Abstract
Dihedral angles are evaluated for the phospholipid ligands of the lipid-binding proteins found in the Protein Data Base (PDB). Phospholipid structures occur with a trans C1–C2 configuration of the glycerol backbone and oppositely extended chains, in addition to the gauche C1–C2 rotamers found in membranes. Headgroup conformations are not restricted to the single bent-down configuration and gauche–gauche configuration of the phosphodiester that is found in phospholipid crystals. Additionally, fully extended headgroups and orientations directed away from the lipid chains are found for phospholipids in the protein binding pockets. On average, the hydrocarbon chains of the protein-bound lipids are conformationally more disordered than in fluid bilayer membranes. However, much of this configurational disorder arises from energetically disallowed skew conformations. This suggests a configurational heterogeneity in the lipids at a single binding site: Eclipsed conformations occur also in some lipid headgroups and glycerol backbones. Stereochemical violations appear for some of the ester carboxyl groups of the protein-bound phospholipids in the PDB, and two glycerol backbones have the incorrect enantiomeric configuration.
Keywords: Lipase, phospholipase, phospholipid transfer protein, saposin, permeability-increasing protein, ultraspiracle protein
Phospholipid-binding proteins are an important component of cellular signalling, trafficking, and metabolism (for a review, see Hurley et al. 2000). The conformations of the phospholipid ligands bound to these various proteins, and their relation to those of phospholipids in the bilayer membrane pool, are therefore of considerable interest. Crystal structures are now available for phospholipids bound to several proteins of the above classes (see Table 1). For phospholipid transfer proteins (Yoder et al. 2001; Roderick et al. 2002) the natural substrate, and for phospholipase A2 inhibitor phospholipids (Scott and Sigler 1994), have been resolved in the X-ray structure. Phospholipids have also been identified bound to lipases (van Tilbeurgh et al. 1993; Brzozowski et al. 2000), to saposin B (Ahn et al. 2003), to catechol-1,2-dioxygenase (Vetting and Ohlendorf 2000), to a flavohaemoprotein (Ollesch et al. 1999), to the endothelial protein C receptor (Oganesyan et al. 2002), and (as a heterologous ligand) to an orphan nuclear receptor (Billas et al. 2001).
Table 1.
Source of coordinates for phospholipids in crystals of soluble proteins
| PDB name | Lipid/proteina | PDB file | Res. (Å)b | References |
| PC2 501 | Ole2PtdCho/ratPI-TPα | 1FVZ | 2.20 | Yoder et al. 2001 |
| PC2 577,578 | Ste2PtdCho/human bpip | 1EWF | 1.70 | Kleiger et al. 2000 |
| PLC 601,701,801 | Lau2PtdCho/Th. lanuginosa lipase | 1EIN | 3.00 | Brzozowski et al. 2000 |
| PLC 1 | Lau2PtdCho/human pancreatic lipase | 1LPA | 3.04 | van Tilbeurgh et al. 1993 |
| DPL 2313 | Lin2PtdCho/human PC-TP | 1LN1 | 2.40 | Roderick et al. 2002 |
| CPL 300,301 | Pal-linPtdCho/human PC-TP | 1LN3 | 2.90 | Roderick et al. 2002 |
| LIO 999,1999 | Pentadec-decPtd(Cho)/Ac 1,2-CTD | 1DMH | 1.70 | Vetting and Ohlendorf 2000 |
| PEH 300 | Ste2PtdEtn/human saposin B | 1N69 | 2.20 | Ahn et al. 2003 |
| EPH 4000 | Ste-palPtdEtn/H. virescens USP | 1G2N | 1.65 | Billas et al. 2001 |
| PTY 606,607 | Pentadec-achPtdEtn/human rsEPCR | 1LQV | 1.60 | Oganesyan et al. 2002 |
| DGG 406(A),(B) | Pal-(cp)palPtd(Etn/Gro)/A. eutrophus FHP | 1CQX | 1.75 | Ollesch et al. 1999 |
a Ste, stearoyl; ole, oleoyl; lau, lauroyl; lin, linoleoyl; pal, palmitoyl; pentadec, pentadecanoyl; dec, decanoyl; ach, arachidoyl; (cp)pal, cis-9,10-methylene-palmitoyl; PtdCho, phosphatidylcholine; PtdEtn, phosphatidylethanolamine; PtdGro, phosphatidylglycerol; PI-TP, phosphatidylinositol transfer protein; bpip, bactericidal permeability-increasing protein; PC-TP, phosphatidylcholine transfer protein; Ac 1,2-CTD, catechol-1,2-dioxygenase/Acinetobacter sp. ADP1; USP, ultraspiracle protein; rsEPCR, recombinant soluble endothelial protein C receptor; FHP, flavohaemoprotein.
b Resolution of structure determination.
The purpose of the present communication is to classify the various phospholipid structures associated with the different phospholipid-binding protein classes, by evaluating dihedral angles. A significant consideration is the extent to which these correspond to energetically allowable rotamers. High-energy single conformers can be an indicator for the presence of conformational heterogeneity. With saposin B, evidence was found for additional conformers, in both the headgroup and acyl chains of the partially ordered phosphatidylethanolamine molecule (Ahn et al. 2003).
Figure 1 ▶ shows LIGPLOT (Wallace et al. 1995) schematic diagrams of different phospholipid ligands associated with various lipid-binding proteins. It is evident that the relative arrangement of the two lipid chains and the orientation of the lipid headgroup vary greatly, depending on the configuration of the protein binding pocket. The chains may be parallel (Fig. 1A,C), splayed (Fig. 1B ▶), or even bent back around the headgroup (Fig. 1D ▶). The headgroup may be bent back (Fig. 1B ▶), extended (Fig. 1C ▶), displaced relative to the chains (Fig. 1A ▶), or strongly coiled (Fig. 1D ▶). Analysis and classification is possible from the dihedral angles for the phospholipid backbone and headgroup.
Figure 1.
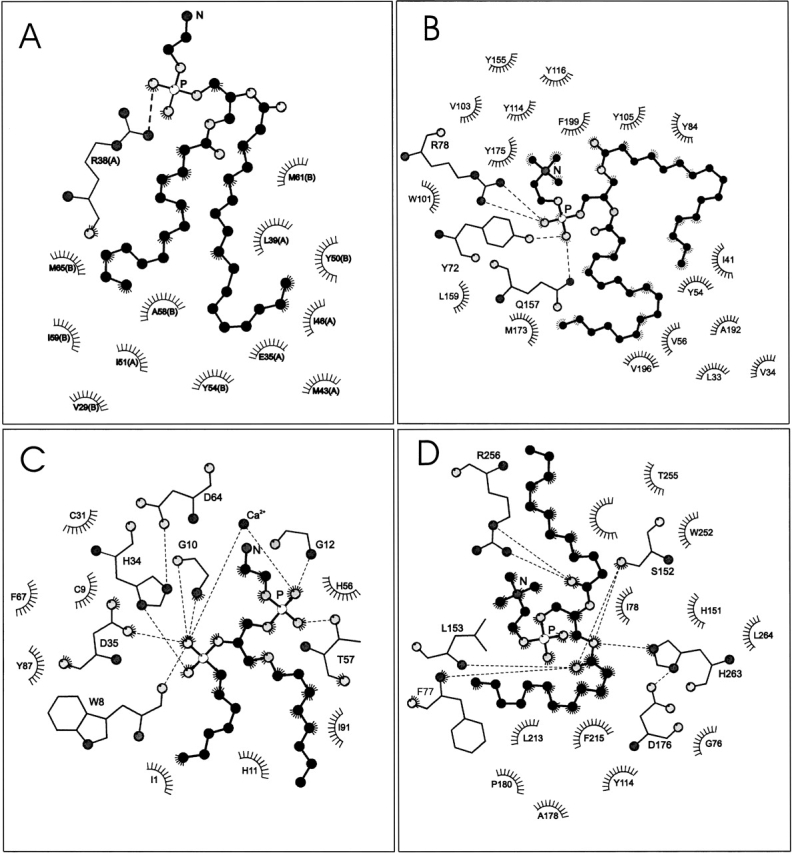
LIGPLOT diagrams (Wallace et al. 1995) of phospholipids associated with various lipid-binding proteins. Neighboring protein residues that are involved in hydrophobic contacts are indicated, and hydrogen bonds are shown explicitly. C-atoms of the phospholipid are shown as black balls. O-atoms are given in light gray, N-atoms in dark gray, and P-atoms are white. (A) Distearoyl phosphatidylethanolamine (PEH 300) bound to human saposin B (PDB:1N69; Ahn et al. 2003). (B) 1-Palmitoyl-2-linoleoyl phosphatidylcholine (CPL 301) bound to human phosphatidylcholine transfer protein (PDB:1LN3; Roderick et al. 2002). (C) 1-O-octyl-2-heptylphosphonyl-sn-glycero-3-phosphoethanolamine (GEL 420) bound to bee venom phospholipase A2 (PDB:1POC; Scott et al. 1990). (D) Dilauroyl phosphatidylcholine (PLC 1) bound to human pancreatic lipase–procolipase complex (PDB:1LPA; van Tilbeurgh et al. 1993).
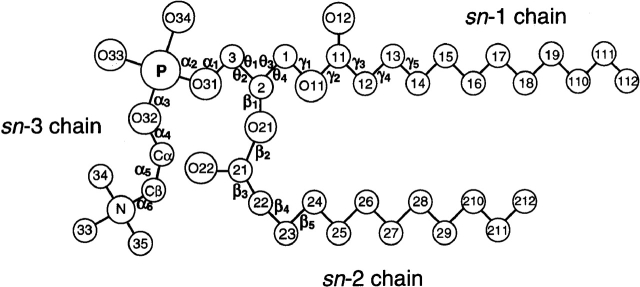
Table 2 lists the headgroup (α1–α5), glycerol backbone (θ1–θ4), and acyl chain (γ1 . . ., β1 . . .) torsion angles for diacyl phosphatidylcholine or phosphatidylethanolamine bound to the proteins listed in Table 1. Corresponding torsion angles for phospholipid inhibitors of phospholipase A2 (PLA2) are listed in Table 3. Of the inhibitors, GEL is a phospholipid transition-state analog with a heptylphosphonyl sn-2 chain, and both INB and DHG have amide-blocked sn-2 chains. These latter phospholipids are bound to different classes of secretory and pancreatic phospholipases A2. For comparison, the torsion angles of phosphatidylcholine (Pearson and Pascher 1979), and phosphatidylethanolamine (Elder et al. 1977) in lamellar single crystals are also included in Table 2. The notation used for defining the phospholipid torsion angles is that given by Pascher et al. (1992; see Fig. 2 ▶). The C-atoms of the glycerol backbone are numbered according to the sn-system. Those of the headgroup are designated Cα and Cβ, outwards from the phosphate.
Table 2.
Torsion angles (°) of diacyl phosphatidylcholines (PC2, PLC, DLP, CPL, LIO) and phosphatidylethanolamine (PEH, EPH, PTY) in protein crystals, and of dimyristoyl phosphatidylcholine (DMPC) and dilauroyl phosphatidylethanolamine (DLPE) alone in lamellar crystalsa
| Lipidb | α1 | α2 | α3 | α4 | α5 | θ1 | θ2 | θ3 | θ4 | β1 | β2 | β3 | β4 | γ1 | γ2 | γ3 | γ4 |
| DMPC A | 163 | 62 | 68 | 143 | −64 | 58 | 177 | −178 | 63 | 82 | 172 | −81 | 45 | −177 | 168 | −173 | 178 |
| DMPC B | 177 | −74 | −47 | −150 | 54 | 168 | −80 | 166 | 51 | 120 | 179 | −134 | 67 | 102 | 176 | 170 | 180 |
| DLPE | −154 | 58 | 66 | 106 | 67 | −52 | 65 | −172 | 69 | 97 | 179 | −119 | 65 | −178 | 173 | 179 | −171 |
| PC2 501 | 175 | −35 | −120 | −167 | 143 | −45 | −154 | −31 | 81 | −139 | −173 | 120 | 108 | 179 | 137 | −30 | −88 |
| PC2 577 | −159 | 167 | 37 | −179 | −27 | 139 | −85 | −80 | 148 | −69 | −4 | −162 | 121 | −161 | −3 | −158 | −72 |
| PC2 578 | −96 | 143 | 104 | 92 | 111 | −144 | −145 | 114 | −68 | −59 | |||||||
| PLC 601 | 178 | −134 | 168 | 150 | 129 | −113 | 20 | 168 | 38 | 56 | −158 | −81 | −175 | 127 | 11 | 100 | −134 |
| PLC 701 | −172 | 147 | 143 | −128 | −167 | −50 | 76 | 172 | 46 | 50 | −165 | −109 | 129 | 159 | 8 | −152 | −139 |
| PLC 801 | −174 | 162 | 107 | −168 | −137 | −57 | 68 | 169 | 41 | 47 | −165 | −113 | 150 | 148 | 15 | −177 | −140 |
| PLC 1 | 157 | 88 | 131 | −128 | 75 | −58 | 72 | −83 | 147 | 32 | −64 | −159 | 145 | 146 | −82 | −101 | −116 |
| DLP 2313 | −148 | 176 | 15 | 177 | 178 | 144 | −84 | 179 | 48 | 78 | 176 | 95 | −97 | −144 | 179 | −50 | 128 |
| CPL 300 | −144 | −177 | 19 | −180 | 174 | 132 | −99 | 180 | 54 | 74 | 178 | 105 | −97 | −111 | 177 | −112 | 112 |
| CPL 301 | −152 | −176 | 12 | 178 | −176 | 139 | −95 | −177 | 56 | 87 | 178 | 87 | −88 | −122 | 175 | −91 | 110 |
| LIO 999 | −141 | (−66 | 179 | 84 | 180)c | 137 | −92 | 0 | −129 | −178 | 1 | 112 | 114 | 177 | 0 | −150 | 180 |
| LIO 1999 | −166 | (−59 | 180 | 79 | 180)c | 129 | −100 | 0 | −130 | 180 | 1 | 124 | 167 | 177 | 0 | −174 | 161 |
| PEH 300 | 177 | 0 | −172 | −137 | 119 | 133 | −105 | 159 | 24 | 61 | 130 | −35 | −180 | −180 | 180 | −96 | 103 |
| EPH 4000 | 173 | 41 | −81 | 143 | 157 | 170 | −71 | −169 | 71 | 114 | −178 | −98 | 180 | −178 | 180 | −122 | 65 |
| PTY 606 | −104 | −63 | −56 | 170 | 83 | 175 | −67 | −177 | 63 | 95 | −173 | −162 | 156 | −177 | −175 | −134 | 42 |
| PTY 607 | −108 | −65 | −72 | 171 | 81 | −178 | −60 | −163 | 78 | 99 | 174 | −171 | 151 | −179 | 176 | −103 | −2 |
| DGG 406(A) | 132 | — | — | — | — | 17 | 141 | 171 | 49 | 57 | 170 | 146 | −166 | −122 | −155 | −91 | −138 |
| DGG 406(B) | −138 | — | — | — | — | −49 | 180 | −68 | 65 | −95 | −136 | 156 | −158 | −155 | 164 | −106 | −94 |
a αi, θi, βi and γi are torsion angles of headgroup, glycerol backbone, sn-2 chain and sn-1 chain, respectively (see Fig. 2 ▶).
b DMPC, dimyristoyl phosphatidylcholine crystal (Pascher et al. 1992); DLPE, dilauroyl phosphatidylethanolamine crystal (Pascher et al. 1992); PC2, distearoyl phosphatidylcholine with phosphatidylinositol transfer protein, PI-TP (PC2 501), bactericidal permeability-increasing protein, bpip (PC2 577 and 578); PLC, didodecanoyl phosphatidylcholine with Thermomyces lanuginosa lipase (PLC 601, 701, 801) and human pancreatic lipase–procolipase complex (PLC 1); DLP, dilinoleoyl phosphatidylcholine with phosphatidylcholine transfer protein, PC–TP; CPL, palmitoyl–linoleoyl phosphatidylcholine with PC–TP; LIO, pentadecanoyl-decanoyl phosphatidylcholine with Acinetobacter calcoaceticus ADP1 catechol-1,2-dioxygenase; PEH, distearoyl phosphatidylethanolamine with saposin B; EPH; phosphatidylethanolamine with Helios virescens ultraspiracle protein; PTY, pentadecanoyl-arachidoyl phosphatidylethanolamine with human recombinant soluble endothelial protein C receptor; DGG, palmitoyl-(cis-9,10-methylene)palmitoyl phosphatidyl(choline/glycerol) with Alcaligenes eutrophus flavohaemoprotein; where lipid identifiers are those from the PDB (see Table 1).
c modeled; no electron density (Vetting and Ohlendorf 2000).
Table 3.
Torsion angles (°) of the inhibitory substrate analogs 1-octyl-2-heptylphosphonyl-sn-glycero-3-phosphoethanolamine (GEL), 1-octadecyl-2-acetamido-2-deoxy-sn-glycero-3-phosphoethyl methyl sulphide (INB), and 2-dodecanoyl-amino-1-hexanol-phosphoglycol (DHG), bound to various phospholipases A2 (PLA2) in crystalsa
| Lipidb | PLA2b | α1 | α2 | α3 | α4 | α5 | θ1 | θ2 | θ3 | θ4 | β1 | β2 | β3 | β4 | γ1 | γ2 | γ3 | γ4 | References |
| GEL930 | cobra | 101 | 86 | 95 | 108 | 154 | 53 | 174 | −169 | 76 | 107 | 103 | −16 | 165 | 102 | −171 | −131 | 117 | White et al. 1990 (PDB:1POB) |
| GEL935 | 67 | 91 | 175 | −121 | 114 | 51 | −177 | 75 | −54 | 129 | 117 | −54 | 158 | 166 | −13 | 148 | 91 | White et al. 1990 (PDB:1POB) | |
| GEL420 | bee | 91 | 94 | −169 | −123 | 133 | 61 | −174 | 173 | 47 | 89 | 121 | −161 | 161 | 109 | −155 | −131 | −172 | Scott et al. 1990 (PDB:1POC) |
| GEL930 | synovial | 96 | 83 | 68 | 169 | −70 | 50 | 173 | 170 | 52 | 101 | 121 | −40 | 173 | 103 | 169 | 160 | −167 | Scott et al. 1991 (PDB:1POE) |
| GEL935 | 95 | 81 | 85 | 177 | −95 | 59 | 176 | −170 | 76 | 106 | 114 | −56 | 166 | 99 | −170 | −143 | 165 | Scott et al. 1991 (PDB:1POE) | |
| GEL150 | pancreas | 118 | 102 | 10 | 172 | 61 | 7 | −179 | −144 | 43 | 62 | 132 | −21 | 172 | 108 | −164 | −59 | −137 | Sekar et al. 1998 (PDB:1MKV) |
| INB201 | synovial | 87 | 81 | 37 | 114 | 76 | 88 | −148 | −113 | 116 | 98 | 177 | −85 | 170 | 172 | −165 | Oh 1995 (PDB:1AYP) | ||
| INB202 | 115 | 61 | 178 | −113 | 170 | 86 | −151 | −151 | 85 | 119 | 178 | 84 | 157 | 152 | 172 | Oh 1995 (PDB:1AYP) | |||
| INB203 | 128 | 64 | 166 | 125 | −166 | 81 | −153 | −92 | 140 | 111 | 177 | −107 | −163 | 170 | 78 | Oh 1995 (PDB:1AYP) | |||
| INB204 | 101 | 75 | −175 | −118 | 166 | 87 | −151 | −163 | 74 | 106 | −176 | 140 | −152 | −175 | 164 | Oh 1995 (PDB:1AYP) | |||
| INB205 | 113 | 63 | 72 | 148 | −63 | 79 | −158 | −101 | 137 | 107 | 177 | −100 | −162 | −173 | 173 | Oh 1995 (PDB:1AYP) | |||
| INB206 | 81 | 79 | 57 | 115 | 70 | 71 | −163 | −162 | 71 | 110 | 178 | 138 | 164 | 162 | −177 | Oh 1995 (PDB:1AYP) | |||
| DHG126(A) | pancreas | 92 | 70 | 74 | 136 | 74 | 85 | −157 | −157 | 83 | 117 | 180 | −83 | −50 | 82 | −169 | Thunnissen et al. 1990 (PDB:5P2P) | ||
| DHG126(B) | 106 | 67 | 75 | −173 | −45 | 61 | −169 | −175 | 54 | 111 | 180 | −85 | −44 | 72 | 62 | Thunnissen et al. 1990 (PDB:5P2P) |
a αi, θi, βi and γi are torsion angles of headgroup, glycerol backbone, sn-2 chain and sn-1 chain, respectively (see Fig. 2 ▶).
b Cobra (Naja naja) venom PLA2/GEL930,935 at 2.00 Å; honey bee venom PLA2/GEL420 at 2.00 Å; human synovial fluid PLA2/GEL930,935 at 2.10 Å; bovine pancreatic PLA2/GEL150 at 1.89 Å; human synovial fluid PLA2/INB 201-6 at 2.57 Å; pig pancreas PLA2/DHG126(A),(B) at 2.40 Å, where lipid identifiers are those in the PDB.
Figure 2.
Designation of phospholipid torsion angles for the headgroup (αi), glycerol backbone (θi), sn-1 chain (γi), and sn-2 chain (βi). For the glycerol backbone: θ1 ≡ C(1)-C(2)-C(3)-O(31), θ2 ≡ O(21)-C(2)-C(3)-O(31), and θ3 ≡ O(11)-C(1)-C(2)-C(3), θ4 ≡ O(11)-C(1)-C(2)-O(21).
Some estimate of the reliability of the torsion angles can be obtained by comparing the data for CPL 300 and CPL 301, which correspond to the two molecules in the asymmetric unit for the human PC-TP. The largest difference between the two sets of torsion angles in Table 2 is 21°, and the mean absolute difference is 7° (N = 17). Extending the data sets up to β14 and γ11, the maximum difference remains in the region 20–30°, with a mean absolute difference of 10° (N = 34). Deviations become larger, however, towards the ends of the hydrocarbon chains. Six of the remaining βn and γn torsion angles have deviations of approximately 60°, corresponding to the difference between adjacent staggered and eclipsed conformers. For one further C–C bond, the torsion angle (γ14) actually reverses sign between CPL 300 and CPL 301. These large deviations indicate an increasing segmental disorder towards the ends of the phospholipid acyl chains, even in the crystal. For the two PTY molecules in the asymmetric unit of EPCR crystals, all torsion angles in Table 2, with the exception of γ3 and γ4, are well within 20° of one another, and the mean absolute deviation is 10° (N = 17). For the two equivalent LIO molecules bound to the 1,2-CTD homodimer, all torsion angles in Table 2, with the exception of β4, are within 25° of one another, and the mean absolute deviation is again 10° (N = 17). For the two DGG molecules in the asymmetric unit of FHP crystals, much larger differences are found between equivalent torsion angles in Table 2. As will be seen later, this arises because one of the two structures is the incorrect glycerol enantiomer. The data for the INB 201–206 inhibitors in Table 3 correspond to the six molecules of synovial PLA2 per asymmetric unit. Thus, each molecule corresponds to only 17% of the total diffracting matter (Oh 1995). Nevertheless, with one exception, the standard deviations of torsion angles for the glycerol backbone and lipid chains in Table 3 are within approximately 30°. This is true also for the headgroup torsion angles α1 and α2, but beyond this the headgroup is apparently disordered, as is the sn-1 chain from γ7 onwards.
Glycerol backbone configuration
Table 4 summarizes configurational data on the glycerol backbone for the bound lipids in Tables 2 and 3. The difference between the complementary torsion angles θ1 and θ2 about the C2–C3 bond, and θ3 and θ4 about the C1–C2 bond, specifies the enantiomeric configuration. For the glycerol sn-3 phosphatidyl configuration, and tetrahedral carbon bonds, one expects that θ1–θ2 = −120° and θ3–θ4 = +120°. This is found to be the case for all lipids except GEL 150 associated with pancreatic phospholipase A2, PC2 501 associated with the phosphatidylinositol transfer protein (PI-TP), and DGG 406(B) associated with Alcaligenes eutrophus flavohaemoprotein (FHP). The latter two lipids possess definitively the incorrect (i.e., sn-1 phosphatidyl) glycerol stereoisomer.
Table 4.
Glycerol backbone configuration of phospholipids in crystals of soluble proteinsa
| Lipidb | Proteinb | θ1–θ2(°) | θ3–θ4(°) | θ4/θ2 | θ3/θ1 |
| GEL 930,935 | cobra PLA2 | −126 ± 5 | 122 ± 7 | sc,−sc/ap | (t,g+)g+ |
| GEL 420 | bee PLA2 | −125 | 126 | sc/ap | tg+ |
| GEL 930,935 | synovial PLA2 | −120 ± 3 | 116 ± 2 | sc/ap | tg+ |
| GEL 150 | pancreas PLA2 | −173 | 173 | sc/ap | s−c |
| INB 202,204,206 | synovial PLA2 | −124 ± 2 | 125 ± 1 | sc/ap | tg+ |
| INB 203,205 | synovial PLA2 | −124 ± 1 | 125 ± 3 | ac/ap | s−g+ |
| INB 201 | synovial PLA2 | −124 | 131 | ac/−ac | s−g+ |
| DGH 126(A,B) | pancreas PLA2 | −124 ± 6 | 125 ± 6 | sc/ap | tg+ |
| PC2 501 | rat PI-TPα | +108 | −112 | sc/ap | g−g− |
| PC2 577 | human bpip | −136 | 132 | ac/−sc | g−s+ |
| PC2 578 | human bpip | − | 121 | ac/− | s−− |
| PLC 601 | Th. lanuginosa lipase | −133 | 131 | sp/sc | ts− |
| PLC 701,801 | Th. lanuginosa lipase | −126 | 127 ± 1 | sc/sc | tg− |
| PLC 1 | human pancreatic lipase | −130 | 130 | ac/sc | g−g− |
| DLP 2313 | human PC-TP | −131 | 130 | sc/−sc | ts+ |
| CPL 300,301 | human PC-TP | −127 ± 1 | 126 ± 1 | sc/−ac | ts+ |
| LIO 999,1999 | Ac 1,2-CTD | −131 | 129 | −ac/−ac | cs+ |
| PEH 300 | human saposin B | −122 | 135 | sp/−ac | ts+ |
| EPH 4000 | H. virescens USP | −119 | 120 | sc/−sc | tt |
| PTY 606,607 | human rsEPCR | −118 ± 1 | 119 | sc/−sc | tt |
| DGG 406(A) | A. eutrophus FHP | −125 | 132 | sc/ac | tc |
| DGG 406(B) | A. eutrophus FHP | 122 | −133 | sc/ap | g−g− |
a Enantiomeric configuration is specified by the relative signs of θ1 and θ2, or of θ3 and θ4. θ4/θ2 gives the relative orientation of the sn-1 and sn-2 chains, and θ3/θ1 specifies the glycerol backbone conformation (see Pascher et al. 1992).
b Nomenclature as defined in Tables 1 to 3.
The relative orientation of the two acyl chains is specified by the torsion angle θ4, and that of the headgroup relative to the chains by the torsion angle θ2 (see Fig. 2 ▶). Following Pascher et al. (1992), the θ4/θ2 configuration is specified in terms of sp (cis), ±sc (gauche±), ±ac (skew±), or ap (trans) conformers with dihedral angles 0° ± 30°, 60° ± 30°, 120° ± 30°, and 180° ± 30°, respectively. The configuration of the glycerol itself is specified by the θ3/θ1 combination of torsion angles. This complementary configuration is also given in Table 4, in terms of the more familar trans (t), gauche (g), skew (s), and cis (c) conformers.
Almost all phospholipids associated with the secretory and pancreatic phospholipases A2 have the θ4/θ2 = sc/ap configuration. This corresponds to staggered glycerol rotamers, and allows approximately parallel alignment of the sn-1 and sn-2 chains (see Fig. 1C ▶). The sole exceptions are INB 203, 205, and INB 201, associated with synovial PLA2, the glycerol backbones of which contain one or two eclipsed rotamers, respectively. The sc/ap configuration is found relatively infrequently in phospholipid crystals, all of which have a lamellar, membrane-like molecular arrangement (Pascher et al. 1992). In the latter case, sc/sc and sc/−sc configurations (as in DLPE and DMPC B, respectively), and to a lesser extent −sc/−sc, are strongly represented because of the intramolecular gauche effect. The sc/ap configuration occurs in one of the molecules (DMPC A) in crystals of dimyristoyl phosphatidylcholine (Pearson and Pascher 1979) and in dilauroyl phosphatidic acid (Pascher et al. 1992). For the former, the headgroup is directed away from the sn-2 chain, viz., the sc/γ/ap configuration for which sn-1 is the leading chain, in the notation of Pascher et al. (1992). This is also the case for the phospholipids bound to PLA2, thus exposing the ester group to hydrolytic attack (see Fig. 1C ▶).
The sc/−sc configuration is found for phosphatidylethanolamine EPH 4000 bound to the Helios virescens ultraspiracle protein (USP), as well as for dilinoleoyl phosphatidylcholine DLP 2313 bound to the phosphatidylcholine transfer protein (PC-TP) and phosphatidylethanolamine PTY 606, 607 bound to the endothelial protein C receptor (rsEPCR). This configuration allows approximately parallel alignment of the lipid chains and appears for one of the molecules (DMPC B) in crystals of dimyristoyl phosphatidylcholine, as the sc/γ/−sc structure with leading sn-1 chain (Pearson and Pascher 1979). EPH 4000 bound to USP has a similar configuration, in which the headgroup is located over the lipid chains. DLP 2313 bound to the PC-TP more resembles the sc/β/−sc structure with leading sn-2 chain and the headgroup directed away from the chains. For PTY bound to EPCR, the headgroup is directed away from the chains, which splay apart further down their length.
The sc/sc configuration, which also allows parallel alignment of the lipid chains, is reported for dilauroyl phosphatidylcholine PLC 701 and 801 bound to Thermomyces lanuginosa lipase. In the latter case, however, the lipid chains are not aligned parallel to one another but instead are oriented in opposite directions. This configuration, which is not found in the crystals of diacyl phospholipids, is achieved by means of an energetically forbidden cis conformation (γ2) for the carboxyl ester group of the sn-1 chain (see Table 2).
The remainder of the phospholipid structures contain at least one eclipsed conformer in the glycerol backbone (or are the incorrect enantiomer). However, PLC 601 and INB 201 have values of θ4/θ2 that approximate to those of the other members of these two series. Palmitoyl-linoleoyl phosphatidylcholine, CPL 300 and 301, bound to the phosphatidylcholine transfer protein lie most closely to the sc/−sc staggered configuration, with the headgroup directed away from the chains (see Fig. 1B ▶), as does DLP 2313 bound to PC-TP. The structure of phosphatidylethanolamine PEH 300 bound to saposin B approximates most nearly to a sc/−sc staggered configuration with parallel aligned chains and the headgroup directed away from the chains (see Fig. 1A ▶).
The structures of phosphatidylcholine PLC1 bound to the pancreatic lipase–procolipase complex and of distearoyl phosphatidylcholine PC2 577 bound to the bactericidal permeability-inducing protein approximate to the ap/sc and ap/−sc staggered configurations in which the sn-1 and sn-2 chains are extended in opposite directions (see Fig. 1D ▶). Such a configuration does not appear in known crystal structures of diacyl phospholipids, but is found as ap/−sc for the extended structure of crystalline N-dihydroxyoctadecanoyl-phytosphingosine (Pascher and Sundell 1992). It is most likely that phosphatidylcholines PLC 601, 701, and 801 bound to Thermomyces lanuginosa lipase that were discussed above also have one of these two configurations (with a trans, rather than cis, sn-1 chain carboxyl ester). In these configurations, the phosphatidylcholine headgroup is predominantly perpendicular to, and in the case of the lipases even encircled by, the lipid chains.
Headgroup conformation
The α1-torsion angle (C3-O) is mostly ap (trans) for the protein-bound phospholipids of Table 2, and ac (skew) for the PLA2 inhibitors in Table 3 (as are also the non-trans conformers in Table 2). The trans rotamer is found in all crystals of phosphatidylethanolamine and its methylated derivatives, including phosphatidylcholine (Pascher et al. 1992). The skew rotamer is found in crystals of phosphatidylglycerol (see Pascher et al. 1987).
Correlated torsion angles α2/α3 = ±sc/±sc are expected on energetic grounds for the O-P-O sequence of phosphate diesters (Gorenstein et al. 1976) and are found without exception in phospholipid crystals. Of the PLA2 inhibitors in Table 3, approximately 60% have the sc/sc configuration for α2/α3 and approximately 40% have the energetically next most favorable sc/ap configuration. Of the protein-bound phospholipids in Table 2, only PTY 606, 607 has the α2/α3 = ±sc/±sc headgroup configuration and none has the ±sc/ap configuration. (The headgroup of LIO is not defined and only modeled in this region.)
In phospholipid crystals, the α4-torsion angle (O–Cα) lies in the range from ap to ±ac, which is also the case for all values of α4 in Tables 2 and 3. The α5-torsion angle (Cα–Cβ) is ±sc in crystals of phosphatidylethanolamine and all N-methylated derivatives, and ap in phosphatidylglycerol crystals. The α5 = ±sc conformation is favored by an internal electrostatic interaction between the phosphate and nitrogen charges, for example, for DMPC and DLPE. These values for α5 are found for most of the protein-bound lipids in Table 3 and some of those in Table 2. An α5 = ap rather than α5 = ±sc conformation can be favored for P–N headgroups by electrostatic interactions with the protein, and is found in several cases in Tables 2 and 3. Nonetheless, there are still an appreciable number of energetically disallowed eclipsed conformers for α5 in the protein-bound phospholipids, especially those in Table 2. In total, 29% of the θ1, θ2, θ3, θ4, and α5 torsion angles for the protein-bound lipids in Tables 2 and 3 are in eclipsed conformations (24% ±ac and 5% sp, compared with 31% ap and 40% ±sc).
Additional to bent-down conformations of the headgroup, which are those found in diacyl phospholipid crystals (Pascher et al. 1992) and in membranes (Seelig 1977), headgroups that are almost fully extended, or are strongly kinked or curled, are found for some protein-bound phospholipids. In the GEL inhibitor lipids bound to PLA2, the headgroup is directed out from the lipid chains and the nitrogen away from the phosphate (see Fig. 1C ▶). Multiple interactions with residues of the binding pocket are responsible for this headgroup orientation. The situation is similar for DHG bound to PLA2, but less well defined—as regards distinct orientation—for the INB inhibitors.
For PLC 601, 701, and 801 phosphatidylcholine bound to Th. lanuginosa lipase, the headgroup is mostly trans (i.e., ap) in conformation and extends directly outwards from the glycerol backbone. A more curled configuration is found, however, for the headgroup of PLC 1 bound to the pancreatic lipase–procolipase complex (see Fig. 1D ▶). For PC2 501 and PC2 577 bound respectively to the PI-transfer protein and the bactericidal permeability-increasing protein, the headgroup although not completely extended is also directed away from the chains. The phosphate group of PC2 is hydrogen bonded to, and interacts electrostatically with, neighboring side chains (Lys 195 and Thr 97 for PI-TP, and Tyr 455 for bpip); for PC2 501, the choline may also participate in electrostatic interaction (with Glu 86). The α-torsion angles of DLP 2313, and CPL 300 and 301, phosphatidylcholines bound to the PC-transfer protein, are essentially all trans, with the exception of α3, which is cis. This causes the headgroup to bend back over the chains (see Fig. 1B ▶). The phosphate group of these lipids is hydrogen bonded to the protein (Tyr 72, Gln 157) and interacts with the neighboring arginine residue (Arg 78). The choline group participates in cation-π interactions with neighboring aromatic side chains (Trp 101, Tyr 114, and Tyr 155).
Phosphatidylethanolamine EPH 4000 bound to the ultraspiracle protein has a headgroup that is bent down towards the chains. This is caused by an H-bond interaction of the amine nitrogen with Gln 338 on the protein. For PEH 300 bound to saposin B, only the phosphate of the phosphatidylethanolamine headgroup interacts electrostatically and hydrogen bonds with the protein (with Arg 38), and the nitrogen is directed away from the chains (see Fig. 1A ▶).
Chain configuration
For the diacyl phospholipids in Table 2, the torsion angles γ2 and β2 represent the carboxyl ester groups (O–CO) of the sn-1 and sn-2 chains, respectively. The conformation should therefore be trans (ap). However, only 56% of the γ2- and β2-torsion angles of the protein-bound phospholipids in Table 2 are trans; 25% are cis, and the remaining 19% are nonplanar. In the case of PLC 601, 701, and 801, it was suggested above that this stereochemical violation could be the result of a misassignment of the backbone θ4-torsion angles.
Figure 3 ▶ shows the complete set of γn and βn chain torsion angles (Cn−2–Cn−1) for the phospholipid ligands of selected proteins. The "allowed" ranges of trans (ap) and gauche (±sc) staggered rotamers are indicated by crosshatching. It is seen that for each system not all torsion angles are in the allowed range. Skew (±ac) conformers, which appear in the ±120° nonhatched regions, are particularly prevalent, although they are only expected adjacent to double bonds because otherwise they are eclipsed. cis conformers (the 0° nonhatched regions) are expected only for double bonds (or cyclopropane rings), and they appear only at the expected positions C9–C10 (n = 11) and C12–C13 (n = 14) for the linoleoyl chains associated with the PC-transfer protein (see Fig. 3A ▶). A cis 9–10 bond is also found in the sn-1 and sn-2 chains of PC2 501 bound to the PI-transfer protein (Fig. 3B ▶). This corresponds to the dioleoyl phosphatidylcholine used to displace bound bacterial phosphatidylglycerol, although it is named distearoyl phosphatidylcholine in the PDB. EPH 4000 bound to USP is designated as 1-palmitoyl-2-oleoyl phosphatidylethanolamine in the PDB. However, neither chain contains a cis bond, and the chainlengths correspond to 1-stearoyl-2-palmitoyl phosphatidylethanolamine, rather than to 1-palmitoyl-2-stearoyl phosphatidylethanolamine. A cis 9–10 conformation (β11) is found for the cyclopropane ring in the sn-2 chain of DGG 406 bound to A. eutrophus FHP.
Figure 3.
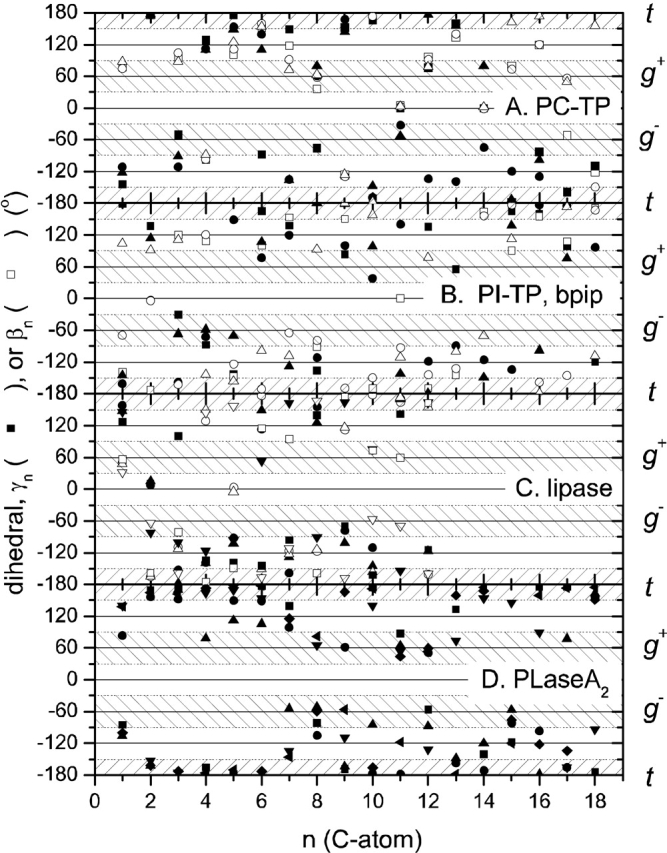
Torsion angles, Cn−3Cn−2Cn−1Cn, in the sn-1 (solid symbols) and the sn-2 (open symbols) chains of protein-bound phospholipids. A. Dilinoleoyl phosphatidylcholine (DLP; ▪,□) or 1-palmitoyl-2-linoleoyl phosphatidylcholine (CPL; •,○;▴,▵) bound to the human phosphatidylcholine transfer protein (PDB:1LNX, 1LN3; Roderick et al. 2002). B. Distearoyl phosphatidylcholine (PC2) bound to the α-isoform of rat phosphatidylinositol transfer protein (▪,□) (PDB:1FVZ; Yoder et al. 2001) or to the human bactericidal permeability-increasing protein (•,○;▴,▵) (PDB:1EWF; Kleiger et al. 2000). C. Dilauroyl phosphatidylcholine (PLC) bound to Th. lanuginosa lipase (▪,□; •,○;▴,▵) (PDB:1EIN; Brzozowski et al. 2000) or to human pancreatic lipase-procolipase complex (▾,▿) (PDB:1LPA; van Tilbeurgh et al. 1993). D. 1-O-octadecyl-2-acetamido-2-deoxy-sn-glycero-3-phosphoethyl methylsulphide (INB) bound to human synovial phospholipase A2 (PDB:1AYP; Oh 1995). Shapes of symbols are used to distinguish the individual lipids in each panel.
Table 5 lists the populations of chain rotamers for all the different lipids bound to the various proteins (see also Fig. 4 ▶). The cis conformers are mostly accounted for by double bonds or a cyclopropane ring. In many cases, however, energetically disallowed eclipsed skew conformations predominate to a far greater extent than would be allowed for by the double-bond, or cyclopropane, content. For phosphatidylethanolamine PEH 300 bound to saposin, this difficulty almost certainly results from the reported conformational heterogeneity. For the other similar situations, the eclipsed conformations probably also represent a superposition of allowed rotamers, rather than a single unique configuration with exceptionally high conformational energy (∼3 kcal/mole per skew conformation). Only 39% of the C–C bonds in Table 5 are in the trans (ap) conformation. This compares with approximately 70% trans bonds for the lipid chains in fluid bilayer membranes (see, e.g., Cevc and Marsh 1987; Moser et al. 1989). Summing all torsion angles >120° and <−120° in Figure 4 ▶ still yields an effective total trans population of only 62%. The postulated conformational heterogeneity makes precise comparisons difficult, but it appears that the chains of the phospholipid ligands are conformationally more disordered in the protein binding pockets than they are in fluid lipid membranes.
Table 5.
Distribution (%) of acyl chain torsion angles for phospholipids in crystals of soluble proteins
| lipida | trans (ap) | eclipsed (±ac) | gauche (±sc) | cis (sp) | Nb |
| GEL | 42 | 47 | 8 | 3 | 60 |
| PC2 | 31 | 51 | 16 | 2 | 90 |
| PLC | 37 | 49 | 11 | 3 | 72 |
| DLP | 27 | 37 | 23 | 13 | 30 |
| CPL | 25 | 43 | 25 | 7 | 56 |
| LIO | 55 | 42 | 3 | 0 | 38 |
| PEH | 48 | 41 | 11 | 0 | 27 |
| EPH | 32 | 39 | 29 | 0 | 28 |
| PTY | 43 | 33 | 21 | 3 | 58 |
| DGG | 50 | 23 | 23 | 4 | 52 |
a Nomenclature as in Tables 1 to 3, which list the proteins involved.
b Total number of torsion angles, βn and γn with n > 3.
Figure 4.
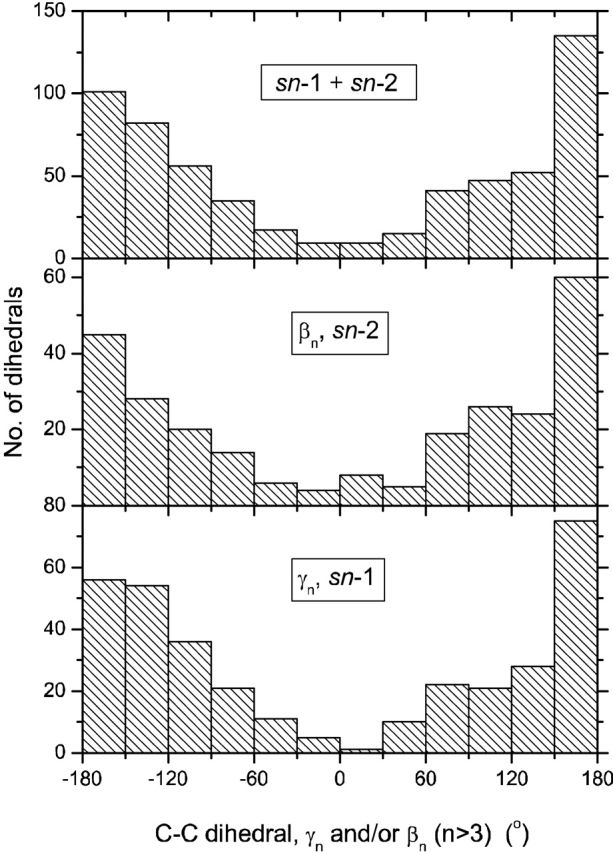
Distribution of torsion angles, γn and βn (n > 3), in the hydrocarbon chains of the protein-bound phospholipids listed in Tables 1 to 3 (with the exception of DHG). From upper to lower: all chains (N = 599); all sn-2 chains (N = 259); all sn-1 chains (N = 340).
Conclusions
The phospholipid ligands of lipid-binding proteins display a considerably greater range of conformations than do phospholipids in crystals. Several of these configurations (e.g., for the lipases and phospholipid transfer proteins) are not compatible with those in the donor or acceptor membranes with which they interact. Configurations are found with oppositely directed lipid chains, rather than the parallel chain stacking required in membranes. Extended lipid headgroups, and other headgroup orientations that are directed away from the lipid chains, appear to optimize polar interactions with residues within the lipid binding pocket, in many cases. These situations are quite different from the bent-down headgroup configuration found in phospholipid crystals and biological membranes. The lipid chains are conformationally disordered within the protein binding site, probably to a greater extent than the dynamic disorder in fluid membranes. Not all of the phospholipid ligand structures in the PDB are stereochemically acceptable, in the sense of incorrect glycerol enantiomer and either nonplanar or cis carboxyl ester conformations. Furthermore, the preponderance of eclipsed C–C bond conformers in the lipid chains strongly suggests the possibility of conformational heterogeneity of the bound lipids. It is, of course, possible that the occurrence of eclipsed conformers also reflects limitations in the accuracy of the crystal structures. However, the former bears no obvious relation to the resolution of the structure determinations (cf. Table 1 and Fig. 3 ▶) and—as already mentioned—there is evidence for conformational heterogeneity from the electron density maps, in at least one case (Ahn et al. 2003).
Acknowledgments
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0396803.
References
- Ahn, V.E., Faull, K.F., Whitelegge, J.P., Fluharty, A.L., and Prive, G.G. 2003. Crystal structure of saposin B reveals a dimeric shell for lipid binding. Proc. Natl. Acad. Sci. 100 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billas, I.M.L., Moulinier, L., Rochel, N., and Moras, D. 2001. Crystal structure of the ligand-binding domain of the ultraspiracle protein USP, the ortholog of retinoid X receptors in insects. J. Biol. Chem. 276 7465–7474. [DOI] [PubMed] [Google Scholar]
- Brzozowski, A.M., Savage, H., Verma, C.S., Turkenburg, J.P., Lawson, D.M., Svendsen, A., and Patkar, S. 2000. Structural origins of the interfacial activation in Thermomyces (Humicola) lanuginosa lipase. Biochemistry 39 15071–15082. [DOI] [PubMed] [Google Scholar]
- Cevc, G. and Marsh, D. 1987. Phospholipid bilayers. Physical principles and models. Wiley-Interscience, New York.
- Elder, M., Hitchcock, M.P., Mason, R., and Shipley, G.G. 1977. A refinement analysis of the crystallography of the phospholipid, 1,2-dilauroyl-DL-phosphatidylethanolamine, and some remarks on lipid–lipid and lipid–protein interactions. Proc. R. Soc. Lond. A 354 157–170. [Google Scholar]
- Gorenstein, D.G., Kar, D., Luxon, B.A., and Momii, R.K. 1976. Conformational study of cyclic and acyclic phosphate esters. CNDO/2 calculations of angle strain and torsional strain. J. Am. Chem. Soc. 98 1668–1673. [DOI] [PubMed] [Google Scholar]
- Hurley, J.H., Tsujishita, Y., and Pearson, M.A. 2000. Floundering about at cell membranes: A structural view of phospholipid signaling. Curr. Opin. Struct. Biol. 10 737–743. [DOI] [PubMed] [Google Scholar]
- Kleiger, G., Beamer, L.J., Grothe, R., Mallick, P., and Eisenberg, D. 2000. The 1.7 Å crystal structure of BPI: A study of how two dissimilar amino acid sequences can adopt the same fold. J. Mol. Biol. 299 1019–1034. [DOI] [PubMed] [Google Scholar]
- Moser, M., Marsh, D., Meier, P., Wassmer, K.-H., and Kothe, G. 1989. Chain configuration and flexibility gradient in phospholipid membranes. Comparison between spin-label electron spin resonance and deuteron nuclear magnetic resonance, and identification of new conformations. Biophys. J. 55 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesyan, V., Oganesyan, N., Terzyan, S., Qu, D.F., Dauter, Z., Esmon, N.L., and Esmon, C.T. 2002. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J. Biol. Chem. 277 24851–24854. [DOI] [PubMed] [Google Scholar]
- Oh, B.-H. 1995. A probe molecule composed of seventeen percent of total diffracting matter gives correct solutions in molecular replacement. Acta Crystallogr. D 51 140–144. [DOI] [PubMed] [Google Scholar]
- Ollesch, G., Kaunzinger, A., Juchelka, D., Schuber-Zsilavecz, M., and Ermler, U. 1999. Phospholipid bound to the flavohemoprotein from Alcaligenes eutrophus. Eur. J. Biochem. 262 396–405. [DOI] [PubMed] [Google Scholar]
- Pascher, I. and Sundell, S. 1992. Molecular arrangements in sphingolipids—Crystal structure of the ceramide N-(2D,3D-dihydroxyoctadecanoyl)-phytosphingosine. Chem. Phys. Lipids 62 79–86. [Google Scholar]
- Pascher, I., Sundell, S., Harlos, K., and Eibl, H. 1987. Conformation and packing properties of membrane lipids: The crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim. Biophys. Acta 896 77–88. [DOI] [PubMed] [Google Scholar]
- Pascher, I., Lundmark, M., Nyholm, P.-G., and Sundell, S. 1992. Crystal structures of membrane lipids. Biochim. Biophys. Acta 1113 339–373. [DOI] [PubMed] [Google Scholar]
- Pearson, R.H. and Pascher, I. 1979. The molecular structure of lecithin dihydrate. Nature 281 499–501. [DOI] [PubMed] [Google Scholar]
- Roderick, S.L., Chan, W.W., Agate, D.S., Olsen, L.R., Vetting, M.W., Rajashankar, K.R., and Cohen, D.E. 2002. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat. Struct. Biol. 9 507–511. [DOI] [PubMed] [Google Scholar]
- Scott, D.L. and Sigler, P.B. 1994. Structure and catalytic mechanism of secretory phospholipase A2. Adv. Protein Chem. 45 53–88. [DOI] [PubMed] [Google Scholar]
- Scott, D.L., Otwinowski, Z., Gelb, M.H., and Sigler, P.B. 1990. Crystal structure of bee-venom phospholipase A2 in a complex with a transition-state analogue. Science 250 1563–1566. [DOI] [PubMed] [Google Scholar]
- Scott, D.L., White, S.P., Browning, J.L., Rosa, J.J., Gelb, M.H., and Sigler, P.B. 1991. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science 254 1007–1010. [DOI] [PubMed] [Google Scholar]
- Seelig, J. 1977. Deuterium magnetic resonance: Theory and application to lipid membranes. Q. Rev. Biophys. 10 358–418. [DOI] [PubMed] [Google Scholar]
- Sekar, K., Kumar, A., Liu, X., Tsai, M.D., Gelb, M.H., and Sundaralingam, M. 1998. Structure of the complex of bovine pancreatic phospholipase A2 with a transition-state analogue. Acta Crystallogr D 54 334–341. [DOI] [PubMed] [Google Scholar]
- Thunnissen, M.M.G.M., Eiso, A.B., Kalk, K.H., Drenth, J., Dijkstra, B.W., Kuipers, O.P., Dijkman, R., de Haas, G.H., and Verheij, H.M. 1990. X-ray structure of phospholipase A2 complexed with a substrate derived inhibitor. Nature 347 689–691. [DOI] [PubMed] [Google Scholar]
- van Tilbeurgh, H., Egloff, M.P., Martinez, C., Rugani, N., Verger, R., and Cambillau, C. 1993. Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography. Nature 362 814–820. [DOI] [PubMed] [Google Scholar]
- Vetting, M.W. and Ohlendorf, D.H. 2000. The 1.8 Å crystal structure of catechol 1,2-dioxygenase reveals a novel hydrophobic helical zipper as a subunit linker. Struct. Fold. Des. 8 429–440. [DOI] [PubMed] [Google Scholar]
- Wallace, A.C., Laskowski, R.A., and Thornton, J.M. 1995. LIGPLOT—A program to generate schematic diagrams of protein ligand interactions. Protein Eng. 8 127–134. [DOI] [PubMed] [Google Scholar]
- White, S.P., Scott, D.L., Otwinowski, Z., Gelb, M.H., and Sigler, P.B. 1990. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science 250 1560–1563. [DOI] [PubMed] [Google Scholar]
- Yoder, M.D., Thomas, L.M., Tremblay, J.M., Oliver, R.L., Yarbrough, L.R., and Helmkamp Jr., G.M. 2001. Structure of a multifunctional protein. J. Biol. Chem. 276 9246–9252. [DOI] [PubMed] [Google Scholar]