Abstract
Purpose
Visual-span profiles are plots of letter-recognition accuracy as a function of letter position left and right of the point of fixation. Legge, Mansfield & Chung (2001) proposed that reduced size of the visual span is a spatial factor limiting reading speed in patients with age-related macular degeneration (AMD). We have recently shown that a temporal property of letter recognition - the exposure time required for a high level of accuracy – is also a factor limiting reading speed in AMD (Cheong, Legge, Lawrence, Cheung & Ruff, 2007). We measured the visual-span profiles of AMD subjects and assessed the relationship of the spatial and temporal properties of these profiles to reading speed.
Methods
Thirteen AMD subjects and 11 age-matched normals were tested. Visual-span profiles were measured by using the trigram letter-recognition method described by Legge et al. (2001). Each individual’s temporal threshold for letter recognition (80% accuracy criterion) was used as the exposure time for measuring the visual-span profile. Size of the visual span was computed as the area under the profile in bits of information transmitted. The information transfer rate in bits per second was defined as the visual-span size in bits divided by the exposure time in sec.
Results
AMD visual-span sizes were substantially smaller (median of 23.9 bits) than normal visual-span sizes in central vision (median of 40.8 bits, p<0.01). For the nine AMD subjects with eccentric fixation, the visual-span sizes (median of 20.6 bits) were also significantly smaller than visual spans of normal controls at 10° below fixation in peripheral vision (median of 29.0 bits, p=0.01). Information transfer rate for the AMD subjects (median of 29.5 bits/sec) was significantly slower than that for the age-matched normals at both central and peripheral vision (median of 411.7 & 290.5 bits/sec respectively, ps<0.01). Information transfer rates were more strongly correlated with reading speed than the size of the visual span, and explained 36% of the variance in AMD reading speed.
Conclusion
Both visual-span size and information transfer rate were significantly impaired in the AMD subjects compared with age-matched normals. Information transfer rate, representing the combined effects of a reduced visual span and slower temporal processing of letters, was a better predictor of reading speed in AMD subjects than was the size of the visual span.
Keywords: low vision, age-related macular degeneration, reading speed, visual span, letter recognition, information transfer rate
1. Introduction
In developed countries, age-related macular degeneration (AMD) is the leading cause of central vision loss among people aged 50 years and over. Central field loss (CFL) is often a consequence of AMD and causes reading difficulty. Seeking assistance to alleviate reading problems is the major goal for the majority of AMD patients attending low vision clinics (Elliott, Trukolo-Ilic, Strong, Pace, Plotkin & Bevers, 1997). Despite appropriate magnifying aids prescribed to compensate for acuity loss, many AMD patients still read very slowly (Legge, Ross, Isenberg & LaMay, 1992, Legge, Rubin, Pelli & Schleske, 1985b, Whittaker & Lovie-Kitchin, 1993). This suggests that there are underlying factors other than acuity limiting reading performance in AMD subjects.
As a consequence of CFL, AMD patients typically use a region of para-central retina for reading, usually within 20° from the damaged fovea. The eccentric location that functions as the fixation reference is commonly termed the preferred retinal locus (PRL) (Timberlake, Mainster, Peli, Augliere, Essock & Arend, 1986). In this paper, “peripheral retina” and “peripheral vision” refer to this paracentral region, consistent with usage in related literature (e.g. Higgins et al. (1996), Latham & Whittaker (1996), Chung et al. (1998), Legge (2007a)). A number of factors have been suggested to explain the reduced reading speed in AMD patients. These factors include impaired oculomotor control (McMahon, Hansen & Viana, 1991, Rubin & Turano, 1994, White & Bedell, 1990, Whittaker, Cummings & Swieson, 1991), poor fixation stability (Crossland, Culham & Rubin, 2004, Whittaker, Budd & Cummings, 1988), shrinkage of the visual span (Legge, Cheung, Yu, Chung, Lee & Owens, 2007b, Legge, Ahn, Klitz & Luebker, 1997, Legge et al., 2001), and slower temporal processing of letter information (Cheong et al., 2007). In this paper, we focus on both the spatial and temporal influences on letter recognition in AMD.
Visual span is defined as “the number of letters that can be recognized reliably without moving the eyes” (Legge (2007), Ch. 3). Legge and colleagues have proposed that the visual span imposes a sensory bottleneck on reading speed (Legge et al., 1997), and that a “shrinking” visual span limits reading performance in low vision (Legge et al., 2007b, Legge et al., 2001). Visual-span profiles are plots of letter-recognition accuracy as a function of distance left and right of the point of fixation. The profiles characterize the visual information available for letter recognition and reading (Legge et al., 2001). For the case of normal central vision, the profiles are centered on the fovea. For people with AMD and stable PRLs, the profiles are centered on the PRL. Legge et al. (2001) found that visual-span size is reduced significantly in normal peripheral vision and these smaller visual spans are associated with slower peripheral reading. This finding led them to hypothesize that AMD patients who must use their non-foveal vision for reading, would have reduced visual spans. The smaller visual spans are expected to result in slower reading in AMD patients (Legge et al., 1997, Legge et al., 2001).
Legge et al.’s hypothesis was indirectly supported by eye-movement studies conducted by Bullimore and Bailey (1995) and by Crossland and Rubin (2006). Consistent with the reduced visual span hypothesis, these eye-movement studies showed that saccade size was substantially reduced in subjects with macular degeneration compared with age-matched healthy readers.
We now turn to temporal processing. Legge et al. (2001) studied the temporal dependence of the visual span by measuring visual-span profiles for a range of exposure times from 25 to 500 ms in normal central and peripheral vision. The visual spans increased in size as exposure time increased up to some limit. In central vision, the visual spans reached their peak amplitudes and breadths in 100 ms or less. In peripheral vision, longer exposure times (e.g. 200 ms at 20° inferior field) were required for visual spans to reach their maximum size. Because AMD subjects with central-field loss rely on their peripheral vision, we would also expect them to require longer exposure times to achieve the maximum spatial extent of their visual spans.
In a recent paper, we have reported that the temporal thresholds for letter recognition in AMD subjects are substantially longer than in normal peripheral vision (Cheong et al., 2007). Reasons for this slower processing of letter information by AMD subjects may include concomitant pathology, fixation instability, and/or crowding (refer to Cheong et al. (2007) for detailed discussion). Whatever the cause, it is likely that the temporal characteristics of test stimuli will have a greater impact on AMD visual-span profiles than on normal visual-span profiles. Because of the wide variation in temporal dependency of letter recognition across our AMD subjects, we measured visual-span profiles for each subject at his/her “temporal threshold”, the exposure time yielding 80% correct for recognizing letters at fixation. We did this instead of measuring visual-span profiles at a single, short exposure time (as we have typically done with normally sighted subjects). This strategy brings to the forefront the temporal, as well as spatial, characteristics of visual spans.
A priori, it seems likely that reading speed would be affected by both spatial and temporal characteristics of the visual span. In order to capture both of these attributes in a single measure, we define the rate of information transfer through the visual span as the visual-span size in bits divided by the exposure time in sec. This quantity takes into account both the spatial extent of the visual span and the time course of processing in the corresponding region of the visual field. We investigated the association of this measure with AMD reading speed.
As a secondary goal, we asked how nearby scotomas would affect the shape of visual-span profiles. The profiles of AMD subjects are expected to be centered at their PRLs. Research has shown that the majority of PRLs in AMD are located below and/or to the left of central-field scotomas (Fletcher & Schuchard, 1997, Fletcher, Schuchard & Livingstone, 1994, Schuchard, Naseer & de Castro, 1999, Sunness, Applegate, Haselwood & Rubin, 1996). See Cheung and Legge (2005) for a review of hypotheses for locations of PRL development. If a visual-span profile, centered on the PRL, extends into a nearby scotoma, we would expect to find a depression in the profile. We looked for these distortions of the visual-span profiles and attempted to predict their occurrence from the presence of scotomas measured with conventional perimetry.
To summarize, in this study, we explored four hypotheses: 1) AMD subjects with central-field loss have smaller visual spans than age-matched normal controls; 2) shrinkage in the size of the visual span, a purely spatial limitation, is an important contributor to reduced reading speed in AMD; 3) reduction in the information transfer rate, combining influences of slower temporal processing and reduced size of the visual span, is more closely associated with slow reading in AMD; and 4) the distortions of visual-span profiles reflect the presence of scotomas adjacent to the PRL.
2. Methods
2.1 Subjects
Thirteen subjects with a primary ocular diagnosis of AMD and age range from 65 to 87 years (mean 78.7 ± 5.5) were recruited from the Low Vision Center of the University of Minnesota and Vision Loss Resources (Minneapolis, MN). Data on temporal thresholds for letter recognition for 12 of these subjects were discussed in detail by (Cheong et al., 2007). All AMD subjects had functional reading vision (≥40 wpm for spot reading skill) and distance acuity of 20/400 (1.3 logMAR) or better, but reported difficulties in reading. We excluded AMD patients with more severe reading or acuity deficits in order 1) to study subjects who continue to read visually; 2) so that RSVP reading words, magnified to exceed the critical print size, would fit on the display screen; and 3) so that the PRL would be within ± 10° from the fovea.
Eleven age-matched normal subjectsa with normal vision (NV) participated in the study (mean age of 74.6 ± 4.9 years). They had visual acuities of 20/25 or better and no reported ocular disease. Prior to the testing, a brief retinal assessment with direct ophthalmoscope was performed for each NV subject to confirm their vision status. Potential subjects with cognitive and neurological limitations (e.g. Parkinson’s and Alzheimer’s diseases) were excluded from the study. Cognitive status was screened by the Mini-Mental State Exam (MMSE) (Folstein, Folstein & McHugh, 1975) and only subjects within the normal range of MMSE score (≥ 25) (Launer, Dinkgreve, Jonker, Hooijer & Lindeboom, 1993) were recruited. All subjects were fluent English speakers and gave signed, informed consent to their participation in the study. This study followed the tenets of the Declaration of Helsinki and was approved by the University of Minnesota Institutional Review Board.
2.2 Apparatus and stimuli
Stimuli for the rapid serial visual presentation (RSVP) reading task and the measurement of visual-span profiles were generated and presented on a Power Mac G4 (model: M8570) with a SONY Trinitron display (model: GDM-FW900; refresh rate: 76 Hz) using Matlab 5.2.1 with the Psychophysics Toolbox extensions. All stimulus letters were rendered in lowercase Courier font and presented as black letters on a white background with contrast of 90%, and background luminance of 100 cd/m2 in the reading task and 90 cd/m2 in the letter-recognition task. Standard letter spacing, in which center-to-center separation of adjacent letters was 1.39 times the height of the lower case x, was used and scaled according to the print size. Angular print size is designated by x-height in degrees.
2.3 Procedure
2.3.1 Vision assessments
Except as indicated below for the tangent field measurements, all vision testing was conducted binocularly. Distance visual acuity with current spectacle prescription was measured with a Lighthouse Distance acuity chart with background luminance of 120 cd/m2 and scored on a per-letter basis in logMAR (Kitchin & Bailey, 1981). Maximum reading speed and critical print size (CPS), the smallest print size yielding maximum reading speed, were measured with the MNREAD chart (Mansfield, Ahn, Legge & Leubker, 1993) with background luminance of 95 cd/m2 and with the appropriate refractive correction. The CPS estimated from the MNREAD Acuity Chart was used to guide selection of the print sizes to be used in RSVP testing (see below). Letter contrast sensitivity was measured using the Pelli Robson chart at 1 m, scored on a per-letter basis and expressed as log contrast sensitivity (Elliott, Bullimore & Bailey, 1991, Pelli, Robson & Wilkins, 1988). Monocular central visual fieldsb were measured using a 5 mm white target on a Tangent screen at 1 m with average background luminance of 80 cd/m2 (Henson, 1993). This method for field measurement is recommended by Lovie-Kitchin and Whittaker (1998) for obtaining a rough estimate of the size and location of the scotoma relative to the PRL. The AMD subjects were instructed to direct their gaze (central or eccentric) to a large fixation target – a letter E (20/150 or 20/300) (Lovie-Kitchin, Bowers & Woods, 2000, Lovie-Kitchin & Whittaker, 1998). During the field measurement, subjects were constantly reminded to maintain their fixation on the E and asked to report when the 5 mm white target (moving at a speed of 5° per second) was missing (refer to Figure 2). Monocular central visual field loss was quantified by the width and height of the scotoma in degrees of visual angle and by solid angle in steradians (Lovie-Kitchin, Mainstone, Robinson & Brown, 1990, Weleber & Tobler, 1986) and categorized into four quadrants: superior, inferior, left and right, according to the location relative to fixation. The shift of the physiological blind spot in the better-eye (i.e. eye with better distance acuity) was used to estimate the PRL location. Three of the 13 AMD subjects had no scotoma for the 5 mm target and are classified as having “no scotoma”. Table 1 summarizes the subjects’ characteristics and clinical vision measures.
Figure 2.

Illustration of the method for estimating monocular scotoma position (measured for the higher-acuity eye) within a visual-span profile (measured under binocular viewing). A large scotoma extending from 3.5° to 10.5° horizontally from the AMD subject’s point of fixation (PRL) is superimposed on the subject’s visual-span profile. The scotoma overlaps letter positions from +2 to +5 in the visual-span profile for characters with x-height of 1.66°. Gray bars in Figure 3 illustrate the positions of scotomas computed in this way.
Table 1.
Summary of vision measures
| Subject | Group | Age | Length of impairment (years) | Distance VA (logMAR) | Log Contrast sensitivity | Monocular scotoma | Estimated PRL (in visual field) | |
|---|---|---|---|---|---|---|---|---|
| (diameter) | (quadrant) † | |||||||
| 1 | AMD | 81 | 4 | 0.24 | 1.10 | 15° × 9° | R,L,S,I | Left 5° & Lower 10° |
| 2 | AMD | 76 | 2 | 0.58 | 1.65 | - | - | Central |
| 3 | AMD | 74 | 3 | 0.44 | 1.25 | 6° × 4° | L,S,I | Central |
| 5 | AMD | 77 | 5 | 0.26 | 1.45 | 7° × 10° | L,I | Upper 3° |
| 7 | AMD | 65 | 3 | 0.12 | 1.90 | - | - | Central |
| 8 | AMD | 81 | 4 | 0.20 | 1.30 | - | - | Central |
| 9 | AMD | 79 | 3 | 0.70 | 0.85 | 18° × 18° | R,L,S,I | Right 10° & Lower 10° |
| 10 | AMD | 87 | 8 | 0.76 | 1.35 | 8.4° × 9° | R,S,I | Left 5° |
| 11 | AMD | 78 | 5 | 0.32 | 1.10 | 2.5 × 1° | L,I | Left 5° |
| 12 | AMD | 79 | 10 | 0.88 | 1.40 | 15° × 25° | R,S,I | Left ‡ |
| 14 | AMD | 81 | 11 | 1.12 | 0.95 | 18° × 21° | R,L,S,I | Left 10° |
| 15 | AMD | 83 | 5 | 0.38 | 1.40 | 10° × 15° | L,S,I | Right 3° |
| 16 | AMD | 85 | 7.5 | 1.00 | 1.40 | 18° × 15° | R,L,S | Left & Lower 12° |
| 17 | NV | 68 | - | -0.10 | 2.00 | - | - | - |
| 18 | NV | 81 | - | 0.02 | 1.95 | - | - | - |
| 19 | NV | 82 | - | 0.04 | 1.85 | - | - | - |
| 20 | NV | 78 | - | -0.12 | 1.85 | - | - | - |
| 21 | NV | 66 | - | -0.04 | 1.85 | - | - | - |
| 22 | NV | 75 | - | 0.08 | 1.90 | - | - | - |
| 23 | NV | 73 | - | -0.18 | 1.95 | - | - | - |
| 24 | NV | 75 | - | -0.10 | 1.90 | - | - | - |
| 25 | NV | 76 | - | -0.12 | 1.90 | - | - | - |
| 26 | NV | 72 | - | -0.12 | 1.85 | - | - | - |
| 27 | NV | 75 | - | -0.12 | 1.85 | - | - | - |
| Mean (SD) | AMD | 78.9
(5.5) |
5.42
(2.84) |
0.54
(0.33) |
1.32
(0.28) |
- | - | - |
| NV | 74.6
(4.9) |
- | -0.07
(0.08) |
1.90
(0.05) |
- | - | - | |
| p-value | 0.10 | - | <0.01 | <0.01 | - | - | - | |
AMD = age-related macular degeneration NV = normal vision
Qualitative categorization of visual field loss: Right (R) bounded by 315° and 45°; Left (L) bounded by 135° and 225°; Superior (S) bounded by 45° and 135°; Inferior (I) bounded by 225° and 315°.
The extent of the PRL could not be estimated for Sub 12 because no physiological blind was mapped due to large right scotoma.
To better reflect the subjects’ habitual reading performance, reading and visual-span measures were assessed binocularly with the appropriate near refractive correction at 40 cm (or a fixed shorter distance if required by the subject’s poorer acuity).
2.3.2 Visual-span measures
2.3.2.1 Temporal thresholds
Individual temporal thresholds for letter recognition at fixation were measured for the AMD subjects. A trigram method was used, comparable in most details to the method for measuring visual-span profiles (see below), except that all stimuli were centered at the subject’s point of fixation, and stimulus exposure time was varied, rather than stimulus position. For details, see Cheong et al. (2007). The data were plotted as psychometric functions--percent correct for the central letter of the trigram as a function of exposure time--and were fit with cumulative Gaussian functions. The exposure time yielding 80% correct was defined as the temporal threshold for the subject. An individual’s temporal threshold (see Table 2) was used as the stimulus duration in measuring the visual-span profile unless the threshold was shorter than 100 ms, in which case 100 ms was used as the stimulus duration (Sub 7 and the normal controls).
Table 2.
Summary of visual span and reading measures
| Subject | Group | Stimuli print size | Visual span | Reading | |||
|---|---|---|---|---|---|---|---|
| Exposure duration (ms) † | Size (bits) | Information transfer rate (bits/sec) | Reading speed (log wpm) | Reading acuity (logMAR) | |||
| (deg) | |||||||
| 1 | AMD | 0.84 | 554.4 | 16.36 | 29.52 | 2.26 | 0.38 |
| 2 | AMD | 1.85 | 596.1 | 36.67 | 92.57 | 1.90 | 0.63 |
| 3 | AMD | 0.42 | 686.5 | 12.50 | 18.20 | 1.71 | 0.28 |
| 5 | AMD | 0.66 | 369.7 | 27.08 | 73.24 | 2.30 | 0.42 |
| 7 | AMD | 0.34 | 100.0 | 40.62 | 406.18 | 2.46 | 0.10 |
| 8 | AMD | 0.58 | 422.5 | 30.52 | 72.15 | 2.44 | 0.30 |
| 9 | AMD | 4.18 | 1293.8 | 30.58 | 23.64 | 1.75 | 1.06 |
| 10 | AMD | 1.66 | 818.5 | 23.91 | 29.21 | 1.75 | 0.92 |
| 11 | AMD | 0.84 | 792.1 | 20.58 | 25.99 | 2.09 | 0.38 |
| 12 | AMD | 3.32 | 1755.7 | 19.42 | 11.06 | 1.86 | 1.12 |
| 14 | AMD | 4.18 | 2033.0 | 18.99 | 9.34 | 1.92 | 1.41 |
| 15 | AMD | 0.84 | 100 | 18.67 | 180.67 | 2.40 | 0.34 |
| 16 | AMD | 3.59 | 409.2 | 27.03 | 66.05 | 2.34 | 1.04 |
| 17 ˆ | NV | 3.5 | 100 | 29.31 | 293.15 | 1.92 | -0.08 |
| 18 ˆ | NV | 3.5 | 100 | 24.25 | 242.49 | 1.72 | 0.06 |
| 19 ˆ | NV | 3.5 | 100 | - | - | - | -0.05 |
| 20 ˆ | NV | 3.5 | 100 | 27.16 | 271.61 | 1.37 | -0.13 |
| 21 ˆ | NV | 3.5 | 100 | 30.21 | 302.07 | 1.54 | 0.01 |
| 22 | NV | 3.5 | 100 | 32.54 | 325.44 | 1.89 | 0.14 |
| 23 | NV | 3.5 | 100 | 26.39 | 263.89 | 2.33 | -0.12 |
| 24 | NV | 3.5 | 100 | 24.76 | 247.55 | 1.95 | -0.10 |
| 25 | NV | 3.5 | 100 | 32.02 | 320.23 | 2.42 | 0.04 |
| 26 | NV | 3.5 | 100 | 28.84 | 288.35 | 2.22 | -0.10 |
| 27 | NV | 3.5 | 100 | 29.26 | 292.59 | 2.23 | -0.12 |
| Mean (SD) | AMD | 1.80
(1.48°) |
764.0
(594.2) |
24.8
(8.2) |
79.8
(108.7) |
2.09
(0.29) |
0.65
(0.41) |
| NV | 3.5° | 100 | 28.5
(2.8) |
284.7
(28.2) |
1.96
(0.35) |
-0.03
(0.09) |
|
| p-value | - | - | 0.20 | <0.01 | 0.43 | <0.01 | |
AMD = age-related macular degeneration NV = normal vision
Letter recognition accuracy for the central letter position as a function of exposure durations was measured for each AMD subject. The exposure time yielding 80% correct was defined as the temporal threshold. For subjects whose temporal threshold was longer than 100 ms, the individual’s temporal threshold was used as the stimulus duration in measuring the visual-span profile; otherwise, 100 ms was used (e.g. Sub 7 and NV).
Among these NV subjects, visual-span profiles and reading were measured at both central and 10° inferior visual field. Only results on 10° inferior visual field were reported in this table.
RSVP reading speed for AMD subjects reflected their reading performance at either their central or preferred retinal location while RSVP reading speed for NV subjects reflected their reading performance at 10° inferior visual field.
For Sub 19, reading speed at 10° inferior vision was not measured because this subject quitted the study due to exhaustion.
2.3.2.2 Visual-span profiles
Visual-span profiles were measured by presenting trigrams (strings of three horizontally arranged letters, randomly selected from the 26 lowercase letters) with middle-letter positions from -6 to +6 (Figure 1). A visual-span profile was measured for a single letter size approximately twice the CPSc for each AMD subject. The CPS was estimated from the RSVP reading assessment (see below), and ranged from 0.34° to 4.18° across subjects (mean of 1.8 ± 1.5°). In previous studies, we have fit visual-span profiles with asymmetric Gaussian functions (cf., (Legge et al., 2001). Due to the presence of central scotomas, some AMD visual-span profiles had distortions and could not be fit with this function. Instead, we connected the data points by line segments in Figures 2 and 3.
Figure 1.
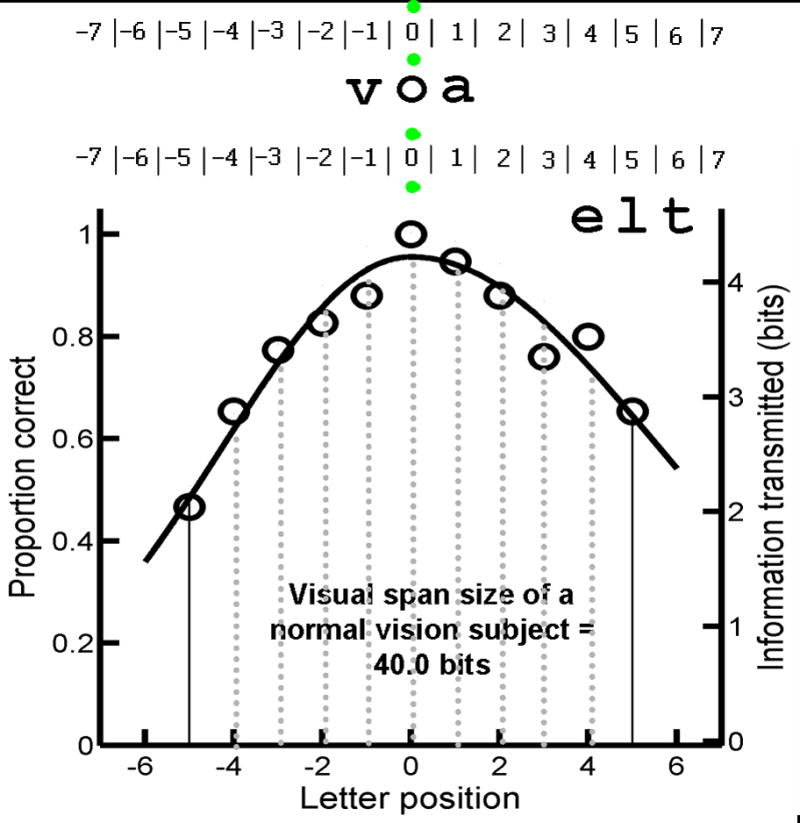
Measurement of Visual-Span Profiles: Trigrams (strings of 3-letters) for measuring the visual span were presented at various positions left or right of fixation. Subjects were instructed to use either their preferred retinal locus (for AMD subjects with central scotomas) or central vision (for AMD subjects without scotomas or normal vision subjects) to fixate the mid point between two vertically separated green fixation dots (shown as grey dots in this figure). Subjects reported aloud all 3 letters of the trigram from left to right. The lower panel shows a sample visual-span profile in which proportion correct (left vertical scale) is plotted as a function of letter position for a normally sighted subject. The right vertical scale shows an approximately linear transformation from percent correct to information transmitted in bits, where 100% correct for identifying 1 of 26 letters corresponds to 4.7 bits of information. The size of the visual span was quantified by summing across the information transmitted in each slot (from letter position -5 to +5).
Figure 3.
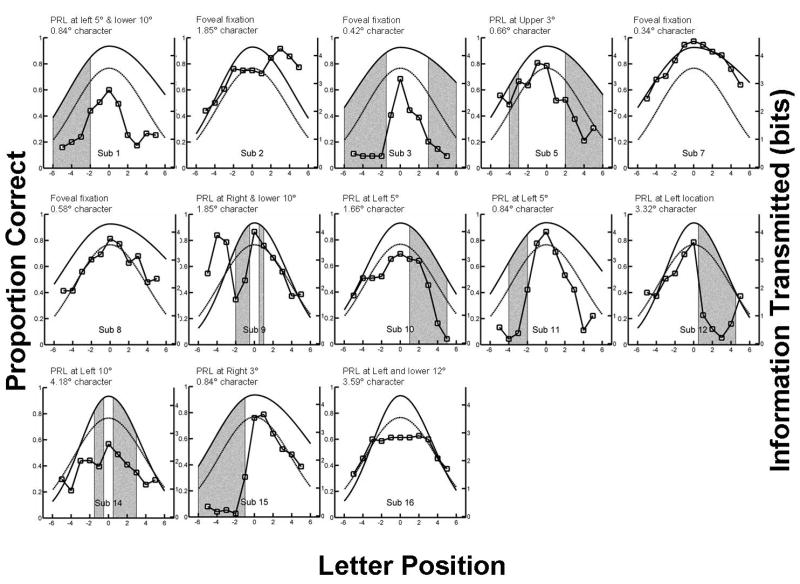
Each panel shows the visual-span profile for an AMD subject (data points are connected by line segments). The dark solid curves show average visual-span profiles for normal central vision at a print size close to the value used for the individual AMD subject. The dashed curves show average visual-span profiles in normal peripheral vision (10° in the lower visual field with print size of 3.5°). The gray bars show the estimated locations of scotomas. Sub 2, 7 and 8 showed no scotoma whereas Sub 16 adopted an inferior-left PRL by shifting the scotoma to her upper visual field, resulting in no scotoma lying along the horizontal meridian where the visual span was measured. Eccentricity of the point of fixation (foveal or PRL) and character size used to measure visual span is shown for each individual subject.
During testing, subjects were instructed to fixate between two vertically separated green dots on the display screen (Figure 1). The separation of the dots ensured that they did not overlap with letters presented at the 0 position. For 9 AMD subjects with an eccentric PRL, it was expected that fixation would be at the PRL. For the NV subjects and four AMD subjects, it was expected that fixation was foveal. Fixation stability for the AMD subjects was monitored with a method similar to Seiple et al. (2001) using a Logitech QuickCam® Pro 5000 (image capture and recording of 640×480 pixel images at a frame rate of 30 Hz). Measurements with NV subjects showed that saccades of 1°, 2° and 3° resulted in 0.30, 0.50 and 0.70 cm physical movement on the monitor respectively. A second experimenter could reliably detect 2° saccades, and less reliably 1° saccades. Trials were rejected when movement of the pupil’s image during a trial was observed (estimated accuracy of 2° or better). This sensitivity is sufficient to reject most trials with eye movements of one letter or more within a trial (average character size was 1.8 ± 1.5° across AMD subjects). In this study, the trial exclusion rate was 19.0 ± 12% (range from 2.7 to 51.2% across AMD subjects).
Subjects reported aloud all 3 letters of the trigram from left to right and the experimenter typed the letters into the computer. A letter was counted as correct only if it was identified correctly in the correct order. Recognition accuracy (proportion correct) at each letter position was calculated by accumulating across trials in which the letters appeared in the three different positions within the trigram. Each visual span measure was based on data from 25 trigrams at each of 13 locations (-6 to +6).
Based on the letter-confusion matrices measured by Beckmann and Legge (2002), percent correct recognition for each letter position was converted to information transmitted, in which 100% accuracy at recognizing one of the 26 letters corresponds to transmission of log2(26) or 4.7 bits of information while the chance level of 3.8% accuracy corresponds to transmission of log2(1) or 0 bits (Legge et al., 2001). The approximately linear transformation from proportion correct to bits of information transmitted is shown on the right vertical axis of the visual-span profile (Figure 1). Size of the visual span is computed as the sum of the information transmitted by letter positions from -5 to +5 of the profile.
For each NV subject, a visual-span profile was measured at 10° in the lower visual field, centered on the midline. The letter size was 3.5° and the exposure time was 100 ms. This print size was adopted because it was approximately twice the CPS in peripheral vision for NV subjects measured in previous studies (Cheung, 2005, Chung, Legge & Cheung, 2004). In addition, for five of the 11 NV subjects, we measured visual-span profiles in central vision for print sizes of 0.5°, 1°, 2° and 4°. This range is approximately matched to the range of print sizes used by the AMD subjects. Asymmetric Gaussian functions (Legge et al., 2001) were fitted to the NV profiles.
2.3.2.3 Superposition of scotomas on AMD visual-span profiles
In order to evaluate the impact of scotomas on visual-span profiles, we needed a method to superimpose scotoma regions from a tangent field on letter positions in a visual-span profile. Tangent field maps were superimposed on visual-span profiles with the assumption that the PRL used for visual-span measurements was the same as the PRL used for tangent-field measurements. For example, Sub 10, who had a large monocular scotoma to the right of the PRL (refer to Section 2.3.1 for the estimation of PRL) from approximately 3.5° to 10.5°, was tested with 1.66° letters in the trigram test. Letters with an x-height of 1.66° have a center-to-center spacing 1.39 times greater than this value for the Courier font, i.e., a center-to-center spacing of 2.3°. The letter in slot zero extended half a letter to the right and half to the left. A letter in slot 1 of the profile would lie between 1.16° and 3.47° from fixation, and a letter in slot 6 would lie between 12.7° and 15.0° from fixation. The visual-span profile, extending from letter position -6 to +6, corresponds to an angular extent on the tangent field from 15.0° left to 15.0° right of the fixation point (Figure 2). From this superposition, letter positions of +2 to +5 in the visual-span profile would entirely overlap with the scotoma position in the field map and would be expected to exhibit reduced performance. Letter positions +1 and +6 would partially overlap with the scotoma. The gray bars on the visual-field profiles in Figure 3 mark the estimated locations of scotomas based on this superposition method.
2.3.3 Reading assessments
RSVP reading speed was measured with the appropriate near refractive correction at 40 cm (or a fixed shorter distance if required by the subject’s lower acuity) over a range of print sizes. Each RSVP sentence was randomly selected from a pool of 2658 sentences prepared by Chung et al. (1998). Each sentence contained 8 to 14 words (mean of 11 ± 1.7 words and a mean word length of 3.94 letters) and contained no punctuation other than a period. Individual words of a sentence were presented sequentially, left-justified at the same location on a monitor for fixed exposure durations. Subjects were required to read the sentence aloud and the experimenter recorded the number of words read correctly. A word was scored as correct when the subject spoke the word correctly, irrespective of the order in the sentence. A detailed description of the method for measuring RSVP reading speed as a function of print size is given by Cheong et al. (2007). In brief, reading speed in which RSVP exposure time yielded 80% of words identified correctly was measured for a minimum of 5 print sizes. A multilevel non-linear mixed effects model (NLME) (Pinheiro & Bates, 2000) was then used to estimate the parameters of an exponential fit to the data yielding maximum reading speed and critical print size (CPS) for the AMD subjects (Cheung, Kallie, Legge & Cheong, 2007). CPS was defined as the smallest print size yielding 80% of the maximum reading speed.
For the NV subjects, peripheral RSVP reading speed was tested for 3.5° characters at 10° in the lower visual field. In addition, RSVP reading speed was tested across a range of print sizes in central vision in five of the 11 NV subjects.
2.4 Data Analysis
SPSS (version 15), Matlab (version 7) including the psignifit toolbox (version 2.5.6), R (version 2.1.0) and MedCalc (version 9.0) were used in the data analyses. Given that our sample size was relatively small, non-parametric statistics without the assumption of data normality was used to examine the differences in visual-span parameters and reading measures in the AMD and NV groups. Spearman correlation and logistic regression were used to investigate the relationship among reading speed, visual span and the location of scotomas. A probability of less than 0.05 was taken to indicate statistical significance.
3. Results
3.1 Size of visual-span profiles in Normal Vision and AMD
Figure 3 shows individual visual-span profiles for 13 AMD subjects (data points connected by line segments). The dark solid curves show average visual-span profiles for normal central vision at a print size close to the value used for the individual AMD subject. The gray dashed curves show average visual-span profiles in normal peripheral vision (10° in the lower visual field with print size of 3.5°). The gray bars show the estimated locations of scotomas (see Methods). Table 2 contains visual-span sizes, information transfer rates, reading speeds and testing parameters for individual subjects.
Group comparisons are summarized in Table 3 and Figure 4. Results are shown for the AMD group as a whole, and also broken down into subgroups with central (N= 4) and eccentric (N= 9) fixation. The nine subjects with eccentric fixation used PRLs which ranged from approximately 3° to 12° away from the fovea (Table 1). The four subjects with central fixation included three with no scotomas as measured on the tangent screen, and Sub 3 who had a ring scotoma with intact central vision.
Table 3.
Summary of the visual span and reading measures in age-related macular degeneration and normal vision
| Age-related macular degeneration | Normal vision | |||||||
|---|---|---|---|---|---|---|---|---|
| All AMD subjects (n=13) | AMD subjects with foveal fixation (n=4) | AMD subjects with eccentric fixation (n=9) | p-value (foveal vs eccentric fixation In AMD) | Central vision (n = 5) | 10° inferior vision (n= 11) | p-value (central vs inferior vision in NV) | ||
| Visual span | Visual-span size (bits) | median: 23.9 (24.8 ± 8.2) (range: 12.5–40.6) | median: 33.6 (30.1 ± 12.4) (range: 12.5–40.6) | median: 20.6 (22.5 ± 4.8) (range: 16.4–30.6) | 0.26 | median: 40.8 (41.2 ± 1.5) (range: 36.7–43.8) | median: 29.0 (28.5 ± 2.8) (range: 24.3–32.5) | 0.001 |
| Temporal threshold (ms) | median: 623.0 (1003.8 ± 926.1) (range: 103.1–3056.3) | median: 548.0 (536.6 ± 27.8) (range: 504.8–556.9) | median: 815.1 (1159.6 ± 1034.4) (range: 103.1– | 0.48 | 13 * | median: 100.3 (97.0 ± 34.2) (range: 60.4–129.9) | 0.001 | |
| Information transfer rate (bits per sec) | median: 29.5 (79.8 ± 108.7) (range: 9.3–406.2) | median: 82.4 (147.3 ± 175.4) (range: 18.2–406.2) | median: 29.2 (49.9 ± 53.8) (range: 9.3–180.7) | 0.26 | median: 407.7 (411.7 ± 15.4) (range: 397.0–437.5) | median: 290.5 (284.7 ± 28.2) (range: 242.5–25.4) | 0.001 | |
| Reading | RSVP reading speed (log wpm) | median: 2.09 (2.09 ± 0.29) (range: 1.71–2.46) | median: 2.17 (2.13 ± 0.38) (range: 1.71–2.46) | median: 2.09 (2.07 ± 0.26) (range: 1.75–2.40) | 0.71 | median: 2.52 (2.54 ± 0.07) (range: 2.45– 2.65) | median: 1.93 (1.96 ± 0.35) (range: 1.37–2.42) | <0.001 |
Bracket in the cell indicates the minimum and maximum.
PRL = preferred retinal location
13 ms represents the upper-bound on the temporal thresholds for the NV subjects at central vision.
Figure 4.
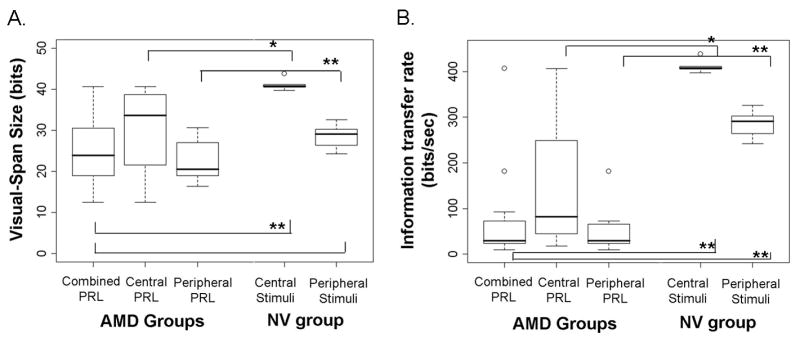
Box plots for visual-span size (panel A) and information transfer rate (panel B). Data are shown for AMD subjects with different fixations: 1) central preferred retinal locus (PRL) (N = 4); 2) peripheral PRL (N = 9); and 3) the combined group (N = 13), and for age-matched normal controls (NV) tested with central stimuli (N = 5) and with peripheral stimuli at 10° in the lower visual field (N = 11).
First, we compared the whole group of AMD visual spans with age-matched visual spans in normal central and peripheral vision. As predicted, the overall AMD group had visual spans that were significantly smaller than NV visual spans when the latter were measured in central vision (median of 23.9 vs 40.8 bits, Mann Whitney Test, U=2.0, p=0.001, Figure 4a). However, AMD visual-span sizes were not significantly different from NV visual-span sizes when the latter were measured at 10° in the lower visual field (median of 23.9 vs 29.0 bits, p=0.17).
Second, separate analyses of visual-span sizes for the AMD subjects with different locations of fixation (central or eccentric) were compared with the corresponding fixation locations for the NV subjects (central or peripheral). Visual-span sizes for the AMD subjects with central fixation (n=4) were marginally smaller than the sizes in normal central vision (median of 33.6 vs 40.8 bits, p=0.06). The visual-span sizes for the AMD subjects with eccentric PRLs (n=9) were significantly smaller than the normal peripheral visual spans (median of 20.6 vs 29.0 bits, p=0.01).
In summary, the visual-span sizes in the AMD subjects were significantly reduced compared with normal central vision. Two factors contributing to the smaller visual spans in AMD are the normal reduction in visual-span size in peripheral vision, and the impact of nearby scotomas (see Sec. 3.5 below).
3.2 Information transfer rate in NV and AMD
Information transfer rate for each subject was computed by dividing the individual size of the visual span in bits by the individual’s exposure time in seconds, Figure 4b). For example, if the size of the visual span is 20 bits, and it is measured with an exposure time of 500 ms, the information transfer rate is 40 bits per sec.
As described in Methods Sec. 2.3.2.1, AMD visual spans were measured with stimulus exposure times determined by the individual’s temporal threshold for letter recognition, whereas the NV visual spans were measured with a fixed exposure time of 100 ms. Because of this procedural difference, the following comparisons between NV and AMD information transfer rates should be considered with caution. The NV values may underestimate maximum information transfer rates since 100 ms may exceed NV temporal thresholds for letter recognition.
Our AMD subjects had a wide range of information transfer rates from 9.34 to 406.2 bits/sec (median of 29.5 bits/sec and mean of 79.8 bits/sec). These values are significantly less than the information transfer rates for normal central vision (median of 407.7 bits/sec, U=2.0, p=0.001) and normal peripheral vision (median of 290.5 bits/sec, U=10, p=0.001). Group comparisons of information transfer rate for AMD subjects with different locations of fixation (foveal or eccentric) and for the NV subjects with corresponding fixation locations (central or peripheral) are summarized in Table 3.
Although slower information transfer rates were found in the AMD subjects with eccentric fixation than those with foveal fixation (median of 29.2 vs 82.4 bits/sec), the difference was not statistically significant (U=10, p=0.48). The lack of statistical significance was probably due to the small sample size in each group and individual variability (Table 3).
3.3 Relationship between reading and visual span measures in AMD
The median reading speed of our AMD subjects (2.09 log wpm) was substantially slower than the median reading speed of age-matched normally sighted controls reading with central vision (2.52 log wpm; the difference in the two samples was significant, U=1.0, p<0.001). The median reading speed for the nine AMD subjects who used an eccentric PRL was 2.09 log wpm, similar to the median value of 1.93 log wpm for peripheral reading (10°) by the eleven age-matched controls (U=37, p=0.51, Table 3). This near equality is likely to be fortuitous, and a consequence of our selection of AMD subjects with relatively mild impairments. The AMD reading speeds reported in the present paper were higher than values reported in many earlier studies, e.g. average values of 1.70 log wpm (Legge et al. (1992, 1985b)) and 1.88 log wpm (Cheong et al. (2005)).
We used nonparametric statistical methods – bootstrap (Efron, 1979, Efron, 1981) with 10,000 resamplings and the randomized permutation test (Fisher, 1935) with 10,000 permutations to examine the relationship between: 1) reading speed and the size of the visual span, and 2) reading speed and information transfer rate in the AMD subjects. Contrary to the hypothesis proposed by Legge et al. (2001), log reading speed did not significantly correlate with visual-span size (r=0.31, pbootstrap>0.05 [or 95% BCa interval = (-0.34, 0.76)]). Individual variations in the size of the visual span explained only 10% of the variance of the maximum reading speed in the AMD subjects. In contrast, log reading speed was significantly and positively correlated with information transfer rate (r=0.60, pbootstrap<0.05 [or 95% BCa interval = (0.75, 0.10)]), indicating that the AMD subjects with faster information transfer rate read faster. Individual information transfer rate explained 36% of the variance of the maximum reading speed in AMD subjects. Excluding the AMD subjects with foveal fixation, the individual information transfer rate improved the regression model, accounting for 49% of the variance in log reading speed.
3.4 Relationship between visual-span profiles and scotomas in AMD
In the Methods section, we described how we superimposed the visual-span profile on the subject’s tangent field map. In most cases, the presence of a scotoma coincided with depressions in the corresponding regions of the visual-span profiles, i.e., regions of reduced letter-recognition accuracy. This can be seen in the profiles for six of the thirteen subjects (Sub 3, 5, 10, 11, 12 and 15) in Figure 3 in which the gray bars, representing the estimated location of the scotomas are coincident with a depression in the profiles. For example, the visual-span profile for Sub 3 has depressions at letter positions of -6 to -1 and +3 to +6, corresponding to the location of a ring scotoma surrounding a spared central island subtending approximately 2° (with corresponding letter positions of -1 to +3).
Sub 16 adopted an inferior-left PRL by shifting the scotoma to her upper visual field, resulting in no scotoma lying along the horizontal meridian where the visual span was measured. However the boundary of this scotoma was just above the fixation location for trigram testing; the accuracy of letter recognition was moderately compromised for letter positions of -2 to +2. For Sub 1, the visual-span profile showed noticeable depressions both left and right of fixation, but only the depression on the left was associated with the estimated location of the scotoma. Visual-span profiles for Sub 9 and 14 were irregular and noisy. As a consequence of poor fixation, a higher proportion of trials was rejected (24% and 51% for Sub 9 and 14 respectively). To compensate for the rejected trials, these subjects were given many additional trials, perhaps resulting in inattention or fatigue and contributing to the noisiness of the data.
The foregoing qualitative analysis is consistent with the idea that letter recognition performance is depressed in portions of the visual span presumed to be superimposed on scotomas.
3.5 Relationship between clinical and visual span measures in AMD
Visual span size was only significantly correlated with scotoma size (in steradians) (r=-0.62, p=0.02), but not with distance visual acuity or contrast sensitivity (ps>0.05). In contrast, the slower information transfer rate in the AMD subjects was strongly correlated with vision loss reflected by distance visual acuity (r=-0.62, p=0.03), contrast sensitivity (r=0.58, p=0.04), and reading acuity (r=-0.57, p=0.04). Consistent with the distribution of PRL locations previously reported (Fletcher & Schuchard, 1997, Schuchard et al., 1999), six of the nine AMD subjects with eccentric fixation adopted PRLs below (and/or to the left of their scotomas in the visual field (Table 1). The information transfer rate in AMD was strongly correlated with scotoma size (in steradians) (r=-0.69, p=0.009), but it was not correlated with the coarsely estimated eccentricity of the PRL (r=-0.47, p=0.12).
4. Discussion
4.1 What are the factors limiting reading speed in AMD?
We began this study with the proposal by Legge et al. (2001) that reduction in the size of the visual span is a major factor limiting AMD reading speed, especially for those who must rely on peripheral vision. Our results showed significant decreases in the AMD visual spans and reading speeds, just as in normal peripheral vision. Contrary to the hypothesis proposed by Legge et al. (2001), the reduced visual-span size was not strongly correlated with reading speed. Our recent results demonstrating slower processing of letter information in AMD (Cheong et al., 2007) led us to consider the combined effects of a reduced visual span and slower temporal processing. We defined the information transfer rate for a given subject as the spatial size of the visual span in bits divided by the exposure time for letter recognition in sec. We found that this new variable was a substantially better predictor of AMD reading speed than visual-span size per se, accounting for 36% of the variance in reading speed (or 49%, if only the subjects with eccentric fixation were considered).
The slower information transfer rate exhibited by the AMD subjects could not be solely attributed to the normal decline in rate of information transmission from foveal vision to 10° peripheral vision. This implies that additional mechanisms such as concomitant retinal pathology (Curcio, Owsley & Jackson, 2000), fixation instability (Crossland et al., 2004) or crowding (Bouma, 1970, Jacobs, 1979, Latham & Whitaker, 1996, Strasburger, Harvey & Rentschler, 1991), must be invoked to explain the slower information transfer rate in the AMD subjects. Discussion of these mechanisms can be found in our partner paper (Cheong et al., 2007).
Although the rate of information transfer is significantly correlated with AMD reading speed, it accounts for less than half of the reading-speed variance. What accounts for the remaining variability? It is certainly possible that our new variable misses some important aspects of visual dysfunction in AMD reading. We comment briefly on three other individual factors potentially contributing to variability. First, because of acuity differences, our AMD subjects were tested with character sizes ranging from 0.34° to 4.18°. It is known that character size influences reading speed over this range for NV subjects (Legge, Pelli, Rubin & Schleske, 1985a) and might well be a source of variability among AMD subjects. Second, AMD subjects frequently reduce the amount of daily reading depending on the severity of their vision loss, facility with magnifiers, and motivation to read. It is possible the variability in reading exposure since the onset of AMD has an influence on reading speed. Third, even with groups of normally sighted subjects, there is substantial individual variability, a major portion of which is probably due to nonvisual factors including individual literacy, cognitive and linguistic skills (Jackson & McClelland, 1979, Jackson & McClelland, 1975). Accordingly, some of the variability in AMD reading speeds is probably due to differences in reading skill which preceded the onset of eye disease.
4.2 Clinical implications of slow information transfer rate in AMD
The definition of information transfer rate highlights the combined effects of spatial and temporal constraints on gathering information about letters in reading. This raises the possibility of a speed-accuracy trade off such that shorter stimulus durations yield smaller visual spans in bits. But, depending on the nature of the relationship between these quantities, the maximum rate of information transfer could occur for an intermediate or even short stimulus duration. Determining the stimulus duration that maximizes the information transfer rate would require measurement of AMD visual spans across a range of exposure times, rather than at one exposure time per subject as in the present study. For example, suppose the visual span sizes for stimulus durations of 200, 400 and 800 ms were 10, 24 and 28 bits respectively. The corresponding information transfer rates would be 50, 60 and 35 bits per sec. In this example, the maximum information transfer rate occurs for an intermediate stimulus exposure of 400 ms.
If such tradeoffs occur, do AMD readers naturally adjust their reading behavior to achieve fixation times that optimize the information transfer rate? If not, would a training program aimed at establishing this behavior be beneficial to AMD reading? A study by Fine and Rubin (1999) of normally sighted subjects reading with simulated central scotomas indicates somewhat prolonged fixation times. But data from subjects with juvenile macular degeneration indicate only slightly prolonged fixation times (Trauzettel-Klosinski, Teschner, Tornow & Zrenner, 1994), and data from AMD subjects indicate no important difference from normal fixation times (Bullimore & Bailey, 1995). It remains possible of course, that AMD subjects maintain normal short fixations in reading through force of habit, even if prolonged fixations might enhance their performance. It may seem paradoxical that prolonging reading fixations might result in faster reading. This could be the case if the extra fixation time allows for capture of information from a substantially wider visual span.
4.3 Relationship between AMD visual-span profiles and location of scotomas
The shapes of some AMD visual-span profiles were distorted from typical visual-span profiles in normal peripheral vision. In most cases, these distortions were associated with scotomas at the corresponding location in the visual field measured by standard perimetry. Despite good correspondence between estimated locations of scotomas and depressions in the visual-span profiles (Figure 3), letter recognition in the scotomatous regions did not drop to chance. Three factors may account for the residual visual function.
First, the scotoma was measured in the better eye on the Tangent screen in order to estimate the location of the PRL, but the visual-span profile was measured with binocular viewing. It is possible that some visual input from the poorer eye might have contributed to the non-zero performance within the presumed scotoma region of the visual span. It is also possible that the AMD subjects used different PRLs in the tangent-screen field test than in the visual span and reading tests. However, we think this possibility is unlikely given the good correspondence between estimated locations of scotomas and depressions in the visual-span profiles (Figure 3).
Second, scotomas were measured with a 5 mm white target on a tangent screen. At the viewing distance of 1 m, the white target subtends 0.28°, compared with test letters ranging from 0.34° to 4.18° for different AMD subjects in the visual-span measurements. Because of the differences in nature and size of the stimuli (dots vs letters), and task (dot detection vs letter recognition), it is possible that the scotomas measured by the Tangent screen would exhibit different extent or severity in the visual span. For example, a field region that is incapable of detecting the small tangent-screen test target might retain sufficient function to perform above chance for large test letters.
Third, it is well known that fixation stability for AMD subjects is significantly impaired (Crossland et al., 2004, Culham, Fitzke, Timberlake & Marshall, 1993, Schuchard & Fletcher, 1994). Although fixation stability was monitored in this study, the accuracy was such that only saccades larger than 1° could be detected. Fixation instability might have allowed some test letters, nominally delivered to the scotomatous region, to be viewed by healthier retina.
5. Conclusions
People with AMD have substantially smaller visual spans and exhibit slower temporal processing of letters than age-matched normally sighted controls. The information transfer rate, defined as the ratio of the size of the visual span in bits to the exposure time in sec for letter recognition, incorporates these two limitations into a single information-processing measure. The information transfer rate is significantly correlated with AMD reading speed and is a better predictor than the size of the visual span.
Acknowledgments
This research was supported by NIH Grant EY 002934 to Gordon E. Legge. We thank Ms Julie Anderson and other colleagues from Vision Loss Resources Minneapolis for assistance in subject recruitment. Some of the data in this paper were presented at the 2006 annual meeting of the Association for Research in Vision and Ophthalmology and 2007 annual meeting of the American Academy of Optometry.
Grant information: NIH Grant EY 002934
Footnotes
Data for six age-matched NV subjects were available from another study in our lab. The visual-span profiles from these subjects were combined with five newly recruited subjects to form the age-matched control group. These subjects self reported having no ocular diseases or cognitive problems.
The monocular field of the poorer eye and the binocular tangent fields were also measured but are not reported in Figure 3.
Reading speed for Sub 3 was compromised at small and large character sizes due to a ring scotoma. The print size which yielded the fastest reading speed in RSVP was adopted as the letter size for temporal threshold and visual span measures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckmann PJ, Legge GE. Preneural limitations on letter identification in central and peripheral vision. Journal of the Optical Society of America A, Optics, Image science, and Vision. 2002;19(12):2349–2362. doi: 10.1364/josaa.19.002349. [DOI] [PubMed] [Google Scholar]
- Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226(5241):177–178. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- Bullimore MA, Bailey IL. Reading and eye movements in age-related maculopathy. Optometry and Vision Science. 1995;72(2):125–138. doi: 10.1097/00006324-199502000-00011. [DOI] [PubMed] [Google Scholar]
- Cheong AMY, Legge GE, Lawrence MG, Cheung SH, Ruff M. Relationship between slow visual processing and reading speed in people with macular degeneration. Vision Research. 2007;47:2943–2965. doi: 10.1016/j.visres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong AMY, Lovie-Kitchin JE, Bowers AR, Brown B. Short-term in-office practice improves reading performance with stand magnifiers for people with AMD. Optometry & Vision Science. 2005;82(2):114–127. doi: 10.1097/01.opx.0000153244.93582.ff. [DOI] [PubMed] [Google Scholar]
- Cheung SH. Plasticity of the visual system following visual impairment. Minneapolis: Department of Psychology, University of Minnesota; 2005. Unpublished PhD Thesis. [Google Scholar]
- Cheung SH, Kallie CS, Legge G, Cheong AMY. Nonlinear mixed-effects modeling of MNREAD data. Investigative Ophthalmology & Visual Science. 2007 doi: 10.1167/iovs.07-0555. In Press. [DOI] [PubMed] [Google Scholar]
- Cheung SH, Legge G. Functional and cortical adaptations to central vision loss. Visual Neuroscience. 2005;22(2):187–201. doi: 10.1017/S0952523805222071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Legge GE, Cheung SH. Letter-recognition and reading speed in peripheral vision benefit from perceptual learning. Vision Research. 2004;44(7):695–709. doi: 10.1016/j.visres.2003.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Mansfield JS, Legge GE. Psychophysics of reading - XVIII. The effect of print size on reading speed in normal peripheral vision. Vision Research. 1998;38(19):2949–2962. doi: 10.1016/s0042-6989(98)00072-8. [DOI] [PubMed] [Google Scholar]
- Crossland MD, Culham LE, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic and Physiological Optics. 2004;24(4):327–333. doi: 10.1111/j.1475-1313.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- Crossland MD, Rubin GS. Eye movements and reading in macular disease: Further support for the shrinking perceptual span hypothesis. Vision Research. 2006;46(4):590–597. doi: 10.1016/j.visres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Culham LE, Fitzke FW, Timberlake GT, Marshall J. Assessment of fixation stability in normal subjects and patients using a scanning laser ophthalmoscope. Clinical Vision Science. 1993;8:551–561. [Google Scholar]
- Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Investigative Ophthalmology and Visual Science. 2000;41(8):2015–2018. [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: Another look at the jackknife. Annals of Statistics. 1979;7:1–26. [Google Scholar]
- Efron B. Nonparametric estimates of standard error: The jackknife, the bootstrap and other methods. Biometrika. 1981;68:589–599. [Google Scholar]
- Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clinical Vision Science. 1991;6(6):471–475. [Google Scholar]
- Elliott DB, Trukolo-Ilic M, Strong JG, Pace R, Plotkin A, Bevers P. Demographic characteristics of the vision-disabled elderly. Investigative Ophthalmology & Visual Science. 1997;38(12):2566–2575. [PubMed] [Google Scholar]
- Fine EM, Rubin GS. Reading with simulated scotomas: attending to the right is better than attending to the left. Vision Research. 1999;39:1039–1048. doi: 10.1016/s0042-6989(98)00208-9. [DOI] [PubMed] [Google Scholar]
- Fisher RA. The Design of Experiment. New York: Hafner; 1935. [Google Scholar]
- Fletcher DC, Schuchard RA. Preferred retinal loci relationship to macular scotomas in a low-vision population. Ophthalmology. 1997;104(4):632–638. doi: 10.1016/s0161-6420(97)30260-7. [DOI] [PubMed] [Google Scholar]
- Fletcher DC, Schuchard RA, Livingstone CL. Scanning laser ophthalmoscope macular perimetry and applications for low vision rehabilitation clinicians. Ophthalmology Clinics of North America. 1994;7:257–265. [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-Mental State: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Henson DB. Visual Fields. New York: Oxford University Press; 1993. [Google Scholar]
- Higgins KE, Arditi A, Knoblauch K. Detection and identification of mirror-image letter pairs in central and peripheral vision. Vision Research. 1996;36(2):331–337. doi: 10.1016/0042-6989(95)00117-i. [DOI] [PubMed] [Google Scholar]
- Jackson MD, McClelland JL. Processing determinants of reading speed. Journal of Experimental Psychology: General. 1979;108(2):151–181. doi: 10.1037//0096-3445.108.2.151. [DOI] [PubMed] [Google Scholar]
- Jackson MD, McClelland L. Sensory and cognitive determinants of reading speed. Journal of Verbal Learning and Verbal Behavior. 1975;14(6):565. [Google Scholar]
- Jacobs RJ. Visual resolution and contour interaction in the fovea and periphery. Vision Research. 1979;19(11):1187–1195. doi: 10.1016/0042-6989(79)90183-4. [DOI] [PubMed] [Google Scholar]
- Kitchin JE, Bailey I. Task complexity and visual acuity in senile macular degeneration. Australian Journal of Optometry. 1981;64(6):235–242. [Google Scholar]
- Latham K, Whitaker D. A comparison of word recognition and reading performance in foveal and peripheral vision. Vision Research. 1996;36:2665–2674. doi: 10.1016/0042-6989(96)00022-3. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Dinkgreve MA, Jonker C, Hooijer C, Lindeboom J. Are age and education independent correlates of the Mini-Mental State Exam performance of community-dwelling elderly? Journal of Gerontology. 1993;48(6):P271–277. doi: 10.1093/geronj/48.6.p271. [DOI] [PubMed] [Google Scholar]
- Legge G, Cheung SH, Chung STL, Lee HW, Gefroh J, Kwon M. Training peripheral vision to reading. In: Rieser JJ, Ashmead DH, Ebner FF, Corn AL, editors. Blindness and brain plasticity in navigation and object perception. Mahwah, NJ: Lawrence Erlbaum Associates; 2007a. [Google Scholar]
- Legge G, Cheung SH, Yu D, Chung ST, Lee HW, Owens DP. The case for the visual span as a sensory bottleneck in reading. Journal of Vision. 2007b;I7(2):1–15. doi: 10.1167/7.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE. Psychophysics of Reading in Normal and Low Vision. Mahwah, NJ & London: Lawrence Erlbaum Associates; 2007. [Google Scholar]
- Legge GE, Ahn SJ, Klitz TS, Luebker A. Psychophysics of reading - XVI. The visual span in normal and low vision. Vision Research. 1997;37(14):1999–2010. doi: 10.1016/s0042-6989(97)00017-5. [DOI] [PubMed] [Google Scholar]
- Legge GE, Mansfield JS, Chung STL. Psychophysics of reading - XX. Linking letter recognition to reading speed in central and peripheral vision. Vision Research. 2001;41(6):725–743. doi: 10.1016/s0042-6989(00)00295-9. [DOI] [PubMed] [Google Scholar]
- Legge GE, Pelli DG, Rubin GS, Schleske MM. Psychophysics of reading - I. Normal vision. Vision Research. 1985a;25(2):239–252. doi: 10.1016/0042-6989(85)90117-8. [DOI] [PubMed] [Google Scholar]
- Legge GE, Ross JA, Isenberg LM, LaMay JM. Psychophysics of reading. XII. Clinical predictors of low-vision reading speed. Investigative Ophthalmology & Visual Science. 1992;33(3):677–687. [PubMed] [Google Scholar]
- Legge GE, Rubin GS, Pelli DG, Schleske MM. Psychophysics of reading - II. Low vision. Vision Research. 1985b;25(2):253–265. doi: 10.1016/0042-6989(85)90118-x. [DOI] [PubMed] [Google Scholar]
- Lovie-Kitchin J, Mainstone J, Robinson J, Brown B. What areas of the visual field are important for mobility in low vision patients? Clinical Vision Science. 1990;4:249–263. [Google Scholar]
- Lovie-Kitchin JE, Bowers AR, Woods RL. Oral and silent reading performance with macular degeneration. Ophthalmic & Physiological Optics. 2000;20(5):360–370. [PubMed] [Google Scholar]
- Lovie-Kitchin JE, Whittaker SG. Vision ’96, Book 1, International Conference on Low Vision Proceedings. Madrid: Organización Nacional de Ciegos Españoles; 1998. Low vision assessment for reading rehabilitation: indications for visual field assessment; pp. 268–275. [Google Scholar]
- Mansfield JS, Ahn SJ, Legge GE, Leubker A. Noninvasive Assessment of the Visual System Technical Digest. Vol. 3. Washington D.C.: Optical Society of America; 1993. A new reading - acuity chart for normal and low vision; pp. 232–235. [Google Scholar]
- McMahon TT, Hansen M, Viana M. Fixation characteristics in macular disease. Relationship between saccadic frequency, sequencing, and reading rate. Investigative Ophthalmology & Visual Science. 1991;32:567–574. [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science. 1988;2(3):187–199. [Google Scholar]
- Pinheiro J, Bates D. Mixed-effects models in S and S-plus. New York: Springer-Verlag; 2000. [Google Scholar]
- Rubin GS, Turano KA. Low vision reading with sequential word presentation. Vision Research. 1994;34(13):1723–1733. doi: 10.1016/0042-6989(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Schuchard RA, Fletcher DC. Preferred retinal locus: a review with applications in low vision rehabilitation. Ophthalmology Clinics of North America. 1994;7:243–256. [Google Scholar]
- Schuchard RA, Naseer S, de Castro K. Characteristics of AMD patients with low vision receiving visual rehabilitation. Journal of Rehabilitation Research and Development. 1999;36(4):294–302. [PubMed] [Google Scholar]
- Seiple W, Holopigian K, Shnayder Y, Szlyk JP. Duration thresholds for target detection and identification in the peripheral visual field. Optometry and Vision Science. 2001;78(3):169–176. doi: 10.1097/00006324-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Strasburger H, Harvey LO, Jr, Rentschler I. Contrast thresholds for identification of numeric characters in direct and eccentric view. Perception and Psychophysics. 1991;49(6):495–508. doi: 10.3758/bf03212183. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Applegate CA, Haselwood D, Rubin GS. Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology. 1996;103(9):1458–1466. doi: 10.1016/s0161-6420(96)30483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake GT, Mainster MA, Peli E, Augliere RA, Essock EA, Arend LE. Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Investigative Ophthalmology & Visual Science. 1986;27(7):1137–1147. [PubMed] [Google Scholar]
- Trauzettel-Klosinski S, Teschner C, Tornow RP, Zrenner E. Reading strategies in normal subjects and in patients with macular scotoma assessed by two new methods of registration. Neuro-ophthalmology. 1994;14:15–30. [Google Scholar]
- Weleber RG, Tobler WR. Computerized quantitative analysis of kinetic visual fields. American Journal of Optometry & Physiological Optics. 1986;101:461–468. doi: 10.1016/0002-9394(86)90648-3. [DOI] [PubMed] [Google Scholar]
- White JM, Bedell HE. The oculomotor reference in humans with bilateral macular disease. Investigative Ophthalmology & Visual Science. 1990;31:1149–1161. [PubMed] [Google Scholar]
- Whittaker SG, Budd J, Cummings RW. Eccentric fixation with macular scotoma. Investigative Ophthalmology & Visual Science. 1988;29(2):268–278. [PubMed] [Google Scholar]
- Whittaker SG, Cummings RW, Swieson L. Saccade control without a fovea. Vision Research. 1991;31:2209–2218. doi: 10.1016/0042-6989(91)90173-3. [DOI] [PubMed] [Google Scholar]
- Whittaker SG, Lovie-Kitchin JE. Visual requirements for reading. Optometry and Vision Science. 1993;70(1):54–65. doi: 10.1097/00006324-199301000-00010. [DOI] [PubMed] [Google Scholar]