Commentary
The paper in this issue of Free Radical Biology and Medicine titled “Faster Plasma Vitamin E Disappearance in Smokers is Normalized by Vitamin C Supplementation” by Richard Bruno and colleagues [1] provides the first direct evidence that vitamins C and E work together as co-antioxidants in vivo.
In the 1920’s and ‘30’s the research of Dr. Albert Szent-Györgyi had vitamin C as a central focus. In his seminal work on vitamin C he provided the first evidence that ascorbic acid (then called hexauronic acid) could function as an antioxidant [2]; he discovered that it was the preferred reducing substrate for the activated form of hemeperoxidases (now known as compound-I) thereby sparing the oxidation of other potential substrates. At this same time Dr. Henry A Mattill was doing research on vitamin E [3]. Although vitamin C is water-soluble and vitamin E oil-soluble, they were encountering somewhat similar problems when trying to determine the exact chemical nature of these two substances; because both substances are excellent reducing agents, they were easily destroyed in the presence of oxidants. In 1936 Olcott and Mattill demonstrated that vitamin E functions as a classic antioxidant [4]. In 1941 Mattill’s group clearly demonstrated that these two substances could work as co-antioxidants to protect fats from oxidation [5]; he observed that tocopherol was necessary for this protection and that it was preserved as long as ascorbate was present. After all the ascorbate was oxidized, tocopherol was subject to loss and the oxidation of the fats/oils ensued. However the chemical mechanism of this protection was not understood.
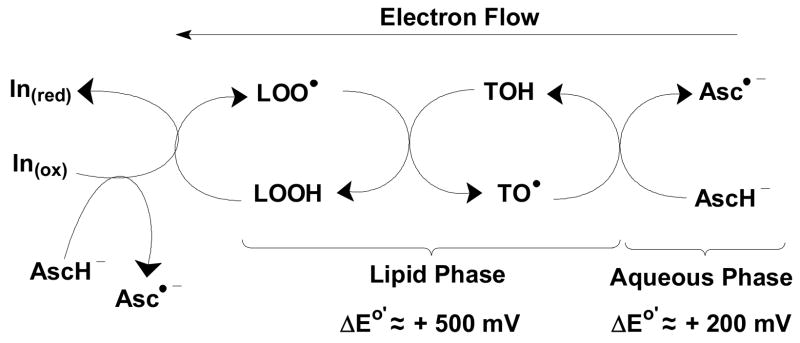
In 1979 J.E. Packer et al. demonstrated a likely mechanism by which these co-antioxidants functioned as partners to protect against lipid oxidation [6]. Using pulse radiolysis, a fast kinetic technique, in a solvent-system that allowed a homogenous solution of both ascorbic acid and tocopherol, they generated the tocopherol free radical (TO•) and observed that it reacted rapidly with ascorbate (AscH−) with the resulting formation of the ascorbate free radical (Asc•−). Thus, the now well-known figure of lipid peroxyl radicals (LOO•) being reduced to lipid hydroperoxide (LOOH) by tocopherol (TOH), with formation of the chromanoxyl radical of tocopherol. TO• is in turn reduced by AscH−, i.e. vitamin E is recycled; enzyme systems and thiols reduce the oxidation products of ascorbate back to AscH−, Figure 1. Their results show that at the heart of the process are one-electron reactions, i.e. ascorbate reacts rapidly with the one-electron oxidation product of tocopherol.
Figure 1.
Ascorbate is an excellent one-electron reducing agent that can spare tocopherol. AscH− can intercept the primary oxidants (In(ox)) that can initiate free radical-mediated lipid oxidation, right. This would spare TOH as the need for its reducing capacity would be lessoned. AscH− can also recycle tocopherol by reducing TO• back to TOH, not allowing TO• to be oxidized further and then in turn go on to form irreversible products and be lost.
Lipid peroxyl radicals are quite oxidizing (E°′ ≈ +1000 mV [7]); the tocopherol radical is less oxidizing (E°′ ≈ +500 mV), but is not innocuous. If not removed it will initiate free radical-mediated lipid oxidation as demonstrated in human low-density lipoprotein (LDL), albeit slowly [8]. The ascorbate free radical is even less oxidizing (E°′ ≈ +280 mV), but rapidly dismutes or is reduced by enzymes and thus the possibility of the initiation of additional oxidations is removed.
In the complex environment of blood plasma, ascorbate had been found to preserve tocopherol and extend the lag phase preceding detectable lipid oxidation when the plasma is subjected to a continuing flux of peroxyl radicals [9, 10]. Because of the rapid reaction of ascorbate with the tocopherol radical (k = 3 x 105 M−1 s−1 [11]), the ascorbate radical can be observed, but the tocopherol radical remains below the limit of detection as long as there is sufficient ascorbate present in plasma [12].
The protection against the loss of vitamin E by C has also been demonstrated in cell culture [13, 14]. Using freshly isolated hepatocytes, it was observed that ascorbate could preserve tocopherol, but it also appeared that each acted independently as an antioxidant. The observations are consistent with the recycling of E by C through one-electron processes, but are not direct evidence supporting this mechanism. Both groups concluded that ascorbate could preserve tocopherol by intercepting the oxidants that would initiate lipid oxidation.
The concept of C and E working as co-antioxidants prompted K.F. Gey to re-examine epidemiological data to see how plasma levels of C and E might correlate with human health [15]. He found that plasma values for C and E for prevention of cardiovascular disease and cancer were: a) ≥50 μM vitamin C; b): ≥30 μM lipid-standardized vitamin E; and c) a vitamin C/vitamin E ratio >1.3–1.5 in plasma for primary prevention. He found that all data published were consistent with the assumption that the functional couple of vitamins C and E is a crucial part of the overall antioxidant network.
The work of Bruno et al. in this issue of FRBM provides the first direct evidence in humans that a chronic oxidative stress, smoking, increases the rate of loss of vitamin E (both α-TOH and γ-TOH) in plasma AND that ascorbate slows this loss in smokers; increasing plasma ascorbate produced no change in the rate of loss of E in non-smokers. Importantly, increasing the plasma level of ascorbate produced no changes in the rate of metabolism of vitamin E via the cytochrome P-450 system in both smokers and non-smokers. This observation provides strong support for the co-antioxidant function of these two antioxidants in vivo. There are two possibilities that can not be deconvoluted for this data for the sparing of E:
AscH− reduces TO• back to TOH, not allowing TO• to lose another electron to form the tocopherol quinone, an oxidation product in which the non-aromatic ring undergoes rupture and is thought to be irreversible; thus, loss of TOH; or
AscH− could reduce the primary oxidants that initiate free radical-mediated lipid peroxidation, removing the burden on TOH and thereby sparing it.
No matter which mechanism, it is clear that AscH− spares tocopherol in smokers. This is not due to changes in the rate of metabolism of TOH by the P-450 system, which points directly to these two reductants acting as co-antioxidants. In the blood this sparing is probably due to the reaction of AscH− with TO•; however in tissue AscH− may be acting as a preventative antioxidant, Figure 1 far left, as well as a recycling agent, Figure 1 far right. That an increase in plasma AscH− did not change the rate of the loss of E in nonsmokers is probably due to the sufficiency of the very robust levels of C (≈60 μM) and the absence of stress. The levels of C in smokers was somewhat less (≈50 μM), but the long-term, chronic oxidative stress of smokers appears to be key to in the increased rate of loss of E. That this loss of TOH is slowed with increased levels of plasma ascorbate, suggests that an organism subjected to oxidative stress should have increased intake of TOH and AscH−. This study does not address this directly, but is clearly suggestive.
References
- 1.Burno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006;40:555–556. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 2.Szent-Györgyi A. Observations on the function of peroxidase systems and the chemistry of the adrenal cortex. Description of a new carbohydrate derivative. Biochem J. 1928;22:1387–1409. doi: 10.1042/bj0221387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf G. The discovery of the antioxidant function of vitamin E: the contribution of Henry A. Mattill. J Nutrition. 2005;135:363–366. doi: 10.1093/jn/135.3.363. [DOI] [PubMed] [Google Scholar]
- 4.Olcott HS, Mattill HA. Antioxidants and the auto-oxidation of fats. VI. Inhibitols. J Am Chem Soc. 1936;58:1627–1630. [Google Scholar]
- 5.Calvin Golumbic HA, Mattill Antioxidants and the Autoxidation of Fats. XIII. The Antioxygenic Action of Ascorbic Acid in Association with Tocopherols, Hydroquinones and Related Compounds. J Am Chem Soc. 1941;63(5):1279–1280. [Google Scholar]
- 6.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 7.Buettner GR. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 8.Bowery VW, Stocker R. Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc. 1993;115:6029–6044. [Google Scholar]
- 9.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. PNAS. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frei B, Roland Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. PNAS. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisby RH, Parker AW. Reaction of ascorbate with the alpha-tocopheroxyl radical in micellar and bilayer membrane systems. Archives of Biochemistry & Biophysics. 1995;317:170–178. doi: 10.1006/abbi.1995.1150. [DOI] [PubMed] [Google Scholar]
- 12.Sharma MK, Buettner GR. Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radic Biol Med. 1993;14:649–653. doi: 10.1016/0891-5849(93)90146-l. [DOI] [PubMed] [Google Scholar]
- 13.Halpner AD, Handelman GJ, Harris JM, Belmont CA, Blumberg JB. Protection by vitamin C of loss of vitamin E in cultured rat hepatocytes. [Journal Article] Archives of Biochemistry & Biophysics. 1998;359(2):305–9. doi: 10.1006/abbi.1998.0914. [DOI] [PubMed] [Google Scholar]
- 14.Glascott PA, Jr, Farber JL. Assessment of physiological interaction between vitamin E and vitamin C. Methods in Enzymology. 1999;300:78–88. doi: 10.1016/s0076-6879(99)00116-0. [DOI] [PubMed] [Google Scholar]
- 15.Gey KF. Vitamins E plus C and interacting conutrients required for optimal health. A critical and constructive review of epidemiology and supplementation data regarding cardiovascular disease and cancer. Biofactors. 1998;7:113–74. doi: 10.1002/biof.5520070115. [DOI] [PubMed] [Google Scholar]