Abstract
A vaccine for the prevention of herpes zoster outbreaks in adults over the age of 60 years has recently been approved. A 76-year-old white female with a history of recurrent left axillary breast cancer undergoing chemotherapy was given a Zostavax® injection by her primary care physician. Eight days later, the patient developed a rash. Given the recent administration of live, attenuated varicella zoster virus (VZV), a diagnosis of disseminated cutaneous herpes zoster was made. The patient was treated successfully with a course of famciclovir for 10 days and cephalexin for 7 days for a secondary bacterial infection. A review of the medical literature disclosed no reports of Zostavax® given to adult cancer patients immunocompromised by systemic chemotherapy. Therefore, we believe this report is the first to describe the consequences of Zostavax® administration to such a host. Clinicians should take care to review contraindications and precautions prior to administering the Zostavax® vaccine.
KEY WORDS: varicella zoster vaccination, adverse reactions, immunocompromised patient
A vaccine for the prevention of herpes zoster (“shingles”) outbreaks has recently been approved by the Food and Drug Administration for use in the United States. It is indicated for adults over age 60 years with a history of primary varicella disease (chickenpox). Zostavax® contains 19,400 plaque forming units of the Oka/Merck strain of live, attenuated varicella zoster virus (VZV), and is administered in a single dose via subcutaneous injection.1 The Advisory Committee of Immunization Practices (ACIP) recommends administration of the vaccine to all individuals without contraindications over the age of 60 years.2 However, the safety and efficacy of Zostavax® have not been evaluated in immunocompromised individuals, (Merck and Company, personal communication, May 17, 2007), and the vaccine is contraindicated in patients with primary or acquired immunodeficiency states, or those receiving immunosuppressive drugs, as shown in Table 1. In this paper, we report a case of disseminated cutaneous herpes zoster following Zostavax® administration to a patient with recurrent breast cancer receiving chemotherapy.
Table 1.
Contraindications, Warnings, and Precautions for Zostavax®1
| Contraindications | Warnings and Precautions |
|---|---|
| History of anaphylactic/anaphylactoid reaction to gelatin, neomycin, or any other component of the vaccine | ZOSTAVAX® is not indicated for the prevention of primary varicella infection (Chickenpox) |
| History of primary or acquired immunodeficiency states | Transmission of vaccine virus may occur rarely between vaccinees and susceptible contacts |
| On immunosuppressive therapy | Defer vaccination in patients with active untreated tuberculosis |
| ZOSTAVAX® is not indicated in women of child-bearing age and should not be administered to pregnant females |
REPORT OF A CASE
A 76-year-old white female with a history of recurrent left axillary breast cancer undergoing chemotherapy with weekly paclitaxel and trastuzumab presented to her primary care physician for a routine follow-up visit. Additional medical problems included type 2 diabetes mellitus, hypertension, stage III chronic kidney disease, and known coronary artery disease. For “health-maintenance” purposes, the patient was given a Zostavax® injection at the time of the visit.
Eight days later, the patient telephoned the treating oncologist’s office to report the development of a rash. The patient described raised, mildly painful, non-pruritic nodules covering her lower abdomen, with intermittent serosanguineous to purulent-appearing discharge. She denied fevers or chills, headache, or cough. She then presented for further evaluation; physical examination findings are shown in Figure 1. Laboratory studies showed a normal white blood cell count at 6.3 × 109/L (reference range (RR) 3.4–10.6 × 109/L); the absolute neutrophil count was normal at 4.31 × 109/L (RR 1.4–6.6 × 109/L); the absolute lymphocyte count was low at 0.97 × 109/L (RR 1.0–3.4 × 109/L).
Figure 1.
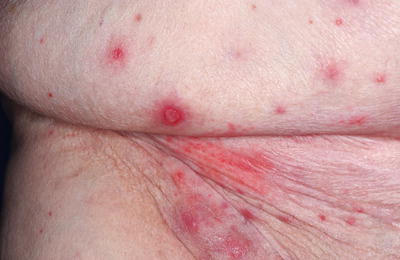
A vesicular eruption on the lower abdomen of a 76-year-old female given live, attenuated varicella zoster virus vaccine.
Given the recent administration of live, attenuated VZV, a diagnosis of disseminated cutaneous herpes zoster was made. Because of their highly characteristic appearance and the timing of disease in relation to the vaccination, it was not deemed necessary to obtain verification with viral culture of the lesions. The patient was commenced on famciclovir at a reduced dose of 500 mg orally once per day (due to the history of impaired renal function) for 10 days. Subsequently, secondary bacterial infection developed, which was treated successfully with a 7-day course of cephalexin. The patient then made a full recovery with no sequelae of herpes zoster.
DISCUSSION
A review of the medical literature disclosed no reports of Zostavax® given to adult cancer patients immunocompromised by systemic chemotherapy. Therefore, we believe this report is the first to describe the consequences of Zostavax® administration to such a host. In this case, disseminated cutaneous herpes zoster resulted, which was successfully managed through treatment with antiviral agents and oral antibiotics.
The Shingles Prevention Study, a double-blind, placebo-controlled trial of 38,546 patients age 60 or older with a history of primary varicella zoster disease, evaluated the safety and efficacy of Zostavax®. Immunocompromised patients, those taking corticosteroids on a regular basis, and patients with a history of herpes zoster were excluded from the study. In the 42-day post-vaccination period of the study, 53 total patients developed a non-injection site zoster-like rash. Of these, 17 patients had been treated with Zostavax®, 36 patients had been treated with placebo. Forty-one specimens were obtained for polymerase chain reaction (PCR) testing; 5 Zostavax® treated patients were found to have wild-type VZV in the lesions; no lesions were found to have the Oka/Merck strain.3 These data suggest that a very small percentage of patients will develop wild-type herpes zoster in the period after administration of the vaccine, perhaps reflecting background incidence in the population. However, the likelihood that an immunocompromised patient will develop herpes zoster specifically related to administration of Zostavax® cannot be determined from the clinical trial experience, and has heretofore been only a theoretical risk. Only one prior report has described the effects of varicella vaccine in an immunocompromised patient; a 36-year-old heart transplant recipient was given the vaccine and, like the patient described here, developed disseminated cutaneous herpes zoster, with polymerase chain reaction (PCR) studies of swab cultures confirming vaccine strain of VZV.4
In June 2007, the Centers for Disease Control and Prevention Advisory Committee of Immunization Practices issued an update on the safety of Zostavax®.5 Through June 1, 2007, 590 reports had been submitted to the Vaccine Adverse Events Reporting System. Forty-four of the reports were serious (requiring hospitalization or resulting in a disabling illness), including 2 deaths. Reports of injection site reactions and rash were most common; there were 145 reports of herpes zoster outbreaks. There were 2 reports of Zostavax® given to immunosuppressed individuals, without further information on the clinical sequelae.
In this case, specimens for VZV PCR testing to identify the viral strain were not obtained from the patient’s lesions. Hence, we are unable to know with certainty that her disease was due to the live, attenuated strain of VZV given in the Zostavax® vaccine, or merely due to reactivation of wild-type VZV. However, the development of clinical herpes zoster within days of administration of the vaccine makes it highly likely in our view that the live, attenuated virus was the cause of our patient’s illness. It should be emphasized that obtaining specimens from suspicious lesions for VZV culture or PCR testing or both can help to confirm the diagnosis of a herpes zoster eruption if the appearance or distribution of a rash is atypical. In addition, clinicians who encounter patients with herpes zoster in the aftermath of Zostavax® vaccination should strongly consider obtaining such testing to assist in further clarifying the safety of the vaccine in general medical practice.
With the recent recommendation by ACIP, clinicians around the country are beginning to vaccinate adults over the age of 60 years for herpes zoster. This patient was fortunate to have developed only cutaneous, not systemic, manifestations of herpes zoster. However, disseminated herpes zoster infections may develop if immunocompromised patients are given Zostavax®. Clinicians should take care to review contraindications and precautions prior to administering the Zostavax® vaccine.
Acknowledgments
The authors wish to thank Jorge Rakela, MD and Keith J. Cannon, MD, Department of Internal Medicine, Mayo Clinic Arizona. The authors received no funding in support of the work described herein.
Conflicts of Interest None disclosed.
References
- 1.Merck and Company. Zostavax® subcutaneous injection product information. 2006. Retrieved November 20, 2007 from: www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi.pdf.
- 2.MMWR Quick Guide. Recommended adult immunization schedule—United States, October 2007–September 2008. MMWR 2007;56:Q1-Q4. Retrieved November 20, 2007 from http://www.cdc.gov/mmwr/pdf/wk/mm5641-Immunization.pdf.
- 3.Oxman M, Levin M, Johnson G, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. [DOI] [PubMed]
- 4.Kraft J, Shaw J. Varicella infection caused by Oka strain vaccine in a heart transplant recipient. Arch Dermatol. 2006;142:943–5. [DOI] [PubMed]
- 5.Centers for Disease Control and Prevention. Update on safety of herpes zoster vaccine. Retrieved November 20, 2007 from: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun07/12-zoster-chavez.pdf.


