Abstract
This study investigates the distribution of fascin in human embryonic, fetal, and normal adult tissues. Tissue microarray technology was used to perform immunohistochemical experiments on human embryos and fetuses at 4–22 weeks of gestation and adult specimens. Fascin was widely expressed in the nervous system. At 4 weeks of gestation, fascin was present in the neural tube. At 8–12 weeks of gestation, homogenous gene expression was seen in cells of the cerebellum and gastrointestinal tract. In later developmental stages and in adults, Purkinje cells of the cerebellum and glandular epithelium of the gastrointestinal tract showed no expression. Fascin was expressed in the cortex and medulla of the adrenal gland at 8–12 weeks of gestation, whereas immunoreactivity decreased from the zona glomerulosa through the zona reticularis and was essentially negative in the adrenal medulla of adults. Significant expression of fascin was seen throughout development in neurons, follicular dendritic cells of lymphoid tissue, basal layer cells of stratified squamous epithelia, mesenchyme, and vascular endothelial cells. Simple columnar epithelia of the biliary duct, colon, ovary, pancreas, and stomach were all negative for fascin expression. These results show that expression of fascin is time specific and highly tissue specific. Parallels between fascin expression in embryogenesis and carcinogenesis are discussed. (J Histochem Cytochem 56:193–199, 2008)
Keywords: fascin, development, embryo, tissue microarray, immunohistochemistry
Fascin, also known as fascin-1, is a 55-kDa globular actin-bundling protein that organizes F-actin into well-ordered, tightly packed parallel bundles in vitro and in cells (Yamashiro-Matsumura and Matsumura 1985). Drosophila melanogaster and echinoderms have a single form of fascin, whereas vertebrate genomes encode three forms: fascin-1 is widely expressed in mesenchymal tissues and the nervous system; fascin-2 is expressed by retinal photoreceptor cells; and fascin-3 is testis specific (Edwards and Bryan 1995; Saishin et al. 1997; Kureishy et al. 2002; Tubb et al. 2002).
Fascin, originally found in extracts of unfertilized sea urchin eggs (Otto et al. 1979), is but one of many fascin proteins, which include the 55-kDa actin-bundling protein of Hela cells and the singed protein of Drosophila (Otto 1994; Edwards and Bryan 1995). Molecular cloning of the sea urchin fascin gene and subsequently those of human, mouse, and Xenopus (Bryan et al. 1993; Duh et al. 1994; Holthuis et al. 1994; Mosialos et al. 1994) revealed that fascin is an evolutionarily conserved protein. In mammals, fascin is abundant in tissues such as the brain and spleen; at the cellular level, it is observed in neuronal and glial cells, microcapillary endothelial cells, and antigen-presenting dendritic cells (Duh et al. 1994; Mosialos et al. 1994; Pinkus et al. 1997). Fascin transcripts are expressed principally in the developing nervous system, developing somites, and other mesoderm-derived tissues such as mesenchyme and limbs during mouse embryogenesis from E8.0 to E16.5 (De Arcangelis et al. 2004). Recently, expression of fascin at high levels has been observed in many transformed cells and carcinoma cells, including hormone receptor–negative breast carcinomas (Grothey et al. 2000; Yoder et al. 2005), and colonic (Jawhari et al. 2003; Hashimoto et al. 2006; Puppa et al. 2007; Vignjevic et al. 2007), gastric (Hashimoto et al. 2004), lung (Pelosi et al. 2003), ovarian (Cao et al. 2005; Kabukcuoglu et al. 2006), skin (Goncharuk et al. 2002), urothelial (Tong et al. 2005), and esophageal squamous cell carcinomas (Xue et al. 2006; Zhang et al. 2006). However, little is known about fascin expression in human fetal and embryonic tissues. In this report, we show the expression of fascin in the human embryo, fetus, and normal adult tissue using tissue microarray technology to better understand the function of this important structural protein in normal development and carcinogenesis.
Materials and Methods
Samples
Forty human samples were used consisting of 3 embryos, 11 fetuses, and 26 postnatal specimens, as summarized in Table 1. Intact embryos and fetuses were acquired from the Gynecology and Obstetrics Department of Shantou Central Hospital. Samples were collected from 14 healthy pregnant women undergoing elective termination of pregnancy at 4–22 weeks of gestation. Specimens were immediately fixed in 4% buffered formalin solution, and subsequently, visible organs were embedded in paraffin blocks. Normal human tissue sections (from autopsy specimens) were acquired from the Department of Forensic Medicine of Shantou University Medical College. These specimens were collected from 2002 through 2005. The following tissues were collected: cerebellum, cerebrum, thymus, lung, trachea, cardiac muscle, esophagus, stomach, large intestine, small intestine, liver, pancreas, kidney, spleen, pituitary, adrenal gland, thyroid gland, prostate gland, uterus, and lymph node. All research was carried out with the permission of local ethics committees.
Table 1.
Description of specimens
| Human stages | Number of cases |
|---|---|
| Embryo | 3 |
| 4 weeks of gestation | 1 |
| 5–8 weeks of gestation | 2 |
| Fetus | 11 |
| 9–12 weeks of gestation | 5 |
| 13–16 weeks of gestation | 3 |
| 17–22 weeks of gestation | 3 |
| Postnatal stages | 26 |
| P1: neonate (<28 days) | 5 |
| P2: 1∼16 years | 6 |
| P3: 17∼39 years | 11 |
| P4: >40 years | 4 |
Construction of Tissue Microarrays
Representative regions of each tissue were selected from hematoxylin and eosin–stained sections and marked on individual paraffin blocks. Samples were chosen from those specimens for which more tissue was available, so that availability of tissue for correlative studies would not be compromised. Two tissue cores were obtained from each specimen measuring 1.8 mm in diameter and ranging in length from 1.0 to 3.0 mm depending on the depth of tissue in the donor block. Each core was precisely arrayed into a new paraffin block. These microarrays were serially sectioned (4 μm) and stained with hematoxylin and eosin to verify tissue sampling and completeness. Unstained sections were baked overnight at 56C in preparation for immunohistochemistry.
Immunohistochemical Staining
Slides were dried in an oven (55–60C) before removing paraffin in several changes of xylene. Slides were hydrated through a series of graded alcohols to water, followed by incubation with 3% hydrogen peroxide for 10 min. For antigen retrieval, slides were autoclaved in 0.01 M citrate buffer (pH 6.0) at 120C for 3 min. Sections were incubated with 10% normal goat serum in PBS for 15 min at room temperature to block nonspecific binding. After rinsing with PBS, slides were incubated overnight at 4C with mouse anti-human fascin monoclonal antibody, clone 55k-2 (1:200 dilution in PBS containing 0.01% Triton X-100; DAKO Biot Co., Glostrup, Denmark). After rinsing with PBS, tissue sections were incubated for 15 min at room temperature with polymer helper solution (Polymer Detection System Kit; GBI Biot Co., Mukilteo, WA) and rinsed with PBS. Slides were incubated for 20 min at room temperature with streptavidin peroxidase–conjugated goat anti-mouse IgG (Polymer Detection System Kit). Subsequently, slides were stained with 0.003% 3,3-diaminobenzide tetrahydrochloride and 0.005% hydrogen peroxide in 0.05 M Tris·HCl (pH 7.2), and counterstained with Mayer hematoxylin, dehydrated, and mounted. Negative controls were prepared by substituting PBS for primary antibody. A metastatic esophageal carcinoma shown previously to have immunoreactivity (Zhang et al. 2006) was used as a positive control in each series of experiments.
Fascin-positive samples were defined as those showing brown signals in the cell cytoplasm. Immunoreactivity was measured semiquantitatively using a scale from − to +++, where − indicates no detectable immunostaining; + represents <25% of the cells are reactive; ++ represents 25∼50% of the cells are reactive; and +++ represents >50% of the cells are reactive. Finally, scales of −, +, ++, and +++ were judged as negative, weakly positive, moderately positive, and strongly positive staining, respectively. Results were reviewed by two pathologists (Z-YS and L-HT) and one observer (F-RZ). When their opinions differed, agreement was reached through careful discussion.
Results
Fascin in Normal Human Tissues
Table 2 summarizes the findings for all tissues studied. As was previously shown in human normal adult tissues, fascin was strongly expressed in vascular endothelial cells, neuronal cells, dendritic cells of lymphoid tissue, the basal layer cells of the stratified squamous epithelia of the skin, the esophagus, and the uterine cervix (Mosialos et al. 1994; Goncharuk et al. 2002; Jawhari et al. 2003; Pelosi et al. 2003). The normal simple columnar epithelia of the biliary duct, colon, ovary, pancreas, and stomach were all negative for fascin. Expression of fascin was homogeneous in postnatal stages; however, differential expression of fascin can be seen during embryonic and fetal development.
Table 2.
Distribution of fascin in normal human tissues determined by immunolabeling
| Findings
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Embryo | Fetus
|
Postnatal stages
|
|||||||||
| Organ/system | Tissue | 4–8 weeks of gestation | 8–16 weeks of gestation | 17–22 weeks of gestation | P1: <28 days | P2: 1∼19 years | P3: 20∼39 years | P4: >40 years | |||
| Nervous system | |||||||||||
| Nerve cell | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ||||
| Nerve fiber | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ||||
| Cardiovascular system | |||||||||||
| Heart | − | − | − | − | − | − | − | ||||
| Artery | − | ++ | − | − | − | − | |||||
| Respiratory system | |||||||||||
| Lung | − | − | − | − | − | − | − | ||||
| Trachea | − | − | − | − | − | − | |||||
| Alimentary system | − | − | − | ||||||||
| Gastrointestinal tract | Mucous layer | ++ | − | − | − | − | − | − | |||
| Submucosa | ++ | ++ | + | + | + | + | + | ||||
| Muscular layer | ++ | ++ | + | − | − | − | − | ||||
| Mantle layer | ++ | ++ | − | − | − | − | − | ||||
| Liver | − | − | − | − | − | − | − | ||||
| Pancreas | − | − | − | − | − | − | − | ||||
| Genitourinary system | |||||||||||
| Kidney | Glomerulus | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |||
| Nephric tubule | − | − | − | − | − | − | |||||
| Urothelium | − | − | − | − | |||||||
| Prostate gland | − | − | − | − | |||||||
| Uterus | − | − | − | − | |||||||
| Endocrine system | |||||||||||
| Adrenal gland | Zona glomerulosa | +++ | +++ | +++ | +++ | +++ | +++ | ||||
| Zona fasciculata | +++ | +++ | ++ | ++ | ++ | ++ | |||||
| Zona reticularis | +++ | +++ | + | + | + | + | |||||
| Thyroid gland | − | − | − | − | |||||||
| Pituitary | − | − | − | − | |||||||
| Immune system | |||||||||||
| Spleen | ++ | ++ | ++ | ++ | ++ | ++ | |||||
| Lymphoid node | + | + | + | + | |||||||
| Thymus | + | + | |||||||||
| Skin | Basal layer | ++ | ++ | ++ | ++ | ++ | |||||
Immunoreactivity was measured semiquantitatively using a scale from − to +++:−, no immunostaining; +, <25% of the cells are reactive; ++, 25∼50% of the cells are reactive; +++, >50% of the cells are reactive. Scales of −, +, ++, and +++ were judged as negative, weakly positive, moderately positive, and strongly positive staining, respectively.
Nervous System
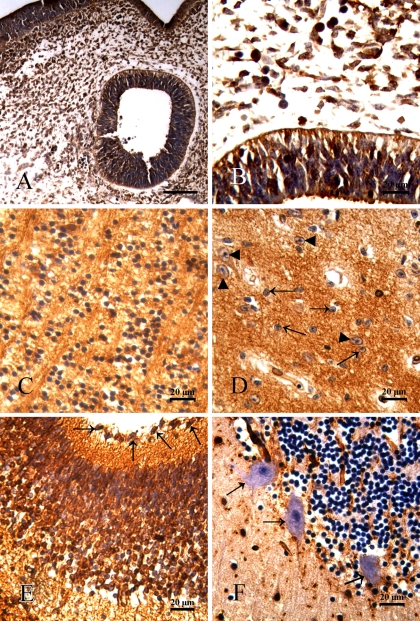
Expression of fascin was strongly positive in nerve fibers and most cells throughout human development. In early embryos (4 weeks of gestation), the medullary epithelium and all nerve cells were fascin positive (Figures 1A and 1B). Fascin was detected in nerve cells and fibers of fetal cerebrum (Figure 1C). In adult tissues, nerve fibers and cytoplasm of stellate cells and glial cells were also fascin detectable (Figure 1D). In the cerebellum, reactivity was found in all cells of the Purkinje cell layer, granular layer, and molecular layer at 8–12 weeks of gestation (Figure 1E); however, the Purkinje cells showed no immunoreactivity in later developmental stages (Figure 1F).
Figure 1.
Expression of fascin in the nervous system. Fascin-positive reactivity was defined as those showing brown spotting. (A) Medullary epithelium and all nerve cells showed significant expression of fascin at 4 weeks of gestation. (B) Medullary epithelium at a higher magnification. (C) Fascin immunoreactivity was observed in nerve fibers and cells of the cerebral cortex at 22-week-old fetus. (D) In adult cerebrum, with the exception of cell nuclei, nerve fibers and cells such as stellate cells (triangles) and glial cells (arrows) were fascin positive. (E) Cerebellum of a 12-week-old fetus. Strong fascin immunoreactivity was seen in nerve fibers and cells of Purkinje cell layer (arrows), granular layer, and molecular layer. (F) Purkinje cells of adult cerebellum showed no immunopositivity (arrows).
Cardiovascular System
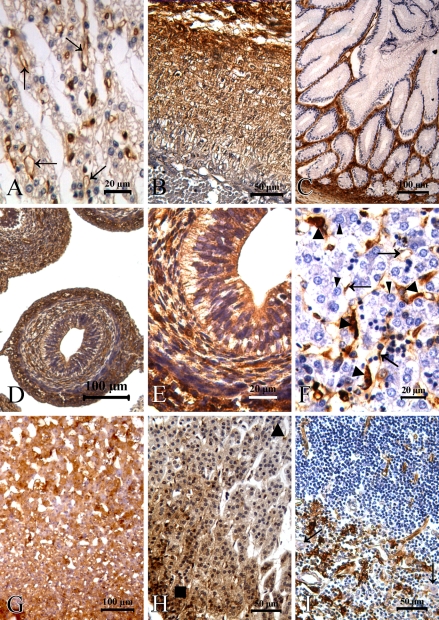
In the cardiovascular system, immunostaining for fascin produced a very strong reactivity in the vascular endothelial cells, whereas few myocardial cells were positive (Figure 2A). Additionally, except for the capillary vessels, the blood vessels showed no staining for fascin. Interestingly, fascin immunoreactivity was confined to the aortic elastic membrane at 22 weeks of gestation (Figure 2B) but was negative in normal adult tissue.
Figure 2.
Fascin distribution in human embryonic, fetal, and normal adult tissue. (A) Vascular endothelial cells (arrows) of the heart showed expression of fascin, whereas cardiocytes were negative at 22 weeks of gestation. (B) Fascin protein was expressed in the elastic membrane of the aorta at 22 weeks of gestation. (C) Glandular epithelium of the intestinal tract was fascin negative at 20 weeks of gestation. (D,E) Immunoreactivity for fascin was shown in cells of the gastrointestinal tract at 8 weeks of gestation. (F) In the liver, expression of fascin was observed in sinusoidal, endothelial (arrows), and Kupffer cells (triangles), whereas normal hepatocytes (arrowheads) were negative at 22 weeks of gestation. (G) Adrenal cortex and medulla showed strong reactivity at 12 weeks of gestation. (H) In the normal adult adrenal gland, fascin immunoreactivity was exhibited in cells of the zona glomerulosa (rectangle) and decreased in the zona fasciculate (triangle). (I) Fascin protein was present in follicular dendritic cells of the thymus and absent in the Hassal corpuscle (arrows).
Gastrointestinal System
A very specific and restricted expression pattern of fascin was seen in the gastrointestinal tract throughout human developmental stages. Fascin protein was expressed in cells of the gastrointestinal tract at 6–8 weeks of gestation (Figures 2D and 2E), whereas the glandular epithelium and muscular layer were negative later in development (Figure 2C). Fascin protein was also detected in basal layer cells of the esophageal squamous epithelium. Liver tissue exhibited focal positivity in sinusoidal, endothelial, and Kupffer cells, whereas normal hepatocytes were negative (Figure 2F). In the pancreas, the acini and tubular epithelium of the glandular pancreatic tissue showed no immunolabeling.
Endocrine System
Examination of endocrine organs showed no fascin reactivity in follicular cells and C cells of the thyroid gland throughout human development. In the anterior pituitary, most cells were fascin negative except for basophilic cells, which showed faint staining. In the adrenal gland, the cortex and medulla showed a strong positive reaction at 8–12 weeks of gestation (Figure 2G). However, in later stages of development, this staining pattern was particularly pronounced in cells of the peripheral cortex, decreased in the inner layers of the cortex, and absent entirely in the medulla (Figure 2H).
Fascin Expression in Other Tissues
An intriguing pattern of fascin expression was seen in the respiratory system. Mesenchyme and vascular endothelial cells in the lung were fascin positive, whereas no definite reactivity was seen in cells of the alveolar lining and trachea. In the kidney, epithelial cells of the acinus renis showed strong positivity throughout human development, whereas nephric tubules were negative. Bladder epithelium, ovary, and prostate tissues were all negative. In the lymph tissues, large cleaved germinal centers restricted to the follicular dendritic cells exhibited a pronounced immunoreactivity (Figure 2I). Fascin protein was present in skeletal muscles and chondroblasts of limbs at 20 weeks of gestation. Mesenchymal fibers in placental tissue were fascin positive, whereas the decidua basalis cells of trophoblastic tissue were negative.
Discussion
The aim of this study was to determine expression of fascin in human embryo, fetus, and adult normal tissues and to show the differential expression among various developmental stages. We found that fascin was widely expressed in the developing nervous system, mesenchymal tissue, and gastrointestinal tract and not exhibited in the liver throughout human embryonic and fetal development, which was extremely similar to fascin transcripts during mouse development (De Arcangelis et al. 2004). These confirmed that fascin is an evolutionarily conserved protein (Kureishy et al. 2002). However, no immunostaining was detectable in cells of the human heart and lung; by contrast, fascin transcripts were detected in the corresponding tissues throughout mouse embryonic development (De Arcangelis et al. 2004). The apparent disagreement can be understood as species differences.
Expression of fascin is highly tissue specific and cell specific throughout human development. In this study, we also found that normal simple columnar epithelia of the biliary duct, colon, ovary, pancreas, and stomach were all negative for fascin (Hashimoto et al. 2004; Lu et al. 2004; Cao et al. 2005; Hashimoto et al. 2006; Okada et al. 2007). However, fascin protein is abundant in tissues such as the brain and acinus renis, and at the cellular level, fascin is specific to neuronal and glial cells, microcapillary endothelial cells, and antigen-presenting dendritic cells, in keeping with previous studies (Mosialos et al. 1994; Goncharuk et al. 2002; Jawhari et al. 2003). A morphological characteristic common to these normal, specialized cells that express high levels of fascin is the development of many membrane protrusions. Fascin contributes to the organization of two major forms of cortical cell protrusions and cytoplasmic microfilament bundles, which mediate cell interactions and migration and contribute to cell architecture and to intracellular movements (Kureishy et al. 2002).
Interestingly, fascin expression was found to be time specific during human growth and development. Fascin immunoreactivity was positive in early embryo stages but negative both during later development and in adult tissue. For example, the Purkinje cells of the cerebellum and the medulla of the adrenal gland (a derivative of neural crest) exhibited strong fascin expression at 8–12 weeks of gestation, whereas the same tissues were negative later in development and in adult tissue. In addition, a pronounced expression pattern for fascin was detected throughout the gastrointestinal tract at early fetal stages, whereas the glandular epithelium and muscular layer were essentially negative in other stages. Although there is no mechanism to explain why fascin protein levels would be upregulated in many normal tissues, as observed at early developmental stages, it could be speculated that fascin mediates cell–cell interactions, cell division, and/or cell growth, because of the high demand for those processes during development.
Recently, several reports have shown that absence of fascin expression or low levels of expression in normal epithelia becomes dramatically altered in many human carcinomas. For example, upregulation of fascin is frequently observed in gastric carcinoma and colorectal carcinoma (Hashimoto et al. 2004,2006; Puppa et al. 2007). According to the work presented here, the glandular epithelium of normal adult gastrointestinal tract is negative for fascin expression, but a pronounced positive straining was observed throughout the gastrointestinal tract at early fetal stages (Figures 2D and 2E). Thus, fascin immunoreactivity can be seen in cells of early fetal stages and in malignant cells. Furthermore, in non–small cell lung carcinoma and invasive pancreatic tumors, fascin expression is consistently associated with poor prognosis and/or tumor metastasis, invasion, or proliferation (Maitra et al. 2002; Pelosi et al. 2003). However, these normal tissues were negative for fascin expression in embryonic, fetal, and adult tissues. These results suggest that there is a specific relationship between fascin expression in some malignant cells and the immunoreactivity seen in the corresponding normal cells at early developmental stages. This implies that there may be some correlation between fascin expression and the processes of embryogenesis and carcinogenesis, although the molecular mechanisms behind this correlation remain unclear.
In conclusion, the immunohistochemical observations in this study are the first systematic demonstration of the immunoreactive presence of fascin in human embryonic, fetal, and normal adult tissues. Through analyses of the differential expression, it was discovered that fascin expression was highly tissue specific and time specific. It was also observed that fascin expression in cells of early developmental stages was similar to that seen in the malignant cells. Therefore, these results may imply that fascin plays an important role in the processes of embryogenesis and carcinogenesis.
Acknowledgments
This work was supported by grants from the National High Technology Research and Development Program of China (863 program, 2006AA02A403), the National Natural Science Foundation of China (30570849, 30672376, and 30772485), Specialized Research Fund for the Doctoral Program of Higher Education of China (20050560002 and 20050560003), and Guangdong Scientific Fund Key Items (37788, 5104541, and 7118419).
We thank Professor X.J. Yu from the Department Forensic Medicine of Shantou University Medical College for providing autopsy specimens.
References
- Bryan J, Edwards R, Matsudaira P, Otto J, Wulfkuhle J (1993) Fascin, an echinoid actin-bundling protein, is a homolog of the Drosophila singed gene product. Proc Natl Acad Sci USA 90:9115–9119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Ji H, Ronnett BM (2005) Expression of mesothelin, fascin, and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int J Gynecol Pathol 24:67–72 [PubMed] [Google Scholar]
- De Arcangelis A, Georges-Labouesse E, Adams JC (2004) Expression of fascin-1, the gene encoding the actin-bundling protein fascin-1, during mouse embryogenesis. Gene Expr Patterns 4:637–643 [DOI] [PubMed] [Google Scholar]
- Duh FM, Latif F, Weng Y, Geil L, Modi W, Stackhouse T, Matsumura F, et al. (1994) cDNA cloning and expression of the human homolog of the sea urchin fascin and Drosophila singed genes which encodes an actin-bundling protein. DNA Cell Biol 13:821–827 [DOI] [PubMed] [Google Scholar]
- Edwards RA, Bryan J (1995) Fascins, a family of actin bundling proteins. Cell Motil Cytoskeleton 32:1–9 [DOI] [PubMed] [Google Scholar]
- Goncharuk VN, Ross JS, Carlson JA (2002) Actin-binding protein fascin expression in skin neoplasia. J Cutan Pathol 29:430–438 [DOI] [PubMed] [Google Scholar]
- Grothey A, Hashizume R, Sahin AA, McCrea PD (2000) Fascin, an actin-bundling protein associated with cell motility, is upregulated in hormone receptor negative breast cancer. Br J Cancer 83:870–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, Imamura M (2004) The prognostic relevance of fascin expression in human gastric carcinoma. Oncology 67:262–270 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Skacel M, Lavery IC, Mukherjee AL, Casey G, Adams JC (2006) Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer 6:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JC, Schoonderwoert VT, Martens GJ (1994) A vertebrate homolog of the actin-bundling protein fascin. Biochim Biophys Acta 1219:184–188 [DOI] [PubMed] [Google Scholar]
- Jawhari AU, Buda A, Jenkins M, Shehzad K, Sarraf C, Noda M, Farthing MJ, et al. (2003) Fascin, an actin-bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro. Am J Pathol 162:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabukcuoglu S, Ozalp SS, Oner U, Bildirici K, Yalcin OT, Oge T, Colak E (2006) Actin bundling protein fascin expression in ovarian neoplasms: comparison of histopathologic features of tumors obtained by the first and secondary cytoreduction surgeries. Eur J Gynaecol Oncol 27:123–128 [PubMed] [Google Scholar]
- Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC (2002) Fascins, and their roles in cell structure and function. Bioessays 24:350–361 [DOI] [PubMed] [Google Scholar]
- Lu Z, Hu L, Evers S, Chen J, Shen Y (2004) Differential expression profiling of human pancreatic adenocarcinoma and healthy pancreatic tissue. Proteomics 4:3975–3988 [DOI] [PubMed] [Google Scholar]
- Maitra A, Iacobuzio-Donahue C, Rahman A, Sohn TA, Argani P, Meyer R, Yeo CJ, et al. (2002) Immunohistochemical validation of a novel epithelial and a novel stromal marker of pancreatic ductal adenocarcinoma identified by global expression microarrays: sea urchin fascin homolog and heat shock protein 47. Am J Clin Pathol 118:52–59 [DOI] [PubMed] [Google Scholar]
- Mosialos G, Yamashiro S, Baughman RW, Matsudaira P, Vara L, Matsumura F, Kieff E, et al. (1994) Epstein-Barr virus infection induces expression in B lymphocytes of a novel gene encoding an evolutionarily conserved 55-kilodalton actin-bundling protein. J Virol 68:7320–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura T, Asakawa K, Hashimoto S, Mochida Y, Suehiro T, Kuwano H (2007) Fascin expression is correlated with tumor progression of extrahepatic bile duct cancer. Hepatogastroenterology 54:17–21 [PubMed] [Google Scholar]
- Otto JJ (1994) Actin-bundling proteins. Curr Opin Cell Biol 6:105–109 [DOI] [PubMed] [Google Scholar]
- Otto JJ, Kane RE, Bryan J (1979) Formation of filopodia in coelomocytes: localization of fascin, a 58,000 dalton actin cross-linking protein. Cell 17:285–293 [DOI] [PubMed] [Google Scholar]
- Pelosi G, Pastorino U, Pasini F, Maissoneuve P, Fraggetta F, Iannucci A, Sonzogni A, et al. (2003) Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer 88:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamashiro S, Mosialos G, Said JW (1997) Fascin, a sensitive new marker for Reed-Sternberg cells of hodgkin's disease. Evidence for a dendritic or B cell derivation? 196. Am J Pathol 150:543–562 [PMC free article] [PubMed] [Google Scholar]
- Puppa G, Maisonneuve P, Sonzogni A, Masullo M, Chiappa A, Valerio M, Zampino MG, et al. (2007) Independent prognostic value of fascin immunoreactivity in stage III–IV colonic adenocarcinoma. Br J Cancer 96:1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saishin Y, Shimada S, Morimura H, Sato K, Ishimoto I, Tano Y, Tohyama M (1997) Isolation of a cDNA encoding a photoreceptor cell-specific actin-bundling protein: retinal fascin. FEBS Lett 414:381–386 [DOI] [PubMed] [Google Scholar]
- Tong GX, Yee H, Chiriboga L, Hernandez O, Waisman J (2005) Fascin-1 expression in papillary and invasive urothelial carcinomas of the urinary bladder. Hum Pathol 36:741–746 [DOI] [PubMed] [Google Scholar]
- Tubb B, Mulholland DJ, Vogl W, Lan ZJ, Niederberger C, Cooney A, Bryan J (2002) Testis fascin (FSCN3): a novel paralog of the actin-bundling protein fascin expressed specifically in the elongate spermatid head. Exp Cell Res 275:92–109 [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, Louvard D, et al. (2007) Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res 67:6844–6853 [DOI] [PubMed] [Google Scholar]
- Xue LY, Hu N, Song YM, Zou SM, Shou JZ, Qian LX, Ren LQ, et al. (2006) Tissue microarray analysis reveals a tight correlation between protein expression pattern and progression of esophageal squamous cell carcinoma. BMC Cancer 6:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S, Matsumura F (1985) Purification and characterization of an F-actin-bundling 55-kilodalton protein from Hela cells. J Biol Chem 260:5087–5097 [PubMed] [Google Scholar]
- Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, Tubbs RR, et al. (2005) The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res 11:186–192 [PubMed] [Google Scholar]
- Zhang H, Xu L, Xiao D, Xie J, Zeng H, Cai W, Niu Y, et al. (2006) Fascin is a potential biomarker for early-stage oesophageal squamous cell carcinoma. J Clin Pathol 59:958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]