Abstract
Background
The inverse correlation between the complexity of a drug regimen and medication adherence is well established. Fixed-dose combination (FDC) therapies are hypothesized to enhance compliance by decreasing the number of required pills.
Objective
The objective of the study is to compare adherence of a FDC [Glucovance®, a FDC of metformin and glyburide] to a 2-pill regimen.
Design
Longitudinal data from a large claims database were used to assess adherence from January 1, 2000, to December 31, 2001. Propensity scoring methods were used to mitigate concerns related to non-random assignment of patients to treatments.
Subjects
The subjects of the study were individuals prescribed metformin or sulfonylurea or both before July 2000, who were prescribed both metformin and sulfonylurea concurrently (either separately or FDC) after August 2000.
Measurements
Adherence was measured by medication possession ratio; the proportion of days on which a patient had medication available.
Results
The FDC enhanced adherence rates by approximately 13% when compared to a 2-pill regimen.
Conclusions
Compared to 2-pill therapy, a FDC resulted in important increases in patient adherence. Economic analyses are warranted to determine whether the clinical benefits attributable to the adherence gains are worth the incremental cost of a FDC.
Key words: adherence, fixed-dose combination drugs, prescription medications
INTRODUCTION
Adherence to prescription drug therapy is a critical component to the management of many common medical conditions. Poor adherence may result in adverse health outcomes, and in some instances, increase aggregate medical care expenditures.1,2 There is an abundant literature demonstrating that patient adherence is inversely associated with the complexity of the prescribed regimen,3 suggesting that simplification can improve adherence. Fixed-dose combination (FDC) drugs—2 or more drugs produced in a single tablet—have been developed to treat one disease with complementary actions (e.g., diabetes mellitus, asthma) or treat multiple clinical conditions (e.g., hypertension and hyperlipidemia). Advocates of FDCs theorize better compliance than with a multi-pill regimen.4,5
Development and marketing of FDCs are becoming increasingly popular, partly due to the fact that for many chronic clinical conditions, evidence-based recommendations require multiple agents to be used simultaneously in complex regimens. However, published studies evaluating the adherence effects of combination agents report mixed results.6,7 The implications of these studies are limited in that the findings were based on multivariable regression analysis. Because drug assignment in these observational studies was not randomized, the treated and nontreated groups may differ, and these differences can lead to biased estimates of treatment effect. Traditional covariance analysis adjustments are inadequate to eliminate this bias.
In this study, we use propensity score methods to assess the adherence effect of Glucovance®, a FDC of metformin and glyburide used to treat hyperglycemia in diabetes mellitus, compared to a multi-pill regimen. The propensity score, defined as the conditional probability of being treated given observed covariates, can be used to balance the two groups, and thus removes bias due to observed covariates and unobserved characteristics to the extent they are correlated with the observed covariates.8
METHODS AND DATA
Study Population
Data from the Medstat MarketScan database (11 million covered lives from 45 large employers), which contains detailed information on medical conditions, insurance coverage, and payments for inpatient, outpatient, and prescription drug services, were used for this study. Continuously enrolled adults over the age of 18 who were prescribed any oral anti-hyperglycemic agents over a 24-month period from January 1, 2000, to December 31, 2001 were eligible for the analysis.
As the FDC evaluated in this study became commercially available in August 2000, the study sample was determined using a 2-step process. First, individuals prescribed metformin or sulfonylurea (the antidiabetic drug category that includes glyburide) or both before July 2000, were identified. Then, those prescribed both metformin and sulfonylurea concurrently (either separately or the FDC) after August 2000 were included in the final sample.
Measuring Adherence
The date of the first claim for metformin and sulfonylurea (either separately or in the FDC) between September 1, 2000 and December 31, 2001 was defined as that subject's index date. Each patient was then followed for 180 days after the index claim. Drug adherence was measured by medication possession ratio (MPR), the proportion of days on which a patient had medication available. To calculate the MPR, each day in the 180-day follow-up period was evaluated as ‘covered’ or ‘not covered’ by a prescription fill or refill. If all days were ‘covered’ by a prescription, then adherence was 100%. This MPR algorithm is similar to the adherence measure described by Bryson and colleagues.9 The days on which a patient prescribed 2 drugs had only one available was included in the analysis; the MPR was reduced by 50% for these partial adherence days.
Propensity Score Method
To compare adherence rates between patients prescribed a single-pill FDC and those taking the same two drugs but with 2 separate prescriptions, one must recognize that in practice, patients were not randomly assigned into the 2 treatment groups. Therefore, differences in observed covariates in the 2 groups may exist, and these differences could lead to biased estimates of treatment effects. The propensity score method has been commonly used to reduce such biases in observational studies.10
A propensity score is the probability of being assigned to the treatment, given a set of observed covariates. Individuals are matched or grouped into strata based on their score. Once the propensity scores and covariates within each stratum are balanced between treatment groups, the treatment assignment within each stratum can be functionally regarded as random.8
The propensity score method is a 2-stage approach. In the first step, the probability of using FDC is estimated by logistic regression model, adjusting the covariates and their interaction terms to balance the propensity score and covariate distributions within each stratum. In the second stage, stratification matching is used to estimate the average treatment effect.11 The average treatment effect is the weighted average of the adherence differences between FDC users and nonuser across the strata.
Covariates
Six predictors for the switch to FDC were chosen based on adherence literature: demographics (age, gender), geographic region (east, north central, west and south), employment status (hourly worker, union worker, retiree, and dependent), health insurance characteristics (average drug co-payment, type of plan including fee-for service, HMO, PPO, and POS), health service utilization during the study period, and comorbidities. The utilization covariate includes inpatient and outpatient use, number of medications, the percentage of brand name medications, the days supplied per medication refill, and the number of refills during the follow-up period. A binary variable was included to distinguish whether the patient took one or both of the medications (metformin and/or sulfonylurea) at baseline. Adherence rates in the baseline period before FDC was calculated and included as a predictor of treatment assignment. Statistical analyses were performed with STATA 9 (College Station, Texas).
Sensitivity Analysis
To assess whether duration of treatment impacted the findings, the follow-up period was changed to 90 and 120 days. In addition, an alternative method, a fixed-effect model that controls for time invariant unobserved factors, was used.
RESULTS
Patient Characteristics
A total of 9,170 diabetic patients were prescribed either the FDC or two-pill regimen in the follow-up period. Twenty-five percent were prescribed the FDC; the remaining 75% were prescribed concurrent 2-pill therapy.
Table 1 demonstrates the patient characteristics for FDC users and 2-pill users. Compared to 2-pill users, FDC users tended to be younger, mainly lived in the south, and were less likely to be hourly workers or retirees. FDC users, on average, had higher out-of-pocket costs for their prescriptions and tended to have a less restricted heath plan.
Table 1.
Comparison of Covariates for FDC Users and Non-FDC Users
| Variables | FDC Users | Non-FDC Users | Unadjusted P Value |
|---|---|---|---|
| Demographics | |||
| Age, year; mean (SD) | 52.4 (0.13) | 54.4 (0.07) | <0.001 |
| 18–44; no. (%) | 284 (12.5%) | 586 (8.5%) | <0.001 |
| 45–54; no. (%) | 878 (38.6%) | 2,524 (36.6%) | 0.115 |
| 55–64; no. (%) | 1,106 (48.6%) | 3,620 (52.5%) | <0.001 |
| 65+; no. (%) | 7 (0.3%) | 97 (1.4%) | <0.001 |
| Male; no. (%) | 1,263 (55.5%) | 3,868 (56.1%) | 0.345 |
| Region | |||
| East; no. (%) | 291 (12.8%) | 1,607 (23.3%) | <0.001 |
| North Central; no. (%) | 594 (26.1%) | 1,793 (26.0%) | 0.918 |
| South; no. (%) | 1,342 (59.0%) | 3,151 (45.7%) | <0.001 |
| West; no. (%) | 46 (2.0%) | 352 (5.1%) | <0.001 |
| Employment | |||
| Hourly; no. (%) | 562 (24.7%) | 1,855 (26.9%) | 0.048 |
| Union; no. (%) | 455 (20.0%) | 1,310 (19.0%) | 0.289 |
| Retiree; no. (%) | 637 (28.0%) | 2,213 (32.1%) | <0.001 |
| Dependent; no. (%) | 3 (0.2%) | 19 (0.3%) | 0.279 |
| Health insurance | |||
| Drug co-payment (dollars); mean (SD) | 13.11 (0.18) | 9.60 (0.07) | <0.001 |
| FFS*; no. (%) | 585 (25.7%) | 1,462 (21.2%) | <0.001 |
| HMO*; no. (%) | 61 (2.7%) | 476 (6.9%) | <0.001 |
| PPO*; no. (%) | 632 (27.8%) | 2,034 (29.5%) | 0.134 |
| POS*; no. (%) | 996 (43.8%) | 2,923 (42.4%) | 0.737 |
| Health services utilization | |||
| Two-drug users before FDC†; no. (%) | 746 (32.8%) | 4,730 (68.6%) | <0.001 |
| Adherence rate before FDC; mean (SD) | 0.833 (0.004) | 0.853 (0.002) | <0.001 |
| No. of medications; mean (SD) | 6.2 (0.10) | 7.1 (0.06) | <0.001 |
| Brand-name medications; no. (%) | 2,157 (94.8%) | 5,523 (80.1%) | <0.001 |
| Average days supply per fill; mean (SD) | 41.8 (0.50) | 50.4 (0.32) | <0.001 |
| No. of refills; mean (SD) | 4.4 (0.04) | 3.6 (0.02) | <0.001 |
| Outpatient encounters; mean (SD)‡ | 19.6 (0.32) | 19.6 (0.21) | 0.926 |
| Hospitalized at least once‡; no. (%) | 141 (6.2%) | 414 (6.0%) | 0.698 |
| Comorbidities | |||
| Hypertension; no. (%) | 1,210 (53.2%) | 3,565 (51.7%) | 0.233 |
| Heart Failure; no. (%) | 123 (5.4%) | 372 (5.4%) | 0.980 |
| Depression; no. (%) | 419 (18.4%) | 1,351 (19.6%) | 0.249 |
| Propensity score mean (SD) | 0.505 (0.006) | 0.157 (0.002) | <0.001 |
| Number of observations | 2,275 | 6,895 | |
*FFS is Fee-For-Service, HMO is Health Management Organization, PPO is Preferred Provider Organization, POS is Point Of Service.
†Two-drug users refers to those who used both metformin and sulfonylurea before FDC.
‡The time frame for inpatient and outpatient encounter is 08/2000–12/2001.
FDC users and 2-pill users did not differ significantly in terms of comorbidities and had similar inpatient and outpatient utilization. FDC users were prescribed fewer medications, tended to use more brand-name drugs, and had shorter days supplied per prescription, but more refills. Compared to 2-pill users, FDC users were less likely to have been prescribed the 2-component medications before the FDC was available. Importantly, patients eventually prescribed the FDC had lower adherence rates (measured as 90-day MPR) during the baseline period.
Propensity score adjustment reduced the magnitude of the covariate imbalance between the groups. When subjects were stratified into 11 strata based on their propensity scores, no statistically significant differences existed between groups within each stratum.
Estimated Treatment Effect
Figure 1 demonstrates the MPR by stratum during the baseline period (Fig. 1a) and after the FDC was available (Fig. 1b). At baseline, the adherence rates in the 2 groups were nearly identical within each stratum. During the follow-up period, FDC users had a significantly higher MPR than 2-pill users, except for stratum 1. The estimated average treatment effect by stratification matching was 0.128, (SE 0.006). In other words, the MPR of FDC users was approximately 13% higher than that of individuals prescribed two pills.
Figure 1.
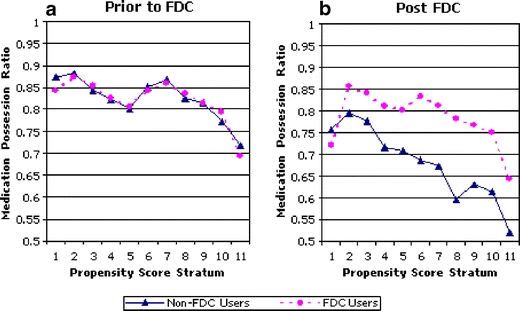
Medication possession ratio by propensity score stratum, before and after the availability of the fixed dose combination product. Legend: The risk stratum denotes the likelihood a patient will switch to the FDC. The line marked by circles illustrates the MPR for patients who switched to the FDC. The line marked by triangles illustrates the MPR for patients who did not switch to the FDC (FDC Fixed dose combination, MPR: medical possession ratio). a MPR by stratum before the FDC was available. b MPR by stratum after the FDC was available.
Changing the follow-up period does not affect the treatment effect significantly. The FDC improved the 120-day MPR by 0.124 (SE 0.007), and 90-day MPR by 0.122 (SE 0.007), similar to the 180-day result. The fixed-effect model revealed that the effect of FDC on MPR was about 0.1, which approximated the propensity score results.
DISCUSSION
Our results demonstrate that after adjusting observed confounders by propensity score method, a FDC increased adherence rates by 12.8% when compared to patients prescribed a 2-pill regimen.
The study has important limitations. First, the propensity score method includes only observed covariates. It cannot solve the nonrandom assignment bias if the switch to the FDC is correlated with unobserved factors that may affect adherence. Second, due to data availability, only short-term effects were measured. Because the initial prescription of the FDC product was likely associated with a clinician visit, the importance of good adherence may have emphasized at that visit which could have resulted in improved adherence unrelated to the switch to the FDC. Therefore, for this and other reasons, the long-term durability of the adherence advantages of FDC drugs is uncertain. Third, the generalizability of these results is limited. The study population included well-insured employees of large companies. Future studies may need to focus on individuals with less generous prescription drug coverage. Also, no information was available on length or severity of disease.
As more than one medication becomes the norm to achieve recommended clinical endpoints for many chronic diseases, the effect of multiple medications on patient adherence becomes especially important. Our findings suggest that compared to 2-pill therapy, a fixed dose combination can yield important improvements in patient adherence. In the case where the acquisition costs of a FDC product exceeds the cost of the individual agents, cost-effectiveness analyses are warranted to determine whether the clinical advantages attributable to the enhanced adherence are worth the incremental expenditures.
Acknowledgment
The authors received neither internal nor external funding for the work represented in this manuscript.
Conflict of Interest Statement None disclosed.
References
- 1.McCombs JS, Nichol MB, Newman CM, Sclar DA. The costs of interrupting antihypertensive drug therapy in a medicaid population. Med Care. 1994;32(3):214–6. [DOI] [PubMed]
- 2.Cantrell C, Eaddy MT, Shah MB, Regan TS, Sokol MC. Methods for evaluating patient adherence to antidepressant therapy: a real-world comparison of adherence and economic outcomes. Med Care. 2006;44(4):300–3. [DOI] [PubMed]
- 3.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26(5):331–42. [DOI] [PubMed]
- 4.Wertheimer AI, Morrison A. Combination drugs: innovation in pharmacotherapy. P&T. 2002;27(1):44–9.
- 5.Leichter SB, Thomas S. Combination medications in diabetes care: an opportunity that merits more attention. Clin Diabetes. 2003;21(4):175–8. [DOI]
- 6.Dezii CM. A retrospective study of persistence with single-pill combination therapy vs. concurrent two-pill therapy in patients with hypertension. Manag Care. 2000;9(9):2–6. [PubMed]
- 7.Melikian C, White TJ, Vanderplas A, Dezii CM, Chang E. Adherence to oral antidiabetic therapy in a managed care organization: a comparison of monotherapy, combination therapy, and fixed-dose combination therapy. Clin Ther. 2002;24(3):460–7. [DOI] [PubMed]
- 8.Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [DOI]
- 9.Bryson CL, Au DH, Young B, McDonell MB, Finh SD. A refill adherence algorithm for multiple short intervals to estimate refill compliance (ReComp). Med Care. 2007;45(6):497–504. [DOI] [PubMed]
- 10.Perkins SM, Tu W, Underhill MG, et al. The use of propensity scores in pharmacoepidemiologic research. Pharmacoepidemiol Drug Saf. 2000;9:93–101. [DOI] [PubMed]
- 11.Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev econ stat. 2002;84(1):151–61. [DOI]


