Summary
Background
Disparities in cancer survival may be related to differences in stage. Segregation may be associated with disparities in stage, particularly for cancers for which screening promotes survival.
Objectives
The objective of the study was to examine whether segregation modifies racial/ethnic disparities in stage.
Design
The design of the study was analysis of Surveillance, Epidemiology, and End Results Medicare data for seniors with breast, colorectal, lung, and prostate cancer (n = 410,870).
Measurements and main results
The outcome was early- versus late-stage diagnosis. Area of residence was categorized into 4 groups: low segregation/high income (potentially the most advantaged), high segregation/high income, low segregation/low income, and high segregation/low income (possibly the most disadvantaged). Blacks were less likely than whites to be diagnosed with early-stage breast, colorectal, or prostate cancer, regardless of area. For colorectal cancer, the black/white disparity was largest in low-segregation/low-income areas (black/white odds ratio [OR] of early stage 0.51) and smallest in the most segregated areas (ORs 0.71 and 0.74, P < .005). Differences in disparities in stage by area category were not apparent for breast, prostate, or lung cancer. Whereas there were few Hispanic–white differences in early-stage diagnosis, the Hispanic/white disparity in early-stage diagnosis of breast cancer was largest in low-segregation/low-income areas (Hispanic/white OR of early stage 0.54) and smallest in high-segregation/low-income areas (OR 0.96, P < .05 compared to low-segregation/low-income areas).
Conclusions
Disparities in stages for cancers with an established screening test were smaller in more segregated areas.
KEY WORDS: race, ethnicity, cancer stage, segregation
INTRODUCTION
Cancer is the second leading cause of death in the United States, and lung, colorectal, prostate, and breast cancer together account for 70% of cancer deaths.1 For each of these common cancers, there is evidence of racial/ethnic disparities in mortality,2–4 and several studies have suggested that disparities in cancer survival are related to differences in cancer stage at the time of diagnosis.4–14
Because stage is such an important determinant of cancer survival,5–9,15 there has been a focus on identifying factors associated with early presentation. For both breast and colorectal cancers, cancer screening has been firmly established as promoting diagnosis at an earlier stage and improving survival.16,17 Whereas screening for prostate cancer may be associated with an earlier stage at diagnosis, there is controversy as to whether screening is associated with improved survival.16 To date, there is no conclusive evidence that lung cancer screening is associated with increased survival.16,18,19 In addition to race/ethnicity, disparities in cancer stage at diagnosis are associated with a variety of other factors including differences in health insurance and access to care,13,20 socioeconomic status,13,21,22 literacy,23 and the perception of cancer-related symptoms and beliefs about cancer.13,24
Racial residential segregation refers to the physical separation of members of 1 racial/ethnic group from those of another group. In the United States, blacks are more segregated than any other racial/ethnic group.25,26 Whereas residential segregation adversely affects the social and economic opportunities of the minority residents who live there,27 less is known about the effect of segregation on health,28–31 particularly cancer care. Segregation may be associated with disparities in cancer stage, particularly for breast and colorectal cancer, where screening promotes early diagnosis, by several mechanisms including differential access to screening services, the targeting of public programs toward specific neighborhoods, and the dissemination of information and education about the importance of screening and early detection of cancer.
The purpose of this study is to examine whether segregation is associated with cancer stage for blacks and Hispanics compared with non-Hispanic whites (hereafter called whites) independent of the income level of the area. We hypothesized that disparities in early-stage diagnosis between blacks or Hispanics and whites would be greater in more segregated areas than less segregated areas for cancers with an established screening test (breast and colorectal) because of more limited access to care but would not differ for cancers without an established role for screening (prostate and lung).
MATERIALS AND METHODS
Data
This analysis is based on data from the Surveillance, Epidemiology, and End Results (SEER) Medicare file. The SEER program collects information on all incident cancer cases for persons with cancer residing in SEER program areas (California, Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico and Utah, rural Georgia, and the metropolitan areas of Detroit, Atlanta, and Seattle). SEER data include cancer stage, primary tumor site, and patient demographics and are linked to Medicare claims data by the National Cancer Institute. Data were available for individuals diagnosed with cancer from 1992 to 2002. A restricted access version of these data was obtained so that the characteristics of each individual’s census tract of residence, from the 1990 U.S. Census, could be appended. This study was reviewed and approved by the Institutional Review Board of Partners Health Care.
Study Sample
Adults who were at least 65 years of age and were diagnosed with colorectal, lung, prostate, or breast cancer as a single primary cancer and who were diagnosed with localized, regional, or distant stage were included in our study. We did not include individuals with in situ cancer as the natural history of in situ cancers was uncertain, particularly during the time period examined (1992–2002), and because in situ cancers are less reliably reported to cancer registries. The sample was limited to individuals whose race/ethnicity was reported as white, black, or Hispanic. Because our analysis was focused on the role of residential characteristics, we excluded individuals with a missing census tract identifier.
Variables
The outcome variable was diagnosis of early- versus late-stage cancer. For breast, colorectal, and lung cancers, early stage was defined as the stages when the cancer is still potentially curable. Prostate cancer is categorized as localized/regional or late in the SEER data. For breast, colorectal, and prostate cancers, early stage was defined as local or regional, and late stage was defined as distant. For lung cancer, early stage was defined as localized and late stage as regional or distant.
Measures of residential segregation reflect both the composition and the spatial distribution of a population across a municipal region. To measure residential segregation, we used the isolation index, a measure of the extent to which a member of 1 racial/ethnic group is likely to be in contact with members of this same group (as opposed to members of other groups).25,32 Isolation may reflect the concentration of multiple disadvantages into an area and the dissemination of information about health-related behaviors and services.33
The isolation index was calculated for each racial/ethnic group separately, using 1990 census tracts and counties. For instance, the isolation index for blacks within a county = ∑ (bi/btotal) × (bi/Ti), where i = 1 of N census tracts in the county, bi = the number of blacks in the census tract i, btotal = the total number of blacks in the county, and Ti = the total population of census tract i.34 We divided large counties with populations greater than approximately 500,000, such as Los Angeles, into smaller, municipal areas based on locally defined neighborhoods and calculated the isolation indices for these municipalities based on the census tract populations within these regions (Ethington et al., submitted for publication). We took this approach because of a growing social science literature that suggests that these areas play a meaningful role in the structure of social processes associated with place of residence, including segregation.35 Municipal areas were defined using information from local urban-planning departments and census maps. There are 468 counties in the dataset, 36 of these were separated into 705 smaller areas. The total number of areas was 1,137.
The isolation index ranges from 0 to 1.0, with a higher number indicating greater segregation.32 We classified areas as high/low isolation for blacks and for Hispanics. High segregation was defined as an isolation index equal to 0.27 or higher, which is approximately the 75% percentile for both black and Hispanic areas in the sample.32 In addition, we used the median per capita income for the census tract to characterize the income level of an individual’s place of residence (dichotomized as high or low income using a threshold of 200% of the 1990 federal poverty threshold for 1 person). Because we wanted to examine the influence of segregation independent of poverty, our principal independent variable categorized areas into 4 groups on the basis of segregation and poverty: low-segregation and high-income areas (potentially the most advantaged areas), high-segregation and high-income areas, low-segregation and low-income areas, and high-segregation and low-income areas (potentially the most disadvantaged areas). Other area variables from the SEER file included an urban/rural indicator. Counties in metropolitan areas or counties with an urban population of 20,000 or more were considered to be urban.
Individual-level independent variables included age (categorized as 65–74, 75–84, or ≥85 years), sex (for individuals with lung or colorectal cancer), race/ethnicity (white, black, Hispanic), marital status (married, not married), Charlson comorbidity score (categorized as 0, 1, 2, ≥3),36 whether an individual was of “low income,” based on eligibility for state assistance with Medicare premiums and copayments (“state buy-in coverage”), year of diagnosis, and indicators of whether or not someone was enrolled in a Medicare managed care plan within the 13 months before diagnosis, at any time after diagnosis, or without Medicare coverage within 13 months before diagnosis (these indicators were used to adjust for individuals for whom we may have less complete information about comorbidity).
Analysis
Characteristics of blacks living in more segregated areas were compared to blacks living in less segregated areas using Chi-square tests. Similar comparisons were done for Hispanics living in areas with high versus low Hispanic segregation and for whites living in areas with high versus low black or Hispanic segregation (separately). Multilevel logistic regression models for the individual-level outcome of early-cancer stage controlling for individual characteristics and area characteristics, clustered by county, were performed using the SAS version 9 (SAS Institute, Carey, NC, USA). Because segregation is calculated separately for blacks and Hispanics, we examined separate models for blacks compared to whites (the reference group) and Hispanics compared to whites. Furthermore, because segregation is strongly confounded by income, we created a 4-level area category variable: low-income–low-segregation areas, low-income–high-segregation areas, high-income–low-segregation areas, and high-income–high-segregation areas. Minority–white comparisons were examined in each of these 4 areas by including interaction terms between race and area category in our multilevel regression models. In this way, we can report the odds of early-stage diagnosis in minorities versus whites for each level of income and segregation, as well as compare the minority–white difference in low-income–low-segregation areas to the minority–white difference in each of the other 3 types of areas. Initial models included only race, area category, and the interaction terms. Final models included these same terms plus the individual and area-level factors mentioned above.
RESULTS
There were 412,482 individuals diagnosed with localized, regional, or distant breast, colorectal, lung, or prostate cancer. A total of 1,615 individuals were excluded because they were missing the census tract identifier needed to attach the area-level indicators, resulting in a final sample size of 410,870 people including 86,723 women with breast cancer, 151,142 men with prostate cancer, 91,497 individuals with colorectal cancer, and 81,508 individuals with lung cancer.
The vast majority of blacks (81.7%) lived in areas with high numbers of other blacks (Table 1). Similarly, the vast majority of Hispanics (72.8%) lived in areas with high percentages of other Hispanics. The majority of whites lived in areas with relatively few blacks (81.0%) and Hispanics (77.6%). Compared with blacks living in a predominantly black area, blacks living in less segregated areas were younger, more likely to be married, less likely to have any chronic health conditions, and less likely to live in a low-income census tract. Compared to Hispanics living in a predominantly Hispanic area, Hispanics living in a less segregated area were less likely to have ever been eligible for state buy-in coverage or live in a low-income census tract but more likely to live in an urban area. Whites living in an area with high black segregation were older, less likely to be married, had more comorbidity, were more likely to have been eligible for state buy-in coverage, and were more likely to live in an urban or low-income area than whites who lived in an area where blacks were less segregated. Similar relationships were observed for whites living in areas with high Hispanic segregation compared to those living in areas with lower Hispanic segregation, except that whites living in areas with high Hispanic segregation had less comorbidity. There were no differences for residence in a low-income area.
Table 1.
Characteristics of the Sample
| Low black segregation | High black segregation | Low Hispanic segregation | High Hispanic segregation | |||||
|---|---|---|---|---|---|---|---|---|
| Black* | White† | Black | White | Hispanic‡ | White† | Hispanic | White | |
| Number | 6,739 | 283,121 | 30,129 | 66,366 | 6,656 | 271,302 | 17,859 | 78,185 |
| Age (years) | ||||||||
| 65–74 | 63.1% | 53.1% | 59.1% | 50.4% | 60.3% | 52.9% | 61.4% | 51.6% |
| 75–84 | 30.4% | 37.5% | 33.3% | 38.9% | 32.1% | 37.6% | 31.5% | 38.4% |
| 85+ | 6.6% | 9.4% | 7.7% | 10.8% | 7.7% | 9.6% | 7.1% | 10.0% |
| Married | 47.3% | 58.6% | 43.4% | 53.0% | 58.8% | 58.0% | 59.0% | 56.0% |
| Charlson comorbidity score | ||||||||
| 0 | 75.5% | 75.7% | 68.8% | 72.9% | 79.1% | 74.0% | 78.2% | 79.1% |
| 1 | 14.0% | 15.7% | 17.9% | 16.9% | 13.3% | 16.7% | 13.3% | 13.3% |
| 2 | 5.1% | 4.9% | 6.6% | 5.7% | 4.2% | 5.3% | 4.5% | 4.2% |
| ≥3 | 5.4% | 3.7% | 6.7% | 4.5% | 3.5% | 4.1% | 3.9% | 3.3% |
| Eligible for state buy-in coverage | ||||||||
| Ever | 30.0% | 10.4% | 29.9% | 11.5% | 26.6% | 9.9% | 41.4% | 12.9% |
| Cancer type | ||||||||
| Breast | 15.6% | 21.9% | 15.5% | 21.8% | 19.4% | 21.6% | 17.9% | 23.1% |
| Colorectal | 19.3% | 22.3% | 19.7% | 23.7% | 23.0% | 22.6% | 21.4% | 22.6% |
| Lung | 19.0% | 19.9% | 20.8% | 21.1% | 15.8% | 20.1% | 14.3% | 20.1% |
| Prostate | 46.1% | 35.9% | 44.0% | 33.4% | 41.7% | 35.7% | 46.4% | 34.3% |
| Residence in | ||||||||
| Urban county | 97.1% | 90.7% | 97.3% | 97.0% | 98.0% | 90.2% | 94.2% | 97.9% |
| Low-income census tract | 38.2% | 23.3% | 71.7% | 25.2% | 21.0% | 23.7% | 61.5% | 23.5% |
Data were missing for marital status (black n = 2,166, Hispanic 1,176, white 16,669.) and for county income (black 6, Hispanic 6, white 64)
*P value < .0001 for age, marital status, comorbidity and low income, and P value < .05 for sex, cancer type from Chi-square test comparing percentages for blacks living in areas with low versus high black segregation.
†P value < .0001 for all variables from Chi-square comparing percentages for whites living in areas with low versus high black segregation; P value < .0001 for all variables except low income from Chi-square comparing percentages for whites living in areas with low versus high Hispanic segregation.
‡P value < .0001 for ever eligible for state buy-in coverage, cancer type, urban area, and low income from Chi-square comparing the percentage of Hispanics living in areas with low versus high Hispanic segregation.
Overall, 94.4% of subjects with breast cancer, 80.0% of subjects with colorectal cancer, 93.7% of subjects with prostate cancer, and 18.7% of subjects with lung cancer were diagnosed at an early stage. For all 4 cancers, whites were more likely to be diagnosed at an early stage than blacks, across all area types (Fig. 1). The white/black disparity in early-stage diagnosis for colorectal cancer was greater for blacks who lived in low-income, less-segregated areas. The white/Hispanic disparity in early-stage diagnosis was smaller than the white/black disparity for breast, colorectal, and prostate cancer but not lung cancer (Fig. 2).
Figure 1.
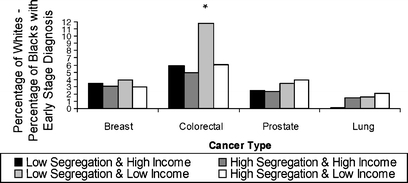
Disparity in early-stage diagnosis by area category for black versus white individuals. Asterisk, P value comparing white–black difference in early-stage diagnosis for the low-segregation and low-income area to: low-segregation and high-income area (P = .03), high-segregation and high-income area (P = .004), and high-segregation and low-income area (P = .001).
Figure 2.
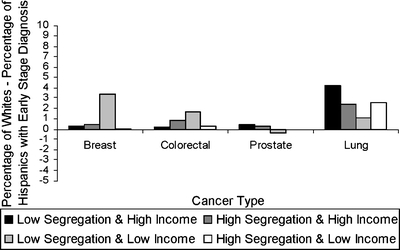
Disparity in early-stage diagnosis by area category for Hispanic versus white individuals.
Table 2 presents the association of the individual-level covariates on early-stage diagnosis. After adjustment for both individual and area-level characteristics, men were less likely than women to be diagnosed at an early stage of colorectal or lung cancer (Table 2). With the exception of colorectal cancer, individuals 85 years and older were less likely to be diagnosed at an early stage compared with individuals between 65 and 74 years of age. Compared with individuals who were never eligible for state buy-in coverage, individuals who had been eligible were more likely to be diagnosed with early-stage colorectal cancer but less likely to be diagnosed with early-stage prostate cancer. Compared to individuals without any comorbidity, individuals with comorbidity were generally more likely to be diagnosed at an early stage.
Table 2.
Association of Individual Characteristics with Early-stage Cancer Diagnosis
| Individual race | Breast | Colorectal | Prostate | Lung | ||||
|---|---|---|---|---|---|---|---|---|
| Black | Hispanic | Black | Hispanic | Black | Hispanic | Black | Hispanic | |
| Odds ratio (relative to whites) | ||||||||
| Age (ref: 65–74 years) | ||||||||
| 75–84 | 0.92* | 0.92* | 1.05* | 1.03 | 0.48* | 0.47* | 1.06 * | 1.07* |
| ≥85 | 0.81* | 0.80* | 1.10* | 1.09* | 0.21* | 0.21* | 0.92* | 0.92* |
| Sex (ref: female) | ||||||||
| Male | 0.90* | 0.90* | 0.83* | 0.82* | ||||
| Ever eligible state buy-in coverage (ref: no) | ||||||||
| Yes | 0.93 | 0.99 | 1.19* | 1.22* | 0.72* | 0.75* | 1.26* | 1.24* |
| Charlson comorbidity score:(ref: none) | ||||||||
| 1 | 1.30* | 1.31* | 1.13* | 1.11* | 1.25* | 1.20* | 1.42* | 1.43* |
| 2 | 1.27* | 1.27* | 1.23* | 1.20* | 0.95 | 0.94 | 1.47* | 1.48* |
| >3 | 1.15 | 1.11 | 1.25* | 1.23* | 0.90 | 0.84* | 1.37* | 1.35* |
Models adjusted for age, race/ethnicity, sex (for individuals with colorectal and lung cancer), marital status, comorbidity, eligibility for state buy-in coverage, cancer type, year of diagnosis, Medicare coverage in the 13 months before diagnosis, HMO coverage after diagnosis, urban/rural indicator, area category (segregation/income), and interaction terms between race and area categories.
*Comparison of blacks or Hispanics to whites significant at P < .05
Table 3 shows the association between area segregation and income category and early-stage diagnosis for blacks compared with whites and for Hispanics compared with whites separately. After adjustment, blacks were less likely than whites to be diagnosed with early-stage breast, colorectal, or prostate cancer, regardless of area characteristics (Table 3). A similar relationship was seen for lung cancer, although it was smaller in magnitude and only significant in highly segregated, low-income areas. For breast and colorectal cancers, the black/white disparity in early-stage diagnosis was the largest in lower-income, less segregated areas and the smallest in areas with more segregation. For colorectal cancer, the black/white disparity was significantly greater in areas with low income and low segregation than in any other type of area. Differences in disparity in early-stage diagnosis were not apparent by area characteristics for prostate or lung cancer. Similarly, the Hispanic/white disparity in early-stage diagnosis was largest in lower-income, less segregated areas compared to other area types for breast and colorectal cancers. Disparity in early-stage diagnosis was less apparent for Hispanics than for blacks.
Table 3.
Area Category and Early-stage Cancer Diagnosis
| Breast | Colorectal | Prostate | Lung | |
|---|---|---|---|---|
| Odds ratio (P value for comparison to whites) | ||||
| Black vs. white individuals | ||||
| Area category | ||||
| Low segregation and low income | 0.57 (.003) | 0.51 (<.001) | 0.62 (<.001) | 0.84 (.16) |
| Low segregation and high income | 0.61 (<.001) | 0.69 (<.001)* | 0.63 (<.001) | 0.96 (.68) |
| High segregation and high income | 0.64 (<.001) | 0.74 (<.001)† | 0.61 (<.001) | 0.80 (.07) |
| High segregation and low income | 0.74 (.001) | 0.71 (<.001)† | 0.65 (<.001) | 0.85 (.005) |
| Hispanic vs. white individuals | ||||
| Area category | ||||
| Low segregation and low income | 0.54 (.01) | 0.87 (.29) | 1.09 (.60) | 0.90 (.55) |
| Low segregation and high income | 0.86 (.29)‡ | 0.93 (.32) | 0.92 (.41) | 0.75 (.004) |
| High segregation and high income | 0.86 (0.25)‡ | 0.90 (0.14) | 0.90 (.20) | 0.81 (.02) |
| High segregation and low income | 0.96 (0.75)* | 0.94 (.34) | 0.98 (.78) | 0.81 (.008) |
Models adjusted for age, race/ethnicity, sex (for individuals with colorectal and lung cancer), marital status, comorbidity, eligibility for state buy-in coverage, cancer type, year of diagnosis, Medicare coverage in the 13 months before diagnosis, HMO coverage after diagnosis, urban/rural indicator, area category (segregation/income), and interaction terms between race and area categories
*Comparison of area category to low segregation and low income significant at P < .05
†Comparison of area category to low segregation and low income significant at P < .005
‡Comparison of area category to low segregation and low income significant at P < .10
CONCLUSIONS
This study confirms disparities in early-stage diagnosis for seniors with breast, colorectal, prostate, and lung cancers, based on a number of individual factors including comorbidity, age, eligibility for state buy-in coverage, and race/ethnicity. Seniors with more comorbidity were generally more likely to be diagnosed early, regardless of cancer type. Individuals age 85 and older were less likely to be diagnosed with early-stage breast, prostate, and lung cancers and more likely to be diagnosed with early-stage colorectal cancer, whereas individuals eligible for state buy-in coverage were more likely to be diagnosed with early-stage colorectal cancer and less likely to be diagnosed with early-stage prostate or lung cancer.
In addition, this paper provides some unexpected findings about the possible role of segregation and poverty on racial/ethnic disparities in cancer stage. As expected, blacks were less likely to be diagnosed with early-stage breast, colorectal, prostate, or lung cancer compared to whites. Whereas we had expected that disparities would be worse in segregated, low-income neighborhoods, this was not the case. For colorectal cancer, the black/white disparity was in fact larger in low-income/less segregated areas than in any other type of area. Whereas there were few Hispanic–white differences in early-stage diagnosis, Hispanics with breast cancer who lived in a low-segregation, low-income area and those with lung cancer who lived in highly segregated areas were less likely to be diagnosed at an early stage. Differences across area type were only significantly worse for women with breast cancer who lived in lower-income, less segregated areas. These unexpected findings may be related to the specific measure of segregation that we used in this analysis, the isolation index. Whereas segregation has been measured in several dimensions,25,26 we selected the isolation measure as it has been hypothesized that this construct may reflect the dissemination of information about health-related behaviors and services and has therefore been used in other health-related studies looking at the potential effects of segregation.29,30,33
We had initially hypothesized that disparities in stage at diagnosis would be greater in more segregated, low-income areas for breast and colorectal cancers, for which access to primary care and cancer screening is critical. This is clearly not the case. Several mechanisms could underlie the smaller black–white disparity in early-stage diagnosis of breast and colorectal cancers in more segregated areas, including higher use of screening by blacks in these communities,37 more education about the importance of seeking care for symptoms that may be related to cancer or personal cancer risk status,38 or perhaps better access to primary care. Blacks who live among higher percentages of other blacks may be more likely to be diagnosed at an earlier stage because these areas have been targeted for early detection outreach. For example, the National Breast and Cervical Cancer Early Detection Program for low-income women may have brought about improved detection in areas where minority and low-income women reside.39 This program may have had a “spillover” effect on women who were covered by Medicare. A phone survey of mammography facilities found that although predominantly black and Hispanic ZIP codes were less likely to have a mammography facility than predominantly white ZIP codes, the mammography facilities in minority areas were much more committed to outreach and service than facilities in white areas.40 Blacks who live in more segregated areas may have more opportunities for discussion about the importance of screening and the evaluation of an abnormal screening test or symptom with people whom they trust. For example, black women may have different cultural beliefs and attitudes about the causes and consequences of breast cancer.13 Minority physicians may be better able to address these beliefs, and minority physicians are more likely to practice in minority communities.41
Our work is consistent with other findings suggesting that blacks and Hispanics who live in more segregated areas may have better access to health care and better health outcomes.42,43 Blacks who lived in areas of New York City where they were the majority racial/ethnic group (i.e., ≥75%) experienced lower mortality compared with individuals of the same race/ethnicity living in ZIP codes where they were not in the majority. Our prior work suggests that blacks and Hispanics may perceive fewer barriers to care when they live in an area with a higher prevalence of people of similar race/ethnicity.44 Geronimus45 postulates that a set of autonomous institutions and social networks in segregated areas may mitigate the effects of discrimination. The literature also suggests that mental health may be enhanced when members of a minority group live in ethnic enclaves.46 In contrast, other work suggests that greater residential segregation is associated with greater all-cause mortality, differences in use of transplantation among patients with end-stage renal disease,31 and poorer self-rated health status for blacks compared with whites.30,47 Segregation has been associated with fewer economic opportunities, worse physical environments, fewer public resources, a scarcity of adequate housing, more pollution and violence, and greater experience of racism, all of which may adversely effect health.25,48–50 Finally, it is not surprising that our findings are stronger for blacks than for Hispanics, as blacks experience the highest levels of residential segregation in the United States.25,26 This literature suggests that the mechanisms that underlie the relationship between segregation and access to health care and health outcomes should be better understood.
Our work has several limitations. First, because of our cross-sectional design, we cannot conclude that these area characteristics caused the observed differences in cancer stage. Second, we cannot directly examine the factors that may mediate the observed differences, for example, the use of screening or diagnostic care or access to educational information. Third, we examined stage at diagnosis for seniors with Medicare coverage. Our findings may not be generalizable to a younger population. Finally, we had limited information about personal socioeconomic status (i.e., eligibility for state buy-in coverage for Medicare). Despite these limitations, this study presents a novel perspective on the role of area characteristics on racial disparities in cancer stage.
There exist widespread disparities in early-stage diagnosis for breast and colorectal cancers and these disparities may be larger in less segregated, low-income areas. Mechanisms for this association should be explored so that appropriate interventions can be designed.
Acknowledgments
We thank Joan Warren, Ph.D., for her helpful comments on an earlier version of this manuscript. This work was supported by the National Cancer Institute (R01 CA112451). We thank Philip J. Ethington, Ph.D., for sharing methods for creating measures of residential segregation in municipal areas.
Conflict of Interest Statement None disclosed.
References
- 1.Fried VM, Prager K, MacKay AP, Xia H. Chartbook on Trends in the Health of Americans: Health, United States, 2003. Hyattsville, MD: National Center for Health Statistics; 2003.
- 2.Ries LAG, Eisner MP, Kosary CL. SEER Cancer Statistics Review, 1973–1999. Bethesda, MD: National Cancer Institute; 2002.
- 3.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–92. [DOI] [PubMed]
- 4.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162(17):1985–93. [DOI] [PubMed]
- 5.Ragland KE, Selvin S, Merrill DW. Black–white differences in stage-specific cancer survival: analysis of seven selected sites. Am J Epidemiol. 1991;133(7):672–82. [DOI] [PubMed]
- 6.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272(12):947–54. [DOI] [PubMed]
- 7.Optenberg SA, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995;274(20):1599–605. [DOI] [PubMed]
- 8.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106–13. [DOI] [PubMed]
- 9.Oakley-Girvan I, Kolonel LN, Gallagher RP, Wu AH, Felberg A, Whittemore AS. Stage at diagnosis and survival in a multiethnic cohort of prostate cancer patients. Am J Public Health. 2003;93(10):1753–9. [DOI] [PMC free article] [PubMed]
- 10.McCarthy EP, Burns RB, Coughlin SS, et al. Mammography use helps to explain differences in breast cancer stage at diagnosis between older black and white women. Ann Intern Med. 1998;128(9):729–36. [DOI] [PubMed]
- 11.Sassi F, Luft HS, Guadagnoli E. Reducing racial/ethnic disparities in female breast cancer: screening rates and stage at diagnosis. Am J Public Health. 2006;96(12):2165–72. [DOI] [PMC free article] [PubMed]
- 12.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. [DOI] [PubMed]
- 13.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279(22):1801–7. [DOI] [PubMed]
- 14.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96(12):2173–8. [DOI] [PMC free article] [PubMed]
- 15.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–57. [DOI] [PubMed]
- 16.Agency for Healthcare Research and Quality. US Preventative Services Task Force. Accessed January 29, 2008, at http://www.ahcpr.gov/clinic/uspstfix.htm.
- 17.American Cancer Society. Cancer Facts and Figures. American Cancer Society; 2006. Accessed January 29, 2008, 2006, at http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf.
- 18.Bach PB, Jett J, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and lung cancer outcomes. Jama. 2007;297(9):953–61. [DOI] [PubMed]
- 19.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–71. [DOI] [PubMed]
- 20.Mandelblatt J, Andrews H, Kao R, Wallace R, Kerner J. Impact of access and social context on breast cancer stage at diagnosis. J Health Care Poor Underserved. 1995;6(3):342–51. [DOI] [PubMed]
- 21.Hoffman RM, Gilliland FD, Eley JW, et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93(5):388–95. [DOI] [PubMed]
- 22.Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health. 1991;81(5):646–9. [DOI] [PMC free article] [PubMed]
- 23.Bennett CL, Ferreira MR, Davis TC, et al. Relation between literacy, race, and stage of presentation among low-income patients with prostate cancer. J Clin Oncol. 1998;16(9):3101–4. [DOI] [PubMed]
- 24.Margolis ML, Christie JD, Silvestri GA, Kaiser L, Santiago S, Hansen-Flaschen J. Racial differences pertaining to a belief about lung cancer surgery: results of a multicenter survey. Ann Intern Med. 2003;139(7):558–63. [DOI] [PubMed]
- 25.Massey DS, Denton NA. Hypersegregation in U.S. metropolitan areas: black and Hispanic segregation along five dimensions. Demography. 1989;26:373–91. [DOI] [PubMed]
- 26.Massey DS. Residential segregation neighborhood conditions in U.S. metropolitan areas. In: Smelser NJ, Wilson WJ, Mitchell F, eds. America Becoming: Racial Trends and Their Consequences. Washington, DC: National Academies; 2001:391–428.
- 27.Cutler DM, Glaeser EL, Vigdor JL. Are ghettos good or bad? Q J Econ. 1997;112:827–72. [DOI]
- 28.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–16. [DOI] [PMC free article] [PubMed]
- 29.Chang VW. Racial residential segregation and weight status among US adults. Soc Sci Med. 2006;63(5):1289–303. [DOI] [PubMed]
- 30.Subramanian SV, Acevedo-Garcia D, Osypuk TL. Racial residential segregation and geographic heterogeneity in black/white disparity in poor self-rated health in the US: a multilevel statistical analysis. Soc Sci Med. 2005;60(8):1667–79. [DOI] [PubMed]
- 31.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146(7):493–501. [DOI] [PubMed]
- 32.Massey DS, Denton NA. The dimensions of residential segregation. Soc Forces. 1988;67(2):281. (35 pages). [DOI]
- 33.Acevedo-Garcia D, Lochner KA, Osypuk TL, Subramanian SV. Future directions in residential segregation and health research: a multilevel approach. Am J Public Health. 2003;93(2):215–21. [DOI] [PMC free article] [PubMed]
- 34.Population Studies Center. Racial Residential Segregation Measurement Project. Population Studies Center, University of Michigan; 2000. Accessed January 29, 2008, at http://enceladus.isr.umich.edu/race/racestart.asp.
- 35.Logan JR, Stults BJ, Farley R. Segregation of minorities in the metropolis: two decades of change. Demography. 2004;4111–22. [DOI] [PubMed]
- 36.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9. discussion 81–90. [DOI] [PubMed]
- 37.MacKinnon JA, Duncan RC, Huang Y, et al. Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiol Biomarkers Prev. 2007;16(4):756–62. [DOI] [PubMed]
- 38.Hughes C, Lerman C, Lustbader E. Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Res Treat. 1996;40:25–35. [DOI] [PubMed]
- 39.Lawson HW, Henson R, Bobo JK, Kaeser MK. Implementing recommendations for the early detection of breast and cervical cancer among low-income women. Oncology (Williston Park). 2000;14(11)1528–30. 638, 641–2 passim. [PubMed]
- 40.Bhargavan M, Sunshine J. Location decisions of mammography facilities: racial/ethnic disparities across zip-codes. In: Proceedings of the Annual Meeting of the Economics of Population Health: Inaugural Conference of the American Society of Health Economists, TBA, Madison, WI, USA, June 4, 2006.
- 41.Komaromy M, Grumbach K, Drake M, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334(20):1305–10. [DOI] [PubMed]
- 42.Inagami S, Borrell LN, Wong MD, Fang J, Shapiro MF, Asch SM. Residential segregation and Latino, black and white mortality in New York City. J Urban Health. 2006;83(3):406–20. [DOI] [PMC free article] [PubMed]
- 43.Fang J, Madhavan S, Bosworth W, Alderman MH. Residential segregation and mortality in New York City. Soc Sci Med. 1998;47(4):469–76. [DOI] [PubMed]
- 44.Haas JS, Phillips KA, Sonneborn D, et al. Variation in access to health care for different racial/ethnic groups by the racial/ethnic composition of an individual’s county of residence. Med Care. 2004;42(7):707–14. [DOI] [PubMed]
- 45.Geronimus AT. To mitigate, resist, or undo: addressing structural influences on the health of urban populations. Am J Public Health. 2000;90(6):867–72. [DOI] [PMC free article] [PubMed]
- 46.Halpern D. Minorities and mental health. Soc Sci Med. 1993;365597–607. [DOI] [PubMed]
- 47.Jackson SA, Anderson RT, Johnson NJ, Sorlie PD. The relation of residential segregation to all-cause mortality: a study in black and white. Am J Public Health. 2000;90(4)615–7. [DOI] [PMC free article] [PubMed]
- 48.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann NY Acad Sci. 1999;896(11):173–88. [DOI] [PubMed]
- 49.Darity WA, Jr. Employment discrimination, segregation, and health. Am J Public Health. 2003;93(2):226–31. [DOI] [PMC free article] [PubMed]
- 50.Centers for Disease Control and Prevention. Neighborhood safety and the prevalence of physical inactivity–selected states, 1996. MMWR Morb Mortal Wkly Rep. 1999;48(7)143–6. [PubMed]


