Abstract
The Reg-related protein family member INGAP (islet neogenesis-associated protein) is a pleiotropic factor enhancing islet neogenesis, neurite growth, β-cell protection, and β-cell function. Using an antibody to the N-termini of INGAP, we have identified that immunoreactivity to INGAP localized to the pancreatic endocrine cells in mouse. INGAP- and insulin-immunoreactive cells are mutually exclusive, with INGAP-immunoreactive cells being preserved after streptozotocin-mediated destruction of β-cells. Glucagon- and INGAP-immunoreactive cells colocalize, although respective antigen expression occurs in different intracellular locations. These data suggest that INGAP-immunoreactive cells include α-cells; however, detection of single INGAP-immunoreactive/glucagon-negative cells indicates that this may not be exclusive. In addition to mouse, detection of islet endocrine cells that were INGAP immunoreactive/glucagon immunoreactive/insulin negative was also observed in islets from human, monkey, and rat. These findings reveal that INGAP and/or related group 3 Reg proteins have a conserved expression in the pancreatic islet. (J Histochem Cytochem 56:183–191, 2008)
Keywords: islet, glucagon, islet neogenesis-associated protein
Diabetes is fundamentally a failure of functional β-cell mass (Bell and Polonsky 2001). Therefore, a curative therapy for diabetes would encompass improved β-cell function and/or replacement of the functional β-cell compartment. Replacement strategies include transplantation of cadaveric islets (Ryan et al. 2002), embryonic stem cell-derived islet-like cells (Jiang et al. 2007), or the endogenous repopulation of islets using trophic factor stimulation of resident precursor adult stem cells (Vinik et al. 2004). Trophic factors include INT (combination of gastrin and epidermal growth factor) therapy (Brand et al. 2002; Suarez-Pinzon et al. 2005), GLP-1 (glucagon-like peptide-1) therapy (De Leon et al. 2003; Drucker 2003; Gallwitz 2006), and INGAP therapy (Rosenberg et al. 2004; Vinik et al. 2004; Pittenger et al. 2007). Interestingly, the gut hormone GLP-1 and stable analogs thereof (Holz and Chepurny 2003) are also potent incretins, promoting glucose-stimulated insulin secretion.
The secreted protein INGAP was initially described as a trophic factor promoting endogenous stimulation of adult pancreatic stem cell differentiation into islets, a process termed islet neogenesis. During islet neogenesis, inductive factors such as INGAP stimulate protodifferentiated cells residing in the pancreatic duct to differentiate, expand, and bud to initially form islet-like clusters (Baggio and Drucker 2002; Bonner-Weir and Sharma 2002; Vinik et al. 2004; Suarez-Pinzon et al. 2005). Endogenous INGAP expression and islet neogenesis occur concurrently (Del Zotto et al. 2000). INGAP is a 16.8-kDa protein and is related to the Reg superfamily of type 2C-lectin proteins (Taylor-Fishwick et al. 2003; Vinik et al. 2004). Organization of the 175 amino acids in INGAP classifies it as a member of the group-three superfamily of Reg-related proteins (Okamoto 1999). In addition to the biological efficacy of the INGAP protein, a pentadecapeptide fragment of the INGAP protein retains neogenic activity (Rosenberg et al. 2004). INGAP or INGAP peptide when administered to rodents (Rosenberg et al. 1996,2004) or dogs (Pittenger et al. 2007) stimulates new islet growth as evidenced by elevated β-cell mass identified in quantitative histology and molecular analyses of insulin. INGAP peptide promotes duct to islet transdifferentiation in vitro (Jamal et al. 2005). INGAP secretion is upregulated in rodent models of injury-induced neogenesis such as the partial duct occlusion model in hamster (Rafaeloff et al. 1997), mouse duct ligation, and the rat partial pancreatectomy models (Song et al. 2005). Further, INGAP therapy reversed established hyperglycemia in rodent models of diabetes (Gold et al. 1998; Rosenberg et al. 2004). The actions of INGAP are, however, not restricted to neogenesis because the molecule has pleiotropic effects. INGAP enhances regrowth of neurites in axotomized dorsal root ganglia (Tam et al. 2002) and corrects sensory dysfunction in streptozotocin (STZ)-induced diabetic mice (Tam et al. 2004). Further, the targeted expression of INGAP in transgenic mice results in either a resistance to chemically induced diabetes (Taylor-Fishwick et al. 2006b) or improved islet function (Taylor-Fishwick DA, unpublished data), depending upon the pancreatic compartment targeted.
Recent data in isolated islets have shown that the INGAP peptide enhances glucose-stimulated insulin secretion (Borelli et al. 2005) with a corresponding upregulation in islet gene expression (Barbosa et al. 2006). These data suggest that INGAP and associated Reg family orthologs may also serve to support the regulation of insulin secretion. In support of this, we have identified INGAP-immunoreactive cells in the endocrine pancreas. This immunoreactivity is restricted to the non-β-cell compartment of the islet in an expression pattern that is conserved across species. These data indicate that Reg family proteins may play an important role in maintaining β-cell function.
Materials and Methods
Animal Studies
Male FVB/N mice were housed in a temperature- and humidity-controlled environment (12:12 light:dark). Blood glucose was measured from the tail vein using a precalibrated Accu-Chek glucometer (Boehringer-Mannheim; Indianapolis, IN). Bolus STZ induction of diabetes was as previously described (Taylor-Fishwick et al. 2006b). For multiple low-dose STZ induction of diabetes, mice were injected in the lower right abdominal quadrant with 50 mg/kg STZ on 5 consecutive days. All experimentation was approved by the Institutional Animal Care and Use Committee, Eastern Virginia Medical School.
Immunohistochemistry
Monkey, human, and rat pancreas sections were supplied by Biochain (Hayward, CA). Harvested mouse pancreata were fixed in 10% formalin (Fisher Chemicals; Fair Lawn, NJ) and paraffin embedded. Deparaffinized sections (5–7 μm) were rehydrated before being blocked, using Superblock (Pierce; Rockford, IL) for up to 30 min. Primary antibodies mouse anti-glucagon (1:1000; Sigma-Aldrich, St Louis, MO), mouse anti-insulin (1:1000; Sigma), rabbit anti-INGAP (1:1000) and rabbit anti-glucagon (Zymed; South San Francisco, CA), rabbit anti-GLP-1 (1:500; Phoenix Pharmaceuticals, Belmont, CA), and rat anti-glial fibrillary acidic protein (GFAP; Zymed) were incubated at room temperature for up to 4 hr. Sections were washed three times in PBS. Nonspecific staining was excluded using isotype-matched irrelevant primary antibodies. Secondary antibodies (anti-mouse TRITC 1:1000, anti-rabbit FITC 1:1000, Sigma or Vector ABC kits: Vector Laboratories, Burlingame, CA) were incubated for 30 min at room temperature. Direct anti-INGAP-FITC staining used a FITC conjugation kit (PanVera; Madison, WI). Stained sections were mounted using Fluoromount-G (Southern Biotechnology; Birmingham, AL) or Vectamount (Vector Laboratories) with or without 1:100,000 DAPI (Sigma). For peptide competition studies, primary antibodies were preincubated for 30 min with a 25-M excess of peptide. Images were captured on a DP-70 camera with a BX-51 microscope (Olympus; Center Valley, PA). Quantitation was achieved using Image Pro 5.0 software (MediaCybernetics; Bethesda, MD). Image deconvolution and 3-D rendering was performed on an Olympus IX70 using Slidebook software with constrained iterative deconvolution module.
Peptide ELISA
INGAP peptide or glucagon was absorbed into 96-well immunosorbent plates (Nunc International; Rochester, NY) for 3 hr at room temperature. Wells were blocked with 1% BSA for 1 hr at 37C. Primary antibodies (rabbit anti-INGAP or rabbit anti-glucagon) were incubated at 1:500, 1:1000, 1:3000, and 1:10,000 for 2 hr at room temperature. Plates were washed three times with PBS-Tw (Sigma) and incubated for 1 hr at room temperature with 1:1000 anti-rabbit FITC conjugate (Sigma). After a wash, fluorescence was read on a Victor 1420 multiplate reader (PerkinElmer; Waltham, MA).
Islet Isolation and Western Blot
Pancreata were minced and placed in HBSS/Hepes buffer containing 0.23 mg/ml liberase R1 (Roche Applied Science; Penzberg, Germany) and 0.1 mg/ml DNase1 (Roche) and digested for 30 min at 37C in an agitating water bath. Upon termination with ice-cold HBSS containing 10% FBS, the digested tissue was filtered through a 380-μm mesh, washed, and separated on an Optiprep gradient (Axis-Shield; Norton, MA). Recovered islets were washed in PBS prior to protein extraction in ice-cold lysis buffer [1% Triton X-100 in PBS containing 1 mM PMSF, 1 mM EDTA, and HALT protease cocktail (Pierce)] for Western blotting. Reg IIIα (Narushima et al. 1997) and Reg IIIδ (Abe et al. 2000) plasmids (generously provided by Prof. Okamoto) were subcloned into pcDNA3.1 expression plasmid (Clontech; Mountain View, CA) and sequenced (DSSF; Iowa State University, Ames, Iowa). Expression plasmids were transfected into 293 cells as previously described (Taylor-Fishwick et al. 2006a). Forty eight hr following transfection, cells were harvested and lysed in lysis buffer. Lysates were prepared for SDS-PAGE and transferred onto PVDF membrane for immunoblotting with 1:10,000 anti-INGAP as previously described (Taylor-Fishwick et al. 2006a).
Results
Immunoreactivity to INGAP Is Localized to Cells in the Islet Mantle
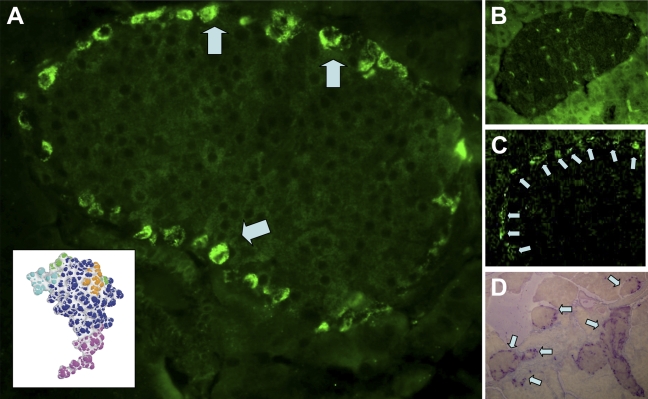
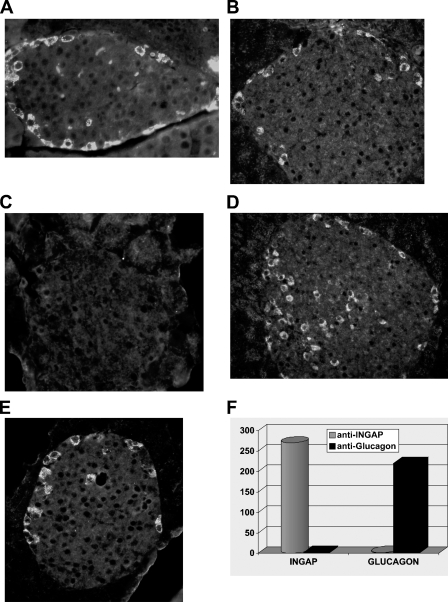
Indirect immunocytochemistry of mouse pancreas tissue sections, using an antibody generated to the N-terminal of INGAP (Figure 1A, inset), detected strong immunoreactive cells around the outer edge of the islets (Figure 1A, arrows). Staining was localized to individual cells as seen by the clustering around unstained nuclei. Incubation of the antibody with a molar excess of antigen displaced the immunoreactive stain (Figure 1B). The signal was specific to the anti-INGAP antibody because no signal was detected in staining protocols that used the second-layer FITC-conjugated antibody in isolation (not shown). The equivalent staining pattern was also detected with an antibody to INGAP directly conjugated to FITC (Figure 1C). The pattern of immunoreactivity to INGAP was observed in the majority of islets and not isolated, as illustrated in Figure 1D, which shows a low-power ×10 micrograph of mouse pancreas.
Figure 1.
Islet neogenesis-associated protein (INGAP)-immunoreactive endocrine cells in the mouse islet. (A) Immunoreactive cells to INGAP present around the islet mantel (arrows). Antibody used was to the N-termini of INGAP (purple region of INGAP molecule, inset). (B) INGAP immunoreactivity is displaced by a 25 molar excess of peptide antigen. (C) Islet mantel cells are detected with an anti-INGAP antibody directly conjugated to FITC (arrows). (D) Alkaline phosphatase detection of INGAP immunoreactivity. Low-power micrograph shows consistent presentation between islets (arrows).
INGAP Immunoreactivity and Insulin Immunoreactivity Are Mutually Exclusive in the Islet
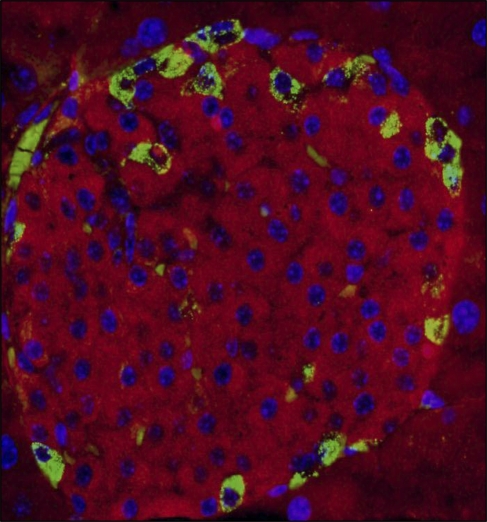
Tissue sections from mouse pancreas were immunostained with antibodies to INGAP and insulin. Identification of antigens in the costained tissue used FITC to detect INGAP and TRITC to detect insulin. For cell body identification, nuclei were also detected with DAPI. Figure 2 shows the merge of the three channels. Insulin-positive β-cells (stained in red, Figure 2) were distinct from INGAP-immunoreactive cells (stained in green, Figure 2).
Figure 2.
INGAP-immunoreactive endocrine cells are not β-cells. Tri-color overlay of mouse islet stained with INGAP (green), insulin (red), and DAPI (blue).
Immunoreactivity to INGAP Colocalizes With Glucagon Immunoreactivity and Is Distinct to GFAP-positive Cells
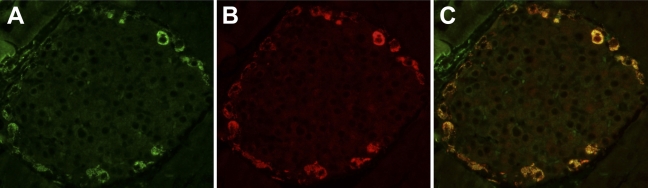
Tissue sections from mouse pancreas were immunostained with antibodies to INGAP and glucagon. For antigen identification in the costained tissue, FITC was used to detect INGAP and TRITC was used to detect glucagon. INGAP-immunoreactive cells (stained green, Figure 3A) were also positive for glucagon immunoreactivity (stained red, Figure 3B). Colocalization is shown in the merged micrographs (Figure 3C). Immunodetection of the GFAP antigen (data not shown) identified cells with a morphology distinct to those cells displaying immunoreactivity to INGAP.
Figure 3.
INGAP-immunoreactive endocrine cells colocalize with α-cells. (A) INGAP-immunoreactive and (B) glucagon-immunoreactive cells in a mouse islet. (C) Merged image of A,B.
STZ-induced Destruction of β-cells Preserves INGAP- and Glucagon-immunoreactive Cells
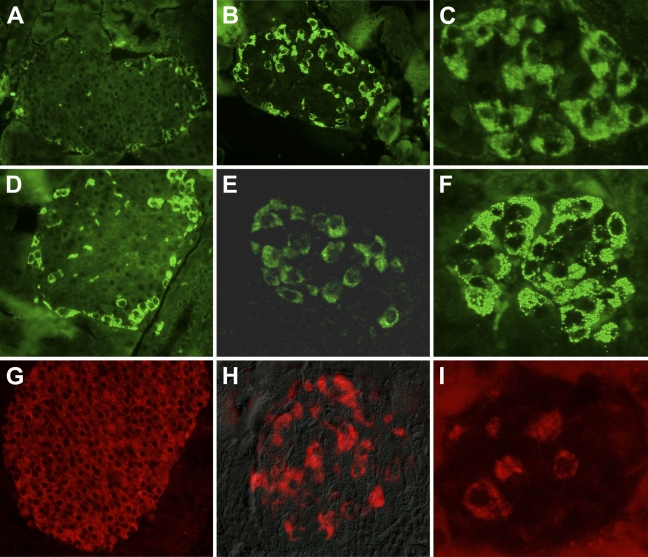
Treatment of the mouse with STZ resulted in β-cell loss and hyperglycemia. Destruction of islet β-cells as detected by insulin immunoreactivity (Figure 4) was more complete using a single high-dose STZ protocol (Figure 4I) than a multiple low-dose STZ model (Figure 4H) when compared with islets from untreated mice (Figure 4G). The number of glucagon-positive cells entering the micrograph field for an islet was directly proportional to the loss of β-cells. With the single high-dose STZ protocol, islets present with a denser organization of glucagon-positive cells (Figure 4F). In islets from mice treated with multiple low-dose STZ, cells immunoreactive to glucagon are more dense than islets from an untreated mouse (Figure 4D) but less dense than islets from mice treated with single high-dose STZ. Islet density of INGAP-immunoreactive cells following treatment with STZ mirrors that seen with glucagon. INGAP-immunoreactive cells are more dense in islets treated with single high-dose STZ (Figure 4C) and have an intermediate density in islets treated with multiple low-dose STZ (Figure 4B) when compared with islets from untreated mice (Figure 4A). Thus, INGAP-immunoreactive cells are not β-cells and do cosegregate with glucagon in these models of STZ-induced diabetes.
Figure 4.
INGAP-immunoreactive endocrine cells map with α-cells in models of β-cell destruction. Islets from control mice (A,D,G) or mice treated with streptozotocin using multiple low dose (B,E,H) or bolus high dose (C,F,I) were stained with glucagon (A–C), INGAP (D–F), or insulin (G–I).
Antigen–Antibody Interactions for Glucagon and INGAP Do Not Cross-react
Ability of the anti-INGAP antibody and the anti-glucagon antibody to interact with glucagon and INGAP was tested (Figure 5). Immunocytochemistry of mouse pancreas revealed that immunoreactivity to INGAP was not displaced by excess glucagon. Similarly, immunoreactivity to glucagon was not displaced by an excess of INGAP peptide but could be displaced by an excess of glucagon. In an antigen-specific ELISA, the antibody to INGAP recognized the INGAP antigen but not glucagon, whereas the antibody to glucagon recognized glucagon but not the INGAP antigen. Thus, the antigens do not cross-react. Further, antibodies to INGAP and GLP-1 do not cross-react (data not shown).
Figure 5.
INGAP immunoreactivity is distinct from glucagon immunoreactivity. Mouse islets stained with glucagon (A,C,E) or INGAP (B,D) in the absence (A,B) or presence of a molar excess of glucagon (C,D) or INGAP (E). (F) Peptide ELISA of anti-INGAP (cylinder) or anti-glucagon (block) recognition of INGAP peptide or glucagon.
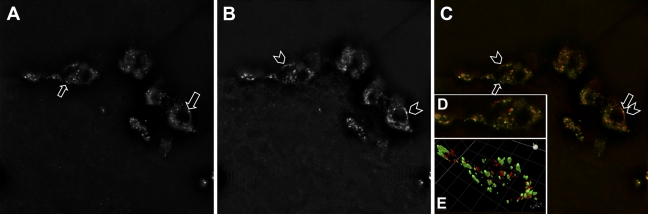
Immunoreactivity to INGAP and Glucagon Reveals Distinct Intracellular Localization
Dual-stain immunocytochemistry of INGAP and glucagon in mouse islets was analyzed on an individual cell basis. High magnification (×100 objective) Z-stacked images were deconvoluted and a representative field was analyzed for INGAP (Figure 6A) or glucagon immunoreactivity (Figure 6B) and their merge (green: INGAP and red: glucagon, Figure 6B). Different regions of fluorescence were observed in the cells as marked (arrows and chevrons, Figures 6A–6C). Additionally, a representative field of a single cell identified with a box in Figure 6C was analyzed by three-dimensional (3-D) rendering (Figure 6D). Cellular locations of the points of fluorescence show different expression patterns between INGAP and glucagon immunoreactivity. These discrete cellular locations for INGAP (green) and glucagon (red) immunoreactivity in the 3-D view are represented graphically in Figure 6E. INGAP- and glucagon-immunoreactive regions within the cell occupy discrete cellular compartments and therefore represent distinct antigen detection.
Figure 6.
INGAP immunoreactivity and glucagon immunoreactivity occur in distinct intracellular locations. High-power immunofluorescence of cells in the mantel of a mouse islet stained with INGAP (A), glucagon (B), and the merge (C). (D) Composite of Z-stacked deconvolved images of a single cell outlined in C. (E) Graphical three-dimensional representation of D.
Comparison of Islets Between Species Identified Conservation of INGAP-immunoreactive Cells
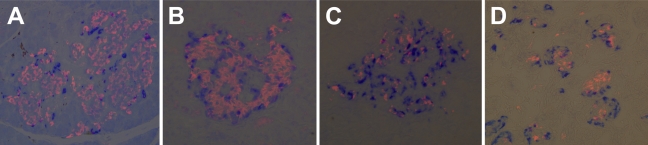
Pancreas sections from hamster, mouse, rat, monkey, and human were stained with antibodies raised against INGAP, glucagon, and insulin. The islet organization of INGAP-immunoreactive endocrine islet cells and insulin-positive β-cells is analogous across species (Figure 7). Consistently, INGAP-immunoreactive cells predominantly colocalized with glucagon-immunoreactive cells (data not shown). In the monkey islet, α- and β-cell patterning is reversed relative to the centric β-cells seen in mouse, hamster, and rat. INGAP immunoreactivity localized to the non-β-cell population of islets in all species studied. In the immature pancreas where the islets are less organized, clusters of isolated INGAP-immunoreactive cells are detected, in addition to those around the mantel of the forming islet (Figure 7D). Small isolated clusters of INGAP-immunoreactive cells are not observed in the adult pancreas. Whereas the majority (∼98%) of INGAP-immunoreactive cells in the adult were also immunoreactive for glucagon, cells were identified that displayed single immunoreactivity for INGAP or glucagon. Intriguingly, detection of single immunoreactive INGAP or glucagon cells varied between species. In hamster islets, INGAP-immunoreactive/glucagon-negative cells were detected, whereas in mouse islets, glucagon-positive/INGAP-negative cells were detected. In contrast, cells showing single immunoreactivity for either glucagon or INGAP were observed in rat (data not shown). More than 40 islets per species were viewed.
Figure 7.
INGAP-immunoreactive endocrine cells are conserved across species. Islets costained with insulin (pink) and INGAP (blue) from rat (A), human (B), monkey (C), and fetal human (D).
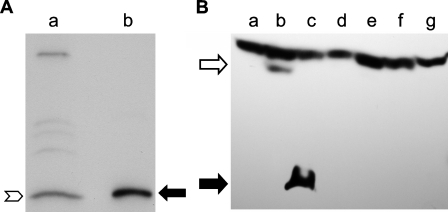
Antigen Detected by INGAP Antibody in Mouse Islets Comigrates With Recombinant INGAP
Islets were isolated from mouse pancreas and shown to be 90% pure as determined by dithizone staining (data not shown). Protein extracts from islet preparations were analyzed by Western blot to determine the size of the antigen detected by the INGAP antibody in mouse islets (Figure 8A). Islet protein (Lane a, Figure 8A) was compared with recombinant INGAP (Lane b, Figure 8A). The antibody to INGAP detected an antigen in the islet protein preparation (chevron, Figure 8A) that comigrated with recombinant INGAP (filled arrow, Figure 8A). Primers to INGAP did not amplify a specific band in mouse islet RNA (data not shown).
Figure 8.
INGAP-immunoreactive proteins. (A) Western blot and anti-INGAP immunostaining of protein extract from purified mouse islets (Lane a) were compared with recombinant INGAP (Lane b). (B) Western blot and anti-INGAP immunostaining of protein extract from 293 cells alone (Lane g) or transfected with expression plasmids for GFP (Lane a), INGAP-GFP (Lane b), INGAP (Lane c), rat Reg I (Lane d), mouse Reg IIIα (Lane e), or mouse Reg IIIδ (Lane f).
Expression plasmids for INGAP, an INGAP–GFP fusion protein, GFP alone, mouse Reg IIIα, mouse Reg IIIδ, and rat Reg I were transfected into 293 cells to determine if the INGAP antibody cross-reacted with known Reg proteins. Lysates from transfected cells were analyzed by Western blot using anti-INGAP antibody (Figure 8B). A band of ∼17 kDa was detected in cells transfected with INGAP (closed arrow, Lane c, Figure 8B). This band comigrated with rINGAP (not shown) and was not detected in control (non-transfected 293) lysate (Figure 8B, Lane g). A single band with a higher mass was detected in lysate from cells transfected with the INGAP–GFP fusion protein (open arrow, Lane b, Figure 8B). The size of this band (∼46 kDa) was consistent with that of INGAP–GFP protein. This band was not detected in lysate from control cells or from cells transfected with GFP alone (Lane a, Figure 8B). No bands specific to the INGAP antibody were detected in the lysates from cells transfected with mouse Reg IIIα, mouse Reg IIIδ, or rat Reg I (Lanes e, f, and d, respectively, Figure 8B). A nonspecific high molecular mass band is present in all lysates.
Discussion
INGAP is a member of the Reg superfamily of proteins (Okamoto 1999; Taylor-Fishwick et al. 2003). It is associated with islet neogenesis, a paradigm in which ligand ligation of the pancreatic duct cell stimulates division and differentiation to new pancreatic endocrine cells (Baggio and Drucker 2002; Bonner-Weir et al. 2004; Paris et al. 2004; Vinik et al. 2004). Expression of INGAP immunoreactivity has previously been described predominantly in the exocrine pancreas (Rafaeloff et al. 1997). The novel description in this study is the identification of INGAP immunoreactivity in normal adult mouse and primate islets and characterization of the immunoreactive cells.
Immunostaining of mouse islets was performed using an anti-peptide antibody raised and affinity purified to the N-terminal 20–40 amino acid region of INGAP. Cells showing high immunoreactivity to INGAP were detected around the outer mantel of the islet. This staining was specific to the INGAP antibody because it was displaced by a molar excess of INGAP(20–40) peptide and was detected by a directly conjugated anti-INGAP antibody, but not with isotype-matched antibody controls. The staining pattern observed with the INGAP antibody was consistent across all islets in the pancreas sections. Using dual-detection costaining studies, the islet cell type expressing INGAP immunoreactivity was identified. Insulin and INGAP immunoreactivity were mutually exclusive; no INGAP-immunoreactive cell expressed insulin. Further, destruction of β-cells using STZ did not eliminate INGAP-immunoreactive cells. The cell types most characterized around the mantel of the mouse islet are the glucagon-expressing α-cells and the Schwann-like GFAP-positive cells (Sunami et al. 2001). Glucagon expression and INGAP immunoreactivity coassociated with the same islet cells in both normal and STZ-induced diabetic mice, whereas the cell morphology of GFAP-positive cells and INGAP-immunoreactive cells were distinct (not shown). Thus, INGAP immunoreactivity is localized to the islet α-cell. The possibility of cross-reactivity between the anti-INGAP antibody and glucagon was excluded. Although the peptide antigens glucagon and INGAP(20–40) inhibited the immunoreactivity associated with the antibodies to glucagon and INGAP, respectively, neither cross-inhibited the immunoreactive signal as seen in both islet staining and a developed peptide ELISA. Conclusively, deconvolved imaging of INGAP and glucagon immunoreactivity within α-cells revealed distinct intracellular locations.
The topology of INGAP immunoreactivity in the mouse islet is similar to that described in the hamster islet (Flores et al. 2003). One distinction was that INGAP immunoreactivity in hamsters was described as occurring in only 40% of glucagon-immunoreactive cells. The observation that INGAP immunoreactivity in the pancreatic islet is conserved across a number of species tested is striking. In addition to mouse and hamster, strong cell-specific INGAP immunoreactivity was detected in rat, human, and monkey islets. In all cases, colocalization of glucagon and INGAP immunoreactivity was predominant (not shown). Intriguingly, identification of cells that displayed single immunoreactivity for either INGAP or glucagon was significantly less than that reported for hamster islets. Further, the occurrence of single positive cells differed with species. Whether INGAP-immunoreactive/glucagon-negative cells represent a novel α-cell subtype, a glucagon-depleted α-cell, or a distinct cell type is a focus of our current investigations. Our recent report of INGAP immunoreactivity in the developing mouse pancreas and its coassociation with islet hormones suggests INGAP immunoreactivity may mark a precursor endocrine cell (Hamblet et al. 2008). Consistently, small clusters of INGAP-immunoreactive cells were detected in fetal human pancreas.
The INGAP protein is a member of the Reg superfamily of proteins, which are classified into four groups based on highly conserved sequence and structural motifs (reviewed in Okamoto 1999). INGAP is a group-three protein (Taylor-Fishwick et al. 2003). The INGAP ortholog detected by the INGAP antibody in the species studied is currently unresolved. Mouse Reg IIIδ shares 72% sequence identity with INGAP (Abe et al. 2000); however, its expression pattern is distinct. Endogenous Reg IIIδ predominates in the exocrine pancreas and is not expressed in normal islets (Terazono et al. 1990). Recently, Gurr et al. developed Reg-specific antibodies that confirm the absence of Reg IIIδ in normal islets and identify the expression of Reg IIIα in the mature islet. The expression pattern for Reg IIIα in the islet mirrors that seen with INGAP in the mature islet, even though the immunogenic peptide has only 53% identity to INGAP20–39 (Gurr et al. 2007). Molecular mass of the antigen detected by the INGAP antibody in isolated mouse islets is consistent with Reg family proteins. However, in Western blot analyses, the INGAP antibody detected rINGAP and a rGFP–INGAP fusion protein but did not detect mouse rReg IIIα, mouse rReg IIIδ, or rReg I.
Islet β-cells are responsive to a 15-amino acid bioactive peptide derived from INGAP (Rosenberg et al. 2004). INGAP peptide enhanced glucose-stimulated insulin secretion in isolated islets (Borelli et al. 2005) and upregulated genes associated with the regulation of insulin secretion (Barbosa et al. 2006). This and other data (Tam et al. 2002; Taylor-Fishwick et al. 2006b) expand the actions of INGAP beyond islet neogenesis and highlight the pleiotropy associated with this molecule and peptide. The action of INGAP peptide on insulin secretion provides a mechanistic rationale for the detection of a species-conserved INGAP immunoreactivity in the non-β-cell compartment of islets. This report shows INGAP and/or other related group-three Reg proteins have a conserved expression in pancreatic islets; this could imply a potential role for these proteins in islet function.
Acknowledgments
This work was supported through research grants from the Diabetes Institutes Foundation, Cosmopolitan International, and Virginia's Commonwealth Health Research Board.
References
- Abe M, Nata K, Akiyama T, Shervani NJ, Kobayashi S, Tomioka-Kumagai T, Ito S, et al. (2000) Identification of a novel Reg family gene, Reg IIIδ, and mapping of all three types of Reg family gene in a 75 kilobase mouse genomic region. Gene 246:111–122 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ (2002) Harnessing the therapeutic potential of glucagon-like peptide-1: a critical review. Treat Endocrinol 1:117–125 [DOI] [PubMed] [Google Scholar]
- Barbosa H, Bordin S, Stoppiglia L, Silva K, Borelli M, Del Zotto H, Gagliardino J, et al. (2006) Islet neogenesis associated protein (INGAP) modulates gene expression in cultured neonatal rat islets. Regul Pept 136:78–84 [DOI] [PubMed] [Google Scholar]
- Bell GI, Polonsky KS (2001) Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414:788–791 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Sharma A (2002) Pancreatic stem cells. J Pathol 197:519–526 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A (2004) The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes 5(suppl 2):16–22 [DOI] [PubMed] [Google Scholar]
- Borelli MI, Stoppiglia LF, Rezende LF, Flores LE, Del Zotto H, Boschero AC, Gagliardino JJ (2005) INGAP-related pentadecapeptide: its modulatory effect upon insulin secretion. Regul Pept 131:97–102 [DOI] [PubMed] [Google Scholar]
- Brand SJ, Tagerud S, Lambert P, Magil SG, Tatarkiewicz K, Doiron K, Yan Y (2002) Pharmacological treatment of chronic diabetes by stimulating pancreatic β-cell regeneration with systemic co-administration of EGF and gastrin. Pharmacol Toxicol 91:414–420 [DOI] [PubMed] [Google Scholar]
- De Leon DD, Deng S, Madani R, Ahima RS, Drucker DJ, Stoffers DA (2003) Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 52:365–371 [DOI] [PubMed] [Google Scholar]
- Del Zotto H, Massa L, Rafaeloff R, Pittenger GL, Vinik A, Gold G, Reifel-Miller A, et al. (2000) Possible relationship between changes in islet neogenesis and islet neogenesis-associated protein-positive cell mass induced by sucrose administration to normal hamsters. J Endocrinol 165:725–733 [DOI] [PubMed] [Google Scholar]
- Drucker DJ (2003) Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 17:161–171 [DOI] [PubMed] [Google Scholar]
- Flores LE, Garcia ME, Borelli MI, Del Zotto H, Alzugaray ME, Maiztegui B, Gagliardino JJ (2003) Expression of islet neogenesis-associated protein in islets of normal hamsters. J Endocrinol 177:243–248 [DOI] [PubMed] [Google Scholar]
- Gallwitz B (2006) Therapies for the treatment of type 2 diabetes mellitus based on incretin action. Minerva Endocrinol 31:133–147 [PubMed] [Google Scholar]
- Gold G, Broderick C, Caragna M, Williams G, Pittenger GL, Rafaeloff R, Reifel-Miller A, et al. (1998) INGAP treatment improves glycemic control in streptozotocin diabetic hamsters. Diabetes 47:A253 [Google Scholar]
- Gurr W, Shaw M, Li Y, Sherwin R (2007) RegII is a β-cell protein and autoantigen in diabetes of NOD mice. Diabetes 56:34–40 [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Shi W, Vinik AI, Taylor-Fishwick D (2008) The Reg family member INGAP is a marker of endocrine patterning in the embryonic pancreas. Pancreas 36:1–9 [DOI] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG (2003) Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem 10:2471–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal AM, Lipsett M, Sladek R, Laganiere S, Hanley S, Rosenberg L (2005) Morphogenetic plasticity of adult human pancreatic islets of Langerhans. Cell Death Differ 12:702–712 [DOI] [PubMed] [Google Scholar]
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt GS, Frisk G, Majumdar AS (2007) Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 25:1940–1953 [DOI] [PubMed] [Google Scholar]
- Narushima Y, Unno M, Nakagawara K, Mori M, Miyashita H, Suzuki Y, Noguchi N, et al. (1997) Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIIIα, RegIIIβ, RegIIIγ. Gene 185:159–168 [DOI] [PubMed] [Google Scholar]
- Okamoto H (1999) The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic β-cells. J Hepatobiliary Pancreat Surg 6:254–262 [DOI] [PubMed] [Google Scholar]
- Paris M, Tourrel-Cuzin C, Plachot C, Ktorza A (2004) Review: pancreatic beta-cell neogenesis revisited. Exp Diabesity Res 5:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger GL, Taylor-Fishwick DA, Johns RH, Burcus N, Kosuri S, Vinik AI (2007) Intramuscular injection of islet neogenesis-associated protein peptide stimulates pancreatic islet neogenesis in healthy dogs. Pancreas 34:103–111 [DOI] [PubMed] [Google Scholar]
- Rafaeloff R, Pittenger GL, Barlow SW, Qin XF, Yan B, Rosenberg L, Duguid WP, et al. (1997) Cloning and sequencing of the pancreatic islet neogenesis associated protein (INGAP) gene and its expression in islet neogenesis in hamsters. J Clin Invest 99:2100–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Lipsett M, Yoon JW, Prentki M, Wang R, Jun HS, Pittenger GL, et al. (2004) A pentadecapeptide fragment of islet neogenesis-associated protein increases beta-cell mass and reverses diabetes in C57BL/6J mice. Ann Surg 240:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Vinik AI, Pittenger GL, Rafaeloff R, Duguid WP (1996) Islet-cell regeneration in the diabetic hamster pancreas with restoration of normoglycaemia can be induced by a local growth factor(s). Diabetologia 39:256–262 [DOI] [PubMed] [Google Scholar]
- Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, et al. (2002) Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 51:2148–2157 [DOI] [PubMed] [Google Scholar]
- Song L, Taylor-Fishwick DA, Park I (2005) Chronological expression of INGAP (islet neogenesis associated protein) and clusterin during pancreas regeneration. Diabetes 54:A650 [Google Scholar]
- Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A (2005) Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet β-cells from pancreatic duct cells and an increase in functional β-cell mass. J Clin Endocrinol Metab 90:3401–3409 [DOI] [PubMed] [Google Scholar]
- Sunami E, Kanazawa H, Hashizume H, Takeda M, Hatakeyama K, Ushiki T (2001) Morphological characteristics of Schwann cells in the islets of Langerhans of the murine pancreas. Arch Histol Cytol 64:191–201 [DOI] [PubMed] [Google Scholar]
- Tam J, Rosenberg L, Maysinger D (2002) Islet-neogenesis-associated protein enhances neurite outgrowth from DRG neurons. Biochem Biophys Res Commun 291:649–654 [DOI] [PubMed] [Google Scholar]
- Tam J, Rosenberg L, Maysinger D (2004) INGAP peptide improves nerve function and enhances regeneration in streptozotocin-induced diabetic C57BL/6 mice. FASEB J 18:1767–1769 [DOI] [PubMed] [Google Scholar]
- Taylor-Fishwick DA, Bowman A, Hamblet N, Bernard P, Harlan DM, Vinik AI (2006b) Islet neogenesis associated protein transgenic mice are resistant to hyperglycemia induced by streptozotocin. J Endocrinol 190:729–737 [DOI] [PubMed] [Google Scholar]
- Taylor-Fishwick DA, Rittman S, Kendall H, Roy L, Shi W, Cao Y, Pittenger GL, et al. (2003) Cloning genomic INGAP: a Reg-related family member with distinct transcriptional regulation sites. Biochim Biophys Acta 1638:83–89 [DOI] [PubMed] [Google Scholar]
- Taylor-Fishwick DA, Shi W, Pittenger GL, Vinik AI (2006a) PDX-1 can repress stimulus-induced activation of the INGAP promoter. J Endocrinol 188:611–621 [DOI] [PubMed] [Google Scholar]
- Terazono K, Uchiyama Y, Ide M, Watanabe T, Yonekura H, Yamamoto H, Okamoto H (1990) Expression of reg protein in rat regenerating islets and its co-localization with insulin in the beta cell secretory granules. Diabetologia 33:250–252 [DOI] [PubMed] [Google Scholar]
- Vinik AI, Rosenberg L, Pittenger GL, Taylor-Fishwick DA (2004) Stimulation of pancreatic islet neogenesis: a possible treatment for type 1 and type 2 diabetes. Curr Opin Endocrinol Diabetes 11:125–140 [Google Scholar]