells. The Km for succinate in Caco-2 cells is around 0.7 mM, consistent with that of hNaDC1. However, the comparison of Na+-dependent succinate and citrate transport in Caco-2 cells indicates that another Na+-dependent citrate transporter, possibly NaCT, may contribute to citrate transport, because the citrate transport activity is higher than would be expected from NaDC1 alone. Na+-dependent succinate transport in Caco-2 cells is approximately double that of citrate transport. In NaDC1 expressed in HRPE cells, succinate transport is about eight fold higher than citrate, whereas in NaCT, succinate transport is less than half of citrate transport. Therefore, NaDC1 appears to be the predominant Na+-dependent transporter in Caco-2 cells, with some contribution by NaCT, particularly to citrate transport.
In addition to the Na+-dependent transport systems, the apical membrane of Caco-2 cells contains one or more Na+-independent citrate transport pathways. Previous studies have shown that organic anion transporters, OAT, transport dicarboxylates such as α-ketoglutarate, glutarate and methylsuccinate in a Na+-independent manner [30,32,33]. RT-PCR analysis showed that mRNA for both OAT2 and OAT4 are found in Caco-2 cells, unlike normal human intestine which does not express OAT1-4 [28,31,35,36]. Functional assays indicate that OAT4 is a possible candidate for the citrate transporter on the apical membrane of differentiated Caco-2 cells since citrate transport was inhibited by bumetanide, taurine and estrone sulfate, all of which are OAT4 substrates [27,32,33]. OAT4 is located on the apical membrane in renal proximal tubule and is thought to contribute to the first step in reabsorption of organic anion substances [32]. OAT2 is also a likely candidate to transport citrate because it was inhibited by PAH [30,39]. We did not find inhibition of sodium-independent citrate transport by succinate although a previous study reported that succinate is transported by hOAT4 [40]. The lack of inhibition could be explained by differences in affinity, but the definitive experiment will be to test whether OAT4 transports citrate. We attempted to verify protein expression by Western blotting with commercially available OAT2 and OAT4 antibodies (Alpha Diagnostics), but the antibodies are not of high enough quality to recognize the native proteins in human kidney homogenate (unpublished observations).
Although there is experimental evidence for transepithelial transport of succinate and citrate in the small intestine, it was not possible to measure transepithelial fluxes of succinate and citrate across Caco-2 cells. The substrates could be metabolized within the cells or the transport rates are too low to measure accurately. The non-metabolizable dicarboxylate, methylsuccinate, was used to evaluate transepithelial transport. Transport of methylsuccinate across the apical membrane into the cell was both Na+-dependent and -independent, possibly mediated by NaDC1 and OAT4, since both are known to transport methylsuccinate [33,38]. NaCT also appears to transport methylsuccinate, although at a much lower rate than citrate (Pajor and Randolph, unpublished observations). It is not known whether other OATs transport methylsuccinate. Transport of methylsuccinate across the basolateral membrane of Caco-2 cells grown on permeable supports is completely sodium-independent and not sensitive to inhibitors such as bumetanide or DIDS, ruling out an OAT transporter and indicating the presence of another sodium-independent transporter. Methylsuccinate is likely to exhibit net secretion, since the transepithelial flux from the basolateral to apical side is greater than from the apical to basolateral.
A model of the possible transport pathways for succinate, citrate and methylsuccinate in Caco-2 cells is shown in Fig.9. The sodium-dependent uptake of di-and tricarboxylates across the brush border membrane into the cells occurs primarily by the activity of NaDC1, with some contribution from NaCT. The energy for the sodium-dependent transport comes from the inwardly-directed electrochemical gradient for sodium, maintained by the Na+/K+-ATPase at the basolateral membrane. One or more sodium-independent transporters for citrate and methylsuccinate, possibly OAT2 and/or OAT4, are also located on the apical membrane. Bidirectional transport of methylsuccinate across the basolateral membrane occurs on an unknown transporter that is not likely to be a member of the OAT family.
Fig. 9.
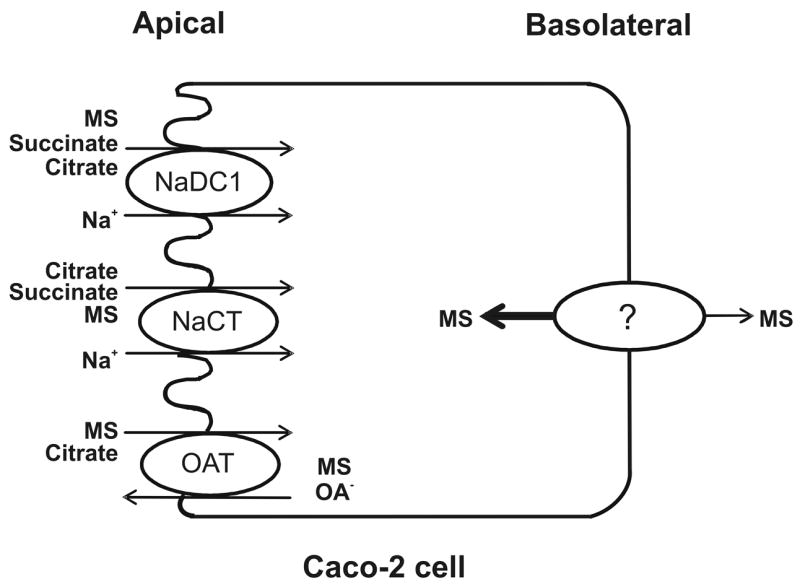
Model for transport of di- and tricarboxylates by Caco-2 cells. The Na+/dicarboxylate cotransporter (NaDC1) on the apical membrane transports succinate, methylsuccinate (MS) and divalent citrate. The Na+-coupled citrate transporter (NaCT) transports trivalent citrate, but also some succinate and methylsuccinate. An organic anion transporter, probably OAT4, transports citrate and methylsuccinate in exchange for organic anions (OA−). The basolateral membrane contains an unknown sodium-independent transporter for methylsuccinate.
In conclusion, this study demonstrates that multiple transport pathways are involved in the transport of citric acid cycle intermediates in differentiated Caco-2 cells. The apical membrane of Caco-2 cells contains at least three transport systems, both Na+-dependent and -independent, for di- and tricarboxylates. The Na+-dependent pathways include NaDC1 and NaCT, and the Na+-independent transporters are possibly the OAT2 or OAT4 organic anion transporters. There is also a Na+-independent transporter on the basolateral membrane. RT-PCR experiments show that Caco-2 cells express different dicarboxylate and organic anion transporters than human small intestine. Although the Caco-2 cells may be useful for studying succinate transport by NaDC1 in vitro, their suitability as a model for studying citrate and methylsuccinate transport is limited since these cells express transporters that are not found in native intestine. The difference between Caco-2 cells and normal intestine could reflect the cancerous state of the Caco-2 cells, resulting in altered metabolism and phenotype [41,42].
Acknowledgments
This research was supported by the National Institutes of Health, grant DK46269. Present address of Jittima Weerachayaphorn: Yale University School of Medicine, Department of Internal Medicine, Section of Digestive Diseases, New Haven, CT 06520-8019.
Abbreviations
- NaDC1
Na+/dicarboxylate cotransporter 1
- NaCT
Na+-coupled citrate transporter
- OAT
organic anion transporter
- TEER
transepithelial electrical resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pajor AM. Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflugers Arch. 2006;451:597–605. doi: 10.1007/s00424-005-1487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pajor AM. Citrate transport by the kidney and intestine. Seminars in Nephrology. 1999;19:195–200. [PubMed] [Google Scholar]
- 3.Rogina B, Reenan RA, Nilsen SP, Helfand SL. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- 4.Fei YJ, Inoue K, Ganapathy V. Structural and functional characteristics of two sodium-coupled dicarboxylate transporters (ceNaDC1 and ceNaDC2) from Caenorhabditis elegans and their relevance to life span. J Biol Chem. 2003;278:6136–6144. doi: 10.1074/jbc.M208763200. [DOI] [PubMed] [Google Scholar]
- 5.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 6.Sakhaee K, Alpern R, Poindexter J, Pak CY. Citraturic response to oral citric acid load. J Urol. 1992;147:975–976. doi: 10.1016/s0022-5347(17)37437-2. [DOI] [PubMed] [Google Scholar]
- 7.Browne JL, Sanford PA, Smyth DH. Transfer and metabolism of citrate, succinate, alpha-ketoglutarate and pyruvate by hamster small intestine. Proc R Soc Lond B Biol Sci. 1978;200:117–135. doi: 10.1098/rspb.1978.0010. [DOI] [PubMed] [Google Scholar]
- 8.Wolffram S, Bisang B, Grenacher B, Scharrer E. Transport of tri- and dicarboxylic acids across the intestinal brush border membrane of calves. J Nutr. 1990;120:767–774. doi: 10.1093/jn/120.7.767. [DOI] [PubMed] [Google Scholar]
- 9.Wolffram S, Hagemann C, Grenacher B, Scharrer E. Characterization of the transport of tri- and dicarboxylates by pig intestinal brush-border membrane vesicles. Comp Biochem Physiol Comp Physiol. 1992;101:759–767. doi: 10.1016/0300-9629(92)90355-t. [DOI] [PubMed] [Google Scholar]
- 10.Wolffram S, Unternahrer R, Grenacher B, Scharrer E. Transport of citrate across the brush border and basolateral membrane of rat small intestine. Comp Biochem Physiol. 1994;109A:39–52. doi: 10.1016/0300-9629(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 11.Wolffram S, Zimmermann W, Scharrer E. Transport of tricarballylate by intestinal brush-border membrane vesicles from steers. Exp Physiol. 1993;78:473–484. doi: 10.1113/expphysiol.1993.sp003699. [DOI] [PubMed] [Google Scholar]
- 12.Hillgren KM, Kato A, Borchardt RT. In vitro systems for studying intestinal drug absorption. Med Res Rev. 1995;15:83–109. doi: 10.1002/med.2610150202. [DOI] [PubMed] [Google Scholar]
- 13.Sambuy Y, de A, Ranaldi IG, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K, Zhuang L, Ganapathy V. Human Na+-coupled citrate transporter: primary structure, genomic organization, and transport function. Biochem Biophys Res Commun. 2002;299:465–471. doi: 10.1016/s0006-291x(02)02669-4. [DOI] [PubMed] [Google Scholar]
- 15.Pajor AM, Randolph KM. Conformationally sensitive residues in extracellular loop 5 of the Na+/dicarboxylate co-transporter. J Biol Chem. 2005;280:18728–18735. doi: 10.1074/jbc.M501265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimelberg HK, O'Connor ER, Sankar P, Keese C. Methods for determination of cell volume in tissue culture. Can J Physiol Pharmacol. 1992;70(Suppl):S323–S333. doi: 10.1139/y92-279. [DOI] [PubMed] [Google Scholar]
- 17.Garthwaite J. Discrepancies in the extracellular space of sympathetic ganglia measured using different isotopes of mannitol and sucrose. J Neurosci Methods. 1979;1:185–193. doi: 10.1016/0165-0270(79)90016-5. [DOI] [PubMed] [Google Scholar]
- 18.Ramond MJ, Martinot-Peignoux M, Erlinger S. Dome formation in the human colon carcinoma cell line Caco-2 in culture. Influence of ouabain and permeable supports. Biol Cell. 1985;54:89–92. doi: 10.1111/j.1768-322x.1985.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 19.Pajor AM, Sun N. Functional differences between rabbit and human Na+-dicarboxylate cotransporters, NaDC-1 and hNaDC-1. Am J Physiol. 1996;271:F1093–F1099. doi: 10.1152/ajprenal.1996.271.5.F1093. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Fei YJ, Kekuda R, Yang-Feng TL, Devoe LD, Leibach FH, Prasad PD, Ganapathy V. Structure, function, and genomic organization of human Na+-dependent high-affinity dicarboxylate transporter. Am J Physiol Cell Physiol. 2000;278:C1019–C1030. doi: 10.1152/ajpcell.2000.278.5.C1019. [DOI] [PubMed] [Google Scholar]
- 21.Pajor AM. Molecular cloning and functional expression of a sodium-dicarboxylate cotransporter from human kidney. Am J Physiol. 1996;270:F642–F648. doi: 10.1152/ajprenal.1996.270.4.F642. [DOI] [PubMed] [Google Scholar]
- 22.Kekuda R, Wang H, Huang W, Pajor AM, Leibach FH, Devoe LD, Prasad PD, Ganapathy V. Primary structure and functional characteristics of a mammalian sodium-coupled high affinity dicarboxylate transporter. J Biol Chem. 1999;274:3422–3429. doi: 10.1074/jbc.274.6.3422. [DOI] [PubMed] [Google Scholar]
- 23.Weerachayaphorn J, Pajor AM. Threonine-509 is a determinant of both substrate and cation affinity in the human Na+/dicarboxylate cotransporter. Biochemistry. doi: 10.1021/bi701417h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue K, Zhuang L, Maddox DM, Smith SB, Ganapathy V. Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J Biol Chem. 2002;277:39469–39476. doi: 10.1074/jbc.M207072200. [DOI] [PubMed] [Google Scholar]
- 25.Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447:666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 26.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 27.Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, Khamdang S, Aleboyeh M, Onozato ML, Tojo A, Enomoto A, Anzai N, Narikawa S, Huang XL, Niwa T, Endou H. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308:1021–1029. doi: 10.1124/jpet.103.059139. [DOI] [PubMed] [Google Scholar]
- 28.Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol. 1999;276:F122–F128. doi: 10.1152/ajprenal.1999.276.1.F122. [DOI] [PubMed] [Google Scholar]
- 29.Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol Renal Physiol. 2003;284:F763–F769. doi: 10.1152/ajprenal.00405.2002. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]) J Pharm Pharmacol. 2005;57:573–578. doi: 10.1211/0022357055966. [DOI] [PubMed] [Google Scholar]
- 31.Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275:4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 32.Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94:297–304. doi: 10.1254/jphs.94.297. [DOI] [PubMed] [Google Scholar]
- 33.Benyajati S, Pritchard JB. Taurine efflux is mediated by renal luminal organic anion transporter, hOAT4 (SLC22A11) FASEB J. 2005;19:A150. Abstr. [Google Scholar]
- 34.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 35.Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 36.Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett. 1998;429:179–182. doi: 10.1016/s0014-5793(98)00585-7. [DOI] [PubMed] [Google Scholar]
- 37.Seithel A, Karlsson J, Hilgendorf C, Bjorquist A, Ungell AL. Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: Comparison between human segments and Caco-2 cells. Eur J Pharm Sci. 2006;28:291–299. doi: 10.1016/j.ejps.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Yao X, Pajor AM. The transport properties of the human renal Na+-dicarboxylate cotransporter under voltage-clamp conditions. Am J Physiol Renal Physiol. 2000;279:F54–F64. doi: 10.1152/ajprenal.2000.279.1.F54. [DOI] [PubMed] [Google Scholar]
- 39.Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001;283:417–422. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- 40.Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, Shiraya K, He X, Cha SH, Takeda M, Miyazaki H, Sakata T, Tomita K, Igarashi T, Kanai Y, Endou H. Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. J Pharmacol Exp Ther. 2005;315:534–544. doi: 10.1124/jpet.105.088583. [DOI] [PubMed] [Google Scholar]
- 41.Anderle P, Rakhmanova V, Woodford K, Zerangue N, Sadee W. Messenger RNA expression of transporter and ion channel genes in undifferentiated and differentiated Caco-2 cells compared to human intestines. Pharm Res. 2003;20:3–15. doi: 10.1023/a:1022282221530. [DOI] [PubMed] [Google Scholar]
- 42.Chung JK, Lee YJ, Kim C, Choi SR, Kim M, Lee K, Jeong JM, Lee DS, Jang JJ, Lee MC. Mechanisms related to [18F]fluorodeoxyglucose uptake of human colon cancers transplanted in nude mice. J Nucl Med. 1999;40:339–346. [PubMed] [Google Scholar]