Abstract
BACKGROUND
Mortality is increased after a hip fracture, and strategies that improve outcomes are needed.
METHODS
In this randomized, double-blind, placebo-controlled trial, 1065 patients were assigned to receive yearly intravenous zoledronic acid (at a dose of 5 mg), and 1062 patients were assigned to receive placebo. The infusions were first administered within 90 days after surgical repair of a hip fracture. All patients received supplemental vitamin D and calcium. The median follow-up was 1.9 years. The primary end point was a new clinical fracture.
RESULTS
The rates of any new clinical fracture were 8.6% in the zoledronic acid group and 13.9% in the placebo group, a 35% risk reduction (P = 0.001); the respective rates of a new clinical vertebral fracture were 1.7% and 3.8% (P = 0.02), and the respective rates of new nonvertebral fractures were 7.6% and 10.7% (P = 0.03). In the safety analysis, 101 of 1054 patients in the zoledronic acid group (9.6%) and 141 of 1057 patients in the placebo group (13.3%) died, a reduction of 28% in deaths from any cause in the zoledronic-acid group (P = 0.01). The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported, and no adverse effects on the healing of fractures were noted. The rates of renal and cardiovascular adverse events, including atrial fibrillation and stroke, were similar in the two groups.
CONCLUSIONS
An annual infusion of zoledronic acid within 90 days after repair of a low-trauma hip fracture was associated with a reduction in the rate of new clinical fractures and improved survival. (ClinicalTrials.gov number, NCT00046254.)
Hip fractures are associated with increased morbidity, functional decline, and death in older adults, as well as increased use of health care services.1,2 Mortality is increased in the year after hip fracture, with reported rates of 15 to 25% and an estimated 9 excess deaths per 100 patients among women 70 years of age or older.2–10
One source of the excess morbidity and cost incurred by patients with hip fractures is new osteoporotic fractures. Such fractures occur at a rate of 10.4 per 100 patients per year, which is 2.5 times as high as the rate in age-matched persons without previous hip fracture.10 However, data suggest that few patients with hip fracture actually receive pharmacologic therapy for osteoporosis.11–13
Zoledronic acid is a potent bisphosphonate that can be administered intravenously once yearly. The drug has been associated with a significant reduction of the risk of vertebral, hip, and nonvertebral fractures in women with postmenopausal osteoporosis.14,15 We present the results of a randomized trial that tested the efficacy and safety of zoledronic acid (at a dose of 5 mg) administered intravenously once yearly for the prevention of new clinical fractures in women and men who had undergone recent surgical repair of a hip fracture.
METHODS
STUDY DESIGN
The Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Recurrent Fracture Trial was an international, multicenter, randomized, double-blind, placebo-controlled trial involving patients with recent hip fracture.16 Patients were randomly assigned to receive either zoledronic acid by intravenous infusion or placebo infusion during a 15-minute period. Study drugs were infused within 90 days after the surgical repair of a hip fracture and every 12 months thereafter for the duration of the study. If the serum 25-hydroxyvitamin D level was 15 ng per milliliter or less or if the level was not available, patients received a loading dose of either vitamin D3 or D2 (at a dose of 50,000 to 125,000 IU given orally or intramuscularly) 14 days before first infusion of a study drug. Thereafter, all patients received daily supplementation with oral calcium (1000 to 1500 mg) and vitamin D (800 to 1200 IU).
Patients were monitored for up to 5 years with quarterly telephone interviews and yearly clinic visits. All study procedures were approved by the local institutional review board at each participating site.
The academic investigators initiated the concept of the study, which was jointly designed with the sponsor. An independent data and safety monitoring board met semiannually to oversee the conduct and safety of the study. In November 2006, after requesting an additional unplanned interim analysis after 185 events had accrued, the data and safety monitoring board recommended that the trial be stopped on the basis of having surpassed the prespecified efficacy boundaries. Data analysis was performed by the sponsor and confirmed by independent statisticians at the Coordinating Center at the University of California at San Francisco, San Francisco.
PATIENTS
All patients who were enrolled in the trial had undergone a hip fracture and were unable or unwilling to take an oral bisphosphonate. All patients signed an informed consent form that stated, “If you or your physician decides that you should take alendronate (Fosamax), risedronate (Actonel), etidronate (Didronel), or teriparatide (Forteo), you should not participate in this study.” Men and women 50 years of age or older were eligible for inclusion within 90 days after surgical repair of a hip fracture sustained with minimal trauma (i.e., a fall from standing height or a lower height). Additional enrollment criteria included being ambulatory before the hip fracture and having both legs.
Concomitant therapy with nasal calcitonin, selective estrogen-receptor modulators, hormone replacement, tibolone, and external hip protectors was allowed at the discretion of the investigator. Previous use of bisphosphonates or parathyroid hormone was allowed after a washout period that varied according to the drug and the duration of its use. Previous use of strontium or sodium fluoride was not allowed. Patients with delirium or dementia were included only after consent had been obtained from both the patient and the legal surrogate.
Exclusion criteria were previous hypersensitivity to a bisphosphonate, a potential for pregnancy, a calculated creatinine clearance of less than 30 ml per minute, a corrected serum calcium level of more than 11.0 mg per deciliter (2.8 mmol per liter) or less than 8.0 mg per deciliter (2.0 mmol per liter), active cancer, metabolic bone disease other than osteoporosis, and a life expectancy of less than 6 months in the investigator’s judgment.
Bone mineral density at the hip and femoral neck and the calculated creatinine clearance were determined at baseline and annually. If patients had a clinical fracture or if measures of bone mineral density at the total hip declined by more than 8% from baseline to month 12 or by more than 10% from baseline to month 24, patients were given the options of continuing in the study, adding an approved medication (calcitonin, hormone-replacement therapy, tibolone, or raloxifene) and continuing in the study, beginning treatment with a prohibited medication (teriparatide or an oral bisphosphonate) and stopping infusions of the study drug but continuing follow-up, or discontinuing active participation in the trial. The study drug was withheld for those patients whose creatinine clearance fell below 30 ml per minute; however, their follow-up continued.
RANDOMIZATION AND BLINDING
Patients were randomly assigned to study groups at a central location through an interactive voice-response system that created randomized permuted blocks according to site. Because the infusion of zoledronic acid sometimes caused an influenza-like syndrome in previous studies,15 patients were given acetaminophen at the time of the study-drug infusion and then as needed for the next 72 hours. Study patients, investigators, steering committee members, the study sponsor, and faculty who adjudicated the clinical and safety end points remained unaware of study-group assignments throughout the trial.
END POINTS
The primary end point was a new clinical fracture, excluding facial and digital fractures and fractures in bone containing metastases. Although the final statistical analysis plan indicated that the mean time to the first fracture would be reported as the primary outcome, because the overall rate of fractures was low, the hazard ratio for fracture was calculated with the use of a Cox proportional-hazards model; these rates were reported as the primary outcome. (For details, see the Supplementary Appendix, available with the full text of this article at www.nejm.org.) Secondary end points included the change in bone mineral density in the nonfractured hip, as measured annually with dual-energy x-ray absorptiometry; new vertebral, nonvertebral, and hip fractures; and prespecified safety end points, including death.
ASSESSMENT OF OUTCOMES
At baseline, most patients underwent lateral radiography of the chest and lumbar spine. A nonvertebral fracture (defined as a skeletal fracture that was not a vertebral, facial, digital, or skull fracture) was confirmed when a radiograph, a radiographic report, or a medical record documented a new fracture. A possible vertebral fracture required blinded review of both baseline and recent radiographs with the use of a semiquantitative technique.17 A new clinical vertebral fracture was defined as new or worsening back pain with a reduction in vertebral body height of 20% (grade 1) or more, as compared with baseline radiographs, or a reduction in vertebral body height of 25% (grade 2) or more if no baseline radiograph was available.
Investigators performed assessments for delayed union of the qualifying hip fracture at each study visit during the first 12 months. Delayed union was defined as persistent pain or an inability to bear weight plus radiographic evidence of any one of the following: a lack of bridging callus over at least two cortices, a persistent fracture line, the appearance of a new fracture line if it had been previously unapparent, or displacement of a previously aligned fracture.
Dual-energy x-ray absorptiometry of the contralateral hip or spine was performed at baseline and annually thereafter. Bone mineral density and T scores were adjusted to correct for site and brand variations of the imaging equipment.18,19
Baseline serum levels of 25-hydroxyvitamin D were measured centrally (by Covance) in a subgroup of 385 patients. Serum calcium and creatinine levels were measured with an autoanalyzer at baseline and subsequently within 4 weeks before each annual infusion of the study drug.
ADVERSE EVENTS AND LABORATORY MEASURES
The site investigator reported adverse events and serious adverse events at each study visit. Such events were categorized with the use of the Medical Dictionary for Regulatory Activities.20 Independent expert committees whose members were unaware of the study-group assignment adjudicated laboratory criteria and targeted adverse events, including ocular events, osteonecrosis of the jaw, avascular necrosis at other skeletal sites, cardiac arrhythmias reported as serious adverse events, deteriorating renal function, hypocalcemia, delayed fracture healing, and primary cause of death.
STATISTICAL ANALYSIS
The trial was event driven and required 211 clinical fractures to have a power of 90%. A two-sided level of significance of 0.05, with two interim analyses performed with the use of an O’Brien–Fleming spending function,21 was needed to detect a 35% reduction in the rate of clinical fracture in the zoledronic acid group, as compared with the placebo group. The trial planned two interim analyses with prespecified stopping rules using O’Brien–Fleming superiority and futility boundaries. After the second interim analysis, the data and safety monitoring board requested a third interim analysis and concluded that the trial had met its efficacy objectives when 185 patients had a confirmed clinical fracture. Between-group differences for the time to the first clinical fracture were determined by the log-rank test in the intention-to-treat population (all randomized patients), with hazard ratios reported on the basis of a proportional-hazards model that included treatment as a variable and were confirmed by the per protocol analysis (which excluded patients with protocol deviations).22 Data were censored for patients without an event on the date when they withdrew consent or died or the date of the last follow-up visit, whichever came first.
Because the protocol called for three annual infusions and because the number of patients who were followed for more than 3 years was small, survival curves were truncated at 36 months. Changes in bone mineral density at the total hip and femoral neck were compared in the two study groups with the use of an analysis-of-variance model adjusted for treatment and region. Four prespecified secondary analyses were performed with the use of the same proportional-hazards methods to assess the time until the first nonvertebral fracture, hip fracture, clinical vertebral fracture, or death. Rates of adverse events were compared with the use of Fisher’s exact test.
Summary statistics for baseline clinical and demographic variables are presented according to the study group. Differences in baseline characteristics between the study groups were evaluated with the use of the chi-square test for discrete variables and t-tests for continuous variables.
RESULTS
BASELINE CHARACTERISTICS AND FOLLOW-UP
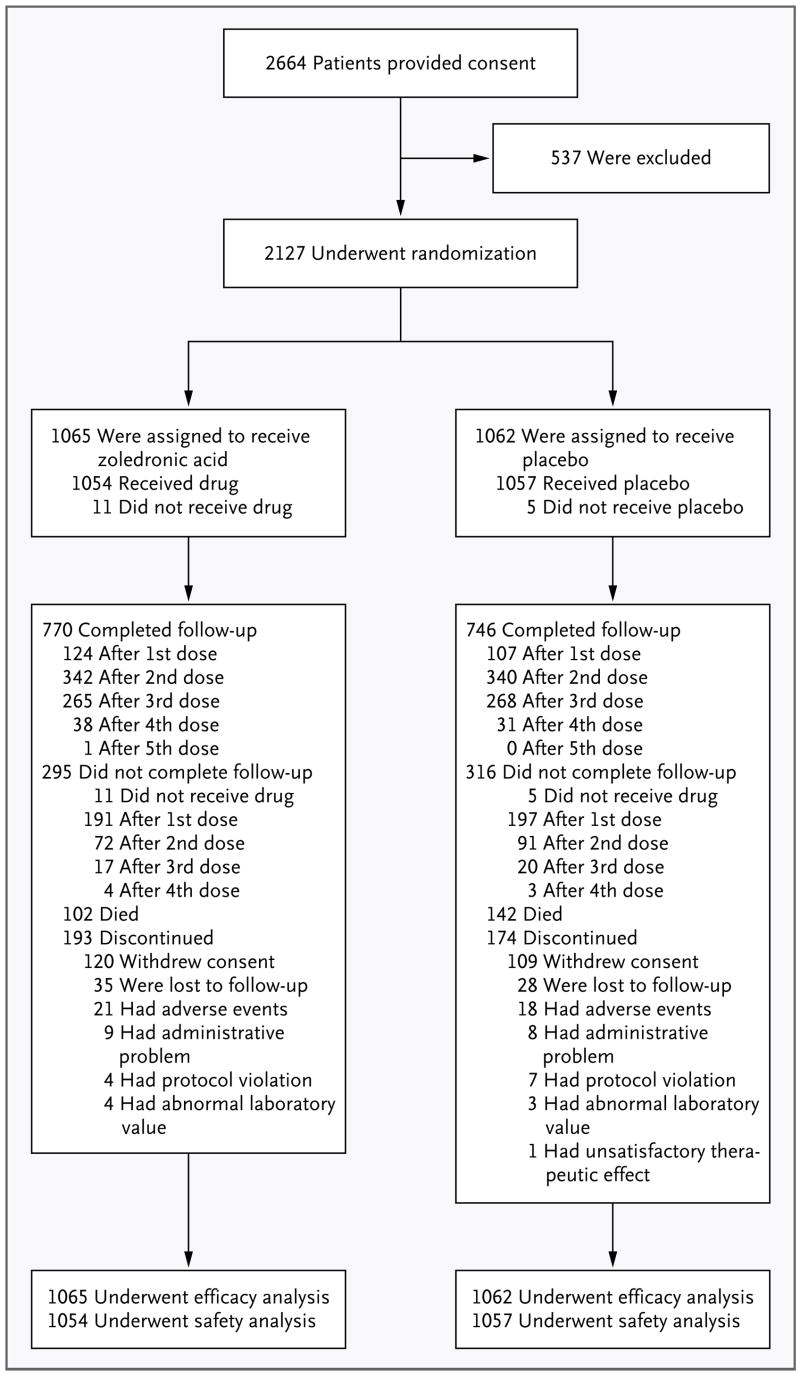
Of a total of 2127 patients, 1065 patients were randomly assigned to receive zoledronic acid, and 1062 patients were assigned to receive placebo; 71.3% of the patients completed the trial (Fig. 1). The median follow-up time was 1.9 years. A total of 3.0% of patients were lost to follow-up, and rate of loss was similar in the two groups. All patients received their intravenous study medication (zoledronic acid or placebo) unless it was withheld because of a decrease in the calculated creatinine clearance to a level below 30 ml per minute.
Figure 1.
Enrollment and Outcomes.
Baseline demographic and clinical characteristics were similar in the two groups (Table 1), with 41.8% of patients having a T score of less than −2.5 SD at the femoral neck.23 The most common coexisting medical conditions in this population at baseline were hypertension, coronary artery disease, osteoarthritis, previous stroke, depression, and diabetes mellitus. Active tachyarrhythmia was present in 5.8% of patients in the zoledronic acid group and in 7.5% of patients in the placebo group.
Table 1.
Baseline Characteristics of the Patients.*
| Variable | Placebo (N = 1062) | Zoledronic Acid (N = 1065) | P Value† |
|---|---|---|---|
| Race or ethnic group — no. (%)‡ | 0.67 | ||
| White | 965 (90.9) | 973 (91.4) | |
| Hispanic | 70 (6.6) | 70 (6.6) | |
| Black | 12 (1.1) | 6 (0.6) | |
| Other | 15 (1.4) | 16 (1.5) | |
| Sex — no. (%) | 0.52 | ||
| Female | 802 (75.5) | 817 (76.7) | |
| Male | 260 (24.5) | 248 (23.3) | |
| Age | |||
| Mean — yr | 74.6±9.86 | 74.4±9.48 | 0.68 |
| Range — no. (%) | |||
| <65 yr | 192 (18.1) | 172 (16.2) | |
| 65–74 yr | 269 (25.3) | 307 (28.8) | |
| 75–84 yr | 449 (42.3) | 446 (41.9) | |
| ≥85 yr | 152 (14.3) | 140 (13.1) | |
| Body-mass index | 24.8±4.5 | 24.7±4.4 | 0.55 |
| Region — no. (%) | 0.92 | ||
| Western Europe | 353 (33.2) | 359 (33.7) | |
| North America | 318 (29.9) | 305 (28.6) | |
| Eastern Europe | 260 (24.5) | 269 (25.3) | |
| Latin America | 131 (12.3) | 132 (12.4) | |
| Bone mineral density — g/cm2 | |||
| Femoral neck | 0.65±0.122 | 0.65±0.127 | 0.25 |
| Total hip | 0.70±0.152 | 0.70±0.153 | 0.84 |
| T score at femoral neck — no. (%) | 0.91 | ||
| −2.5 or less | 437 (41.1) | 451 (42.3) | |
| More than −2.5 to −1.5 | 375 (35.3) | 360 (33.8) | |
| More than −1.5 | 121 (11.4) | 123 (11.5) | |
| Missing data | 129 (12.1) | 131 (12.3) | |
| Patients who received concomitant osteoporosis therapy — no. (%) | 125 (11.8) | 99 (9.3) | 0.07 |
Plus–minus values are means ±SD. The body-mass index is the weight in kilograms divided by the square of the height in meters. Percentages may not total 100 because of rounding.
Continuous variables were compared with the use of a two-sample t-test. Categorical variables were compared with the use of a chi-square test.
Race or ethnic group was reported by the patients.
FRACTURES
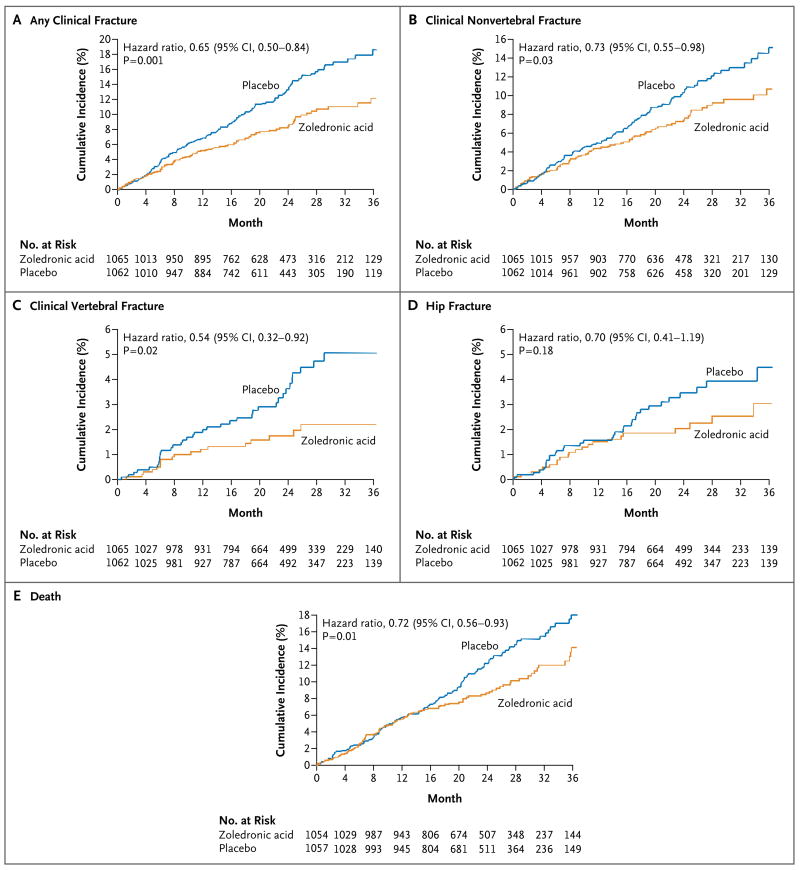
A total of 424 new clinical fractures occurred in 231 patients during the follow-up period. Zoledronic acid was associated with a rate of new clinical fractures of 8.6%, as compared with 13.9% in the placebo group, an absolute risk reduction of 5.3% and a relative reduction of 35% (Table 2 and Fig. 2). Among patients who had a fracture, the mean time to clinical fracture was 39.8 months in the zoledronic acid group and 36.4 months in the placebo group. The risk reduction was very similar in the intention-to-treat and per-protocol populations. The rates of a new clinical vertebral fracture were 1.7% in the zoledronic-acid group and 3.8% in the placebo group (P = 0.02); the rates of a new clinical nonvertebral fracture were 7.6% and 10.7%, respectively (P = 0.03). New hip fractures occurred in 27% of patients in the zoledronic acid group and in 3.5% of those in the placebo group, a nonsignificant reduction in relative risk of 30%. In a post hoc analysis, significant divergence in the fracture-free survival curves between the two groups for all clinical fractures was seen as early as 12 months (P=0.02 by the log-rank test) (Fig. 2).
Table 2.
Rates of Fracture and Death in the Study Groups.*
| Variable | Placebo (N = 1062) | Zoledronic Acid (N = 1065) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| no. (cumulative rate or %) | ||||
| Fracture | ||||
| Any | 139 (13.9) | 92 (8.6) | 0.65 (0.50–0.84) | 0.001 |
| Nonvertebral | 107 (10.7) | 79 (7.6) | 0.73 (0.55–0.98) | 0.03 |
| Hip | 33 (3.5) | 23 (2.0) | 0.70 (0.41–1.19) | 0.18 |
| Vertebral | 39 (3.8) | 21 (1.7) | 0.54 (0.32–0.92) | 0.02 |
| Death | 141 (13.3) | 101 (9.6) | 0.72 (0.56–0.93) | 0.01 |
Rates of clinical fracture were calculated by Kaplan–Meier methods at 24 months and therefore are not simple percentages. Because of variable follow-up, the number and percentage of patients who died are provided on the basis of 1057 patients in the placebo group and 1054 patients in the zoledronic acid group in the safety population.
Figure 2.
Time to Primary or Secondary End Point.
BONE MINERAL DENSITY
Bone mineral density at the total hip increased in the zoledronic acid group by 2.6% at 12 months, 4.7% at 24 months, and 5.5% at 36 months and declined in the placebo group by 1.0%, 0.7%, and 0.9%, respectively. Bone mineral density at the femoral neck increased in the zoledronic acid group by 0.8% at 12 months, 2.2% at 24 months, and 3.6% at 36 months and declined in the placebo group by 1.0%, 0.7%, and 0.9%, respectively. The differences in bone mineral density at the total hip and femoral neck between the zoledronic acid group and the placebo group were significant (P<0.001 for all comparisons). On the basis of prespecified bone safety measures, 26 patients in the zoledronic acid group had a clinically important loss of bone mineral density, of whom 4 patients were withdrawn from the trial (15.4%), and 126 patients in the placebo group had a clinically important loss of bone mineral density, of whom 21 patients were withdrawn from the trial (16.7%).
DEATH
In the safety analysis, a total of 242 of 2111 patients (11.5%) died during the study, of whom 101 of 1054 (9.6%) were in the zoledronic acid group and 141 of 1057 (13.3%) were in the placebo group (hazard ratio for the zoledronic acid group, 0.72; 95% CI, 0.56 to 0.93; P = 0.01). One patient in each study group died without having received the assigned study drug. The adjudication committee determined that 11 deaths (1.0%) were from cardiovascular disease and 7 deaths (0.7%) were from cerebrovascular disease in the zoledronic acid group, as compared with 18 deaths (1.7%) and 7 deaths (0.7%), respectively, in the placebo group.
ADVERSE EVENTS
Adverse events were reported in 867 patients (82.3%) in the zoledronic acid group and in 852 patients (80.6%) in the placebo group (Table 3). Serious adverse events occurred with similar frequency in the two groups (38.3% in the zoledronic acid group and 41.2% in the placebo group). More patients in the zoledronic acid group than in the placebo group reported pyrexia (8.7% vs. 3.1%), myalgia (4.9% vs. 2.7%), bone pain (3.2% vs. 1.0%), or musculoskeletal pain (3.1% vs. 1.2%). More patients in the placebo group (11.4%) reported having fallen than in the zoledronic-acid group (9.7%).
Table 3.
Adverse Events in the Safety Population.*
| Event | Placebo (N = 1057) | Zoledronic Acid (N = 1054) | P Value† |
|---|---|---|---|
| General — no. (%) | |||
| Any adverse event | 852 (80.6) | 867 (82.3) | 0.34 |
| Any serious adverse event | 436 (41.2) | 404 (38.3) | 0.18 |
| Death‡ | 141 (13.3) | 101 (9.6) | 0.01 |
| Discontinuation of follow-up owing to adverse event | 18 (1.7) | 21 (2.0) | 0.63 |
| Renal event — no./total no. (%) | |||
| Increase in serum creatinine >0.5 mg/dl | 50/900 (5.6) | 55/886 (6.2) | 0.62 |
| Calculated creatinine clearance <30 ml/min | 65/891 (7.3) | 72/882 (8.2) | 0.53 |
| Five typical symptoms ≤3 days after infusion — no. (%)§ | |||
| Myalgia | 9 (0.9) | 33 (3.1) | <0.001 |
| Influenza-like symptoms | 3 (0.3) | 6 (0.6) | 0.34 |
| Headache | 9 (0.9) | 16 (1.5) | 0.17 |
| Arthralgia | 23 (2.2) | 33 (3.1) | 0.18 |
| Pyrexia¶ | |||
| Any event | 9 (0.9) | 73 (6.9) | <0.001 |
| After first infusion | 7 (0.7) | 72 (6.8) | <0.001 |
| After second infusion | 2 (0.3) | 3 (0.4) | 0.68 |
| After third infusion | 0 | 3 (0.9) | 0.25 |
| Cardiovascular or cerebrovascular event — no. (%) | |||
| Atrial fibrillation | |||
| Any event | 27 (2.6) | 29 (2.8) | 0.79 |
| Serious adverse event | 14 (1.3) | 12 (1.1) | 0.84 |
| Stroke | |||
| Serious adverse event | 38 (3.6) | 46 (4.4) | 0.37 |
| Fatal event | 6 (0.6) | 9 (0.9) | 0.45 |
| Myocardial infarction | 17 (1.6) | 13 (1.2) | 0.58 |
| Death from cardiovascular causes | 52 (4.9) | 36 (3.4) | 0.10 |
All adverse events were reported before adjudication.
P values for all comparisons except death were calculated with the use of Fisher’s exact test. P values for death were computed with the use of the log-rank test.
Two patients (one in the zoledronic acid group and one in the placebo group) died without having received the study drug and were excluded from the safety population.
The five symptoms listed were the most frequently cited in Black et al.15 and other studies.
Patients could report more than one event. A total of 753 in the placebo group and 739 in the zoledronic acid group underwent a second infusion, and 322 in the placebo group and 325 in the zoledronic acid group underwent a third infusion.
The incidence of cardiovascular events was similar in the two groups. A total of 24 patients in the zoledronic acid group (2.3%) and 39 patients in the placebo group (3.7%) had a serious adverse event of arrhythmia; these events were confirmed by adjudication in 19 patients in the zoledronic acid group (1.8%) and 28 patients in the placebo group (2.6%). Atrial fibrillation occurred in 12 patients in the zoledronic acid group (1.1%) and in 14 patients in the placebo group (1.3%) (Table 3). The incidence of renal adverse events was similar in the two study groups, including events among patients with a baseline creatinine clearance of 30 to 60 ml per minute.
Although no specific case-finding effort was made, no cases of osteonecrosis of the jaw were reported or confirmed by the central adjudication committee after a search of the database. The incidence of delayed union of the qualifying hip fracture was 34 (3.2%) in the zoledronic acid group and 29 (2.7%) in the placebo group (risk ratio for the zoledronic acid group, 1.17; 95% CI, 0.72 to 1.90; P = 0.61).
Three patients in the zoledronic acid group (0.3%) and no patient in the placebo group had adjudicated hypocalcemia. Four patients in the zoledronic acid group (0.4%) and one patient in the placebo group (0.1%) had ocular events that were considered possibly or probably related to a study drug according to an expert review.
DISCUSSION
Patients with hip fracture represent an important population to target for the prevention of secondary fractures. Data have been needed to show that available osteoporosis therapies are effective in such patients. Because the patients in our study were older and had more coexisting conditions and a higher risk of falls than patients in many clinical trials of treatment for osteoporosis, our findings contain helpful information for clinicians and for patients who have had a hip fracture.
Poor adherence to oral bisphosphonate therapy has been shown to compromise the efficacy of this treatment for fracture reduction and to increase medical costs24,25; such findings have been particularly notable in frail older adults.26 The once-yearly intravenous regimen that we used offers another option for affected patients.
Vitamin D deficiency is frequently observed in older patients and is associated with an increased risk of hypocalemia when bisphosphonates are administered before a normal vitamin D level has been achieved.27 Within 2 months after the initiation of our study (after one patient had been randomly assigned), a loading dose of vitamin D was administered to any patient with a vitamin D deficiency 2 weeks before the administration of a study drug. Since very high rates of vitamin D deficiency were observed in the first 385 patients who underwent randomization, a subsequent protocol amendment provided for a loading dose of vitamin D in all patients, regardless of the level of serum 25-hydroxyvitamin D. This approach might explain the low rate of hypocalcemia (in three patients) seen in our study.
Although some observers have questioned the appropriateness of a placebo-controlled trial involving patients at high risk for fracture, patients who were enrolled in our trial could not tolerate or would not take an oral bisphosphonate. In addition, the trial allowed for open-label concomitant use of several approved osteoporosis therapies. Furthermore, few data are available to guide treatment in frail older patients with osteoporosis; a previous risedronate trial was not successful in reducing hip fracture in patients over the age of 80 years.28 Finally, many studies show that patients with osteoporotic fractures are frequently not given therapy to prevent further fractures.29,30 We therefore believed that clinical equipoise existed and that a positive result would probably improve the care provided to patients after hip fracture.
Increased rates of death after hip fracture are well described. Indeed, mortality in our study was about three times as high as that reported in the recently completed study of zoledronic acid in postmenopausal women.15 We observed a reduction of 28% in the risk of death in the zoledronic acid group. The reduction in the risk of death observed in the zoledronic acid group may have been related in part to a reduction in new fractures after the initial hip fracture. However, further investigation is needed to understand more fully the reason for the reduction in the risk of death, which is probably multifactorial.
The safety profile for zoledronic acid indicated few areas of concern. There were transient post-infusion symptoms, as previously reported in patients receiving intravenous bisphosphonates,15 which may have been attenuated by the routine administration of acetaminophen. We did not find an increased incidence of renal adverse events, despite high baseline rates of mild-to-moderate chronic kidney disease. Although an increased risk of atrial fibrillation was unexpectedly observed in one zoledronic acid study involving postmenopausal women,15 we found no increased incidence of atrial fibrillation. One concern related to bisphosphonate treatment of patients with peripheral fractures has been a possible adverse effect on fracture healing. We observed no significant difference in delayed union of fractured bone between the two study groups.
Our study had several limitations. The study patients were, on average, slightly younger and healthier than are patients with hip fracture in the general population, as suggested by data regarding 1-year mortality. However, patients in our study ranged widely in age (up to 98 years), and some had cognitive impairment. Because we did not evaluate spinal radiographs unless symptoms of a possible vertebral fracture developed, we probably underestimated the prevalence of new vertebral fractures.
In conclusion, our findings indicate that treatment with zoledronic acid after a hip fracture is associated with reduced rates of new clinical factures and death from all causes.
Acknowledgments
Supported by Novartis.
Dr. Lyles reports receiving grant support from Novartis, the Alliance for Better Bone Health (Sanofi-Aventis and Procter & Gamble), and Amgen, consulting fees from Novartis, Procter & Gamble, Merck, Amgen, GTx, GlaxoSmithKline, Eli Lilly and Bone Medical, and being listed as an inventor on a U.S. patent application (20050272707) covering methods for preventing or reducing secondary fractures after hip fracture and on another provisional patent application for medication kits and formulations for preventing, treating, or reducing secondary fractures after a previous fracture; Dr. Colón-Emeric, receiving consulting fees from Novartis and research grants from Novartis and the Alliance for Better Bone Health; Dr. Magaziner, receiving consulting fees from Amgen, Merck, Aventis, and GTx, lecture fees from Merck and Pfizer, and grant support from Novartis and Merck; Dr. Adachi, receiving consulting fees from Amgen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi-Aventis, and Servier and grant support from Amgen, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, and Roche; Dr. Pieper, receiving research support from Novartis; Dr. Mautalen, receiving consulting and advisory board fees from Novartis; Dr. Hyldstrup, receiving advisory board fees from Novartis, Eli Lilly, and Nycomed, lecture fees from Merck, Eli Lilly, Nycomed, Novartis, Novo Nordisk, and Sevih, and grant support from Eli Lilly, Novartis, Pfizer, Nycomed, Roche, and GlaxoSmithKline; Dr. Recknor, receiving consulting fees from Procter & Gamble, Roche, and Eli Lilly, lecture fees from Procter & Gamble, Eli Lilly, Roche, GlaxoSmithKline, Merck, and Aventis, and grant support from Procter & Gamble; Dr. Nordsletten, receiving consulting and advisory board fees from Novartis and DePuy, lecture fees from Wyeth, and grant support from Biomet; Ms. Lavecchia and Drs. Zhang, Mesenbrink, Abrams, Orloff, and Eriksen, being employees of and owning stock in Novartis; Dr. Horowitz (now an employee of Savient Pharmaceuticals), being an employee of Novartis during the design and initiation of the study and Dr. Boonen, receiving consulting, advisory board, or lecture fees from Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Sanofi-Aventis, and Servier and grant support from Amgen, Eli Lilly, Novartis, Pfizer, Procter & Gamble, Sanofi-Aventis, and Roche–GlaxoSmithKline. No other potential conflict of interest relevant to this article was reported.
We thank Kathleen Betchyk, B.S.N., R.N., Peter Burckhardt, M.D., John Caminis, M.D. (now employed at NPS Pharmaceuticals), Robert P. Heaney, M.D., Cheri Janning, R.N., John A. Kanis, M.D., Joel Krasnow, M.D. (now employed at Roche), and Theresa Rosario-Jansen, Ph.D. (now employed at Savient Pharmaceuticals), and colleagues who performed the data-replication analysis: Lisa Palermo, M.A., Trisha Hue, M.P.H., Steven R. Cummings, M.D., and Dennis M. Black, Ph.D.
APPENDIX
The following investigators participated in the HORIZON Recurrent Fracture Trial: Steering Committee — K.W. Lyles (chair), J. Adachi, S. Boonen, L. Hyldstrup, J.S. Magaziner, C. Mautalen, L. Nordsletten, C. Pieper, C. Recknor, K. Abrams (Novartis), E.F. Eriksen (Novartis), P. Mesenbrink (Novartis); Past Steering Committee — F. Hartl (Novartis), T. Rosario-Jansen (Novartis), J. Caminis (Novartis); Clinical End Point Committee — C. Colón-Emeric, N. Major, S. Olson; Data and Safety Monitoring Board — L. Raisz (chair), P. Bauer, J. Compston, D. DeMets, R. Hirschberg, O. Johnell (deceased), S. Ralston, R. Wallace; Data and Safety Monitoring Board Consultant —M. Farkouh; Duke Clinical Research Institute — K. Moore.
The following investigators are listed according to clinical site: Argentina — E. Albiero, J.L. Aparicio, G. Arroyo, C. Bartolucci, D. Bertolaccini, E.J. Buabse, M. Caubet, E.M. Cavillon, J. Chahla, R. Diaz, A. Ferrari, C.A. Gobbi, I. Gorosito, M.S. Larroude, G. Macias, Z. Man, M.S. Moggia, E. Mysler, L. Nardin, L. Suarez, G. Tate, S. Cobas, C. Mautalen, E. Vega, L. Schuman, C. Sedlinsky; Austria — A. Fahrleitner, S. Kudlacek, G. Leb, G. Peham, C. Piswanger-Soelkner, F. Singer, R. Thun-Hohenstein, R. Willvonseder; Belgium — A.-H. Batens, J. Bentin, S. Boonen, Y. Boutsen, J.-P. Devogelaer, R. Driesen, S. Goemaere, P. Haentjens, J. Kaufman, R. Joos, P. Milants, A. Mindlin, F. Raeman, B. Temmerman, A. Van Couter, J. Van Den Bergh, A. Vanderborght, E. Vangheluwe, R. Van Hoeywegen, A. Verbruggen, R. Witvrouw, H.-G. Zmierczak; Brazil — B.-H. Albergaria, V.Z. Borba, M.L. Castro, A.C. de Rescende, M.P. de Souza, S. Eis, C. Kulak, E. Meirelles, E. J. Moana, P. Papler, G. Sguizzato, D. Zaninelli; Canada — F. Abuzgaya, J. Adachi, D. Armstrong, G. Biore, E. Bogoch, J. Brown, V. Bykerk, D. Choquette, R. Crilly, E. Dessouki, G.A. Ecker, R. Faraawi, B. Galway, D. Hanley, A.B. Hodsman, A. Jovaisas, S. Kaiser, A. Karapilis, J. Karsh, D. Kendler, A.A. Khan, L. Komer, R. Kremer, B.W. LeBlanc, R. McDougal, F. Morin, W.P. Olszynski, A. Papaioannou, D. Puskas, L.-G. Ste-Marie, D. Stevens, J. Stewart, J. Wade; Colombia — W. Arbelaez, J. Espinosa, J.J. Jaller Raad, J. Montaña, J. Perez; Czech Republic — L. Benes, M. Bis, M. Bobula, E. Dokoupilova, M. Filipovic, Z. Fojtik, J. Jensovsky, P. Kasalicky, Z. Malek, P. Novosad, J. Rosa, A. Skrabal, M. Sugarek, V. Vyskocil; Denmark — K. Brixen, C. Christiansen, J. Christiansen, B.R. Christoffersen, P. Eskildsen, P. Gebuhr, J. Gram, P. Hermann, M. Hitz, L. Hyldstrup, T.W. Jenen, J.-E.B. Jensen, B. Langdahl, N. Nissen, H. Perrild, S. Pors Nielsen, P. Riegels-Nielsen, L. Scheirbeck, H.A. Sørensen, M.-B. Tanderup Petersen; Finland — H. Aro, J. Heikkinen, M. Helin, H. Kurikka, J. Salmi, M. Välimäki, S. Vepsäläinen, H. Von Plato; France — C.-L. Benhamou, P. Delmas, E. Fontanges, S. Loiseau-Peres, E. Vignot; Greece — G. Basthekis, T. Karachalios, G.P. Lyritis, E. Papakitsou, P. Soukakos; Guatemala — H. Briones Alvarado, C. Davila Mohr, R. Gonzalez, L. Ramirez, E. Rosal Palomo; Norway — H. Apold, W. Figved, F. Frihagen, J. Halse, L. Nordsletten, E.S. Øfjord, B. Robstad, A. Skag, U. Syversen, M. Westberg; Peru — A. Calvo, C. Diaz, J. Garcia, R. Huamanchumo, G. Leon, T. Miraval, C. Pastor, S. Quevedo, J. Saly Rosas, M. Segami, R. Vera; Poland — M. Chrobot, B. Franczuk, J. Klimczak, M. Klimczak, T. Niedzwiedzki, M. Szuscik, W. Szwarczyk; Russia — A. Abolin, A.I. Afaunov, A. Afinogenova, A. Balashov, A.D. Batutina, K. Belova, I. Bondar, I. Brusin, G. Burachevskaya, M.A. Cherednikova, V. Danilyak, A. Dreval, R.E. Dubinskaya, L. Dvoretsky, I. Egorova, I. Elizarov, V. Emelyanov, O.B. Ershova, V. Esenamanov, L. Gabbasova, E.S. Geydeshman, E.M. Gulyaeva, A. Ivleva, O.V. Kamerer, F.D. Khot, O. Khrustalev, S. Kondrichina, L. Konenkova, M. Korolev, E. Koroleva, A. Kravtsov, E.N. Kuranova, V.V. Kurnosenkov, G.V. Kuropatkin, A.I. Kuzin, L. Kuznetsova, L.N. Laskovaya, S. Levashov, V. Logachev, V. Lomovtseva, V. Marasaev, L. Marchenkova, L. Matkevich, O.K. Maximenko, B. Moskvicheva, A. Myasnikov, S.M. Novichkov, M. Okoyomov, O.D. Ostroumova, S. Petrosov, N.A. Petrova, E. Philippova, E.G. Pikhlak, V. Pilyaev, E. Poliakova, O. Pososhkova, Y. Prigarina, D.V. Pryuts, B.V. Ramashevsky, L.B. Reznikova, M. Rubin, N.M. Sadchikova, A.M. Savintsev, V.A. Sergeev, E. Sergeeva, A.V. Shevchenko, S.B. Shoustov, O.I. Shumilova, L. Silin, A. Sizikov, I.E. Starygina, A. Stoyanov, V. Tereshchenkov, O.N. Tulkin, G.U. Usova, E. Vasilieva, L. Velitchenko, N. Vesikova, E. Volkova, V. Yakusevich, B. Zarkeshev, I. Zazerskaya, E. Zonova, A. Zykova; Slovakia — L. Baqi, Z. Killinger, Z. Kmecová, A. Letkovska, P. Maresch, P. Masaryk, J. Payer, S. Selcanova, E. Stenova, A. Svec; Spain — A. Cuxart, E. Duarte, G. Encabo, E. Pages, S. Rodriguez; Sweden — U. Bergstrom, P. Curman, O. Ljunggren, J.-O. Magnusson, H. Mallmin, M. Palmér, O. Svensson, K.-G. Thorngren, O. Törring; Switzerland — S. Cuenot, P. Frey-Rindova, K. Gasser, D. Hans, M.-A. Krieg, O. Lamy, M. Lietz, K. Lippuner, A. Popp, R. Rizzoli, R. Theiler, A. Trombetti, B. Uebelhart; Turkey — U. Akarirmak, K. Aktuglu, S. Alper, F. Atamaz, S. Bayram, O. El, F. Erdogan, S. Gulbahar, S. Kanyilmaz, V. Karatosun, Y. Kirazli, S. Kucukoglu, S. Oncel, E.O. Senocak, N. Tercan, S. Tuzun; United Kingdom — R. Addison, N. Arden, A. Black, M. Brown, M. Bull, C. Cooper, E. Dennison, R. Eastell, A. McLellan, M. Oscosta, D. Reid, P.J. Ryan, M.D. Stone, G. Summers, J. Turton, J. Walsh, J. Wass; United States — J. Abruzzo, R. Ackerman, R. Acus, J.F. Aloia, R. Altman, S. Baak, E. Barengolts, U. Barzel, S. Berven, N.C. Binkley, S.J. Birge, R. Bockman, D. Bodenner, H.G. Bone, M. Borofsky, M. Bosse, S. Broy, K. Buehler, P.K. Burke, H. Camel, C. Cefalu, D. Chesler, J. Christensen, R. Cobden, C. Corsi, R. Downs, S. Dubois, R.S. Duffett, L. Dulipsingh, M.J. Econs, M.H. Edwards, B.J. Edwards, J. Ervin, R. Ettlinger, W. Feng, F. Flandry, C.G. Fox, R. Fraback, J. Garino, M.F. Gloth, M. Gollapudi, M. Grabois, R.M. Griffin, B. Gruber, S. Hakki, W. Hall, D. Healey, R.C. Henderson, W. Henry, M.C. Hermann, D. Hillard-Sembell, V.A. Hirth, M.C. Hochberg, S.D. Hodges, S. Honig, L.H. Hunninghake, C. Irby, S. Jan de Beur, H.L. Katzeff, D.P. Kiel, M. Kimball, M. King, M. Kleerekoper, R. Knight, N. Koval, G. Krick, K. Krohn, J. Lake, M. Leboff, S. Lederman, A.M. Lee, S. Leichter, P. Levin, C. Libanati, A. Licata, J.S. Lindberg, R. Lindsay, R.G. Loeffler, J.E. Loveless, J. Maby, N. Marcus, S. Martinez, B. Mason, V. Matkovic, M. McClung, M. McDermott, N. Mezitis, P. Miller, S.S. Miller, J.R. Minkoff, M.R. Mitchell, A. Moses, J. Mukand, S. Nattrass, J. Nuckolls, M. O’Grady, M.D. Ohl, J. O’Keefe, W.D. O’Riordan, D. Orwig, E.S. Orwoll, M. Pahor, G.M. Palmieri, G. Pennington, H. Perry, A. Pfister, T.L. Poling, T. Powell, K.M. Prestwood, D.S. Rao, T.H. Real, G. Rechtine, C.P. Recknor, R. Reese, J. Renne, H.F. Riegler, S.G. Robbins, G. Rodgers, R. Ronquist, T.W. Rooney, M. Rosemore, C.J. Rosen, J. Roth, C.D. Rubin, K.G. Saag, L. Savage, C. Schmitt, C.R. Schneyer, S.L. Schwartz, E.N. Schwartz, J. Seaquist, S.C. Sharp, S.J. Siff, S. Silverman, J.A. Simon, C. Simonelli, D. Slovik, D. Spratt, S. Srivastsa, M. Stachniw, J. Stock, R. Swanson, R.J. Takacs, D. Tange, S.B. Tanner, M. Trice, J.R. Tucci, R. Valente, D. Vaziri, T.J. Weber, S.J. Wimalawansa, G. Woodson, M. Zaidi, T. Zizic.
References
- 1.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871–3. doi: 10.2105/ajph.80.7.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boonen S, Autier P, Barette M, Vanderschueren D, Lips P, Haentjens P. Functional outcome and quality of life following hip fracture in elderly women: a one-year prospective controlled study. Osteoporos Int. 2004;15:87–94. doi: 10.1007/s00198-003-1515-z. [DOI] [PubMed] [Google Scholar]
- 3.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55:M498–M507. doi: 10.1093/gerona/55.9.m498. [DOI] [PubMed] [Google Scholar]
- 4.Adachi JD, Ioannidis G, Berger C, et al. The influence of osteoporotic fractures on health related quality of life in community-dwelling men and women across Canada. Osteoporos Int. 2001;12:903–8. doi: 10.1007/s001980170017. [DOI] [PubMed] [Google Scholar]
- 5.Hannan EL, Magaziner J, Wang JJ, et al. Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes. JAMA. 2001;285:2736–42. doi: 10.1001/jama.285.21.2736. [DOI] [PubMed] [Google Scholar]
- 6.Ray NF, Chan JK, Thamer M, Melton LJ., III Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, Campion G, Melton LJ., III Hip fractures in the elderly: a worldwide projection. Osteoporos Int. 1992;2:285–9. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 8.Davidson CW, Merrilees MJ, Wilkinson TJ, McKie JS, Gilchrist NL. Hip fracture mortality and morbidity — can we do better? N Z Med J. 2001;114:329–32. [PubMed] [Google Scholar]
- 9.Magaziner J, Lydick E, Hawkes W, et al. Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health. 1997;87:1630–6. doi: 10.2105/ajph.87.10.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colón-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fracture: data from two longitudinal studies. Osteoporos Int. 2003;14:879–83. doi: 10.1007/s00198-003-1460-x. [DOI] [PubMed] [Google Scholar]
- 11.Gardner MJ, Brophy RH, Demetrakopoulos D, et al. Interventions to improve osteoporosis treatment following hip fracture: a prospective, randomized trial. J Bone Joint Surg Am. 2005;87:3–7. doi: 10.2106/JBJS.D.02289. [DOI] [PubMed] [Google Scholar]
- 12.Solomon DH, Finkelstein JS, Katz JH, Mogun H, Avorn J. Underuse of osteoporosis medications in elderly patients with fractures. Am J Med. 2003;115:398–400. doi: 10.1016/s0002-9343(03)00357-7. [DOI] [PubMed] [Google Scholar]
- 13.Colon-Emeric C, Lyles KW, House P, et al. Randomized trial to improve fracture prevention in nursing home residents. Am J Med. doi: 10.1016/j.amjmed.2007.04.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–61. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 15.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 16.Colón-Emeric CS, Caminis J, Suh TT, et al. The HORIZON Recurrent Fracture Trial: design of a clinical trial in the prevention of subsequent fractures after low trauma hip fracture repair. Curr Med Res Opin. 2004;20:903–10. doi: 10.1185/030079904125003683. [DOI] [PubMed] [Google Scholar]
- 17.Genant HK, Wu CY, Van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int. 2001;12:438–44. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 19.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–90. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 20.Medical dictionary for regulatory activities (MedDRA) Reston, VA: Northrup Grumman; 2003. [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 23.Prevention and management of osteoporosis. World Health Organ Tech Rep Ser. 2003;921:1–164. [PubMed] [Google Scholar]
- 24.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–8. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013–22. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 26.van Eijken M, Tsang S, Wensing M, de Smet PA, Grol RP. Interventions to improve medication compliance in older patients living in the community: a systemic review of the literature. Drugs Aging. 2003;20:229–40. doi: 10.2165/00002512-200320030-00006. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 28.McClung MR, Geussens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 29.Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109:326–8. doi: 10.1016/s0002-9343(00)00457-5. [DOI] [PubMed] [Google Scholar]
- 30.Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med. 2002;162:2217–22. doi: 10.1001/archinte.162.19.2217. [DOI] [PubMed] [Google Scholar]