Abstract
Background
With positive results from diabetes prevention studies, there is interest in convenient ways to incorporate screening for glucose intolerance into routine care and to limit the need for fasting diagnostic tests.
Objective
The aim of this study is to determine whether random plasma glucose (RPG) could be used to screen for glucose intolerance.
Design
This is a cross-sectional study.
Participants
The participants of this study include a voluntary sample of 990 adults not known to have diabetes.
Measurements
RPG was measured, and each subject had a 75-g oral glucose tolerance test several weeks later. Glucose intolerance targets included diabetes, impaired glucose tolerance (IGT), and impaired fasting glucose110 (IFG110; fasting glucose, 110–125 mg/dl, and 2 h glucose < 140 mg/dl). Screening performance was measured by area under receiver operating characteristic curves (AROC).
Results
Mean age was 48 years, and body mass index (BMI) was 30.4 kg/m2; 66% were women, and 52% were black; 5.1% had previously unrecognized diabetes, and 24.0% had any “high-risk” glucose intolerance (diabetes or IGT or IFG110). The AROC was 0.80 (95% CI 0.74–0.86) for RPG to identify diabetes and 0.72 (0.68–0.75) to identify any glucose intolerance, both highly significant (p < 0.001). Screening performance was generally consistent at different times of the day, regardless of meal status, and across a range of risk factors such as age, BMI, high density lipoprotein cholesterol, triglycerides, and blood pressure.
Conclusions
RPG values should be considered by health care providers to be an opportunistic initial screening test and used to prompt further evaluation of patients at risk of glucose intolerance. Such “serendipitous screening” could help to identify unrecognized diabetes and prediabetes.
KEY WORDS: type 2 diabetes, prediabetes, impaired glucose intolerance, impaired fasting glucose, screening
Type 2 diabetes mellitus affects 21 million Americans, and the numbers are projected to be 48 million by 2050.1 The disease increases the risk of cardiovascular, renal, and ophthalmologic complications, and limb amputations, and patients with diabetes have a 10- to 14-year reduction in their life expectancy.2,3 The annual total cost of diabetes in the USA was estimated at $132 billion in 2002;4 as the incremental costs of care for patients with diabetes begin to rise during an 8-year period before diagnosis, such estimates are likely too low.5
Diabetes in its earliest stages is asymptomatic, and clinical recognition is estimated to occur 8–12 years after onset of dysglycemia.6,7 As a consequence of the delay in diagnosis, individuals with newly diagnosed diabetes often exhibit early evidence of complications.8,9 Moreover, although effective treatment of diabetes can reduce the development of complications10,11, factors such as loss of beta cell mass12 can make it difficult to normalize glucose levels13,14, and many patients do not meet national standards for control. However, treatment may be more effective if initiated early in the natural history15, and there is now unequivocal evidence that diabetes itself can be delayed or prevented if glucose intolerance is detected at the stage of “prediabetes”—IGT or IFG.16,17
Detection of IGT, IFG, and early diabetes is, thus, clearly desirable, and the National Institutes of Health and the American Diabetes Association have accordingly recommended screening to identify both diabetes and prediabetes.18 However, most healthcare systems and most individual practitioners do not screen for early diabetes or prediabetes in any formal way.19 As systematic strategies for screening are still in evolution, it is important to take full advantage of the information in tests for glycemia that are already in widespread use—such as random plasma glucose (RPG). It is also important to limit the need for tests performed in the fasted state, which patients are reluctant to schedule.20 In a recent study, 70% of primary care patients had some measure of glycemia over a 3-year period, and 95% of the assessments were RPG;19 RPGs are already being performed nearly routinely in adults at risk for diabetes, and these results could be used more by clinicians to identify a need for further more definitive screening. Many clinicians regard RPG as informative only if values are over 200 mg/dl in the presence of typical symptoms of diabetes.21 We asked whether lower levels of RPG could be used as an opportunistic “serendipitous screening” strategy to identify individuals with diabetes or prediabetes.
METHODS
Subjects
Between 1 January 2005 and 31 December 2006, 990 subjects from age 18 to 75 took part in the “Screening for Impaired Glucose Tolerance” (SIGT) study. Invitation to participate was extended to employees of the Grady Health System, Emory HealthCare, and Emory University and Morehouse Schools of Medicine, as well as members of the community. Inclusion criteria were no prior diagnosis of diabetes, not pregnant or nursing, not taking glucocorticoids, and being well enough to be able to have worked during the previous week (without requiring actual employment). During the 2-year period, 2,377 individuals expressed initial interest in the study and were appended to the recruitment database, 2,258 could be contacted, 1,302 were scheduled for visits, and 1,060 completed first visits. Of these, 39 declined second visits, 17 were scheduled for but had not had second visits by 31 December 2006, 997 completed both visits, and 990 had data that were largely complete; 989 subjects were used for some analyses.
Protocol
The study was approved by the Emory Institutional Review Board and was performed in General Clinical Research Centers at Emory University Hospital and Grady Memorial Hospital. Random plasma glucose samples were obtained at the first visit, which did not require a prior overnight fast and was scheduled during the work day. The second visit was scheduled within 3 weeks and included a 75-g oral glucose tolerance test (OGTT) begun before 11 am after an overnight fast.
Height was measured with a stadiometer after shoes were removed. Weight was measured using digital scales with subjects in light clothing. Blood pressure was measured with digital manometers after subjects had been seated quietly for 5 minutes. Waist circumference was measured halfway between the costal margin and the iliac crest by trained research interviewers, and sex-adjusted values were expressed relative to cutoffs for the “metabolic syndrome” as defined by the National Cholesterol Education Program (NCEP)22 criteria. Subjects also reported their demographic information and family history of diabetes.
Measurements
Plasma samples for glucose were obtained using sodium fluoride/oxalate preservative. These and fasting samples for lipids were centrifuged, separated, and frozen within 30 minutes. Chemical analyses were performed in the central clinical laboratory of the Grady Health System using the Beckman–Coulter LX-20 (Brea, CA). High-density lipoprotein (HDL) cholesterol levels were expressed relative to “metabolic syndrome” cutoffs as above.
Analysis
Normal glucose tolerance (NGT) was defined by the American Diabetes Association (ADA) criteria (fasting glucose <100, 2-hour glucose <140 mg/dl); impaired fasting glucose100 (IFG100) and impaired fasting glucose110 (IFG110) by fasting glucose 100–109 and 110–125 mg/dl, respectively, with 2-hour glucose <140 mg/dl; impaired glucose tolerance (IGT) by 2-hour glucose 140–199 with fasting glucose ≤125 mg/dl; and diabetes (DM) by fasting glucose ≥126 or 2-hour glucose ≥200 mg/dl. We focused particularly on diabetes and abnormal glucose tolerance110 (AGT110, diabetes or IGT or IFG110), as such levels confer increased mortality.23,24
Receiver operating characteristic (ROC) curve analysis was used to evaluate the discriminative effectiveness of random plasma glucose. The area under the ROC curve (AROC) is the index of effectiveness, with 1.0 indicating perfect discrimination and 0.5 chance discrimination. Bootstrap methodology was applied to assess the over-optimism of sample-calculated AROCs.25 In this application, 200 bootstrap replicates were drawn (with replacement) to calculate the maximum absolute error in AROCs. Locally weighted least squares regression (LOWESS) was used to assess the relationship between random plasma glucose and postprandial time. To assess the performance of RPG in subgroups with different pretest probability of glucose intolerance, we examined the detection of diabetes/AGT110 in groups with differences in risk factors such as age, body mass index (BMI), family history of diabetes in a first-degree relative, etc. For each subgroup, we determined the likelihood of having RPG exceeding a cutoff, the likelihood of diabetes/dysglycemia, and AROC for detection of diabetes.
Statistical analyses were conducted using S-Plus, version 7 (Insightful, Inc. Seattle, WA) and Stata, version 9 (Stata Corporation, College Station, TX).
RESULTS
The 990 study subjects had an average age of 48 years and BMI of 30.4 kg/m2; 54% were black and 66% were women, and they had an average RPG of 99 mg/dl (Table 1). Those with NGT tended to be younger and less overweight, with an average RPG of 93 mg/dl. Those with abnormal glucose tolerance were older and heavier but had similar distribution of gender and race. However, those with IFG only were less likely to be black or women, and those with IGT only were more likely to be women.
Table 1.
Subject Demographics
| Group | Number of patients | Age (yr) | BMI kg/m2 | Black (%) | Sex % F | RPG mg/dl |
|---|---|---|---|---|---|---|
| All | 990 | 48 | 30.4 | 52 | 66 | 98.7 |
| NGT | 619 | 45 | 29.3 | 54 | 73 | 93.1 |
| IFG100–109 | 133 | 50 | 30.6 | 38 | 45 | 104.4 |
| IFG110–125 | 29 | 52 | 33.3 | 41 | 45 | 109.3 |
| IGT only | 78 | 51 | 31.2 | 56 | 73 | 100.2 |
| IGT + IFG100–109 | 51 | 53 | 33.2 | 61 | 53 | 108.1 |
| IGT + IFG 110–125 | 30 | 54 | 34.3 | 57 | 57 | 114.5 |
| Diabetes | 50 | 54 | 34.5 | 60 | 54 | 125.6 |
| Any IGT or any IFG 110 or diabetes (any glucose intolerance110, AGT110) | 238 | 53 | 33.0 | 56 | 59 | 110.1 |
Figure 1 shows greater variability and higher levels of RPG in the first 3–4 hours after meals in subjects with either NGT or AGT110 (diabetes or IGT or IFG110, see Methods). Only 4.7% of NGT subjects had values as high as 125 mg/dl at any postprandial time, whereas 18.5% of AGT110 subjects had values that high (p < 0.001). However, there was substantial overlap in RPG between subjects with NGT and those with glucose intolerance.
Figure 1.
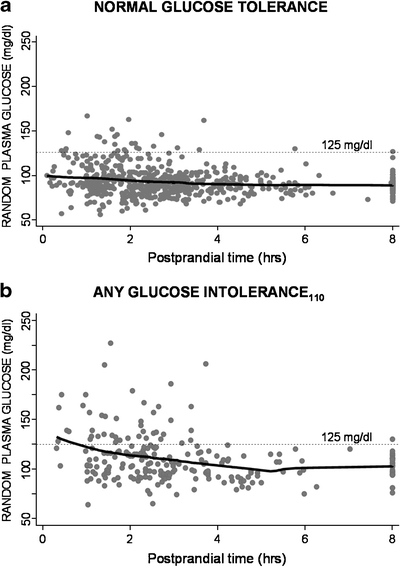
Distribution of RPG values according to time after meals (hours). a Subjects with NGT (fasting plasma glucose < 100 and 2-hour glucose < 140 mg/dl during OGTT); b Subjects with abnormal glucose tolerance110 (diabetes or IGT or IFG110).
Despite such overlap, RPG was a strong indicator of unrecognized glucose intolerance. We used ROC analysis to evaluate the ability of RPG to identify prediabetes110 (any IGT or IFG110), AGT110, and diabetes (Fig. 2). The area under the ROC curve (AROC) for RPG was 0.66 (95% CI 0.62–0.70) for prediabetes110, 0.72 (95% CI 0.68–0.75) for AGT110, and 0.80 (95% CI 0.75–0.86) for diabetes—all significantly greater than chance (all p < 0.001); RPG identified IFG110 (AROC 0.75) better than IGT (0.67). The maximum absolute errors (bias) for the AROCs were small, 0.01–0.03.
Figure 2.
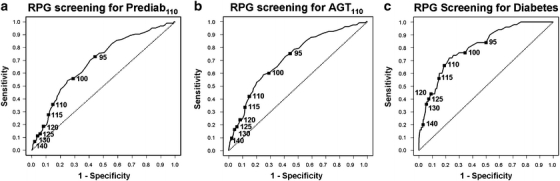
ROC curves showing performance of RPG in identifying prediabetes110 (any IGT or IFG110), AGT110 (diabetes or prediabetes110), and diabetes.
Table 2 shows AROCs at different times after meals and times of day; sensitivities correspond to 70% specificity cutoffs at those times (approximately 112 mg/dl for diabetes and 100 for AGT110; see below). The AROC for RPG to identify unrecognized diabetes was unaffected by postprandial time or time of day. In contrast, the AROC for AGT110 was also unaffected by postprandial time but reduced later in the day (p < 0.0078 for trend; values after 3:30 pm were excluded because of small numbers of subjects in that group).
Table 2.
Impact on RPG AROC of Time after Meals and Time of Day
| Glucose intolerance | Postprandial time (h) | Time of day | Sensitivity | Specificity | AROC | 95% CI | Number of patients |
|---|---|---|---|---|---|---|---|
| AROC for RPG by time after meals | |||||||
| Diabetes | <2 | 75 | 70 | 0.775 | 0.642–0.909 | 308 | |
| 2–4 | 87 | 70 | 0.900 | 0.844–0.957 | 409 | ||
| >4 | 53 | 71 | 0.738 | 0.613–0.864 | 273 | ||
| Any glucose intolerance110 | <2 | 59 | 70 | 0.717 | 0.648–0.786 | 308 | |
| 2–4 | 62 | 71 | 0.752 | 0.697–0.806 | 409 | ||
| >4 | 64 | 69 | 0.714 | 0.642–0.786 | 273 | ||
| AROC for RPG by time of day | |||||||
| Diabetes | 7:30–9:29 am | 87 | 70 | 0.871 | 0.737–1.000 | 203 | |
| 9:30–11:29 am | 76 | 70 | 0.799 | 0.687–0.912 | 272 | ||
| 11:30–1:29 pm | 77 | 70 | 0.806 | 0.706–0.906 | 225 | ||
| 1:30–3:29 pm | 73 | 69 | 0.817 | 0.696–0.938 | 266 | ||
| 3:30+ pm | 0 | 68 | 0.409 | – | 26 | ||
| Any glucose intolerance110 | 7:30–9:29 am | 77 | 69 | 0.802 | 0.737–0.867 | 203 | |
| 9:30–11:29 am | 70 | 69 | 0.764 | 0.697–0.830 | 272 | ||
| 11:30–1:29 pm | 63 | 69 | 0.696 | 0.613–0.779 | 225 | ||
| 1:30–3:29 pm | 45 | 70 | 0.640 | 0.566–0.714 | 266 | ||
| 3:30+ pm | 17 | 76 | 0.456 | 0.178–0.734 | 26 | ||
Shown are AROCs corresponding to different times of day and times after meals and sensitivities and specificities corresponding to ∼70% specificity cutoffs at those times (70% specificity cutoffs are approximately 112 for diabetes and 100 for AGT110—see Table 4).
Table 3 shows the performance of RPG as a screen in subgroups with higher or lower pretest probability of having diabetes or glucose intolerance. The likelihood of unrecognized diabetes or AGT110 was significantly increased by well-accepted risk factors for glucose intolerance. Each factor also contributed to the probability of having RPG >125 mg/dl but did not affect the AROC for RPG to identify unrecognized diabetes. There was no significant difference in AROC whether the risk factor was present or absent and no trend with respect to markers of insulin resistance; AROC was somewhat higher with increased waist circumference but also with normal triglycerides. Thus, the presence of risk factors was associated with a higher prevalence of high glucose levels and would increase the positive predictive value of RPG when used as a screening test but did not modify the ability of the screen to detect diabetes. Results were similar for the impact of risk factors on the RPG AROC to detect AGT110 (not shown).
Table 3.
Impact of Diabetes “Risk Factors” on RPG AROC to Detect Diabetes; Likelihood of RPG > 125 mg/dl, and Likelihood of Diabetes or Any Glucose Intolerance110
| Group | AROC | 95% CI | Number of patients | Likelihood of RPG >125 OR (95% CI) | Likelihood of diabetes | Likelihood of any glucose intolerance110 |
|---|---|---|---|---|---|---|
| All | 0.805 | 0.746–0.864 | 989 | |||
| Black | 0.834 | 0.761–0.906 | 517 | 0.56 (0.36–0.87) | 1.39 (0.78–2.46) | 1.23 (0.92–0.165) |
| White | 0.776 | 0.675–0.876 | 472 | |||
| Female | 0.833 | 0.759–0.907 | 652 | 0.60 (0.39–0.93) | 0.50 (0.33–1.04) | 0.68 (0.50–0.92) |
| Male | 0.751 | 0.646–0.855 | 337 | |||
| Age, <40 | 0.807 | 0.632–0.981 | 234 | 1.00 | 1.00 | 1.00 |
| Age, 40–55 | 0.734 | 0.614–0.855 | 460 | 1.96 (1.02–3.74) | 1.86 (0.71–4.90) | 2.53 (1.59–4.03) |
| Age, >55 | 0.837 | 0.764–0.910 | 295 | 2.33 (1.19–4.57) | 4.60 (1.70–15.50) | 4.66 (2.90–7.50) |
| BMI, <25 | 0.782 | 0.651–0.913 | 221 | 1.00 | 1.00 | 1.00 |
| BMI, 25–35 | 0.782 | 0.670–0.895 | 542 | 1.36 (0.75–2.47) | 2.31 (0.82–6.47) | 3.14 (1.95–5.05) |
| BMI, >35 | 0.809 | 0.732–0.887 | 226 | 1.71 (0.83–3.30) | 6.47 (2.16–26.03) | 4.70 (2.80–7.86) |
| Trig, >150 | 0.698 | 0.533–0.862 | 129 | 1.80 (1.04–3.12) | 1.96 (0.99–3.90) | 2.55 (1.73–3.75) |
| Trig, <150 | 0.821 | 0.756–0.886 | 860 | |||
| HDL low | 0.798 | 0.718–0.877 | 454 | 1.35 (0.88–2.09) | 1.82 (1.03–3.24) | 1.99 (1.48–2.68) |
| HDL normal | 0.812 | 0.722–0.902 | 535 | |||
| Family Hx+ | 0.792 | 0.714–0.870 | 487 | 1.66 (1.07–2.59) | 2.27 (1.25–4.14) | 1.63 (1.22–2.20) |
| Family Hx− | 0.814 | 0.725–0.903 | 502 | |||
| SBP, >130 | 0.804 | 0.703–0.905 | 239 | 1.77 (1.12–2.79) | 2.69 (1.52–4.76) | 1.99 (1.45–2.73) |
| SBP, <130 | 0.797 | 0.721–0.873 | 750 | |||
| Waist+ | 0.805 | 0.742–0.868 | 736 | 1.15 (0.74–1.77) | 3.40 (1.78–6.78) | 3.04 (2.22–4.17) |
| Waist− | 0.672 | 0.431–0.914 | 253 |
To assess the performance of RPG in subgroups with different pretest probability of glucose intolerance, we examined detection of diabetes/AGT110 in groups with differences in risk factors such as age, BMI, family history of diabetes in a first-degree relative, etc. For each subgroup, shown are the likelihood of having RPG > 125 mg/dl, the likelihood of diabetes/dysglycemia, and AROC for detection of diabetes. With regard to the black race, many blacks were female and had a family history of diabetes in a first-degree relative. In our dataset, women were somewhat less likely to fail the cutoff or to have glucose intolerance; in multivariate analyses adjusted for age, BMI, gender, and family history, black race contributed independently to the risk of the different categories of glucose intolerance (all p < 0.03) and also to risk of having RPG > 125 (p = 0.012).
Table 4 shows the effect of different RPG cutoffs on sensitivity, specificity, and positive predictive value (PPV). The lower cutoffs provide greater sensitivity but reduced PPV because specificity is lower—more false positives; sensitivity is also prevalence independent, but PPV responds to the underlying presence of the abnormalities in the population. In the entire dataset, 62.5% had NGT, 5.0% had diabetes, and 24.0% had AGT110. For identification of diabetes, an intermediate cutoff of 125 mg/dl provided 93% specificity, 40% sensitivity, and 22% positive predictive value. The same cutoff provided 94% specificity, 18% sensitivity, and 49% positive predictive value for AGT110. Use of a lower cutoff would increase the sensitivity for detection of glucose intolerance, but a lower percentage of those with a positive test would prove to have AGT110 or diabetes. Conversely, screening with progressively higher RPG cutoffs would provide greater likelihood that diabetes and AGT110 would be found among those who screened positive (higher positive predictive value) at the cost of reduced sensitivity. Even balance between sensitivity and specificity and a higher product of sensitivity × (1-specificity) would be provided by cutoffs of 100–110 mg/dl for diabetes and 95–100 mg/dl for any glucose intolerance110. However, with these lower cutpoints, positive predictive value would be lower, especially for the detection of diabetes, and cost-effectiveness might be reduced as well.26 With the intermediate cutoff, 125 mg/dl, 89 subjects would screen positive, and these would include 40% of those with diabetes and 18% of those with AGT110 in the entire dataset.
Table 4.
Effect of Different RPG Cutoffs on Sensitivity, Specificity, Positive Predictive Value, and RPG AROC
| Glucose intolerance | Cutoff (mg/dl) | Sensitivity (%) | Specificity (%) | PPV (%) | AROC (95% CI) |
|---|---|---|---|---|---|
| Diabetes | 0.805 (0.746–0.864) | ||||
| 95 | 84 | 50 | 8 | ||
| 100 | 76 | 66 | 10 | ||
| 110 | 66 | 81 | 16 | ||
| 115 | 56 | 85 | 17 | ||
| 120 | 44 | 90 | 18 | ||
| 125 | 40 | 93 | 22 | ||
| 130 | 36 | 94 | 25 | ||
| 140 | 20 | 97 | 26 | ||
| Any glucose intolerance110 | 0.717 (0.681–0.754) | ||||
| 95 | 75 | 56 | 35 | ||
| 100 | 60 | 71 | 40 | ||
| 110 | 42 | 85 | 47 | ||
| 115 | 34 | 88 | 48 | ||
| 120 | 24 | 92 | 48 | ||
| 125 | 18 | 94 | 49 | ||
| 130 | 16 | 96 | 55 | ||
| 140 | 10 | 98 | 59 |
Lower cutoffs provide greater sensitivity but reduced positive predictive value (PPV) since specificity is lower – more false-positives.
DISCUSSION
We found that RPG functioned well (AROC 0.80) as a screen for undiagnosed diabetes. The performance of the test was good at different times of the day regardless of meal status, and performance was maintained in the presence or absence of risk factors such as older age, higher BMI, low HDL cholesterol, or high triglycerides or blood pressure. A cutoff of 125 mg/dl had 93% specificity and 40% sensitivity for diabetes. Performance was lower to find abnormal glucose intolerance110 (AGT110, diabetes or IGT, or IFG110), with AROC 0.72. However, AROCs for RPG to detect diabetes and AGT110, respectively, were better than those for detection of coronary artery disease by evaluation of coronary artery calcium and treadmill stress tests, respectively.27 As RPG is included in routine blood chemical analyses, such glucose values could be used to prompt follow-up studies with definitive oral glucose tolerance tests and help to identify early diabetes and prediabetes.
There have been previous considerations of diabetes screening via RPG, but some early workers did not report exclusion of individuals with known diabetes.28 The Australian Diabetes Screening Study found overlap between normal and diabetes similar to that in the present study and concluded that a cutoff of 99 mg/dl should be used for screening but did not report sensitivity, specificity, or AROC.29,30 Johnson et al.31 reported that a RPG of 130 mg/dl would provide 87% specificity and 63% sensitivity, and Zhang et al.32 reported that a capillary glucose of 120 mg/dl would provide 89% specificity and 68% sensitivity, consistent with the present findings, but did not perform ROC analysis. Rolka et al.33 found that a capillary glucose of 120 mg/dl would provide 88–89% specificity and 70–80% sensitivity to detect diabetes, and 90–91% specificity and 41–47% sensitivity for AGT110 but did not report AROC.
Engelgau et al.34 reported that the performance of random capillary glucose to identify diabetes was better shortly after meals, a finding not reproduced in the present study. However, as the difference between RPG in diabetes vs. NGT tended to be larger in the first few hours after meals (Fig. 1), it is possible that the test will be more useful shortly after meals. Better performance has been reported with multivariate equations that include postprandial time as well as random capillary glucose, age, sex, and BMI,35 but such information is not usually captured when chemistry analyses are performed. Questionnaires based on symptoms and risk factors have also been developed as inexpensive strategies to identify patients who need further evaluation. Although some questionnaires have been reported to provide AROC for diabetes ~0.80,36–38 comparable to that of screening with RPG, most provide 0.70–0.75,39–41 and some that initially appeared promising had lower performance when applied to a separate population in the same city.42
In the USA, it has been estimated that diabetes goes on for 10 years before clinical recognition,43 and longer delay leads to higher glucose levels at the time of diagnosis.44 Delay likely explains why many patients have diabetes complications at the time of diagnosis,7,45,46 with increased cardiovascular risk,47 and exhibit an increase in cardiovascular events, health care system resource use, and costs before diagnosis.48–50 Consistent with such observations, earlier diagnosis appears to lead to improved outcomes.51 Patients may also be easier to manage if they are recognized earlier in their natural history and have had less loss of β-cell mass and function;12,52,53 although national guidelines for management are not achieved13,14 and providers often fail to intensify therapy when indicated,54–56 conventional therapies seem to be more effective if initiated earlier in the natural history,15 and detection at the earliest stage of glucose intolerance—“prediabetes”—is essential to permit initiation of efforts aimed to prevent or delay development of diabetes.16,17 Such considerations argue for screening, especially in patients at high risk.19,57,58
The use of RPG in routine chemistry profiles as “serendipitous screening” for diabetes may also be cost effective. For example, use of the 125-mg/dl cutoff in the 990-patient dataset would have resulted in 89 positive tests. If all of these were followed by oral glucose tolerance tests, the added direct costs to identify the included 20 individuals with diabetes—40% of those in the entire dataset—would have been $1,601 at current Medicare rates ($17.99 per OGTT, CPT 82951).59 The projected added cost of $80 per true positive compares favorably with values reported in analyses of the cost effectiveness of diabetes screening.31,32
Although screening should be cost effective,26,31,32,60 especially if targeted to patients with risk factors such as hypertension61, there is little systematic screening at present. While screening programs are being developed, our findings indicate that (a) the plasma glucose values included in routine chemistry profiles could be used as part of a screening strategy, and (b) if individuals with values > 125 mg/dl had follow-up glucose tolerance tests (ordered infrequently in the USA but more often in other countries62), the approach might identify many patients with previously unrecognized diabetes and prediabetes.
Limitations of the study include the lack of racial/ethnic groups other than whites and blacks, and the possibility of selection bias because participation was entirely voluntary; the prevalence of undiagnosed diabetes in our subjects was higher than that in NHANES III [5.1% vs. 2.7%,63]. Some subjects may have participated because they were aware of risk factors such as obesity or a family history of diabetes, and our study constituted free screening. However, while any potential selection bias might limit generalizability of positive predictive values, it should have less impact on the use of RPG as a screening test; we found that the presence of abnormal risk factors increased the likelihood of both diabetes and having RPG >125 mg/dl, but the risk factors generally had no effect on the performance of the test. The strengths of the study include the use of a substantial number of generally healthy subjects, the inclusion of subjects both above and below the age recommended for screening18, inclusion of a large proportion of women and blacks, and evaluation across a range of diabetes risk factors.
At present, over 5 million Americans have undiagnosed diabetes and many more have “prediabetes,”64,65 but most health care systems in the USA do not have programs to screen systematically for these problems. While various screening protocols are being evaluated, more use could be made of the RPG measurements that are included in the many blood chemistry profiles that Americans have in the process of routine health evaluations; one large laboratory service provider reported ~2.5 million glucose tests per year in metropolitan Atlanta alone (J. Ritchie, personal communication). Our findings demonstrate that RPG could be considered by practitioners to be part of an opportunistic “serendipitous screening” algorithm—to prompt further evaluation with a glucose tolerance test. Whether lower cutoffs are chosen to optimize sensitivity or higher cutoffs to favor specificity, use of an RPG-based strategy could help to identify patients who now have undiagnosed diabetes and prediabetes.
Acknowledgment
This work was supported in part by DK07298, DK062668, RR017643, DK066204, and RR00039. We thank Jane Caudle, Jack Kaufman, Eileen Osinski, Elizabeth Barrera, Jade Irving, and Aisha Bobcombe for their assistance. Portions of this work were presented at the Scientific Sessions of the American Diabetes Association in Washington, DC (2006).
Conflict of interest Statement None disclosed.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- NHANES-III
National Health and Nutrition Examination Survey III
- AGT110
any glucose intolerance110 (dysglycemia)
- T2DM
type 2 diabetes mellitus
- ROC
receiver-operating-characteristic
- AROC
area under the ROC curve
- IGT
impaired glucose tolerance
- IFG
impaired fasting glucose
- IFG110 or IFG110–125
IFG with fasting plasma glucose 110–125 mg/dl
- Dysglycemia
type 2 diabetes or IGT or IFG110
- WHO
World Health Organization
- NCEP
National Cholesterol Education Program
- HDL
high density lipoprotein
References
- 1.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–6. [DOI] [PubMed]
- 2.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21:C11–4. [DOI] [PubMed]
- 3.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–90. [DOI] [PubMed]
- 4.American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917–32. [DOI] [PubMed]
- 5.Brown JB, Nichols GA, Glauber HS, Bakst AW. Type 2 diabetes: incremental medical care costs during the first 8 years after diagnosis. Diabetes Care. 1999;22:1116–24. [DOI] [PubMed]
- 6.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–9. [DOI] [PubMed]
- 7.Thompson TJ, Engelgau MM, Hegazy M, Ali MA, Sous ES, Badran A, et al. The onset of NIDDM and its relationship to clinical diagnosis in Egyptian adults. Diabet Med. 1996;13:337–40. [DOI] [PubMed]
- 8.Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 2000;116:297–303. [DOI] [PubMed]
- 9.Goldschmid MG, Domin WS, Ziemer DC, Gallina DL, Phillips LS. Diabetes in Urban African-Americans. II. High prevalence of microalbuminuria and nephropathy in African-Americans with diabetes. Diabetes Care. 1995;18:955–61. [DOI] [PubMed]
- 10.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–32. [DOI] [PMC free article] [PubMed]
- 11.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [DOI] [PubMed]
- 12.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. [DOI] [PubMed]
- 13.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. [DOI] [PubMed]
- 14.Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006;144:465–74. [DOI] [PubMed]
- 15.Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl SJ, Brown JB. Treatment escalation and rise in HbA1c following successful initial metformin therapy. Diabetes Care. 2006;29:504–9. [DOI] [PubMed]
- 16.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed]
- 17.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–105. [DOI] [PubMed]
- 18.American Diabetes Association, NIH/NIDDK. The prevention or delay of type 2 diabetes. Diab Care. 2002;25:742–9. [DOI] [PubMed]
- 19.Ealovega MW, Tabaei BP, Brandle M, Burke R, Herman WH. Opportunistic screening for diabetes in routine clinical practice. Diabetes Care. 2004;27:9–12. [DOI] [PubMed]
- 20.Leiter LA, Barr A, Belanger A, Lubin S, Ross SA, Tildesley HD, et al. Diabetes screening in Canada (DIASCAN) study: prevalence of undiagnosed diabetes and glucose intolerance in family physician offices. Diabetes Care. 2001;24:1038–43. [DOI] [PubMed]
- 21.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl 1):S37–42. [DOI] [PubMed]
- 22.American Heart Association. http://www.americanheart.org/presenter.jhtml?identifier = 4756. Accessed 10 January 2008.
- 23.Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care. 2005;28:2626–32. [DOI] [PubMed]
- 24.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Bittner V, Cauley JA, et al. Impaired fasting glucose and cardiovascular outcomes in postmenopausal women with coronary artery disease. Ann Intern Med. 2005;142:813–20. [DOI] [PubMed]
- 25.Harrell FE Jr. Regression modeling strategies with applications to linear models, logistic regression and survival analysis. New York: Springer; 2001.
- 26.CDC Diabetes Cost-Effectiveness Study Group. The cost-effectiveness of screening for type 2 diabetes. JAMA. 1998;280:1757–63. [DOI] [PubMed]
- 27.Shavelle DM, Budoff MJ, Lamont DH, Shavelle RM, Kennedy JM, Brundage BH. Exercise testing and electron beam computed tomography in the evaluation of coronary artery disease. J Am Coll Cardiol. 2000;36:32–8. [DOI] [PubMed]
- 28.West KM, Kalbfleisch JM. Sensitivity and specificity of five screening tests for diabetes in ten countries. Diabetes. 1971;20:289–96. [DOI] [PubMed]
- 29.Hilton DJ, Welborn TA, O’Rourke PK, Reid CM. Forget to fast. Diabetes Care. 2002;25:2122. [DOI] [PubMed]
- 30.Welborn TA, Reid CM, Marriott G. Australian Diabetes Screening Study: impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Metabolism. 1997;46:35–9. [DOI] [PubMed]
- 31.Johnson SL, Tabaei BP, Herman WH. The efficacy and cost of alternative strategies for systematic screening for type 2 diabetes in the U.S. population 45–74 years of age. Diabetes Care. 2005;28:307–11. [DOI] [PubMed]
- 32.Zhang P, Engelgau MM, Valdez R, Cadwell B, Benjamin SM, Narayan KMV. Efficient cutoff points for three screening tests for detecting undiagnosed diabetes and pre-diabetes: an economic analysis. Diabetes Care. 2005;28:1321–5. [DOI] [PubMed]
- 33.Rolka DB, Narayan KMV, Thompson TJ, Goldman D, Lindenmayer J, Alich K, et al. Performance of recommended screening tests for undiagnosed diabetes and dysglycemia. Diabetes Care. 2001;24:1899–903. [DOI] [PubMed]
- 34.Engelgau MM, Thompson TJ, Smith PJ, Herman WH, Aubert RE, Gunter EW, et al. Screening for diabetes mellitus in adults: the utility of random capillary blood glucose measurements. Diabetes Care. 1995;18:463–6. [DOI] [PubMed]
- 35.Tabaei BP, Herman WH. A multivariate logistic regression equation to screen for diabetes: development and validation. Diabetes Care. 2002;25:1999–2003. [DOI] [PubMed]
- 36.Lindstrom J, Tuomilehto J. The diabetes risk score. Diabetes Care. 2003;26:725–31. [DOI] [PubMed]
- 37.Glümer C, Carstensen B, Sandbæk A, Lauritzen T, Jørgensen T, Borch-Johnsen K. A Danish diabetes risk score for targeted screening: the Inter99 study. Diabetes Care. 2004;27:727–33. [DOI] [PubMed]
- 38.Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Mohlig M, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care. 2007;30:510–15. [DOI] [PubMed]
- 39.Spijkerman AMW, Yuyun MF, Griffin SJ, Dekker JM, Nijpels G, Wareham NJ. The performance of a risk score as a screening test for undiagnosed hyperglycemia in ethnic minority groups: data from the 1999 health survey for England. Diabetes Care. 2004;27:116–22. [DOI] [PubMed]
- 40.Franciosi M, De BG, Rossi MC, Sacco M, Belfiglio M, Pellegrini F, et al. Use of the diabetes risk score for opportunistic screening of undiagnosed diabetes and impaired glucose tolerance: the IGLOO (Impaired Glucose Tolerance and Long-Term Outcomes Observational) study. Diabetes Care. 2005;28:1187–94. [DOI] [PubMed]
- 41.Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, et al. A risk score for predicting incident diabetes in the Thai population. Diabetes Care. 2006;29:1872–77. [DOI] [PubMed]
- 42.Ruige JB, De Neeling JND, Kostense PJ, Bouter LM, Heine RJ. Performance of an NIDDM screening questionnaire based on symptoms and risk factors. Diabetes Care. 1997;20:491–6. [DOI] [PubMed]
- 43.Screening for Type 2 Diabetes Mellitus. AHRQ Systematic Evidence Review number 19. http://www.ahrq.gov/downloads/pub/prevent/pdfser/diabser.pdf. Accessed 28 February 2007.
- 44.Samuels TA, Cohen D, Brancati FL, Coresh J, Kao WHL. Delayed diagnosis of incident type 2 diabetes mellitus in the ARIC study. Am J Manag Care. 2006;12:717–24. [PubMed]
- 45.Thaler LM, El-Kebbi IM, Ziemer DC, Gallina DL, Dunbar VG, Phillips LS. High prevalence of albuminuria among African-Americans with short duration of diabetes. Diabetes Care. 1998;21:1576–7. [DOI] [PubMed]
- 46.Henricsson M, Nyström L, Blohmé G, Östman J, Kullberg C, Svemsson M, et al. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS). Diabetes Care. 2003;26:349–54. [DOI] [PubMed]
- 47.Smith NL, Barzilay JI, Kronmal R, Lumley T, Enquobahrie D, Psaty BM. New-onset diabetes and risk of all-cause and cardiovascular mortality: the Cardiovascular Health Study. Diabetes Care. 2006;29:2012–17. [DOI] [PubMed]
- 48.Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–34. [DOI] [PubMed]
- 49.Gulliford MC, Charlton J, Latinovic R. Increased utilization of primary care 5 years before diagnosis of type 2 diabetes: a matched cohort study. Diabetes Care. 2005;28:47–52. [DOI] [PubMed]
- 50.Nichols GA, Glauber HS, Brown JB. Type 2 diabetes: incremental medical care costs during the 8 years preceding diagnosis. Diabetes Care. 2000;23:1654–9. [DOI] [PubMed]
- 51.Colagiuri S, Cull CA, Holman RR. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: U.K. Prospective Diabetes Study 61. Diabetes Care. 2002;25:1410–7. [DOI] [PubMed]
- 52.Festa A, Williams K, D’Agostino R Jr., Wagenknecht LE, Haffner SM. The natural course of {beta}-cell function in nondiabetic and diabetic individuals: The Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114–20. [DOI] [PubMed]
- 53.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic {beta}-cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006;55:1074–9. [DOI] [PubMed]
- 54.Phillips LS, Branch WT Jr., Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, et al. Clinical inertia. Ann Int Med. 2001;135:825–34. [DOI] [PubMed]
- 55.Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care. 2003;9:213–7. [PubMed]
- 56.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. [DOI] [PubMed]
- 57.Cowie CC, Harris MI, Eberhardt MS. Frequency and determinants of screening for diabetes in the U.S. Diabetes Care. 1994;17:1158–63. [DOI] [PubMed]
- 58.Clark HD, van Walraven C, Code C, Karovitch A, Keely E. Did publication of a clinical practice guideline recommendation to screen for type 2 diabetes in women with gestational diabetes change practice. Diabetes Care. 2003;26:265–8. [DOI] [PubMed]
- 59.CMS Clinical Laboratory Fee Schedule—December 1, 2006. http://www.cms.hhs.gov/ClinicalLabFeeSched/02_clinlab.asp. Accessed 28 February 2007.
- 60.Zhang P, Engelgau MM, Valdez , Benjamin SM, Cadwell B, Narayan KMV. Costs of screening for pre-diabetes among U.S. adults. Diabetes Care. 2003;26:2536–42. [DOI] [PubMed]
- 61.Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med. 2004;140:689–99. [DOI] [PubMed]
- 62.Hilton DJ, O’Rourke PK, Welborn TA, Reid CM. Diabetes detection in Australian general practice: a comparison of diagnostic criteria. Med J Aust. 2002;176:104–7. [DOI] [PubMed]
- 63.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little R, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. A third national nutrition examination study, 1988–1994. Diabetes Care. 1998;21:518–24. [DOI] [PubMed]
- 64.Centers for Disease Control and Prevention (CDC). Prevalence of diabetes and impaired fasting glucose in adults—United States, 1999–2000. MMWR. 2003;52:833–7. [PubMed]
- 65.CDC National Diabetes Fact Sheet; 2002. http://www.cdc.gov/diabetes/pubs/estimates.htm. Accessed 28 February 2007.


