Abstract
Chemotactic cytokines (chemokines) have been traditionally defined as small (10–14 kDa) secreted leukocyte chemoattractants. However, chemokines and their cognate receptors are constitutively expressed in the central nervous system (CNS) where immune activities are under stringent control. Why and how the CNS uses the chemokine system to carry out its complex physiological functions has intrigued neurobiologists. Here, we focus on chemokine CXCL12 and its receptor CXCR4 that have been widely characterized in peripheral tissues and delineate their main functions in the CNS. Extensive evidence supports CXCL12 as a key regulator for early development of the CNS. CXCR4 signaling is required for the migration of neuronal precursors, axon guidance/pathfinding and maintenance of neural progenitor cells (NPCs). In the mature CNS, CXCL12 modulates neurotransmission, neurotoxicity and neuroglial interactions. Thus, chemokines represent an inherent system that helps establish and maintain CNS homeostasis. In addition, growing evidence implicates altered expression of CXCL12 and CXCR4 in the pathogenesis of CNS disorders such as HIV-associated encephalopathy, brain tumor, stroke and multiple sclerosis (MS), making them the plausible targets for future pharmacological intervention.
Keywords: CXCL12, CXCR4, Chemokines, Chemokine receptors, CNS development, Neuromodulation, CNS disorders
1. Introduction
Chemokines are small, mostly secreted proteins that were initially implicated in the regulation of leukocyte trafficking during host defense and pathological immune responses. Chemokines can be classified into CXC, CC, C or CX3C subfamilies based on the arrangement of the two cysteine residues near the N-terminus (Zlotnik A and Yoshie O, 2000). Chemokine receptors are G protein-coupled receptors (GPCRs) with seven-transmembrane domains that are highly conserved in evolution (Fredriksson R et al, 2003; Kawasawa Y et al, 2003; DeVries ME et al, 2006). By binding to their cognate receptors, chemokines activate a series of downstream signaling pathways to guide the movement of leukocytes to target tissues or organs (Rot A and von Andrian UH, 2004). Directional movement of leukocytes through a concentration gradient of chemokine is defined as “chemotaxis”. So far, 53 human chemokines and 23 chemokine receptors have been cloned or characterized (http://cytokine.medic.kumamoto-u.ac.jp/). Under a new systematic nomenclature the chemokines are named according to subfamilies (such as CC or CXC), along with letter “L” to indicated “ligand” and a number that corresponds to the order of cloning and characterization. Thus, SDF1 is now termed CXCL12 and the receptor nomenclature follows this family assignment. CXC receptors respond to CXC chemokines (for the most part). To gain receptor designations, a GPCR must bind chemokines with high affinity and specificity and must signal (mobilize Ca2+, for example).
As an important member of the CXC subfamily, CXCL12 has been widely explored in the hematopoietic system. In 1993, a CXCL12 cDNA clone was first isolated from a murine bone-marrow stroma cell line by the signal sequence trap method and the gene was originally named SDF1 (stromal cell-derived factor 1) (Tashiro K et al, 1993). Several years later, two groups simultaneously and independently identified its receptor, an orphan GPCR called LESTR/fusin (Bleul CC et al, 1996a; Oberlin E et al, 1996). LESTR/fusin was also the first identified coreceptor for human immunodeficiency virus (HIV-1) infection of CD4+ lymphocytes (Feng Y et al, 1996) and administration of CXCL12 inhibited infection by T-tropic HIV-1 of HeLa-CD4 cells (Bleul CC et al, 1996a; Oberlin E et al, 1996). According to its ability to bind and respond to CXCL12, the designation CXCR4 was adopted to replace LESTR/fusion.
CXCL12/CXCR4 plays pleiotropic functions in the peripheral immune system. CXCL12 is a highly efficacious chemoattractant for lymphocytes and monocytes but not neutrophils (Bleul CC et al, 1996b). CXCL12/CXCR4 not only regulates the development of T and B lymphocytes but also contributes to the survival of mature lymphocytes and in the generation of memory T cells (Klein RS and Rubin JB, 2004). Recent studies indicate that CXCL12/CXCR4 enhances the inflammatory infiltration of neutrophils or lymphocytes in diverse models and settings involving acute inflammation or fulminant infection (Wald O et al, 2004; Ding Z et al, 2006; Petty JM et al, 2007). CXCL12 is an important regulator for homing of hematopoietic progenitor cells (HPCs) to the bone marrow (BM) microenvironment (Lapidot T et al, 2005). Deficient development of blood cells and heart were also described in CXCL12 knockout (KO) mice (Nagasawa T et al, 1996). Interestingly, similar phenotypes were observed in CXCR4 KO mice too (Tachibana K et al, 1998; Zou YR et al, 1998), suggesting that the interaction between CXCL12 and CXCR4 may be one- to one- relationship. Recent results from studying an alternative non-signaling CXCL12 receptor, termed CXCR7 (Burns JM et al, 2006), suggest that the CXCL12/CXCR4 relationship is not entirely exclusive.
CXCL12/CXCR4 is involved in the embryonic development of diverse peripheral tissues such as blood vessels, muscle, primordial germ cell (PGC) and sensory lateral line etc (Nagasawa T et al, 1996; Tachibana K et al, 1998; Zhu YR et al, 1998; David NB et al, 2002; Odemis V et al, 2005; Sapede D, 2005; Haas P and Gilmour D 2006). In addition, CXCL12/CXCR4 participates in peripheral pathogenetic processes. For instance, CXCL12/CXCR4 may in part regulate T cell accumulation in rheumatoid synovial tissues (Buckley CD et al, 2000; Nanki T et al, 2000). CXCR4 is the most widely expressed chemokine receptor in many different cancers and the effects of CXCL12 on CXCR4-bearing tumor cells include a wide diversity of functions (growth, differentiation) besides migration (Zlotnik A, 2006). CXCL12 stimulates survival and growth of neoplastic cells in a paracrine fashion, which can be further enhanced by CXCL12-induced angiogenesis (Burger JA and Kipps TJ, 2006).
The central nervous system (CNS) is an immunologically specialized site where entry of immune cells is tightly regulated. Immune responses are not initiated in the CNS due to the absence of endogenous antigen-presenting cells (Ransohoff RM et al, 2003). Both CXCL12 and CXCR4 are constitutively expressed in the CNS (Tham TN et al, 2001; Banisadr G et al, 2003; Tissir F et al, 2004), but do not mediate leukocyte recruitment to that organ under physiological conditions. Although deficient CNS development has been reported in either CXCL12 or CXCR4 KO mice (Zou YR et al, 1998, Lu M et al, 2002; Lieberam I et al, 2005), the detailed roles of CXCL12/CXCR4 in the adult CNS remain under active investigation. In this review, we will discuss several established functions of CXCL12/CXCR4 in the CNS.
2. CXCL12/CXCR4 signaling pathways
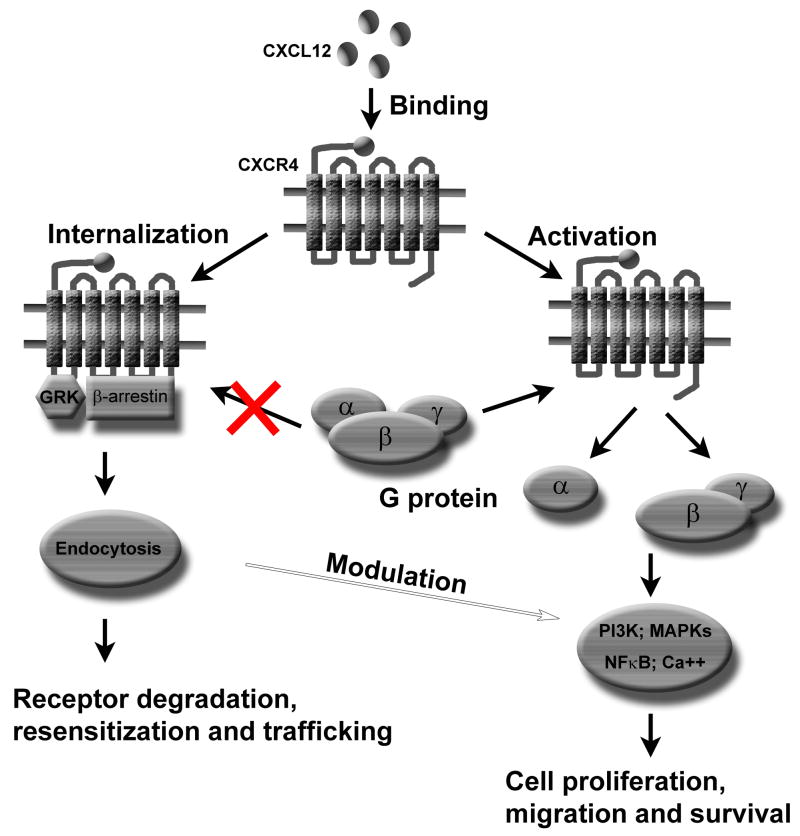
Similar to other chemokines, CXCL12 binds its receptor CXCR4 to activate a series of down-stream signaling pathways (Fig. 1). The interaction between CXCL12 and CXCR4 can be blocked by a bicyclam antagonist AMD31000 (Schols D et al, 1997). Initial binding of CXCL12 to the extracellular components of CXCR4 modifies the tertiary structure of the receptor, allowing the intracellular part to activate heterotrimeric G-proteins (Rot A and von Andrian UH, 2004). Signaling pathways triggered by CXCL12 are mediated both by pertussis toxin (PTX)-sensitive Gαi and by Gq components. Activated heterotrimer G proteins exchange guanosine diphosphate (GDP) for guanosine triphosphate (GTP) and dissociate into α- and βγ-subunits respectively (al-Aoukaty A et al, 1996; Kuang Y et al, 1996). The a subunit further activates phospholipase C (PLC), causing the generation of diacylglycerol (DAG) and inositol 1, 4, 5 trisphosphate (IP3). IP3 binds its specific receptor in the endoplasmic reticulum and releases Ca2+ from intracellular stores. Transient Ca2+ elevation is an important consequence of ligand-induced chemokine receptor activation (Boutet A et al, 2001; Gillard SE et al, 2002). Binding of CXCL12 to CXCR4 also induces the activation of phosphoinositide-3 kinase (PI3K) that activates downstream AKT, PyK2 and nuclear transcriptional factor NFκB signaling pathways (Bajetto A et al, 2001; Han Y et al, 2001a; Fernandis AZ et al, 2004; Lee BC et al, 2004; Alvarez S et al, 2005; Kukreja P et al, 2005; Florio T et al, 2006). G-protein dependent activation of CXCR4 also induces several MAPKs signaling pathways such as ERK, JNK and P38 (Bajetto A et al, 2001; Han Y et al, 2001a; Han Y et al, 2001b; Fernandis AZ et al, 2004; Alvarez S et al, 2005). In addition, CXCL12/CXCR4 activates the Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway in a PTX independent manner (Vila-Coro AJ et al, 1999; Soriano SF et al, 2003; Soldevila G et al, 2004).
Fig. 1. CXCL12/CXCR4 signaling pathways and their regulation.
Binding of CXCL12 to CXCR4 activates multiple G-protein-mediated signaling pathways (such as PI3K, MAPKs and NFκB) and induces the release of intracellular Ca2+. These signaling pathways are involved in cell proliferation, migration and survival or apoptosis. Activation of CXCR4 leads to GRK/β-arrestin-mediated receptor desensitization and prevents CXCR4 from further interacting with G proteins. GRK/β-arrestin promotes clathin-dependent endocytosis that is coupled to receptor trafficking, degradation and resensitization. β-arrestin2 may modulate CXCR4-mediated activation of p38 MAPK signaling pathway.
CXCL12/CXCR4 signaling is regulated by G protein-coupled receptor kinases (GRKs) and β-arrestins (Fig. 1). CXCL12 activation of CXCR4 desensitizes CXCR4 though GRK-mediated receptor phosphorylation and β-arrestin binding (Cheng ZJ et al, 2000; Fong AM et al, 2002; Vroon A, 2004). β-Arrestin2 enhances CXCR4-mediated activation of p38 MAPK that is critically involved in CXCR4-mediated chemotaxis (Sun Y, et al, 2002).
3. Expression of CXCL12 and CXCR4 in the CNS
Both CXCL12 and CXCR4 are constitutively expressed in the developing and mature CNS. In early developmental stages, expression of CXCR4 is mainly detected in the ventricular zone (VZ), subventricular zone (SVZ) and marginal zone (MZ), which are specialized niches for precursors to survive and proliferate. In the mature CNS, CXCL12 or CXCR4 is detected in a remarkable diversity of differentiated cells. In some cases, expression of CXCL12 complements that of CXCR4 and abnormal elevation of both CXCL12 and CXCR4 has been a prominent feature in varied pathological states.
3.1 Constitutive expression
In the developing mouse CNS, expression of CXCR4 starts as early as embryonic day 8.5 (E8.5) and is sustained until adulthood (McGrath KE et al, 1999; Tissir F et al, 2004; Lieberam I et al, 2005). CXCR4 expression is mainly detected in the ventral part of the neural tube during early neural development. However, CXCL12 expression can be found in mesenchymal cells. Beginning from E15.5, both CXCL12 and CXCR4 are expressed in the cortex, olfactory bulb, hippocampus and cerebellum as well as the meninges and endothelia. CXCR4 immunoreactivity has been reported in virtually all CNS cells: neurons, astrocytes, microglia, oligodendrocytes and endothelial cells (Lavi E et al, 1997; Jazin EE et al, 1997; Bajetto A et al, 1999; McGrath KE et al, 1999; van der Meer P et al, 2000; Tham TN et al, 2001; Banisadr G et al, 2002; Banisadr G et al, 2003; Krumbholz M et al, 2006), although some observations have not been validated by multiple consistent findings.
3.2. Complementary expression of CXCL12 and CXCR4
In developing and mature CNS, there is a complementary expression pattern for CXCL12 and CXCR4 that is consistent with their roles in mediating migration, proliferation and survival (Fig. 2). In mouse cerebellum after birth, expression of CXCR4 is observed in the granule cell precursors in the external granular layer (EGL) while expression of CXCL12 is detected in the pial surface adjacent to the EGL (Ma Q et al, 1998; Zou YR et al, 1998; Reiss K et al, 2002). In dentate gyrus (DG) and cortex, expression of CXCR4 is detected in the migrating neuronal precursor cells in the cortical primordium and MZ, while CXCL12 is expressed in the associated pia matter (Bagri A et al, 2002; Lu M et al, 2002).
Fig. 2.
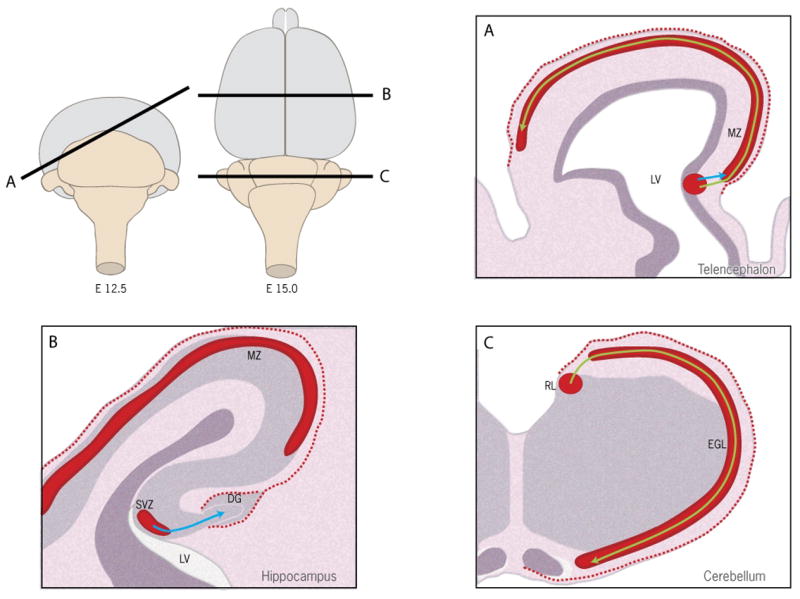
Roles of CXCL12/CXCR4 in brain development. The cartoon shows a schematic view of developing mouse brain (E12.5 and E15). CXCL12/CXCR4 regulates the migration of neuronal precursors during early brain development. (A) At embryonic day 12.5 (E12.5), CXCR4+ Cajal-Retzius (CR) cells are generated from the ventricular zone (VZ) near the lateral ventricle (LV) of the telencephalon and migrate to the marginal zone (MZ) through short range radial migration (blue arrow). CXCR4+ CR cells further spread to the whole telencephalon through long range tangential migration (yellow arrow). CXCL12 secreted from the meninges (dashed red line) is required for the migration of CR cells; (B) In E15 hippocampus, CXCR4+ dentate precursors are generated from subventricular zone (SVZ) near the lateral ventricle (LV). CXCL12 secreted from the meninges (dashed red line) guides the migration (blue line) of dentate precursors to the dentate gyrus (DG); (C) In E15 cerebellum, CXCR4+ granule cell precursors that are generated from the subventricular zone (SVZ) of rhombic lip (RL) migrate tangentially (yellow arrow) to cover most of the cerebellar surface forming the external granule cell layer (EGL). CXCL12 (dashed red line) secreted from meninges maintains cell proliferation and prevents premature exit of granular cell precursors from the EGL.
3.3. Inducible expression
Although CXCL12 is constitutively expressed in the adult CNS, its expression is normally controlled at a relatively low level. CXCL12 can be briefly up-regulated under some pathathological situations such as HIV 1-asociated dementia, brain tumor, ischemia (stroke) and Neuroinflammation (Rempel SA et al, 2000; Rostasy K et al, 2003; Hill WD et al, 2004; Metelitsa LS et al, 2004; Miller JT et al, 2005; Peng H et al, 2006; Tabatabai G et al, 2006). Astrocytes and vascular endothelial cells in the parenchyma have been suggested as two primary cell sources for inducible CXCL12. In ischemia, the local hypoxic microenvironment activates transcription factor hypoxia-inducible factor-1 (HIF-1) that regulates CXCL12 gene expression in endothelial cells, resulting in selective expression of CXCL12 in ischemic tissue in direct proportion to reduced oxygen tension (Ceradini DJ et al, 2004). In acquired immunodeficiency syndrome (AIDS) patients, quinolinic acid (QUIN) is an important neurotoxin associated with AIDS dementia complex (ADC) and mainly released from mononuclear phagocytes (which may be infected by HIV-1 or stimulated in paracrine fashion by cytokines released from infected cells). QUIN up-regulates the expression of CXCL12 and CXCR4 in astrocytes (Croitoru-Lamoury J et al, 2003a; Guillemin GJ et al, 2003). In multiple sclerosis (MS), CXCL12 protein is elevated and detected on astrocytes and blood vessels (Ambrosini E et al, 2005; Krumbholz M et al, 2006). In tumors, expression of both CXCL12 and CXCR4 increases with increasing tumor grade and over expression of CXCR4 was observed in the neovascular endothelial cells (Rempel SA et al, 2000).
Up-regulation of either CXCL12 or CXCR4 relies on other regulators. HIF-1 and VEGF enhance the expression of CXCR4 in glioblastoma (Zagzag D et al, 2006). IL-1β induces CXCL12 in astrocytes by ERK and PI3K signaling pathways (Calderon TM et al, 2006; Peng H et al, 2006). Our lab demonstrated that CXCR4 expression by primary mouse astrocytes is suppressed by exposure to TNF-α (Han Y et al, 2001a). In contrast, other labs found that TNF-α up-regulates the expression of CXCR4 (Croitoru-Lamoury J et al, 2003b), suggesting cytokine-dependent CXCR4 regulation is a complicated process.
4. Roles of CXCL12/CXCR4 in the development of CNS
4.1 Migration of Neuronal progenitor Cells
Brain cortex exhibits a highly laminar organization arising during early development when newborn neurons migrate from proliferative VZ and spread into other cortical layers. There are two basic migration patterns: short range radial migration, in which neurons use the radial glial scaffold to migrate to their final destination; and long range tangential migration dependent on substrates other than radial glial cells (Marin O and Rubenstein JL, 2003). CXCL12/CXCR4 directly regulates the migration of cortical neuronal precursors, including cortical Cajal-Retzius cells (CR), cerebellar granule precursor cells (GPC) and dentate gyrus GPC, a function which is essential for the formation of complex laminated cortex structures during early development (Fig. 2).
4.1.1. Cortical Cajal-Retzius (CR) cells
In the developing brain cortex, Cajal-Retzius (CR) cells are a transient population of neurons located in the marginal zone (MZ), directly adjacent to the meninges (Derer P and Derer M, 1990; Marin-Padilla M, 1990; Del Rio JA et al, 1995; Verney C and Derer P, 1995). The major function of CR cells is to regulate cortical lamination by secreting the glycoprotein reelin, which maintains the radial glial scaffold that governs the migration of granule cells (D’Arcangelo G et al, 1995; Ogawa M et al, 1995; Super H et al, 2000). Recent studies suggest three major CR cell sources in the embryonic telencephalon, including the cortical hem, the septum and the ventral pallium (Takiguchi-Hayashi K et al, 2004; Bielle F et al, 2005; Yoshida M et al, 2006). CR cells initially radially spread from VZ to MZ and subsequently migrate long distances tangentially along the cortical surface (Takiguchi-Hayashi K et al, 2004; Bielle F et al, 2005; Muzio L and Mallamaci A, 2005; Yoshida M et al, 2006).
Expression of CXCR4 is detected in the MZ CR cells from preplate (E13.5) to early postnatal stage (P3) of neocortical development (Stumm RK et al, 2003; Borrell V and Marin O, 2006) while expression of CXCL12 is detected in the meninges (Stumm RK et al, 2003; Borrell V and Marin O, 2006). In either CXCL12−/− or CXCR4−/− mice, there is obvious ectopic dispersion of CR cells that are displaced into deeper cortical layers (Stumm RK et al, 2003; Paredes MF et al, 2006), which suggests that CXCL12/CXCR4 may influence the early localization of CR cells. Recently, Borrel and coworkers (2006) demonstrated that meninges themselves are the important substrate for CR cell tangential migration. They performed a series of transplant assays by which green fluorescent protein (GFP) positive cortical hem tissues were transplanted into wild type brain slices. After 48 hours, they observed the consistent CR cell tangential migration in association with the intact meninges rather than the cortical neuroepithelium (Borrell V and Marin O, 2006). Further, they demonstrated both in vivo and in vitro that CXCL12 triggers the migration of CR cells by binding CXCR4.
Paredes et al (2006) demonstrated similar roles of CXCL12 in the development of CR cells by studying methylazoxymethanol (MAM) treated rats. MAM is a DNA-alkylating agent that causes the accumulation of defects during DNA replication of dividing cells (Kisby GE et al, 1999). They observed disruption of the MZ in the E15 embryos of MAM treated Sprague Dawley (SD) rats, with the MZ becoming thicker. Further, many reelin-positive CR cells redistributed to the deeper cortical layers, a similar phenotype to that observed in CXCL12−/− and CXCR4−/− mice (Stumm RK et al, 2003; Paredes MF et al, 2006). However, MAM treating does not directly cause the abnormal distribution of CR cells, which occupy the MZ before drug administration at E15. Furthermore, exogenous CXCL12 rescued the developmental defects caused by MAM, suggesting that loss of CXCL12 might lead to the defective migration of CR cells. Additional studies showed that MAM causes meningeal injury and defects in the meningeal basement membrane leads to the loss of CXCL12 expression by leptomeninges. Therefore, CXCL12/CXCR4 directly regulates the tangential migration of CR cells and contributes to the formation of laminated cortex. In addition, CXCL12 is expressed in the subventricular zone/intermediate zone (SVZ/IZ) and CXCL12 secreted from the proliferating precursors of projection neurons has been suggested to regulate the invasion of interneurons during corticogenesis (Tiveron MC et al, 2006).
4.1.2. Cerebellar granule precursor cells
In the developing cerebellum, there is a subpial proliferative zone called external granular layer (EGL), occupied by granule cell precursors and other glial progenitor cells after their generation from the VZ of the rhombic lip (Jensen KF and Altman J, 1982; Hausmann B and Sievers J, 1985). Starting from E10, granule cell precursors migrate tangentially to cover most of the cerebellar surface at E15, following a lateromedial and posteroanterior trajectory (Sotelo C, 2004). After transient proliferation in the EGL, granule cell precursors exit the cell cycle and become postmitotic granule cells. Postmitotic granule cells migrate radially inward to form the internal granule layer (IGL) at the later stages (Altman J and Bayer SA, 1985).
Expression of CXCR4 had been implicated in the developing EGL (Zou YR et al, 1998; Tissir F et al, 2004) and CXCR4 protein can be found in cultured granule progenitor cells in vitro (Klein RS et al, 2001; Reiss K et al, 2002). In complementary fashion, CXCL12 is expressed in the meninges (Tham TN et al, 2001; Klein RS et al, 2001; Zhu YR, 2002; Reiss K et al, 2002). Zou et al (1998) first reported the abnormal migration of granule cell precursors in the cerebellar anlage of CXCR4−/− mice during first postnatal week. They found ectopic clusters of dividing granule cells, beneath the Purkinje cell layer or intermingled with Purkinje cells. Similar abnormal phenotypes were observed in the CXCL12−/− cerebellum (Ma Q et al, 1998).
A widely accepted hypothesis for explaining these phenotypes in the CXCR12−/− and CXCR4−/− mice is that CXCL12 prevents granular cell precursors from prematurely exiting the EGL (Zou YR et al, 1998). In vitro, CXCL12 was found to be a chemotactic factor for cells isolated from EGL (Klein RS et al, 2001) and CXCL12-induced chemotaxis was negatively regulated by an EphB receptor whose ligation activates a PDZ-RGS3 protein (Lu Q et al, 2001). Within EGL, Reiss et al (2002) demonstrated that CXCL12 binds to and is concentrated by heparan sulfate proteoglycans (HSPG), using a similar mechanism to that employed in the CXCL12-mediated adhesion of leukocytes to the endothelial surface. In addition, CXCL12 may also enhance the proliferation of granule cell precursors mediated by Sonic hedgehog (SHH) through the activation of down-stream signaling pathways that eventually lead to increased intracellular cAMP (Klein RS et al, 2001). Therefore, CXCL12 has two basic functions in cerebellogenesis: one is to attract and maintain the granule cell precursors within the EGL; a second function is to promote granule cell precursor proliferation. Besides ephrin B signaling, there are many other signaling pathways such as brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), neuregulin (NRG), astrotactin and tenascin, tissue plasminogen activator, platelet-activating factor (PAF), cyclin-dependent kinase 5(Cdk-5), 9-O-acetyl GD3 and somatostatin that have been suggested in the regulation of cerebellar granule cell migration (Komuro H and Yacubova E, 2003). However, the detailed relationships between these components and CXCL12/CXCR4 have not been addressed.
Another possible mechanism to explain the abnormal distribution of granule cell precursors in either CXCL12−/− or CXCR4−/− mice is that CXCL12 may directly guide the tangential migration of granule cell precursors in the EGL along the cerebellar surface during early gestation. In support of this possibility, CXCR4 is expressed in the germinal region of the rhombic lip, the only region from which the cerebellar granule cell precursors are generated (Wingate RJ and Hatten ME, 1999; Tissir F et al, 2004). Previous studies demonstrated that selective destruction of the meninges in this region causes reduction of the superficial basal lamina, a misrouting of cell migration within the VZ and the abnormal development of radial glia (Hartmann D et al, 1992; Sievers J et al, 1994; Hartmann et al, 1998).
4.1.3 Dentate gyrus (DG) granule precursor cells
The early development of dentate granule cells initiates with radial migration of cells from the dentate neuroepithelium to the dentate gyrus anlage. The migrating stream contains both precursor cells and postmitotic neurons that determine the morphogenesis of the primary dentate gyrus and hillus. As development proceeds, most precursor cells in the hillus exit the cell cycle to become postmitotic granule cells. Only a small proportion of granule cell precursors remain within the subgranular zone (SGZ), a thin border between the dentate GCL and hillus (Pleasure, SJ et al, 2000). Dentate GCL represents an important site of adult neurogenesis. During development, dentate CR cells migrate within the MZ from the supra-granular blade, where they can be observed from perinatal stages onward, toward the developing infra-granular blade (Super H et al, 1998). Dentate CR cells influence the migration of granule cells in the dentate gyrus by acting on the radial glial scaffold (Frotscher M et al, 2003).
In early developing DG, CXCL12 is constitutively expressed in the meninges overlying the hippocampus and in the hippocampal primordium itself near the hippocampal fissure, overlying the developing DG (Bagri A et al, 2002; Lu M et al, 2002). During the first postnatal weeks, expression of CXCL12 is detected in meninges, CR cells and maturing DG granule cells (Berger O et al, 2007). In adulthood, expression of CXCL12 is detected in the blades of DG and some cells in the hillus (Lu M et al, 2002). In CXCR4−/− mice, ectopic dispersion and abnormal migration of granule cells were observed. Abnormal development of the DG in CXCR4−/− mice was potentially caused by deficient migration of granule cells since over-expression of CXCL12 in vitro disrupted the migration of granule cells (Bagri A et al, 2002; Lu M et al, 2002).
4.1.4. Others
CXCL12/CXCR4 has also been suggested in the development of other tissues or organs such as peripheral nervous systems (PNS), gonadotropin-releasing hormone (GnRH) neurons, primordial germ cells (PGCs), lateral line, facial motor neurons and muscle. Recent studies demonstrated that CXCL12 regulates the migration of sensory neuron precursors in the dorsal root ganglia (DRG) (Belmadani A et al, 2005). CXCR4 null mice exhibited small and malformed DRGs and CXCL12 acted as a chemoattractant for the cells from neural tube explants (Belmadani A et al, 2005). Similarly, Knaut et al (2005) reported that trigeminal sensory ganglia (TgSG) neurons were ectopically located in the CXC4b morphant zebrafish embryos (embryos injected with morpholine-conjugated antisense oligonucleotides) while both the number of TgSG neurons and axon projections were normal. In CXCR4−/− mice, most GnRH neurons also failed to exit the vomeronasal organ (VNO) at E13, and comparatively few GnRH neurons reached the forebrain (Schwarting GA et al, 2006). Primordial germ cells (PGCs) are the founders of sperm or oocytes. In zebrafish, knocking down CXCL12 or receptor CXCR4 results in severe defects in PGC migration (Doitsidou M et al, 2002). Inhibition of PI3K signaling in PGCs slows down their migration and leads to abnormal PGC morphology as well as to reduced stability of filopodia (Dumstrei K et al, 2004). PGCs undergo directed migration through tissues of embryos in CXCL12−/− mice, but the numbers of PGCs in the gonads are significantly reduced without evident effects on the proliferation of PGCs (Ara T et al, 2003).
The posterior lateral line (PLL) is a mechanosensory system located caudally in fish and amphibians. Recent studies demonstrated that inactivation of CXCL12 and CXCR4 prevents PLL primordium migration, resulting in the formation of defective PLL (David NB et al, 2002; Li Q et al, 2004; Haas P and Gilmour D, 2006). The migration of motor neurons of the facial nucleus from rhombomere 4 to 6 was also affected in CXCL12 morphants (Sapede D et al, 2005). In CXCR4 mutant mice, the number of muscle progenitors that colonized the anlage of the tongue and the dorsal limb was reduced. Changes in the distribution of the muscle progenitor cells were accompanied by increased apoptosis, indicating that CXCR4 signals provide not only attractive cues but also control survival possibly through interacting with Gab1, an adaptor protein that transduces signals elicited by tyrosine kinase receptors (Vasyutina E et al, 2005).
4.2. Axon guidance and pathfinding
The development of the CNS depends not only on the well-regulated migration of precursor cells but also the establishment of complete circuitry by extending neuritic processes (axons and dendrites) to target neurons to form synaptic connections. Axonal growth cones are guided by axonal guidance cues (Tessier-Lavigne and Goodman, 1996). CXCL12 serves as a guidance cue to regulate axon projection, interacting with other axon guidance cues.
Wu et al (2001) first reported that slit-2, a secreted protein previously known as a mediator of repulsion in axon guidance and neuronal migration, also inhibits leukocyte chemotaxis induced by CXCL12. The inhibitory effects of slit-2 could be reconstituted by CXCR4 and slit receptor Roundabout (Robo). This crosstalk between CXCL12 and slit-2 in leukocytes raised the question whether chemokines play a role in axon guidance. This line of research showed that CXCL12 mediates several important axon guidance cues and also directs relative axon repulsion. For instance, CXCL12 reduces the repellent activities of slit-2 on cultured retinal ganglion cell axons, of semaphorin 3A on dorsal root ganglion sensory axons, and of semaphorin 3C on sympathetic axons (Chalasani SH et al, 2003). In zebrafish, antisense knockdown of CXCL12 or CXCR4 induced retinal axons to follow aberrant pathways within the retina. Further, retinal axons deviated from their normal pathways and extended to cells ectopically expressing CXCL12 within the retina (Li Q et al, 2005). However, these effects may be indirect since CXCL12 does not have detectable attractive or repellent effects on retinal or DRG axons by itself (Chalasani SH et al, 2003). In cultured retinal axons, CXCL12 mediates reduced responsiveness to slit-2 (Chalasani SH et al, 2007). In vivo, reducing CXCL12 signaling rescues retinal axon pathfinding errors in zebrafish morphants that have a partial functional loss of robo as compared with morphants that completely lack robo (Chalasani SH et al, 2007), indicating that guidance or repulsive effects of CXCL12 relies on other axon guidance cues. However, the detailed molecular mechanism is not well known.
Chalasani (2003) has suggested that modulation of intracellular cyclic nucleotides may influence the guidance effects of CXCL12 towards axons. In slit-2 induced growth cone collapse experiments, either PTX or CXCR4 antagonist AMD3100 could block the effects of CXCL12. cAMP antagonist RpcAMPS also abrogated the modulation mediated by CXCL12. RpcAMPS failed to affect slit-2-induced collapse. Together, the results indicated that binding of CXCL12 to CXCR4 elevates intracellular cAMP level and modulates axonal projection. Rho-GTPase associated with CXCL12 signaling has also been implicated in axon outgrowth (Arakawa Y et al, 2003). In zebrafish, CXCL12 activates two distinct Rho-dependent pathways with opposite consequences. At low concentrations, CXCL12 stimulates a Rho-dependent pathway that facilitates axon elongation. In contrast, Rho/ROCK activation stimulated by a higher concentration of CXCL12 repressed axon formation and induced arrest in axon length (Arakawa Y et al, 2003).
Lieberam et al (2005) demonstrated that CXCL12/CXCR4 is required for the ventral trajectory of mouse spinal motor neurons (vMN). Briefly, there are two types of motor neurons (ventral motor neurons-vMNs and dorsal motor neurons-dMNs), choosing different pathways to project axons to peripheral muscle (Gutherie S and Lumsden A, 1992). Expression of CXCR4 was only detected in the axonal growth cones of vMN but not dMN. Interestingly, many CXCR4−/− vMN axons adopted a dMN-like trajectory despite preservation of their vMN transcriptional identity and initial axon projection (Lieberam I et al, 2005).
In zebrafish, peripheral sensory efferent projection requires intact CXCL12/CXCR4 signaling (Gilmour D et al, 2004; Sapede D et al, 2005). The peripheral PLL in zebrafish receives axonal afferent inputs from two different efferent nuclei in the hindbrain (Metcalfe WK et al, 1985). Although migration of PLL was inhibited in CXCL12 or CXCR4 morphants, the sensory axons remained associated to the primordium, which suggests efferent projection and migration of PLL are two independent phenomena (David NB et al, 2002). By generating a series of genetic mosaic embryos from zebrafish, Gilmouir D et al (2004) demonstrated that PLL, a migrating cell cluster, provides similar cues that are sufficient to steer its associated nerve throughout the entire extension process.
Burns et al (2006) identified an orphan GPCR, RDC1 as a second receptor for CXCL12, designated CXCR7. To date, limited evidence is available regarding the functional roles of CXCR7. In particular, knockdown studies in zebrafish suggest that CXCR7 is active during vasculogenesis. In CXCR7 morphant zebrafish, abnormal distribution of neuromasts were oberserved, suggesting CXCR7 is required for guidance of primordium migration (Dambly-Chaudiere C et al, 2007). However, the underlying mechanism remains to be further addressed.
5. Roles of CXCL12/CXCR4 in neural progenitor cells (NPCs)
NPCs or neural stem cells are self-renewing multi-potential cells that give rise to residual neurons, astrocytes and oligodendrocytes in embryonic and mature CNS (Gage FH et al, 1995; Brustle, O et al, 1997). It has been suggested recently that NPCs are associated with embryonic/adult neurogenesis and tissue repair. In mature CNS, SVZ/olfactory bulb system and dentate gyrus SGZ has been suggested as the two major neurogenic regions that host NPCs constitutively for adult neurogenesis. Thored P et al (2006) demonstrated that endogenous NPCs continuously supply the injured adult brain with new neurons, which suggests novel self-repair strategies to improve recovery after stroke. The expression pattern of CXCR4 in the neurogenic regions of postnatal brain is consistent with the distribution of NPCs (Tran PB et al, 2007). In vitro, expression of CXCR4 has been widely reported in cultured NPCs isolated from different regions of adult brain (Ji JF et al, 2004; Peng H et al, 2004; Tran, PB et al, 2004; Dziembowska M et al, 2005; Tran PB et al, 2005). Interestingly, important functions of CXCL12/CXCR4 have been demonstrated in hematopoietic progenitors, including homing to the bone marrow; trafficking in the circulation; survival; and proliferation (Lataillade JJ et al, 2004). It is worth noting that similar roles for CXCL12/CXCR4 have been suggested for adult NPCs.
First, CXCL12/CXCR4 regulates the migration of adult NPCs. In vitro, administration of CXCL12 directly induces adult NPC chemotaxis (Reiss K et al, 2002; Imitola J et al, 2004; Peng H et al, 2004). Imitola et al (2004) demonstrated that human NPCs migrate in vivo (including from the contralateral hemisphere) toward an infarcted area, where local astrocytes and endothelium up-regulate CXCL12. Robin et al (2006) found that cultured NPCs harvested from the normal adult SVZ, when they were overlaid onto stroke brain slices, exhibited significantly increased migration. Blocking CXCL12 by a neutralizing antibody against CXCR4 significantly attenuated stroke-enhanced NPC migration. Several signaling pathways may mediate CXCL12 induced NPC migration, including IP3; extracellular signal-regulated kinases1/2; AKT, c-Jun N-terminal kinase; intracellular Ca2+ and reduction of cAMP (Peng H et al, 2004). Second, CXCL12 stimulates the proliferation of adult NPCs (Tran PB et al, 2004; Dziembowska M et al, 2005; Tran PB et al, 2005). Exposure of CXCL12 to quiescent NPCs enhances proliferation, promotes chain migration and transmigration (Imitola J et al, 2004). CXCL12 enhances the proliferation of NPCs in vitro, dependent on the ERK1/2 and PI-3 kinase pathways (Gong X et al, 2006). CXCL12 may promote survival, but also maintains NPCs in a quiescent state (Krathwohl MD and Kaiser JL, 2004).
6. Roles of CXCL12/CXCR4 in the adult CNS
6.1. Neuromodulation
Constitutive expression of CXCR4 in mature neurons suggested that CXCL12/CXCR4 might modulate neurotransmission. Limatola C et al (2000) first reported that Purkinje neurons in cerebellar slices responded to CXCL12 application by a slow inward current followed by an increase of both intracellular Ca2+ and spontaneous synaptic activity. The same group reported that the amplitude of evoked excitatory postsynaptic currents (EPSC) of Purkinje neurons was reversibly reduced by CXCL12 application (Ragozzino D et al, 2002). They suggested that neuromodulatory effects of CXCL12 were indirect, caused by glutamate release from glial cells. Recent studies found that CXCL12 controls melanin-concentrating hormone neuron excitability not only through indirect glutamate/GABA release but also through direct G protein activated K+ channel (GIRK) current activation (Guyon A et al, 2005; Guyon A et al, 2006). By blocking CXCR4, antagonist human Immunodeficiency virus (HIV)-1 gp120 inhibits the long-term potentiation (LTP) in the CA1 region of rat hippocampus through indirect release of gamma-aminobutyric acid type A (GABAA) while no effects were observed on basal synaptic transmission (Dong J and Xiong H, 2006). CXCL12 affects the electrical activity of arginine vasopressin (AVP) neurons, resulting in changes in AVP release (Callewaere C et al, 2006). Chemokines and gp120 have been suggested to facilitate pain pathways via direct actions on chemokine receptors expressed by nociceptive neurons (Oh SB et al, 2001). Szabo et al (2002) reported that chemokine administration into the periaqueductal gray matter inhibits opioid-induced analgesia. Some neurons express CXCR4 and μ-opioid receptors (MORs). Treatment of CXCR4+/MOR+ neurons with the selective MOR agonist DAMGO or peptide endomorphin-1 inhibited intracellular signaling pathways (ERK1/2 and Akt) activated by CXCL12. Furthermore, DAMGO abolished the neuroprotective effect of CXCL12 in N-methyl-d-aspartate (NMDA) neurotoxicity studies. (Patel JP et al, 2006).
6.2. Neurotoxicity/Neuroprotection
Similar experiments to those described in the previous section led to the proposal that neuronal apoptosis induced by HIV-1 gp120 and CXCL12 was mediated by the chemokine receptor CXCR4 (Hesselgesser J et al, 1998). More recent studies showed that gp120 stimulates p53 activity and induces expression of the p53 pro-apoptotic target Apaf-1 in cultured neurons. Gp120 regulates p53 phosphorylation and expression of other p53-responsive genes, such as MDM2 and p21. Meanwhile, CXCL12 promotes neuronal survival, increases p53 acetylation and p21 expression in neurons (Khan MZ et al, 2005). CXCL12 rescues neurons from apoptosis by stimulating the time-dependent increase of total Rb expression while decreasing the nuclear content of phosphorylated (Ser780/Ser795) Rb and the transcriptional activity of E2F-1. Gp120 exerts opposite effects at the nuclear level (Khan MZ et al, 2003). BDNF and FGF reduced the levels of CXCR4, thereby protecting from gp120-mediated neuronal apoptosis (Sanders VJ et al, 2000; Bachis A et al, 2004). However, TNF-α may render neuronal cells vulnerable to the apoptotic effects of HIV by increasing the cell surface expression of CXCR4 (Rostasy K et al, 2005). Gp120 injected into the rat striatum or hippocampus is sequestered by neurons and subsequently retrogradely transported to distal neurons that project to these brain areas. Both retrograde transport of gp120 and apoptosis are dependent on CXCR4 (Bachis A et al, 2006).
Recent studies indicate that gp120 may induce both neuronal apoptosis and axonal degeneration in cultured rat DRG sensory neurons (Melli G et al, 2006). Binding of gp120 to CXCR4 on sensory axons may directly cause the local axonal degeneration. In addition, gp120 triggered the release of chemokine CCL5 from Schwann cells, which caused TNF α-mediated neuronal apoptosis and neuritic degeneration in vitro (Kurry P et al, 2003).
6.3. Intercellular communication
Besides neurons, expression of CXCR4 is detected on the microglia and astrocytes in the mature CNS (Tanabe S et al, 1997; Ohtani Y et al, 1998). CXCL12 induces the migration of mouse microglial cells but not astrocytes (Tanabe S et al, 1997). In vitro, CXCL12 stimulates astrocyte proliferation, associated with sequential activation of a G-protein-PI-3Kinase-ERK1/2 signaling cascade (Bajetto A et al, 2001; Barbero S et al, 2002; Bonavia R et al, 2003). Our lab demonstrated that CXCL12 activates NF-κ B, stimulates production of chemokines and cytokines, and induces cell death in primary astrocytes, effects that depend on ongoing secretion of TNF-α(Han Y et al, 2001a) cAMP and IL-6 have been suggested to induce CXCL12-dependent chemotaxis of astroglia by upregulating the expression of CXCR4 (Odemis V et al, 2002). CXCL12/CXCR4 have been suggested as regulator of complex intercellular neuro-glial or glial-glial interactions. For instance, CXCL12 induces the production of TNF-α in astrocytes. Autocrine/paracrine TNF-α from astrocytes not only results in the glutamate release that directly signals to the astrocytes, but also causes astrocyte derangement when activated microglia cooperate to dramatically enhance release of TNF-α in response to CXCR4 stimulation (Bezzi P et al, 2001).
7. Pathological implications of CXCL12/CXCR4 in human CNS disorders
7.1. HIV-associated encephalopathy
HIV-associated encephalopathy has been one of the most common causes of morbidity associated with infection by HIV-1 (Price RW and Sidtis JJ, 1990) in the absence of particularly effective anti-retroviral treatments. Pathology of HIV-associated encephalopathy is characterized by the loss of subsets of neurons in the cortex, limbic system and basal ganglia (Glass JD et al, 1998). CXCR4 was the first identified coreceptor for T trophic HIV virus that infects CD4+ T-lymphocytes (Feng Y et al, 1996; Lapham CK et al, 1996). CXCR4 is constitutively expressed in the human brain by neurons and microglia. Microglia are the key cell type infected by HIV-1 in the CNS (Gonzalez-Scarano F and Martin-Garcia J, 2005). HIV-1 virus may also enter the CNS via infected monocytes (probably infected by using the CCR5 co-receptor) that traverse the blood brain barrier to replenish perivascular macrophages. Up-regulation of CXCR4 expression has been reported in macrophage/microglia and neurons in AIDS patients (Sanders VJ et al, 1998; Vallat AV et al, 1998; Van der Meer P et al, 2000; Petito CK et al, 2001). HIV-1-infected microglia or macrophages regulate astrocyte CXCL12 production through IL-1β (Peng H et al, 2006), providing ligand for upregulated CXCR4. The abnormal expression of CXCL12 and CXCR4 is consistent with the possibility this complex interactions might contribute to the pathogenesis of HIV-associated encephalopathy.
One line of research suggests that gp120 and CXCL12 could directly induce neuronal degeneration and apoptosis through binding to CXCR4, but that cell death can be reduced through heterologous desensitization of CXCR4 (Hesselgesser J et al, 1998; Meucci O et al, 1998; Corasaniti MT et al, 2001; Bachis A and Mocchetti I, 2003). Another protection mechanism implicates BDNF, which reduced expression of CXCR4, an effect which correlated with the ability of BDNF to reduce gp120 internalization and neuronal apoptosis. Moreover, BDNF directly blocked the neurotoxic effect of CXCL12 (Bachis A et al, 2003; Bachis A and Mocchetti I, 2005). However other studies demonstrated that CXCL12 failed to prevent gp120 neurotoxicity, and in fact induced neuronal apoptosis acting indirectly through glial stimulation (Kaul M and Lipton SA, 1999). Bardi G et al (2006) recently demonstrated as expected that CXCL12 down-regulated CXCR4 as is typical for ligand engagement of GPCRs. Interestingly, gp120 did not change CXCR4 membrane levels. Nevertheless, gp120 was still able to activate intracellular signaling and proapoptotic pathways via CXCR4. High expressing alleles of CXCL12 are associated with protection from HIV-1 pathogenesis (Winkler C et al, 1998). In other studies, HIV-1-infected monocyte-derived macrophage secretions, virus or CXCL12 induced a Gi protein-linked decrease in cAMP and increased IP3 and intracellular calcium. Such effects were partially blocked by antibodies to CXCR4 or removal of virus from MDM fluids. Changes in G-protein-coupled signaling correlated with, but were not directly linked, to increased neuronal synaptic transmission, caspase 3 activation and apoptosis (Zheng J et al, 1999). In anther line of research, Jana A and Pahan K (2004) recently suggested that gp120 may induce neuronal apoptosis through the CXCR4-NADPH oxidase-superoxide-neutral sphingomyelinase-ceramide pathway. Indirect effects of CXCR4 signaling on neuronal viability have also been explored. In particular, gp120 or CXCL12 can indirectly induce the release of glutamate from activated astrocytes, which can lead to excitotoxic neuronal injury (Bezzi P et al, 2001). They found that binding of gp120 or CXCL12 via CXCR4 in astrocytes activates the intracellular calcium-dependent ERK signaling pathway that leads to the release of TNF-α. Second, in a paracrine/autocrine fashion, TNF alpha binds to TNFR1 on astrocytes, which results in the production of prostaglandin E2 (PgE2) by inducible cyclooxygenase 2 (COX-2). PgE2 leads to increased intracellular calcium, triggering the release of glutamate. At the same time, gp120 or CXCL12 binds microglial CXCR4 to stimulate the production of TNF-α. Interestingly, Taylor DL et al (2005) demonstrated that induction of microglial TNF-α is dependent on the stimulation of mGlu2, which is highly correlated with the microglial neurotoxic phenotype. Further, they found that TNF-α can induce neuronal apoptosis by directly binding to its receptor TNFR1 on the surface of neurons. Bachis A et al (2006) recently reported that cleaved caspase-3 and terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL), hallmarks of apoptosis, were seen in neurons internalizing and transporting gp120. The retrograde transport of gp120 and apoptosis were mediated by the chemokine receptor CXCR4. Besides that, Zhang et al (2003) reported HIV-infected macrophages secrete matrix metalloproteinase-2 (pro-MMP-2), which can be activated by neuronal membrane-type 1 matrix metalloproteinase (MT1-MMP). Activated MMP2 cleaves CXCL12 into a truncated CXCL12 (5–67) by removing its N-terminal tetrapeptide. Truncated CXCL12 (5–67) caused neurodegeneration potentially through binding another chemokine receptor CXCR3 (Zhang et al, 2003; Vergote D et al, 2006).
In summary, signaling to CXCR4 by gp120 is proposed to activate multiple pathways that converge, directly or indirectly on neuronal injury.
7.2. Brain tumors
Brain tumors encompass neoplasms that originate in the brain itself (primary brain tumors) or involve the brain as a metastatic site (secondary brain tumors). The majority of recent research related to CXCR4 focused on primary brain tumors. Up-regulated CXCL12 and CXCR4 were observed in primary brain tumors including glioblastoma; astrocytoma; and medulloblastomas (Sehgal A et al, 1998a; Sehgal A et al, 1998b; Schuller U et al, 2005; Liu G et al, 2006; Woerner BM et al, 2005). While CXCR4 was expressed in tumor cells and some endothelial cells, CXCL12 was present in endothelial cells and infiltrating microglia. By using an antibody that can distinguish activated CXCR4 phosphorylated on serine 339, phospho-CXCR4 was present in tumor cells and vascular endothelial cells in all grades of astrocytoma (Woerner BM et al, 2005). A significant correlation of high CXCR4 mRNA levels and presence of the neurotrophin receptor p75NTR or expression of ATOH1 and GLI1 suggested that CXCR4 is a reliable marker for tumors derived from the cerebellar external granular layer (Schuller U et al, 2005). In astrocytomas, CXCL12 and CXCR4 expression were colocalized when both were expressed, and CXCL12 and CXCR4 expression increased with increasing tumor grade. Two proteins were expressed in regions of angiogenesis and degenerative, necrotic, and microcystic changes (Rempel SA et al, 2000). Expression of CXCL12 was associated with a significantly shorter time to tumor progression (TTP). The data suggest the possible prognostic value for CXCL12 as an angiogenesis- and tumor-growth-related chemokine on TTP in low-grade gliomas (Salmaggi A et al, 2005). CXCL12 induced a significant increase of DNA synthesis in primary human glioblastoma cell cultures and chemotaxis in a glioblastoma cell line (Bajetto A et al, 2006). CXCR4 was increased by VEGF and bFGF, suggesting an angiogenic amplification loop. In tumor microenvironments, CXCL12 is proposed to modulate the proliferation and survival of both endothelial and tumor cells (Salmaggi A et al, 2004). Activation of CXCR4 is critical for the growth of both malignant neuronal and glial tumors. Systemic administration of CXCR4 antagonist AMD 3100 inhibits growth of intracranial glioblastoma and medulloblastoma xenografts in small-animal models by increasing apoptosis and decreasing the proliferation of tumor cells (Rubin JB et al, 2003).
7.3. Stroke
Stroke is the acute neurologic injury that occurs as a result of brain ischemia due to thrombosis, embolism, or systemic hypoperfusion or due to intracerebral hemorrhage or subarachnoid hemorrhage. In stroke animal models, CXCL12 is increased and released primarily by activated astrocytes and endothelial cells (Ceradini DJ et al, 2004; Hill WD et al, 2004). The elevation of CXCL12 is associated with the infiltration of monocytes to the areas of ischemic injury and potentially mediates neuroinflammatory pathogenesis (Hill WD et al, 2004). Dexamethasone (DEX) down-regulates CXCR4 and exerts neuroprotection in neonatal rats by influencing the inflammatory cascade induced by hypoxia-ischemia in the neonatal brain (Felszeghy K et al, 2004). Previously, Stumm RK (2002) reported that neuronal CXCL12 was transiently downregulated and neuronal CXCR4 was transiently upregulated in the nonlesioned cerebral cortex in response to ischemia. Although the detailed pathological consequences are not clear, the effects would markedly reduce signaling through CXCR4.
Interestingly, CXCL12/CXCR4 has been suggested to regulate the recruitment of hematopoietic NPCs to the injured site, further enhancing the tissue repair during neuronal regeneration. Ceradini DJ et al (2004) demonstrated that up-regulated CXCL12 expression increases the adhesion, migration and homing of circulating CXCR4-positive NPCs to ischemic tissue. Blockade of CXCL12 in ischemic tissue or CXCR4 on circulating NPCs impairs the recruitment of NPCs to sites of injury. Yamaguchi et al (2003) suggested that locally delivered CXCL12 augments vasculogenesis and subsequently contributes to ischemic neovascularization in vivo by augmenting endothelial progenitor cell (EPC) recruitment in ischemic tissues.
7.4. Multiple sclerosis (MS)
MS is an inflammatory demyelinating disorder, which is characterized pathologically by multifocal areas of demyelination with loss of oligodendrocytes and astroglial scarring in the CNS (Weiner H, 2004). Experimental allergic encephalomyelitis (EAE) is a murine CD4+ Th1-cell and Th17-cell mediated demyelinating disease of the CNS that serves as a model for inflammatory aspect of human MS (Swanborg RH, 1995; Kleinschek MA et al, 2007). CXCL12 is present in the cerebrospinal fluid (CSF) from patients with MS and other inflammatory neurological disorders (Pashenkov M et al, 2003; Corcione A et al, 2004; Krumbholz M et al, 2006). In active MS lesions, CXCL12 levels were high on astrocytes throughout the lesion areas and on some monocytes/macrophages within vessels and perivascular cuffs, with lesser staining on EC. In silent MS lesions, CXCL12 was detected on EC and astrocytes, particularly hypertrophic astrocytes near the lesion edge (Calderon TM et al, 2006; Krumbholz M et al, 2006). In vitro, IL-1β or myelin basic protein (MBP) induced CXCL12 in astrocytes by signaling pathways involving ERK and PI3-K while human umbilical vein endothelial cell (HUVEC) did not produce CXCL12 after treatment with MBP or IL-1β (Calderon TM et al, 2006). Cultured human astrocytes stimulated with IL-1β and TNF produced CXCL12 and other chemokines (Ambrosini E et al, 2005). In EAE animals, increased level of CXCR4 was reported in CNS parenchymal cells (Jiang Y et al, 1998; Glabinski AR et al, 2000). These descriptive observations were extended by McCandless et al (2006) who demonstrated that inhibition of CXCR4 activation during the induction of EAE led to loss of the typical intense perivascular cuffs, which were replaced with widespread white matter infiltrationof mononuclear cells, worsening the clinical severity of the disease. However, the detailed roles of CXCL12 in CNS autoimmunity remain to be comprehensively determined.
8. Summary
Chemokine CXCL12 critically regulates an enormous diversity of processes in both CNS and peripheral immune system, including migration; survival; proliferation; and intercellular communication. Although many functional features appear to be shared in common between the CNS and the immune system, the detailed roles of CXCL12 in the CNS are not simply duplicated from the peripheral immune system. To the contrary, the involvement of CXCL12 and CXCR4 suggests that this ligand-receptor pair is required for CNS homeostasis. Additionally, CXCL12 and CXCR4 have been implicated in the pathogenesis of various neurodegenerative and neuroinflammatory diseases, making them valuable targets for drug development.
Acknowledgments
Work in the Ransohoff lab is supported by National Institutes of Health grants, by grants and fellowships from the National Multiple Sclerosis Society, the Dana Foundation, the Robert Packard Foundation for ALS Research at Johns Hopkins and the Nancy Davis Centers Without Walls. We thank Jeff Loerch for excellent technical assistance in graphics.
Abrreviations
- ADC
AIDS dementia complex
- AIDS
acquired immunodeficiency syndrome
- AKT
v-akt murine thymoma viral oncogene homolog
- AVP
arginine vasopressin
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- CDK-5
cyclin-dependent kinase 5
- CNS
central nervous system
- COX-2
cyclooxygenase 2
- CR cell
Cajal-Retzius cell
- DG
dentate gyrus
- EAE
experimental allergic encephalomyelitis
- EC
endothelial cell
- EGL
external granular layer
- EPC
endothelial progenitor cell
- EPSC
excitatory postsynaptic currents
- ERK
extracellular signal-regulated kinase
- Gαi
inhibitory Gα protein isotypes
- GCL
granule cell layer
- GnRH
gonadotropin-releasing hormone
- GPC
cerebellar granule precursor cells
- GPCR
G protein-coupled receptor
- GTP
guanosine triphosphate
- GRK
G protein-coupled receptor kinase
- HIF-1
hypoxia-inducible factor-1
- HIV-1
human Immunodeficiency virus
- HPC
hematopoietic progenitor cell
- HSPG
heparan sulfate proteoglycans
- HUVEC
human umbilical vein endothelial cell
- IGL
internal granule layer
- IL-1β interleukin 1
beta
- IL-6
interleukin 6
- IP3
inositol 1, 4, 5 trisphosphate
- IZ
intermediate zone
- JAK/STAT
janus kinase-signal transducer and activator of transcription
- JNK
Jun N-terminal kinase
- LTP
long-term potentiation
- MAM
methylazoxymethanol
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MMP-2
metalloproteinase-2
- MOR
μ-opioid receptor
- MS
multiple sclerosis
- MT1-MMP
membrane-type 1 matrix metalloproteinase
- MZ
marginal zone
- NKκB
nuclear factor kappa-B
- NMDA
N-methyl-d-aspartate
- NPC
neural progenitor cell
- PGC
primordial germ cell
- PgE2
prostaglandin E2
- PI3K
phosphoinositide-3 kinase
- PLC
phospholipase C
- PLL
posterior lateral line
- PNS
peripheral nervous systems
- PTX
bordetella pertussis toxin
- PyK2
protein tyrosine kinase 2 beta
- QUIN
quinolinic acid
- SGZ
subgranular zone
- SHH
sonic hedgehog
- SVZ
subventricular zone
- TgSG
trigeminal sensory ganglia
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- VEGF
vascular endothelial growth factor
- VNO
vomeronasal organ
- VZ
ventricular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Aoukaty A, Schall TJ, Maghazachi AA. Differential coupling of CC chemokine receptors to multiple heterotrimeric G proteins in human interleukin-2-activated natural killer cells. Blood. 1996;87:4255–60. [PubMed] [Google Scholar]
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. II. Translocation and regional distribution of the deep neurons. J Comp Neurol. 1985;231:27–41. doi: 10.1002/cne.902310104. [DOI] [PubMed] [Google Scholar]
- Alvarez S, Serramia MJ, Fresno M, Munoz-Fernandez M. Human immunodeficiency virus type 1 envelope glycoprotein 120 induces cyclooxygenase-2 expression in neuroblastoma cells through a nuclear factor-kappaB and activating protein-1 mediated mechanism. J Neurochem. 2005;94:850–61. doi: 10.1111/j.1471-4159.2005.03267.x. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–15. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol. 2003;161:381–91. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1) Proc Natl Acad Sci U S A. 2003;100:5319–23. doi: 10.1073/pnas.0730719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–22. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Ann N Y Acad Sci. 2005;1053:247–57. doi: 10.1196/annals.1344.022. [DOI] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. The chemokine receptor CXCR4 and not the N-methyl-D-aspartate receptor mediates gp120 neurotoxicity in cerebellar granule cells. J Neurosci Res. 2004;75:75–82. doi: 10.1002/jnr.10826. [DOI] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–80. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–60. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Barbero S, Bonavia R, Piccioli P, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001;77:1226–36. doi: 10.1046/j.1471-4159.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Barbieri F, Dorcaratto A, Barbero S, Daga A, Porcile C, Ravetti JL, Zona G, Spaziante R, Corte G, Schettini G, Florio T. Expression of CXC chemokine receptors 1–5 and their ligands in human glioma tissues: role of CXCR4 and SDF1 in glioma cell proliferation and migration. Neurochem Int. 2006;49:423–32. doi: 10.1016/j.neuint.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Costa A, Schettini G. Expression of chemokine receptors in the rat brain. Ann N Y Acad Sci. 1999;22(876):201–9. doi: 10.1111/j.1749-6632.1999.tb07640.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–71. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003;18:1593–606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- Barbero S, Bajetto A, Bonavia R, Porcile C, Piccioli P, Pirani P, Ravetti JL, Zona G, Spaziante R, Florio T, Schettini G. Expression of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1 in human brain tumors and their involvement in glial proliferation in vitro. Ann N Y Acad Sci. 2002;973:60–9. doi: 10.1111/j.1749-6632.2002.tb04607.x. [DOI] [PubMed] [Google Scholar]
- Bardi G, Sengupta R, Khan MZ, Patel JP, Meucci O. Human immunodeficiency virus gp120-induced apoptosis of human neuroblastoma cells in the absence of CXCR4 internalization. J Neurovirol. 2006;12:211–8. doi: 10.1080/13550280600848373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger O, Li G, Han SM, Paredes M, Pleasure SJ. Expression of SDF-1 and CXCR4 during reorganization of the postnatal dentate gyrus. Dev Neurosci. 2007;29:48–58. doi: 10.1159/000096210. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–12. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996a;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996b;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Pirani P, Florio T, Schettini G. Chemokines and their receptors in the CNS: expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicol Lett. 2003;139:181–9. doi: 10.1016/s0378-4274(02)00432-0. [DOI] [PubMed] [Google Scholar]
- Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–93. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- Boutet A, Salim H, Leclerc P, Tardieu M. Cellular expression of functional chemokine receptor CCR5 and CXCR4 in human embryonic neurons. Neurosci Lett. 2001;311:105–8. doi: 10.1016/s0304-3940(01)02149-8. [DOI] [PubMed] [Google Scholar]
- Brustle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RD. In vitro-generated neural precursors participate in mammalian brain development. Proc Natl Acad Sci U S A. 1997;94:14809–14. doi: 10.1073/pnas.94.26.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–9. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Desarmenien MG, Mechighel P, Kitabgi P, Rostene WH, Melik Parsadaniantz S. The chemokine SDF-1/CXCL12 modulates the firing pattern of vasopressin neurons and counteracts induced vasopressin release through CXCR4. Proc Natl Acad Sci U S A. 2006;103:8221–6. doi: 10.1073/pnas.0602620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23:1360–71. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Sabol A, Xu H, Gyda MA, Rasband K, Granato M, Chien CB, Raper JA. Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo. J Neurosci. 2007;27:973–80. doi: 10.1523/JNEUROSCI.4132-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, Wu GX, Pei G. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275:2479–85. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Piccirilli S, Paoletti A, Nistico R, Stringaro A, Malorni W, Finazzi-Agro A, Bagetta G. Evidence that the HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci Lett. 2001;312:67–70. doi: 10.1016/s0304-3940(01)02191-7. [DOI] [PubMed] [Google Scholar]
- Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, Gambini C, Mancardi GL, Uccelli A, Pistoia V. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:11064–9. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croitoru-Lamoury J, Guillemin GJ, Dormont D, Brew BJ. Quinolinic acid up-regulates chemokine production and chemokine receptor expression in astrocytes. Adv Exp Med Biol. 2003a;527:37–45. doi: 10.1007/978-1-4615-0135-0_4. [DOI] [PubMed] [Google Scholar]
- Croitoru-Lamoury J, Guillemin GJ, Boussin FD, Mognetti B, Gigout LI, Cheret A, Vaslin B, Le Grand R, Brew BJ, Dormont D. Expression of chemokines and their receptors in human and simian astrocytes: evidence for a central role of TNF alpha and IFN gamma in CXCR4 and CCR5 modulation. Glia. 2003b;41:354–70. doi: 10.1002/glia.10181. [DOI] [PubMed] [Google Scholar]
- Dambly-Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- David NB, Sapede D, Saint-Etienne L, Thisse C, Thisse B, Dambly-Chaudiere C, Rosa FM, Ghysen A. Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci U S A. 2002;99:16297–302. doi: 10.1073/pnas.252339399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio JA, Martinez A, Fonseca M, Auladell C, Soriano E. Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb Cortex. 1995;5:13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M. Cajal-Retzius cell ontogenesis and death in mouse brain visualized with horseradish peroxidase and electron microscopy. Neuroscience. 1990;36:839–56. doi: 10.1016/0306-4522(90)90027-2. [DOI] [PubMed] [Google Scholar]
- DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–15. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- Ding Z, Jia SH, Marshall JC, Downey GP, Waddell TK. Up-regulation of functional CXCR4 expression on human lymphocytes in sepsis. Crit Care Med. 2006;34:3011–7. doi: 10.1097/01.CCM.0000247719.37793.43. [DOI] [PubMed] [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–59. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res. 2006;83:489–96. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- Dumstrei K, Mennecke R, Raz E. Signaling pathways controlling primordial germ cell migration in zebrafish. J Cell Sci. 2004;117:4787–95. doi: 10.1242/jcs.01362. [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–69. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Banisadr G, Rostene W, Nyakas C, Haour F. Dexamethasone downregulates chemokine receptor CXCR4 and exerts neuroprotection against hypoxia/ischemia-induced brain injury in neonatal rats. Neuroimmunomodulation. 2004;11:404–13. doi: 10.1159/000080151. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–67. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- Florio T, Casagrande S, Diana F, Bajetto A, Porcile C, Zona G, Thellung S, Arena S, Pattarozzi A, Corsaro A, Spaziante R, Robello M, Schettini G. Chemokine stromal cell-derived factor 1alpha induces proliferation and growth hormone release in GH4C1 rat pituitary adenoma cell line through multiple intracellular signals. Mol Pharmacol. 2006;69:539–46. doi: 10.1124/mol.105.015255. [DOI] [PubMed] [Google Scholar]
- Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci. 2002;99:7478–83. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Haas CA, Forster E. Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cereb Cortex. 2003;13:634–40. doi: 10.1093/cercor/13.6.634. [DOI] [PubMed] [Google Scholar]
- Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–92. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Gillard SE, Lu M, Mastracci RM, Miller RJ. Expression of functional chemokine receptors by rat cerebellar neurons. J Neuroimmunol. 2002;124:16–28. doi: 10.1016/s0165-5728(02)00005-x. [DOI] [PubMed] [Google Scholar]
- Gilmour D, Knaut H, Maischein HM, Nusslein-Volhard C. Towing of sensory axons by their migrating target cells in vivo. Nat Neurosci. 2004;7:491–2. doi: 10.1038/nn1235. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, O’Bryant S, Selmaj K, Ransohoff RM. CXC chemokine receptors expression during chronic relapsing experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2000;917:135–44. doi: 10.1111/j.1749-6632.2000.tb05377.x. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL. Viral load in HIV-associated dementia. Ann Neurol. 1998;44:150–1. doi: 10.1002/ana.410440131. [DOI] [PubMed] [Google Scholar]
- Gong X, He X, Qi L, Zuo H, Xie Z. Stromal cell derived factor-1 acutely promotes neural progenitor cell proliferation in vitro by a mechanism involving the ERK1/2 and PI-3K signal pathways. Cell Biol Int. 2006;30:466–71. doi: 10.1016/j.cellbi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Croitoru–Lamoury J, Dormont D, Armati PJ, Brew BJ. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia. 2003;41:371–81. doi: 10.1002/glia.10175. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Lumsden A. Motor neuron pathfinding following rhombomere reversals in the chick embryo hindbrain. Development. 1992;114:663–73. doi: 10.1242/dev.114.3.663. [DOI] [PubMed] [Google Scholar]
- Guyon A, Rovere C, Cervantes A, Allaeys I, Nahon JL. Stromal cell-derived factor-1alpha directly modulates voltage-dependent currents of the action potential in mammalian neuronal cells. J Neurochem. 2005;93:963–73. doi: 10.1111/j.1471-4159.2005.03083.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelsi D, Rovere C, Rostene W, Parsadaniantz SM, Nahon JL. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J Neurochem. 2006;96:1540–50. doi: 10.1111/j.1471-4159.2006.03659.x. [DOI] [PubMed] [Google Scholar]
- Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–80. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001a;108:425–35. doi: 10.1172/JCI12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang J, He T, Ransohoff RM. TNF-alpha down-regulates CXCR4 expression in primary murine astrocytes. Brain Res. 2001b;888:1–10. doi: 10.1016/s0006-8993(00)02924-3. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Sievers J, Pehlemann FW, Berry M. Destruction of meningeal cells over the medial cerebral hemisphere of newborn hamsters prevents the formation of the infrapyramidal blade of the dentate gyrus. J Comp Neurol. 1992;320:33–61. doi: 10.1002/cne.903200103. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Ziegenhagen MW, Sievers J. Meningeal cells stimulate neuronal migration and the formation of radial glial fascicles from the cerebellar external granular layer. Neurosci Lett. 1998;244:129–32. doi: 10.1016/s0304-3940(98)00154-2. [DOI] [PubMed] [Google Scholar]
- Hausmann B, Sievers J. Cerebellar external granule cells are attached to the basal lamina from the onset of migration up to the end of their proliferative activity. J Comp Neurol. 1985;241:50–62. doi: 10.1002/cne.902410105. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–8. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–40. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazin EE, Soderstrom S, Ebendal T, Larhammar D. Embryonic expression of the mRNA for the rat homologue of the fusin/CXCR-4 HIV-1 co-receptor. J Neuroimmunol. 1997;79:148–54. doi: 10.1016/s0165-5728(97)00117-3. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Altman J. Radiosensitivity of the granule cell line and other cell types of the immature rat cerebellar cortex. Exp Neurol. 1982;77:113–28. doi: 10.1016/0014-4886(82)90148-0. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Salafranca MN, Adhikari S, Xia Y, Feng L, Sonntag MK, deFiebre CM, Pennell NA, Streit WJ, Harrison JK. Chemokine receptor expression in cultured glia and rat experimental allergic encephalomyelitis. J Neuroimmunol. 1998;86:1–12. doi: 10.1016/s0165-5728(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett. 2004;355:236–40. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasawa Y, McKenzie LM, Hill DP, Bono H, Yanagisawa M RIKEN GER Group, GSL Members. G protein-coupled receptor genes in the FANTOM2 database. Genome Res. 2003;13:1466–77. doi: 10.1101/gr.1087603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MZ, Brandimarti R, Musser BJ, Resue DM, Fatatis A, Meucci O. The chemokine receptor CXCR4 regulates cell-cycle proteins in neurons. J Neurovirol. 2003;9:300–14. doi: 10.1080/13550280390201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MZ, Shimizu S, Patel JP, Nelson A, Le MT, Mullen-Przeworski A, Brandimarti R, Fatatis A, Meucci O. Regulation of neuronal P53 activity by CXCR 4. Mol Cell Neurosci. 2005;30:58–66. doi: 10.1016/j.mcn.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Rubin JB. Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol. 2004;25:306–14. doi: 10.1016/j.it.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–70. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H, Blader P, Strahle U, Schier AF. Assembly of trigeminal sensory ganglia by chemokine signaling. Neuron. 2005;47:653–66. doi: 10.1016/j.neuron.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Komuro H, Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol Life Sci. 2003;60:1084–98. doi: 10.1007/s00018-003-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22:109–18. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–11. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Kabel H, Hugon J, Spencer P. Damage and repair of nerve cell DNA in toxic stress. Drug Metab Rev. 1999;31:589–618. doi: 10.1081/dmr-100101937. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Wu Y, Jiang H, Wu D. Selective G protein coupling by C-C chemokine receptors. J Biol Chem. 1996;271:3975–8. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res. 2005;65:9891–8. doi: 10.1158/0008-5472.CAN-05-1293. [DOI] [PubMed] [Google Scholar]
- Kury P, Koller H, Hamacher M, Cornely C, Hasse B, Muller HW. Cyclic AMP and tumor necrosis factor-alpha regulate CXCR4 gene expression in Schwann cells. Mol Cell Neurosci. 2003;24:1–9. doi: 10.1016/s1044-7431(03)00132-5. [DOI] [PubMed] [Google Scholar]
- Lapham CK, Ouyang J, Chandrasekhar B, Nguyen NY, Dimitrov DS, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–5. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Domenech J, Le Bousse-Kerdiles MC. Stromal cell-derived factor-1 (SDF-1)\CXCR4 couple plays multiple roles on haematopoietic progenitors at the border between the old cytokine and new chemokine worlds: survival, cell cycling and trafficking. Eur Cytokine Netw. 2004;15:177–88. [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, O’Connor M, Hoxie JA, Gonzalez-Scaran F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–42. [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–38. [PubMed] [Google Scholar]
- Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron. 2005;47:667–79. doi: 10.1016/j.neuron.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Limatola C, Giovannelli A, Maggi L, Ragozzino D, Castellani L, Ciotti MT, Vacca F, Mercanti D, Santoni A, Eusebi F. SDF-1alpha-mediated modulation of synaptic transmission in rat cerebellum. Eur J Neurosci. 2000;12:2497–504. doi: 10.1046/j.1460-9568.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Shirabe K, Kuwada JY. Chemokine signaling regulates sensory cell migration in zebrafish. Dev Biol. 2004;269:123–36. doi: 10.1016/j.ydbio.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Li Q, Shirabe K, Thisse C, Thisse B, Okamoto H, Masai I, Kuwada JY. Chemokine signaling guides axons within the retina in zebrafish. J Neurosci. 2005;25:1711–7. doi: 10.1523/JNEUROSCI.4393-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–5. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–83. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Three-dimensional structural organization of layer I of the human cerebral cortex: a Golgi study. J Comp Neurol. 1990;299:89–105. doi: 10.1002/cne.902990107. [DOI] [PubMed] [Google Scholar]
- McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–64. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–8. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK. Sensory neuron growth cones comigrate with posterior lateral line primordial cells in zebrafish. J Comp Neurol. 1985;238:218–24. doi: 10.1002/cne.902380208. [DOI] [PubMed] [Google Scholar]
- Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, Groshen S, Wilson SB, Seeger RC. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–21. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–56. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- Miller JT, Bartley JH, Wimborne HJ, Walker AL, Hess DC, Hill WD, Carroll JE. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–41. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nanki T, Hayashida K, El-Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, Lipsky PE. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165:6590–8. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- Odemis V, Lamp E, Pezeshki G, Moepps B, Schilling K, Gierschik P, Littman DR, Engele J. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30:494–505. doi: 10.1016/j.mcn.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Odemis V, Moepps B, Gierschik P, Engele J. Interleukin-6 and cAMP induce stromal cell-derived factor-1 chemotaxis in astroglia by up-regulating CXCR4 cell surface expression. Implications for brain inflammation. J Biol Chem. 2002;277:39801–8. doi: 10.1074/jbc.M200472200. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–35. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani Y, Minami M, Kawaguchi N, Nishiyori A, Yamamoto J, Takami S, Satoh M. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neurosci Lett. 1998;249:163–6. doi: 10.1016/s0304-3940(98)00425-x. [DOI] [PubMed] [Google Scholar]
- Paredes MF, Li G, Berger O, Baraban SC, Pleasure SJ. Stromal-derived factor-1 (CXCL12) regulates laminar position of Cajal-Retzius cells in normal and dysplastic brains. J Neurosci. 2006;26:9404–12. doi: 10.1523/JNEUROSCI.2575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashenkov M, Soderstrom M, Link H. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J Neuroimmunol. 2003;135:154–60. doi: 10.1016/s0165-5728(02)00441-1. [DOI] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–29. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co-receptor expression in patients with AIDS. J Neuropathol Exp Neurol. 2001;60:377–85. doi: 10.1093/jnen/60.4.377. [DOI] [PubMed] [Google Scholar]
- Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME, Suratt BT. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–57. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000;20:6095–105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Sidtis JJ. Evaluation of the AIDS dementia complex in clinical trials. J Acquir Immune Defic Syndr. 1990;3(Suppl 2):S51–60. [PubMed] [Google Scholar]
- Ragozzino D, Renzi M, Giovannelli A, Eusebi F. Stimulation of chemokine CXC receptor 4 induces synaptic depression of evoked parallel fibers inputs onto Purkinje neurons in mouse cerebellum. J Neuroimmunol. 2002;127:30–6. doi: 10.1016/s0165-5728(02)00093-0. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Reiss K, Mentlein R, Sievers J, Hartmann D. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience. 2002;115:295–305. doi: 10.1016/s0306-4522(02)00307-x. [DOI] [PubMed] [Google Scholar]
- Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6:102–11. [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–34. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA. SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62:617–26. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Gorgun G, Kleyner Y, Garcia A, Kramer M, Melanson SM, Mathys JM, Yiannoutsos C, Skolnik PR, Navia BA. Tumor necrosis factor alpha leads to increased cell surface expression of CXCR4 in SK-N-MC cells. J Neurovirol. 2005;11:247–55. doi: 10.1080/13550280590952763. [DOI] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Salmaggi A, Gelati M, Pollo B, Frigerio S, Eoli M, Silvani A, Broggi G, Ciusani E, Croci D, Boiardi A, De Rossi M. CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. J Neurooncol. 2004;67:305–17. doi: 10.1023/b:neon.0000024241.05346.24. [DOI] [PubMed] [Google Scholar]
- Salmaggi A, Gelati M, Pollo B, Marras C, Silvani A, Balestrini MR, Eoli M, Fariselli L, Broggi G, Boiardi A. CXCL12 expression is predictive of a shorter time to tumor progression in low-grade glioma: a single-institution study in 50 patients. J Neurooncol. 2005;74:287–93. doi: 10.1007/s11060-004-7327-y. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Everall IP, Johnson RW, Masliah E. Fibroblast growth factor modulates HIV coreceptor CXCR4 expression by neural cells. HNRC Group. J Neurosci Res. 2000;59:671–9. doi: 10.1002/(SICI)1097-4547(20000301)59:5<671::AID-JNR10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–6. [PubMed] [Google Scholar]
- Sapede D, Rossel M, Dambly-Chaudiere C, Ghysen A. Role of SDF1 chemokine in the development of lateral line efferent and facial motor neurons. Proc Natl Acad Sci U S A. 2005;102:1714–8. doi: 10.1073/pnas.0406382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–8. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller U, Koch A, Hartmann W, Garre ML, Goodyer CG, Cama A, Sorensen N, Wiestler OD, Pietsch T. Subtype-specific expression and genetic alterations of the chemokinereceptor gene CXCR4 in medulloblastomas. Int J Cancer. 2005;117:82–9. doi: 10.1002/ijc.21116. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Henion TR, Nugent JD, Caplan B, Tobet S. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J Neurosci. 2006;26:6834–40. doi: 10.1523/JNEUROSCI.1728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Keener C, Boynton AL, Warrick J, Murphy GP. CXCR-4, a chemokine receptor, is overexpressed in and required for proliferation of glioblastoma tumor cells. J Surg Oncol. 1998a;69:99–104. doi: 10.1002/(sici)1096-9098(199810)69:2<99::aid-jso10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Ricks S, Boynton AL, Warrick J, Murphy GP. Molecular characterization of CXCR-4: a potential brain tumor-associated gene. J Surg Oncol. 1998b;69:239–48. doi: 10.1002/(sici)1096-9098(199812)69:4<239::aid-jso9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW, Gude S, Berry M. A time course study of the alterations in the development of the hamster cerebellar cortex after destruction of the overlying meningeal cells with 6-hydroxydopamine on the day of birth. J Neurocytol. 1994;23:117–34. doi: 10.1007/BF01183866. [DOI] [PubMed] [Google Scholar]
- Soldevila G, Licona I, Salgado A, Ramirez M, Chavez R, Garcia-Zepeda E. Impaired chemokine-induced migration during T-cell development in the absence of Jak 3. Immunology. 2004;112:191–200. doi: 10.1111/j.1365-2567.2004.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano SF, Serrano A, Hernanz-Falcon P, Martin de Ana A, Monterrubio M, Martinez C, Rodriguez-Frade JM, Mellado M. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol. 2003;33:1328–33. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]
- Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–78. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–30. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Ma L, Pei G. β-Arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–19. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Super H, Del Rio JA, Martinez A, Perez-Sust P, Soriano E. Disruption of neuronal migration and radial glia in the developing cerebral cortex following ablation of Cajal-Retzius cells. Cereb Cortex. 2000;10:602–13. doi: 10.1093/cercor/10.6.602. [DOI] [PubMed] [Google Scholar]
- Super H, Martinez A, Del Rio JA, Soriano E. Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. J Neurosci. 1998;18:4616–26. doi: 10.1523/JNEUROSCI.18-12-04616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanborg RH. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin Immunol Immunopathol. 1995;77:4–13. doi: 10.1016/0090-1229(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99:10276–81. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai G, Frank B, Mohle R, Weller M, Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12. Brain. 2006;29:2426–35. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, Takamatsu M, Hasegawa H, Suzuki-Migishima R, Yokoyama M, Nakanishi S, Tanabe Y. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–95. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Heesen M, Yoshizawa I, Berman MA, Luo Y, Bleul CC, Springer TA, Okuda K, Gerard N, Dorf ME. Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J Immunol. 1997;159:905–11. [PubMed] [Google Scholar]
- Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–3. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Jones F, Kubota ES, Pocock JM. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci. 2005;25:2952–64. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tham TN, Lazarini F, Franceschini IA, Lachapelle F, Amara A, Dubois-Dalcq M. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. Eur J Neurosci. 2001;13:845–56. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–8. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–33. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Ren D, Miller RJ. The HIV-1 coat protein gp120 regulates CXCR4-mediated signaling in neural progenitor cells. J Neuroimmunol. 2005;160:68–76. doi: 10.1016/j.jneuroim.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Vallat AV, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith TW, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–78. [PMC free article] [PubMed] [Google Scholar]
- Van der Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005;19:2187–98. doi: 10.1101/gad.346205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, Jhamandas JH, Overall CM, Power C. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:19182–7. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Derer P. Cajal-Retzius neurons in human cerebral cortex at midgestation show immunoreactivity for neurofilament and calcium-binding proteins. J Comp Neurol. 1995;359:144–53. doi: 10.1002/cne.903590110. [DOI] [PubMed] [Google Scholar]
- Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez-A C, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–710. [PubMed] [Google Scholar]
- Vroon A, Heijnen CJ, Raatgever R, Touw IP, Ploemacher RE, Premont RT, Kavelaars A. GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo. J Leukoc Biol. 2004;75:698–704. doi: 10.1189/jlb.0703320. [DOI] [PubMed] [Google Scholar]
- Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, Franitza S, Weiss I, Avniel S, Boaz P, Hanna J, Zamir G, Eid A, Mandelboim O, Spengler U, Galun E, Peled A. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004;34:1164–74. doi: 10.1002/eji.200324441. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Immunosuppressive treatment in multiple sclerosis. J Neurol Sci. 2004;15,223(1):1–11. doi: 10.1016/j.jns.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Wingate RJ, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, Dean M, Honjo T, Tashiro K, Yabe D, Buchbinder S, Vittinghoff E, Goedert JJ, O’Brien TR, Jacobson LP, Detels R, Donfield S, Willoughby A, Gomperts E, Vlahov D, Phair J, O’Brien SJ. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC) Science. 1998;279:389–93. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- Woerner BM, Warrington NM, Kung AL, Perry A, Rubin JB. Widespread CXCR4 activation in astrocytomas revealed by phospho-CXCR4-specific antibodies. Cancer Res. 2005;65:11392–9. doi: 10.1158/0008-5472.CAN-05-0847. [DOI] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Zh, Zhang Xc, Rao Y. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–52. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–45. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–32. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–71. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–20. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]