Summary
Research on the neuronal control of locomotion in leeches spans almost four decades. Recent advances reviewed here include discoveries that 1) interactions between multiple hormones modulate initiation of swimming, 2) stretch receptors associated with longitudinal muscles interact with the central oscillator circuit via electrical junctions, 3) intersegmental interactions, according to theoretical analyses, must be relatively weak compared to oscillator interactions within ganglia, and 4) multiple interacting neurons control the expression of alternative modes of locomotion. The innovative techniques that facilitated these advances include optical recording of membrane potential changes, simultaneous intracellular injection of high and low molecular weight fluorescent dyes, and detailed modeling via an input-output systems engineering approach.
Introduction
A central tenet of neuroethology is that animal behavior results from the cellular properties of, and synaptic interactions among sensory neurons, interneurons, and motoneurons. Neuroethologists study the nervous system with the aim of identifying cellular properties and delineating synaptic interactions that are the neuronal substrates of specific behaviors. A major component of this effort, which aims to discover general principles concerning the neuronal control of animal movements, is the study of sensory mechanisms, central oscillatory circuits, and motoneuron effectors that generate rhythmic locomotion. Investigation of such repetitious movements is particularly tractable because the underlying neuronal circuits tend to be well-delimited and the behavior is readily quantified. Although the list is by no means exhaustive, essential elements of the conceptual schema informing research on rhythmic movements include hormonal regulation of behavior, sensory feedback generated by executed movements, and behavioral choice. Because of the species specificity and high complexity of neuronal circuits, the overall neuroethological research program is necessarily comparative, involving diverse species ranging from annelids to mammals, with approaches ranging from behavioral observations to mathematical modeling [1-5]. Individual labs typically focus on one, or perhaps two species. In addition to the leech, preparations that have enjoyed especial attention for study of rhythmic movements include the mollusks Tritonia and Melibe; arthropod species of locust, crayfish, and stick insects; and vertebrate preparations of lamprey, Xenopus, neonatal rodents, and cats.
Studies on the neuronal substrates of leech behavior began with 19th-century observations of leech anatomy and embryology, continued with behavioral and physiological studies in the next century, and now goes on with numerous studies on leech embryology, development, pharmacology, and neurophysiology. As a result, sensory and motor systems in the leech are among the best understood in nature, providing a foundation for increasingly quantitative models that explicitly reveal the interplay between central oscillators and sensory feedback in the execution of leech behavior [2]. This review focuses on advances in leech locomotion during the past 3 years, from 2004 to 2007. Because progress in our understanding of locomotion in leeches has been most pronounced in these areas, we review: 1) hormonal control of locomotion initiation; 2) integration of sensory feedback with central circuits to express effective movements; 3) functional characteristics of intersegmental coordination through computer simulation and mathematical analysis; and 4) decision-making mechanisms whereby sensory input is channeled to activate swimming or crawling, but not both at once. Through comparative studies in other species, these areas represent foci of opportunity, where further research can lead to deep and broad insights in the neuronal origins of animal behavior.
Hormonal control of swim initiation
The head ganglia provide the highest level of control for leech locomotion. In particular, the subesophageal ganglion includes somata of several neurons whose activity levels are correlated with swim initiation or termination in isolated nerve cords (Fig.1). Serotonin, as well as octopamine application to the isolated nerve cord induces spontaneous swimming oscillations. New studies demonstrate that such spontaneous swimming activity is particularly strongly induced when a mixture of serotonin and octopamine is applied and then washed out [6]. This manipulation enhanced not only swim initiation, revealed by an increased occurrence of spontaneous episodes of swimming, but also swim maintenance expressed as an increase in the number of cycles in swim episodes. Inhibitory effects of local drug application to the head ganglia may be mediated via a swim-gating neuron (cell 204) and perhaps a trigger neuron (Tr1), both of which are inhibited by focal application of serotonin to the head ganglion [7]. Another cephalic (cell SE1) also directly excites swim-gating cells, oscillator cells, and swim-related MNs and because it (like cell 204) projects to most, if not all, ganglia, it too may mediate hormone-induced inhibition and excitation [8].
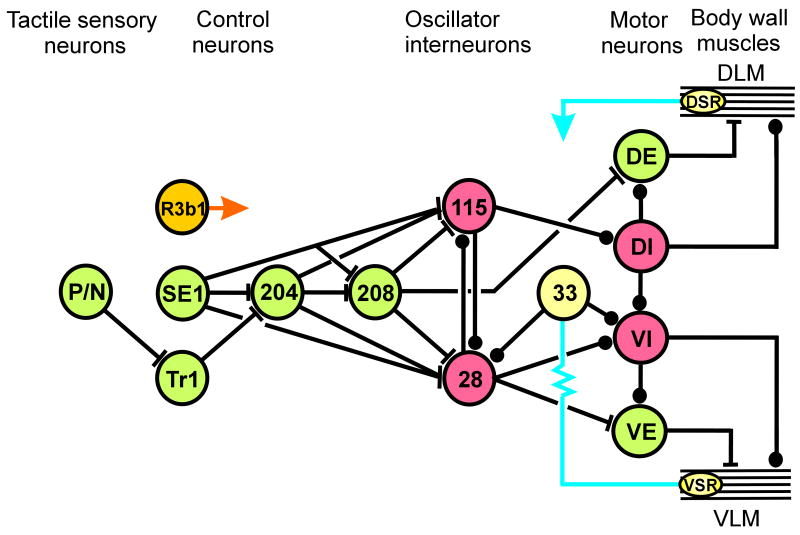
Figure 1.
Neurons and circuits. Subset of the neuronal circuits that control and generate leech locomotion. The cascade from left to right begins with swim-initiating sensory neurons, which are found in each segment. The somata of control neurons R3bi, SE1, and Tr1 are found in the subesophageal ganglion, but their axons project to the posterior end of the nerve cord. The somata of swim-gating cell 204 are restricted to segmental ganglia 10 - 16, whereas those of oscillator interneurons, are found in most, if not all, segmental ganglia. These cells all have extensive intersegmental projections. Finally, motor neurons and the stretch receptors (VSR and DSR) are local, with processes restricted to individual segments. Abbreviations and symbols: P - pressure-sensitive neuron; N - nociceptive neuron; R3b1 - decision neuron, SE1 - swim excitor neuron; Tr1 - trigger neuron; 204 - swim-gating neuron; 208, 33, 115, 28, and 33 - subset of the central oscillator neurons; DE - dorsal excitor; DI - dorsal inhibitor; VI - ventral inhibitor; VE - ventral excitor; DSR - dorsal stretch receptor; VSR - ventral stretch receptor; DLM - dorsal longitudinal muscle; VLM - ventral longitudinal muscle; filled circles - inhibitory synapses, bars - excitatory synapses; and resistor - non-rectifying electrical interaction. Green denotes excitatory, and red denotes inhibitory neurons. The direct targets of R3b1 and DSR are not identified. For the sake of simplicity some identified neurons and many synaptic interactions are not depicted in this diagram.
Initiation of swimming in leeches by stimulation of tactile sensory neurons habituates with repeated stimulation. As in other animals, the response also exhibits sensitization and dishabituation. Recent studies [9] reveal that the dishabituation by tactile stimulation reverses habitation induced by repetitive electrical stimulation. This effect is mimicked by intra-crop application of serotonin. Moreover the dishabituating action of serotonin and of mechanical stimulation is reversed by inhibitors of cAMP synthesis, but mimicked by injection of the membrane permeable cAMP analog, 8-bromocyclic AMP. Hence, it appears that the elevation of serotonin, naturally released by Retzius cells when tactile mechanosensory neurons are activated, increases the responsiveness of leeches to swim-inducing sensory input. Likely sites of action for the facilitating action of serotonin, whether at sensory terminals or swim initiating interneurons, are not revealed by these experiments. In particular, because the head brain was disconnected and only swim latency was measured, these data leave unaddressed questions regarding the role of serotonin in swim maintenance or connections within the head brain. Delay in habituation of swim initiation when stroking the body wall in a nearly isolated preparation was seen when only two ganglia – far from the recording site – were exposed to exogenous serotonin [10].
Results from these pharmacological manipulations can help explain the results of behavioral and physiological experiments on the propensity of swimming in leeches that were tested after their exposure to artificial blood (active ingredient - L-arginine) [11]. These experiments showed that leech swimming is elicited more readily when the animals have been exposed to weak concentrations of the artificial blood. Even more interesting was the finding that high concentrations of artificial blood applied to the anterior leech inhibited swim initiation, perhaps because this corresponds to a real-life scenario where the leech actually is very near its prey and swimming would take it away from, rather than towards a meal. Behavioral choice experiments on leeches exposed to a variety of experimental manipulations are now greatly facilitated by the development of an automated optical monitoring system designed specifically for tracking leech behaviors [12, 13].
A deeper understanding of the mechanisms through which swimming behavior is modulated arises from recent studies of neurohormonal effects in lamprey and toads. As in leeches, serotonin is an important modulator of swimming locomotion in lamprey. In the latter, two levels of action occur in both excitatory and inhibitory spinal interneurons: at the cellular level (via a reduction in the amplitude of a slow afterhyperpolarization), and at the circuit or synaptic level (via a reduction In synaptic strength) [14]. Similarly, glutamate, acting via metabotropic receptors, can act as a modulator, rather than as a fast-acting excitatory neurotransmitter. In Xenopus tadpoles, differential activation of Group I, II, and III, metabotropic glutamate receptors in paralyzed, fictively swimming preparations altered swim cycle, motoneuron burst durations and burst amplitudes [15]. These findings are interesting because, although glutamate is commonly known to mediate excitatory interactions via ionotropic receptors, the modulatory role of metabotropic glutamate receptor is less widely appreciated.
Integration of sensory feedback with central circuits
The leech ventral nerve cord includes circuits (central pattern generator – CPG) that generate swim-like activity even in the absence of all sensory input. In the intact animal, these circuits, together with sensory feedback, shapes the swimming movements. One such input arises from stretch receptors associated with ventral longitudinal muscles [16]. One of these receptors (ventral stretch receptors – VSRs), are inhibited by increases in muscle tension, generated either by MN stimulation or stretching the body wall. The VSR membrane potential oscillates (up to 10 mV peak-to-trough) in concert with rhythmic muscle contractions. These cells are interconnected with CPG neurons by strong electrical coupling, so that VSR membrane potential can affect cycle-by-cycle modulation [17]. Additional neurons, with morphology similar to the VSR were studied through injection of fluorescent (Alexa Fluor hydrazide) dyes. The unique terminal microanatomy of these axons within nerve cord ganglia makes them individually identifiable [18]. One of these is a putative dorsal stretch receptor (DSR) that could provide input from dorsal longitudinal muscle. Rhythmic currents injected into the VSR can entrain on-going swimming over a large frequency range (0.9–1.8Hz); moreover, current pulses injected into the VSR shift the phase of the swimming rhythm [19]. These results predict that the VSR, and likely the DSR, play a major part in generating effective swim movements.
A novel technique for identifying functional neuronal circuits is through intracellular injections of two fluorescent dyes, one with a relatively low molecular weight (neurobiotin) that readily diffuses through gap junctions to reveal electrical coupling, the other with a higher weight (Alexa Fluor 488) that remains within the injected neuron [20]. Application of this technique to swim-related MNs revealed that these are each dye-coupled to about 25 neurons, half of which are small, candidate interneurons (INs). Dye-coupling was found to be a reliable indicator of electrical connections. None of the MNs is dye coupled to any putative stretch receptor terminals.
An ongoing investigation of the mechanical properties of leech body wall is part of a larger project to unite central oscillator circuits with execution of leech swimming movements (Fig. 2). The overall aim is to predict behavioral dynamics from models of system elements, of which tension generated by muscles is a critical element. Two types of experiments have established length-tension relationships for leech muscles: step-stretch manipulations [21] and imposed sinusoidal length changes near the length observed during actual swimming [22]. Step-stretch data were described well by a mathematical model comprising three nonlinear springs, two in series with nonlinear dashpots. Rhythmic length changes, similar to those of swimming leeches were predicted by a static model, comprising one nonlinear spring. Serotonin application, to mimic concentrations in intact leeches, reduced steady-state and peak tensions, without alterations in tension dynamics. Experiments in which leeches were anesthetized with ethanol suggested that passive tension in the obliquely striated muscles of leeches results largely from a resting tonus [22].
Figure 2.
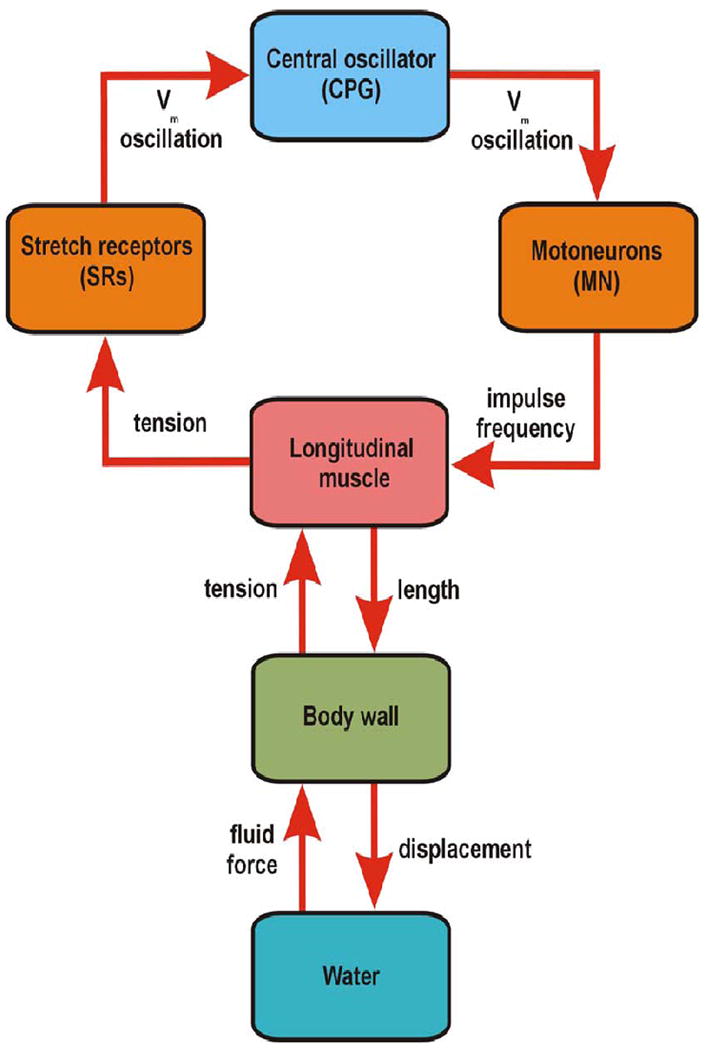
Systems overview. The system that generates and executes swimming locomotion in leeches comprises many complex subsystems. The central oscillator (central pattern generator – CPG) includes mostly inhibitory intersegmental interneurons that drive inhibitory and excitatory segmental motor neurons. These command, or countermand local, antiphasic contractions of segmental dorsal and ventral longitudinal muscles. Muscle contractions, which are phase-delayed along the body, cast the body wall into a rearward traveling body wave of about length wavelength. Movement of the body against the water medium generates forces (fluid force) that are essential elements in the realization of the sinusoidal swimming undulations and in turn, critically affect the tension in longitudinal muscles. These tensions are transduced by peripheral stretch receptors (SRs) associated with the longitudinal muscles. Giant, non-spiking axons convey graded rhythmic membrane potentials to the central oscillator through strong non-rectifying electrical interactions with at least one interneuron, thereby completing a loop of interactions. Both motor neuron impulse frequencies and muscle lengths, strongly influenced by fluid-body wall interactions, determine muscle tensions during each sector of the swim cycle.
Such studies on the properties of muscle are essential for filling the gap between models of central oscillator mechanisms and output (see below) and a full model of swimming behavior that includes the mechanical properties of the animal as well as the forces generated by body-fluid interactions. Progress towards a greater understanding of such mechanical properties was provided by a recent theoretical study in which the swimming animal is viewed as an elliptical elastic rod with imposed time-dependent curvature changes to generate a traveling wave [23]. This study includes both mathematical analyses of forces on the continuous rod and numerical simulations of a rod comprising up to 80 discrete segments, to simulate swimming in lamprey. The model included motoneuron activity, the effects of body stiffness (analogous to passive and active muscle tensions), and fluid forces.
Computer simulation and mathematical analysis
The central oscillator generating the swimming rhythm in leeches was initially modeled topologically with essential intrasegmental and intersegmental components [24]. A series of three successively more sophisticated and refined modeling studies [25–27], have helped to define the nature of intersegmental coordination in the swim oscillator circuits. The initial study demonstrated feasibility of a 5-cell recurrent cyclic inhibition (RCI) model that spanned two segments [24]. The next refinement demonstrated that intersegmental interactions between intrasegmental IN phase groups could generate the rearward progressing body wave by generating intersegmental phase lags of about 10 degrees. In this phase-coupled model, detailed intra- and intersegmental interactions were replaced by segmental phase oscillators linked by intersegmental interactions during four phases of the cycle [25]. Use of phase-response curves derived from models of identified intersegmental interactions was a further refinement that substantiated the earlier conclusions [26]. Recently, a systems-level model brought new insights into the origins of intersegmental phase lags in swimming leeches. This mathematical model is of intermediate complexity based on the premise that a high level of biophysical realism at the segmental level is not critical for capturing the intersegmental phase lag property [27]. Instead of utilizing detailed synaptic interactions, segmental oscillators were simulated by a ring of 3 inhibitory neurons, which, when connected by the identified intersegmental synaptic interactions, achieved realistic intersegmental phase relationships. Models parameters were developed from the input/output relationships in physiological data yielding synaptic amplitudes, time constants, and impulse conduction data. This novel approach generates quantitative predictions of cycle period, intersegmental phase lags, and oscillation amplitude, and also reveals explicitly the relationships between model parameters and system properties [27].
Decision making
A simple notion about how nervous systems accomplish behavioral choice is that the decision-making neurons function as “command neurons,” whose only role in life is to be activated by a particular pattern of processed sensory input, along with internal states and memories, and to, in turn, activate the central pattern-generators for a particular behavior [28]. A decision is accomplished by a competition among command neurons for different behaviors (e.g., they might inhibit one another) that culminates in the expression of a single behavior. This notion was tested in the leech by recording from swim command neurons – both gating and trigger interneurons – while eliciting shortening. Surprisingly, three out of four of these interneurons were excited during shortening [29]. Another complication in the simple “competition among command neurons” view was the finding of a neuron in the subesophageal ganglion, named R3b1, whose activation sometimes commands swimming and other times crawling [30]. This locomotory choice is strongly influenced by the depth of water experienced by the intact body of the animal: in shallow water, R3b1 normally elicits crawling and in deep water, it usually elicits swimming. These interactions among swimming, crawling, and shortening led to the idea that command neurons are actually multifunctional--they help to choose among several different behaviors--and which behavior occurs is determined by the combination of neurons activated [31]. Hormonal control of the choice between swimming and crawling in leeches may be mediated by dopamine, which has an inhibitory effect on the expression of spontaneous swimming but not crawling [32].
To find the neurons making the decision to swim or crawl, a stimulus was delivered to the isolated nervous system that produced either swimming or crawling, randomly, at about equal probability while recording from most of the neurons in a segmental ganglion using voltage-sensitive dyes [33]. Most neurons in the ganglion were active during both behaviors, but about 50% of them were equally active during both behaviors. In fact, only a small number of them (3-5%) showed different activity in the interval between the delivery of the stimulus and the expression of a definitive motor pattern. The subtle differences in activity of the earliest reliable predictors of the ensuring activity were detected only by analyzing their covarying activity. Depolarizing and hyperpolarizing one of these neurons, cell 208, biased the choice between swimming and crawling. In sum, the experimental results on behavioral choice, suggest that decision-making in the leech is a sequential process (Fig. 3), with a general decision (“do something”) made first, followed by progressively more specific decisions as the specific behavior is selected.
Figure 3.
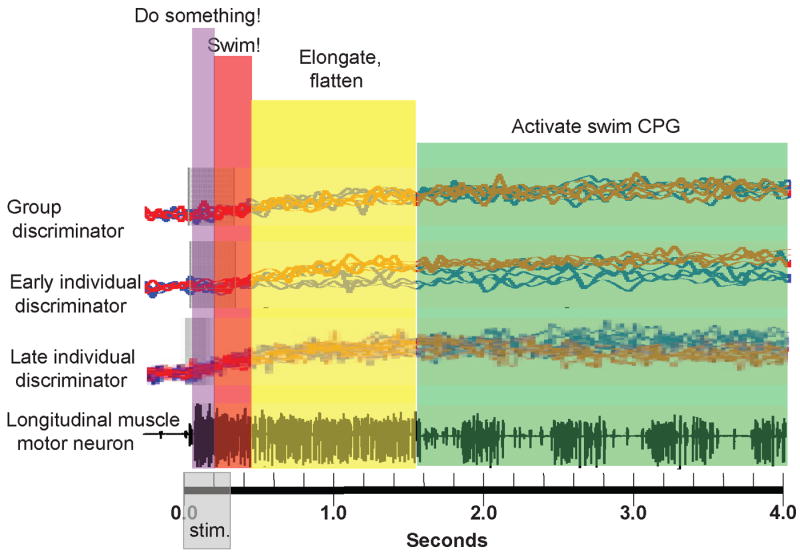
Hypothesized series of choices made by a leech nervous system in deciding to swim. The bottom trace is an extracellular recording from a nerve in the middle of an isolated leech nervous system; the largest spikes are from a motor neuron (DE-3) that excites dorsal longitudinal muscles. A burst of electrical pulses during the time indicated by “stim.,” delivered to another nerve, activated DE-3 for about 1.5 seconds, followed by the swim motor program, indicated by a series of four-spike bursts. The top traces (“Group discriminator”) are 9 overlain optical trajectories from one neuron when the stimulation led to swimming (blue traces) or crawling (red traces). The traces from this neuron did not, by themselves, discriminate swimming from crawling, but taken together with several other neurons, they did predict which behavior would occur during the time period marked by the red vertical stripe. Other neurons, typified by the second set of traces (“Early individual discriminator”) did predict which behavior would occur, but only at later times, marked by the yellow vertical stripe. These neurons are likely to be involved in the elongation and flattening movements made by the leech as it prepares to swim. Still other neurons (“Late individual discriminator”) became active during the production of the motor pattern (green vertical stripe); these are likely to be the neurons responsible for generating the swim motor program. It should be noted that the DE-3 motor neuron began to fire even earlier than the earliest discriminators (violet stripe), which is thought to mean that there were decision-making neurons active before the swimming/crawling decision was made, readying the nervous system for either behavior. The time after the delivery of the stimulus, therefore, is thought to consist of four kinds of decisions: (1) “do something;” (2) “swim or crawl” (swim, in the nerve recording shown); (3) “prepare the body,” by elongating and flattening it; and, finally, (4) make the appropriate motor pattern. Examples of all but the first kind of interneuron have been identified. (This figure is based upon data from references 30 and 33.)
One view of the multifunctional nature of neurons is that the same neuronal circuitry can be used for different behaviors, an idea first proposed by Peter Getting, in his studies of swimming in the sea slug Tritonia [34]. A recent study of swimming and crawling in the leech showed that most neurons in the segmental ganglion that oscillate in phase with the swimming motor program also oscillate in phase with the much slower and much differently coordinated crawling motor program [35]. This suggests that, as in the stomatogastric ganglion of lobsters and crabs [36], the multifunctional neuronal neurons in the leech can be reconfigured into different behavioral circuits. A recent review [37] compared decision-making in leeches and mollusks and concluded that such reconfiguring may be a major contributor to deciding which behavior is selected in a variety of leech and molluscan behaviors.
Conclusions
Although there is good understanding of the mechanisms whereby intersegmental synaptic interactions generate intersegmental phase lags during swimming activity in the isolated nervous system of the leech, much remains unclear on other fronts. For example, the synaptic interactions that underlie behavioral choice are unknown. Similarly, specific sites of action for hormonal control of swim initiation have yet to be described. The neuronal mechanisms underlying the initiation of sustained animal movements – including leech swimming – by brief stimuli remains a puzzle. A long-standing hypothesis that sustained excitation arises from reciprocal excitatory synaptic interactions has recently received much delayed support from whole-cell clamp experiments Xenopus tadpoles, where electrophysiological and modeling studies showed that these interactions could underlie sustained excitatory locomotory drive [38]. Similarly positive feedback between neurons in the lamprey brainstem are thought to act in concert with plateau potentials in the reticulospinal command system to cause protracted depolarization in response to brief stimulation [39]. Such reciprocal excitation or cellular processes may also underlie maintenance of swimming in the leech.
The use of novel techniques described in this brief review, optical recording, injection of diffusing and localized fluorescent dyes, mathematical analysis, and computer simulations, should provide new insights. Studies on the ontogeny of leech locomotion [40] together with the new technique for optical tracking of freely behaving leeches [12, 13], should fuel further progress in understanding of leech locomotion. More broadly, comparative studies on the leech (annelid), snails (mollusk), crayfish (arthropod), lamprey (vertebrate), and mammalian neonatal rodents should lead to deep and broad insights into the neuronal bases of locomotion in all animals.
Acknowledgments
Supported by grants from the National Science Foundation (Research grant IOS-0615631 to WOF), the National Institutes of Health (Research grants NS46057 to WOF; MH43396 and NS35336 to WBK), and Microsoft Research Labs (WBK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of the review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;763:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 2.Kristan WB, Jr, Calabrese R, Friesen WO. Neuronal control of leech behavior. Prog in Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]; **A review of what is known about behaviorally relevant neuronal circuits in the leech central nervous system.
- 3.Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals. 2004;13:50–69. doi: 10.1159/000076158. [DOI] [PubMed] [Google Scholar]
- 4.Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 5.Büschges A. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiology. 2005;93:1127–1135. doi: 10.1152/jn.00615.2004. [DOI] [PubMed] [Google Scholar]
- 6*.Crisp KM, Mesce KA. Beyond the central pattern generator: amine modulation of decision-making neural pathways descending from the brain of the medicinal leech. J Exp Biol. 2006;209:746–56. doi: 10.1242/jeb.02204. [DOI] [PubMed] [Google Scholar]; Serotonin is long known to powerfully modulate the expression of swimming locomotion in the leech. This study shows that serotonergic neurons in the leech brain are excited by stimuli that initiate swimming activity and when swimming is spontaneous. Intracellular recordings from command-like neurons in leech brain revealed that serotonin application inhibited high-level locomotory control neurons and led, via a reduction in drive to segmental swim-gating neurons, to a reduction of the excitatory drive to the swim oscillator circuit. Although the overall effect of serotonin is to facilitate swimming activity, serotonin may act via brain neurons to limit swimming activity at the level of the head brain.
- 7.Crisp KM, Mesce KA. To swim or not to swim: regional effects of serotonin, octopamine and amine mixtures in the medicinal leech. J Comp Physiol A. 2003;189:461–470. doi: 10.1007/s00359-003-0424-0. [DOI] [PubMed] [Google Scholar]
- 8.Brodfuehrer PD, Parker HJ, Burns A, Berg M. Regulation of the segmental swim-generating system by a pair of identified interneurons in the leech head ganglion. J Neurophysiol. 1995;73:983–992. doi: 10.1152/jn.1995.73.3.983. [DOI] [PubMed] [Google Scholar]
- 9.Zaccardi ML, Traina G, Cataldo E, Brunelli M. Sensitization and dishabituation of swim induction in the leech Hirudo medicinalis: role of serotonin and cyclic AMP. Behav Brain Res. 2004;153:317–326. doi: 10.1016/j.bbr.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Alkatout BA, Marvin NM, Crisp KM. Serotonin delays habituation of leech swim response to touch. Behav Brain Res. 2007;182:145–149. doi: 10.1016/j.bbr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 11*.Brodfuehrer PD, Tapyrik L, Pietras N, Zekavat G, Convery M. Modification of leech behavior following foraging for artificial blood. J Comp Physiol A. 2006;192:817–25. doi: 10.1007/s00359-006-0119-4. [DOI] [PubMed] [Google Scholar]; The authors of this study make the interesting case that the specific mode of locomotion evoked by electrical stimulation in intact leeches is modulated by prior exposure to artificial blood. Following exposure to this chemical, electrical stimulation of the rear of the leech increased the likelihood of swimming and crawling behaviors. In semi-intact preparations, exposure to full strength artificial blood, unlike a 10% solution, reduced likelihood that nerve stimulation would evoke swimming activity. Hence, the behavioral responses of the leech are selectively modified by prior exposure to chemicals in their environment with effects differing, in an apparently adaptive way, as concentrations are altered.
- 12.Garcia-Perez E, Mazzoni A, Zoccolan D, Robinson HPC, Torre V. Statistics of decision making in the leech. J Neurosci. 2005;25:2597–2608. doi: 10.1523/JNEUROSCI.3808-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzoni A, Garcia-Perez E, Zoccolan D, Graziosi S, Torre V. Quantitative characterization and classification of leech behavior. J Neurophysiol. 2005;93:580–593. doi: 10.1152/jn.00608.2004. [DOI] [PubMed] [Google Scholar]
- 14*.Biró Z, Hill RH, Grillner S. 5-HT Modulation of identified segmental premotor interneurons in the lamprey spinal cord. J Neurophysiol. 2006;96:931–935. doi: 10.1152/jn.00309.2006. [DOI] [PubMed] [Google Scholar]; As in many other vertebrates and invertebrates, serotonin is an important modulator of behavior in lamprey. This paper describes experiments on neuromodulation of excitatory and inhibitory spinal interneurons that generate and phase motoneuron output during lamprey swimming. The main findings are that serotonin affects both cellular properties of the interneurons (reducing slow afterhyperpolarization) and properties of their synaptic interactions with motoneurons (reducing synaptic amplitude). This study begins to explain the effects of serotonin on the expression of lamprey swimming movements.
- 15*.Chapman RJ, Sillar KT. Modulation of a spinal locomotor network by metabotropic glutamate receptors. Eur J Neurosci. 2007;26:2257–2268. doi: 10.1111/j.1460-9568.2007.05817.x. [DOI] [PubMed] [Google Scholar]; Serotonin is an important neuromodulator of animal behaviors, but this study demonstrates that glutamate, acting via metabotropic receptors, can play a similar role. The paper describes experiments on Xenopus tadpoles in which three types of metabotropic glutamate receptors were activated by specific agonists. Different agonists increased or decreased cycle period of fictive swimming, some altered motoneuron burst durations, some did not, and some altered the amplitude of motor bursts. These findings are interesting because, although glutamate is commonly known to mediate excitatory interactions via ionotropic receptors, the modulatory role of metabotropic glutamate receptor is less widely appreciated.
- 16.Blackshaw SE. Morphology and distribution of touch cell terminals in the skin of the leech. J Physiol Lond. 1981;320:219–228. doi: 10.1113/jphysiol.1981.sp013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cang J, Yu X, Friesen WO. Sensory modification of leech swimming: interactions between ventral stretch receptors and swim-related neurons. J Comp Physiol A. 2001;187:569–579. doi: 10.1007/s003590100229. [DOI] [PubMed] [Google Scholar]
- 18**.Fan RJ, Friesen WO. Morphological and physiological characterization of stretch receptors in leeches. J Comp Neurol. 2006;494:290–302. doi: 10.1002/cne.20818. [DOI] [PubMed] [Google Scholar]; Eight pairs of putative stretch receptors are closely associated with longitudinal muscles in the leech body wall. Through the injection of Alexa Fluor hydrazide, this study presents the morphology of terminal arborizations of 7 of the eight. In addition morphology and physiology demonstrated that one of these a dorsal stretch receptor associated with dorsal longitudinal muscle. At least two of these axons undergo membrane potential oscillations that are phase locked to the swimming rhythm expressed in nerve cord-body wall preparations and, at a different phase angle, also in isolated nerve cords. These two are phasically modulated by muscle tension oscillations and hence could be a source of cycle-by-cycle input to the swim oscillator network.
- 19.Yu X, Friesen WO. Entrainment of leech swimming activity by the ventral stretch receptor. J Comp Physiol A. 2004;190:939–949. doi: 10.1007/s00359-004-0549-9. [DOI] [PubMed] [Google Scholar]
- 20.Fan RJ, Marin-Burgin A, French KA, Friesen WO. A dye mixture (Neurobiotin and Alexa 488) reveals extensive dye-coupling among neurons in leeches; physiology confirms the connections. J Comp Physiol. 2005;191:1157–1171. doi: 10.1007/s00359-005-0047-8. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RJA, Skierezynski BA, Meyer JK, Skalak R, Kristan WB., Jr Mapping motor neuron activity to overt behavior in the leech. I. Passive biomechanical properties of the body wall. J Comp Physiol A. 1996;178:637–654. doi: 10.1007/BF00227377. [DOI] [PubMed] [Google Scholar]
- 22*.Tian J, Iwasaki T, Friesen WO. Muscle function in animal movement: Passive mechanical properties of leech muscle. J Comp Physiol A. 2007;193:1205–1219. doi: 10.1007/s00359-007-0278-y. [DOI] [PubMed] [Google Scholar]; This paper advances our understanding of muscle function during animal movements by describing passive muscle tensions caused by step-stretch experiments and by realistic rhythmic changes in length, such as occur during leech swimming locomotion. Although tensions generated by step changes in length are complex, requiring models that comprise three nonlinear springs, and two nonlinear dashpots, tensions generated by rhythmic stretching are described well by a single non-linear spring, without any dynamics. Hence, this paper supports the surprising conclusion that passive muscle tensions during rhythmic locomotion may not require the use of Hill-type models for muscle dynamics.
- 23**.McMillen T, Holmes P. An elastic rod model for anguilliform swimming. J Math Biol. 2006;53:843–886. doi: 10.1007/s00285-006-0036-8. [DOI] [PubMed] [Google Scholar]; This paper describes a very thorough modeling study of the mechanics underlying anguilliform swimming, in which the animal is viewed as an elliptical elastic rod. Time-dependent curvature changes are imposed onto the rod to generate a traveling wave that, through fluid interactions, simulates animal swimming movements. The study includes both mathematical analyses of forces on the continuous rod and numerical simulations of a rod comprising up to 80 discrete segments. The investigation includes the effects of stiffness (analogous to passive and active muscle tensions), body shape, and even motor neuron patterns on swim execution. This study is an important theoretical contribution to an essential step in the formulation of complete models of swimming locomotion. These will comprise central oscillators, sensory feedback, body and fluid mechanics, initiation and termination control mechanisms, and neuromodulation.
- 24.Friesen WO, Stent GS. Generation of a locomotory rhythm by a neural network with recurrent cyclic inhibition. Biol Cybernetics. 1977;28:27–40. doi: 10.1007/BF00360911. [DOI] [PubMed] [Google Scholar]
- 25.Pearce RA, Friesen WO. A model for intersegmental coordination in the leech nerve cord. Biol Cybernetics. 1988;58:301–311. doi: 10.1007/BF00363939. [DOI] [PubMed] [Google Scholar]
- 26.Cang J, Friesen WO. Model for intersegmental coordination of leech swimming: Central and sensory mechanisms. J Neurophysiol. 2002;87:2760–2769. doi: 10.1152/jn.2002.87.6.2760. [DOI] [PubMed] [Google Scholar]
- 27**.Zheng M, Iwasaki T, Friesen WO. Systems-level modeling of neuronal circuits for leech swimming. J Comput Neurosci. 2007;22:21–38. doi: 10.1007/s10827-006-9648-7. [DOI] [PubMed] [Google Scholar]; Using a systems approach, this paper presents a mathematical model of intermediate complexity to analyze the segmental and intersegmental circuits that generate the swimming rhythm of the leech. Using electrophysiological synaptic and impulse conduction data from physiological experiments, the time dependent and static properties are modeled for individual ganglia. The segmental models are then linked by identified intersegmental interactions to form a coordinated system. This novel approach, with an intermediate level of biophysical realism, generates quantitative predictions of period, phase, and amplitude, and also reveals the relationships between model parameters and system properties.
- 28.Davis WJ, Mpitsos GJ, Siegler MVS, Pinneo JM, Davis KB. Neuronal substrates of behavioral hierarchies and associative learning in pleurobranchaea. Amer Zool. 1974;14:1037–1050. [Google Scholar]
- 29.Shaw BK, Kristan WB., Jr The neuronal basis of the behavioral choice between swimming and shortening in the leech: control is not selectively exercised at higher circuit levels. J Neurosci. 1997;17:786–795. doi: 10.1523/JNEUROSCI.17-02-00786.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esch T, Mesce KA, Kristan WB., Jr Evidence for sequential decision making in the medicinal leech. J Neurosci. 2002;22:11045–11054. doi: 10.1523/JNEUROSCI.22-24-11045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esch T, Kristan WB., Jr Decision-making in the leech nervous system. Integr Comp Biol. 2002;42:716–724. doi: 10.1093/icb/42.4.716. [DOI] [PubMed] [Google Scholar]
- 32.Crisp KM, Mesce KA. A cephalic projection neuron involved in locomotion is dye coupled to the dopaminergic neural network in the medicinal leech. J Exp Biol. 2004;207:4535–4542. doi: 10.1242/jeb.01315. [DOI] [PubMed] [Google Scholar]
- 33**.Briggman KL, Abarbanel HDI, Kristan WB., Jr Optical imaging of neuronal populations during decision-making. Science. 2005;307:896–901. doi: 10.1126/science.1103736. [DOI] [PubMed] [Google Scholar]; Stimulating a single nerve in an isolated nervous system in exactly the same way again and again produced either swimming or crawling in the whole nerve cord. The neurons that predicted swimming or crawling earliest also biased the choice when they were depolarized or hyperpolarized. The activity of these neurons individually did not predict the outcome; only as a group of 6 or more were they reliable predictors. This study strongly suggests that the choice between these two behaviors is a subtle one, made by the concerted activity of a neuronal population.
- 34.Getting PA, Dekin MS. Tritonia swimming: A model system for integration within rhythmic motor systems. In: Selverston AI, editor. Model Neural Networks and Behavior. Plenum, Press; 1985. pp. 3–20. [Google Scholar]
- 35**.Briggman KL, Kristan WB., Jr Imaging dedicated and multifunctional neural circuits generating distinct behaviors. J Neurosci. 2006;26:10925–10933. doi: 10.1523/JNEUROSCI.3265-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used voltage-sensitive dyes to record from 317 of the 400 neurons in a leech segmental ganglion while the swimming and crawling central pattern generators were active. Of these 317, 188 oscillated in phase with crawling and 90 oscillated in phase with swimming. Of the 90 swim-phased neurons, 84 of them were also phased-locked to crawling. This implies that many of the neurons in the swim CPG are also used in the crawl CPG. This suggests a very high degree of shared neurons (multifunctionality) in the two pattern generators.
- 36.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 37.Kristan WB, Jr, Gillette R. Decision-making in small neuronal networks. In: North G, Greenspan R, editors. Invertebrate Neurobiology. Cold Spring Harbor Laboratory Press; 2007. pp. 533–554. [Google Scholar]
- 38**.Li WC, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: feedback excitation. J Neurosci. 2006;26:4026–4035. doi: 10.1523/JNEUROSCI.4727-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors investigated the long-held, but weakly supported view that sustained locomotory movements induced by even very brief stimulation arise from reciprocal excitatory interactions in neuron circuits. Using whole-cell patch recording techniques they demonstrated that such mutually excitatory interactions exist in the caudal hindbrain and rostral spinal cord of Xenopus tadpoles. Electrophysiological and modeling studies were used to show that these interactions could be the source of sustained excitatory drive to maintain swimming activity. The paper provides the best evidence to date that excitatory synaptic feedback can lead to prolonged excitation in locomotory networks.
- 39.Grillner S, Wallén P. Cellular bases of a vertebrate locomotor system-steering, intersegmental and segmental co-ordination and sensory control. Brain Res Brain Res Rev. 2002;40:92–106. doi: 10.1016/s0165-0173(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 40.French KA, Chang J, Reynolds S, Gonzalez R, Kristan WB, III, Kristan WB., Jr Development of swimming in the medicinal leech; the gradual acquisition of a behavior. J Comp Physiol. 2005;191:813–21. doi: 10.1007/s00359-005-0003-7. [DOI] [PubMed] [Google Scholar]