Abstract
Osteoarthritis is a chronic, debilitating joint disease characterized by progressive destruction of articular cartilage. Recently, a number of studies have identified a chondroprogenitor cell population within articular cartilage with significant potential for repair/regeneration. As yet, there are few robust biomarkers of these cells. In this study, we show that monoclonal antibodies recognizing novel chondroitin sulfate sulfation motif epitopes in glycosaminoglycans on proteoglycans can be used to identify metabolically distinct subpopulations of cells specifically within the superficial zone of the tissue and that flow cytometric analysis can recognize these cell subpopulations. Fluorochrome co-localization analysis suggests that the chondroitin sulfate sulphation motifs are associated with a range of cell and extracellular matrix proteoglycans within the stem cell niche that include perlecan and aggrecan but not versican. The unique distributions of these sulphation motifs within the microenvironment of superficial zone chondrocytes, seems to designate early stages of stem/progenitor cell differentiation and is consistent with these molecules playing a functional role in regulating aspects of chondrogenesis. The isolation and further characterization of these cells will lead to an improved understanding of the role novel chondroitin sulfate sulfation plays in articular cartilage development and may contribute significantly to the field of articular cartilage repair. (J Histochem Cytochem 56:125–138, 2008)
Keywords: biomarker, chondroitin sulfate, sulfation motif epitopes, proteoglycans, chondroprogenitor, stem cell niche, immunohistochemistry, confocal microscopy, flow cytometry
Articular cartilage is an avascular, hypocellular hyaline connective tissue that has a limited capacity for self repair. The mature tissue consists of four distinct zones, variously referred to as (a) superficial/tangential; (b) intermediate/mid/transitional; (c) deep/radial; and (d) calcified. The cellular morphology, extracellular matrix (ECM) composition, and collagen fiber organization of each zone differs dramatically throughout the tissue depth, and together they contribute to the overall function of articular cartilage in resisting biomechanical load (reviewed by Poole et al. 2001).
Because of the poor reparative ability of articular cartilage, once damaged, tissue destruction often progresses spatiotemporally from the articular surface to the subchondral bone. This leads, inevitably, to a loss of zonal tissue architecture and painful, limited joint movement, made manifest as degenerative joint disease or osteoarthritis (OA). Although there are a number of promising cell-based strategies to repair articular cartilage [e.g., autologous chondrocyte implantation (ACI); Carticel; Genzyme, Cambridge, MA] (Brittberg et al. 1994), there are none thus far that adequately regenerate (a) the correct hyaline cartilage tissue phenotype and (b) the correct zonal tissue organization. Fundamentally, this is because of the fact that the implanted chondrocytes lack the required developmental repertoire to fulfill their intended purpose (Hayes et al. 2007), a deficiency that could potentially be overcome if a cartilage progenitor/stem cell was used as an alternative cell source (Dowthwaite et al. 2004).
Recently, Dowthwaite et al. (2004) have isolated a progenitor cell from the superficial zone of immature bovine articular cartilage through its high affinity to fibronectin. This cell, previously hypothesized to drive the appositional growth of the tissue (Archer et al. 1994; Hayes et al. 2001b) and positive for the cell fate receptor Notch-1 (Hayes et al. 2003; Dowthwaite et al. 2004), has been shown to exhibit high colony-forming efficiency, high expansion potential, and phenotypic plasticity (Dowthwaite et al. 2004; Martin et al. 2005; Melero-Martin et al. 2006a,b), thus showing significant therapeutic potential for cell-based articular cartilage repair procedures such as ACI. It is important to point out, however, that although this stem cell population expresses the cell fate receptor Notch-1 on its surface, expression of this receptor per se does not specifically designate a chondroprogenitor cell, because it is also present on other non-stem/progenitor cells within the cartilage tissue (Hayes et al. 2003; Dowthwaite et al. 2004).
In humans, stem cells with similar growth characteristics have also been isolated from both normal and OA articular cartilage by fluorescence activated cell sorting (FACS) through immunoreactivity to CD105 (endoglin) and CD166 (ALCAM; neurolin; DM-GRASP; SC-1); cell surface markers whose coexpression defines mesenchymal stem cells (MSCs) in bone marrow and perichondrium (Alsalameh et al. 2004). Although the precise niche of these cells from within the cartilage volume was not clear from the study mentioned earlier, a similar study using immature bovine articular cartilage indicated that these markers are also associated with superficial zone cell subpopulations (Richardson et al. 2006). Additionally, positivity for CD9 (cell differentiation antigen), CD44 (hyaluronic acid receptor), CD54 (ICAM-1), CD90 (Thy-1), and CD166, in various triplicate combinations, has recently been used to sort populations of cells with growth characteristics of progenitors from human OA cartilage (Fickert et al. 2004). Thus, at present, although there is considerable evidence of a stem/progenitor cell niche within articular cartilage, there is still no definitive biomarker of these cells.
In previous studies (Sorrell et al. 1988,1990; Caterson et al. 1990), we identified several monoclonal antibodies (MAbs) that recognize specific disaccharide/oligosaccharide sulfation motif (SM) epitopes within the linear framework of chondroitin sulfate (CS) glycosaminoglycans [GAGs; i.e., MAbs 3B3(−), 7D4, 6C3, and 4C3]. In these studies, we showed specific immunolabeling patterns at sites of lymphopoeisis in the developing Bursa of Fabricius that corresponded with distinct stem, progenitor, and stromal cell populations. Some of these MAbs have also been used to identify cellular repair responses in the pathogenesis of degenerative joint diseases such as OA (Caterson et al. 1990,1995; Carney et al. 1992; Ratcliffe et al. 1993; Visco et al. 1993; Slater et al. 1995). Interestingly, in these latter studies, MAbs 7D4 and 3B3(−) specifically identified clusters of proliferating chondrocytes in fibrillated OA cartilage tissue. More recently, these cell clusters have also been shown positive for both Notch 1 and CD166 (Hiraoka et al. 2006; Redman et al. 2006), which are cell surface markers that are synonymous with the stem cell niche (SCN), within the superficial zone of healthy articular cartilage (Hayes et al. 2003; Alsalameh et al. 2004; Dowthwaite et al. 2004; Richardson et al. 2006).
Numerous studies have now shown the importance of CS and heparan sulfate (HS) GAGs on cell and ECM proteoglycans (PGs) in the binding of a variety of cell signaling molecules (e.g., growth and differentiation factors, cytokines, chemokines, and enzymes) (Deepa et al. 2002; Kawashima et al. 2002; Bao et al. 2004; Nandini et al. 2004; Rapp et al. 2005; Tiedemann et al. 2005). Significantly, it is the presence of specific sulfated saccharide motifs within the GAG chains that allow the binding and regulation of many of these signaling molecules, thereby regulating the intracellular signaling pathways that drive cell behaviors such as cell proliferation, differentiation, and matrix synthesis (Tiedemann et al. 2005; Johnson et al. 2007).
In this study, we set out to examine the possibility that novel SMs on CS-containing PGs may be associated with the stem/progenitor cell niche of articular cartilage and whether MAbs toward these SMs could be used for cell sorting purposes. Using a bovine model, as used previously (Dowthwaite et al. 2004), we report that (a) monoclonal antibodies 3B3(−), 4C3, and 7D4 can be used to identify metabolically distinct subpopulations of cells from within the SCN of articular cartilage (the latter two MAbs showing significant potential for cell sorting), which, we believe, designate early stages of stem/progenitor cell differentiation; and (b) that within this niche, these SMs seem to be associated with the CS GAG chains on a range of PGs, which include perlecan and aggrecan but not versican. Overall, our data suggest that, within the superficial zone of articular cartilage, novel CS sulfation confers functional specialization to a range of PGs within the SCN and may be involved in regulating cell behavior.
Materials and Methods
Source of Tissue
Articular cartilage was obtained from the lateral and medial condyles of hock joints of 7-day-old bovines.
Immunohistochemistry
Full-thickness cartilage tissue was fixed overnight in cold 75% ethanol, washed and cryoprotected in PBS containing 5% sucrose for 1 hr, and snap frozen onto cryostat chucks in Cryo-M-Bed tissue mountant (Bright Instruments; Cambridgeshire, UK). Frozen sections were cut and labeled by standard indirect immunofluorescence procedures with a panel of antibodies recognizing CS SM epitopes and CS chain PGs (see Table 1 for antibody details). All immunoreagents were diluted in 0.05 M PBS containing 0.1% TWEEN 20 (Sigma-Aldrich; Dorset, UK), which was also used as a wash buffer. For controls, sections were first enzymatically deglycosylated by pretreatment with 0.5 U/ml chondroitinase ABC (Sigma-Aldrich) in 100 mM Tris acetate buffer (pH 7.4) for 1 hr at 37C to eliminate the CS chains recognized by MAbs 3B3, 7D4, and 4C3.
Table 1.
Antibody details
| Antibody (dilution) | Clone (isotype) | Specificity | Source/reference |
|---|---|---|---|
| 3B3 (1:100) | Monoclonals (IgM, κ) | Distinct “native” CS sulphation motifs | Sorrell et al. 1988; Caterson et al. 1990 |
| 7D4 (1:100) | |||
| 4C3 (1:100) | |||
| 6C3 (1:100) | |||
| 1948(1:100) | Monoclonal (IgG2a) | Perlecan core protein (domain 4) | Chemicon |
| 6B4 (1:100) | Monoclonal (IgG, κ) | Aggrecan (interglobular domain) | Abcam |
| 12C5 (1:5) | Monoclonal (IgG1) | Versican (HA-binding region) | DSHB |
| PR1(1:20) | Monoclonal (IgG) | Biglycan (core protein) | Rees et al. 2000 |
| DS1 (1:5) | Monoclonal (IgG1) | Decorin (core protein) | DSHB |
CS, chondroitin sulphate; HA, hyaluronic acid; DSHB, Developmental Studies Hybridoma Bank.
Fluorescent Labeling of CS SM Epitopes
Sections were washed, treated with blocking serum (Dakopatts Ltd.; High Wycombe, UK) at 1:20 dilution for 30 min at room temperature, and incubated overnight at 4C with primary antibody (Table 1). Monoclonal antibodies 12C5 (Asher et al. 1991) and DS1 (Poole et al. 1986) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA. After washing, sections were incubated with FITC-conjugated goat anti-mouse F(ab)2 fragments (Dakopatts) for 30 min, counterstained with propidium iodide (0.5 μg/ml; Molecular Probes, Invitrogen, Paisley, UK) for 5 min, and mounted under coverslips in Vectashield mountant (Vector Laboratories; Peterborough, UK). Control sections that were incubated with either PBS or 10 μg/ml mouse immunoglobulins (Sigma-Aldrich) instead of primary antibody, with or without the enzymatic digestion step, showed no nonspecific labeling with either primary or secondary antibody.
Dual Labeling of CS SMs and PG Core Proteins
Cryosections of articular cartilage were dual labeled for different PG/CS epitope combinations using isotype-specific secondary antibodies. Because we had shown aggrecan, versican, and perlecan core proteins to be prominent within the superficial zone, suggestive of potential association with the CS SM epitopes under study, we consequently focused on these PGs, in particular, for dual-labeling experiments. Briefly, sections were first blocked in goat serum (1:20; Dakopatts) for 30 min at room temperature before overnight incubation at 4C with a MAb toward each PG core protein (Table 1). Sections were washed in buffer and incubated with goat anti-mouse F(ab)2 IgG Alexa 488 secondary antibody (1:400; Molecular Probes) for 1 hr at room temperature. After a second blocking step, sections were incubated with the anti-CS SM IgM antibody for 2 hr at room temperature (Table 1), washed in buffer, and incubated with a goat anti-mouse IgM (μ chain)-specific Alexa 594 sec secondary antibody (1:400; Molecular Probes) for 1 hr at room temperature. For nuclear context, some tissue sections were fluorescently stained with Hoechst 33258 (1 μg/ml; Sigma-Aldrich) before mounting in Vectashield mountant (Vector Laboratories). Dual-labeled sections were viewed under conventional epifluorescence illumination or by confocal laser scanning microscopy (see below).
Epifluorescent Image Analysis
To identify potential associations between different PG core proteins and different CS SM epitopes, the labeling patterns of green (Alexa 488) and red (Alexa 594) fluorochromes were compared using the Image J public domain Java image processing software (http://rsb.info.nih.gov/ij/). Epifluorescent images were split into their red, green, and blue components, and the degree of red/green overlap highlighted using the Image J Co-localization plug in (written by Pierre Bourdoncle, Institut Jacques Monod, Service Imagerie, Paris; http://rsb.info.nih.gov/ij/plugins/co-localization.html). Two points were considered co-localized in the image if their respective intensities were higher than the threshold value (100) of their channels and if the ratio of their intensity was higher than the ratio setting value (50%). Applying this criteria, regions of red-green co-localization were highlighted on the original epifluorescence images as a white overlay mask.
Confocal Laser Scanning Microscopy
Confocal microscopy of dual-labeled tissue sections was performed using a Leica TCS SP2 AOBS confocal laser scanning microscope (Leica; Wetzlar, Germany). Series of optical sections (z-stacks) were taken through dual-labeled tissue sections at a spacing of ∼0.5 μm using a ×63 oil immersion objective. To eliminate the possibility of spectral cross-talk, fluorochromes were scanned sequentially using scan parameters optimized for the excitation and detection of either Alexa 488 (green) or Alexa 594 (red) fluorochromes. The frequency distributions of fluorescent intensities from green and red fluorochromes were analyzed using the proprietary Leica Confocal Software (Leica; Wetzlar, Germany) and presented both as scatter plots (cytofluorograms) and pixel intensity profiles.
Flow Cytometry
To obtain enriched populations of superficial zone cells for flow cytometry, thin slices of cartilage (∼300 μm thick) were taken from the articular surface and digested sequentially in 7 U/ml pronase (Roche; Hertfordshire, UK) for 3 hr at 37C, followed by overnight digestion in 100 U/ml type II collagenase (Worthington Biochemical Co., Berkshire, UK). Suspensions of cells were washed in PBS and indirectly labeled with Alexa 488 (Molecular Probes) at 4C using monoclonal antibodies 3B3(−), 4C3, and 7D4 as described in the preceding section, but with the following modifications: all immunoreagents were diluted in 0.05 M PBS (pH 7.4) without TWEEN, and all antibody incubation steps were for 15 min. Mouse IgG (Sigma-Aldrich) was used as an isotype control antibody. Labeled suspensions of cells were analyzed on a flow cytometer (FACSCalibur; Becton Dickinson UK, Plymouth, UK) with CellQuest software (Becton Dickinson UK). Twenty thousand to 30,000 events were registered per sample, and analysis of whole cells was performed using appropriate scatter gates to avoid cellular debris and aggregates. Propidium iodide (Molecular Probes) was used in unstained samples to monitor the viability of the chondrocytes and acted as a guide for the scatter gate. The mean fluorescence intensity (MFI) of the positive cell population analyzed using the data acquired from flow cytometry histograms was used as an indication of the density of monoclonal antibody binding to the chondrocytes. MFI values were obtained by subtraction of the MFI of the negative control population from the MFI of the positively stained population.
Results
CS SM Epitopes
The immunohistochemical (IHC) labeling patterns revealed that all three CS SM MAbs strongly immunolocated distinct and unique SM epitopes in the microenvironment of superficial zone chondrocytes (Figures 1A, 1D, and 1G). The IHC labeling pattern for MAb 3B3(−) was the most subtle of the three antibodies (Figures 1A–1C). It identified a CS SM within the pericellular milieu of a small subpopulation of chondrocytes within the superficial zone, exhibiting a distinctive punctuate staining pattern in histological sections (Figure 1A). Punctate pericellular staining of cells was also observed within the zone of calcified cartilage matrix but not in the overlying uncalcified tissue (Figure 1C). The staining pattern observed with MAb 7D4 (Figures 1D–1F) was distinct from that of 3B3(−) and more widespread. MAb 7D4 strongly identified a CS SM both at the articular surface, as a thin band of immunolabel, and within the pericellular matrix of the superficial zone to a depth of two to three cells (Figure 1D). No immunolabeling was present in deeper zones of the cartilage tissue (Figures 1E and 1F); however, a specific population of cells surrounding sites of vascular ingression, possibly microvascular pericytes, were positive for the CS epitope recognized by this antibody (Figure 1F, inset). The CS epitope recognized by MAb 4C3 (Figures 1G–1I) extended to a greater depth from the articular surface than that recognized by MAb 7D4 (Figure 1G). This antibody also labeled the pericellular matrix of cells in the calcified cartilage matrix but not the overlying uncalcified cartilage tissue (Figure 1I). Comparison of sections with negative controls labeled with either naive immunoglobulin or secondary antibody alone showed no nonspecific labeling (Figures 2A–2C). Similarly, after deglycosylation with chondroitinase ABC, the specific labeling patterns were lost (data not shown). Dual-labeling studies confirmed that the three CS SM epitopes had distinct, but overlapping, distributions within the superficial zone (data not shown).
Figure 1.
Distribution of novel chondroitin sulfate (CS) sulfation motif (SM) epitopes (green labeling) in immature bovine articular cartilage after immunohistochemical (IHC) labeling with monoclonal antibodies 3B3(−) (A–C), 7D4 (D–F), and 4C3 (G–I). Cell nuclei shown in red. Arrowheads in A,D,G denote pericellular labeling associated with subpopulations of superficial zone cells. Inset in F shows IHC labeling of microvascular pericytes in the calcified zone with monoclonal antibody (MAb) 7D4. Bar = 50 μm.
Figure 2.
Distribution of large aggregating proteoglycans (PGs) (hyalectins), aggrecan, and versican (green labeling) in immature bovine articular cartilage. Cell nuclei shown in red. (A–C) Unstained IHC negative control (mouse immunoglobulin). (D–F) Aggrecan is detectable throughout the cartilage matrix, but is particularly prominent pericellularly within the superficial zone (arrowheads) and also around chondrocytes at the mineralization front within the calcified zone. (G–I) IHC labeling of versican occurs specifically within the interterritorial matrix compartment of the superficial zone. Apart from at sites of vascular ingression (asterisk), versican is absent from the underlying cartilage zones. Bar = 50 μm.
CS PGs
Lecticans
Aggrecan was highly prominent within the pericellular matrix of both the superficial and deep/calcified zones (Figures 2D and 2F), suggesting potential overlap with the CS SM epitopes under study. This lectican was also diffusely distributed throughout the tissue depth within the interterritorial matrix (Figure 2D–2F). In contrast, versican was present only within the superficial zone (Figure 2G) and absent from the underlying cartilaginous strata (Figures 2H and 2I). Unlike aggrecan, however, it was absent from the pericellular matrix compartment, occurring instead within the interterritorial matrix (Figure 2G), further supported by confocal microscopic examination (see below). Weak immunolabeling for versican was also seen at sites of vascular ingression in the calcified zone of the tissue (Figure 2I).
HS-PG2
Perlecan was strongly detectable in the pericellular milieu throughout the cartilage depth with diffuse weak labeling of the interterritorial matrix (Figures 3A–3C). Pericellular labeling of this PG was particularly prominent in the superficial zone (Figure 3A), suggesting potential overlap with the CS SM epitopes under study.
Figure 3.
Distribution of HSPG2, perlecan, and the small leucine-rich PGs, biglycan, and decorin (green labeling) in immature bovine articular cartilage. Cell nuclei shown in red. (A–C) Perlecan has a strong pericellular presence throughout the tissue but is particularly prominent in the superficial zone (arrowheads). (D–F) Biglycan is detectable throughout the uncalcified cartilage matrix, but within the calcified zone, it has a mainly pericellular distribution. (G–I) Decorin also occurs throughout the uncalcified cartilage matrix, but within the calcified zone, it is present mainly within the territorial matrix compartment. Bar = 50 μm.
Small Leucine-rich PGs
Biglycan (Figures 3D–3F) and decorin (Figures 3G–3I) were both distributed relatively homogeneously throughout the matrix in the upper two thirds of the cartilage depth; however, they had quite distinct distributions in the lower third of the tissue. Biglycan was highly prominent in the pericellular matrix of the deep and calcified zones (Figure 3F), whereas decorin seemed to be associated more with the territorial and interterritorial matrix compartments (Figure 3I).
Fluorochrome Co-localization Analysis
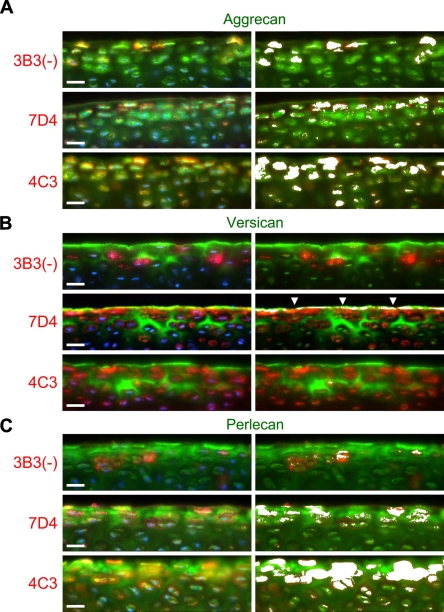
Dual-labeling studies indicated that both aggrecan (Figure 4A) and perlecan (Figure 4C), but not versican (Figure 4B), had potential association with the CS SM epitopes 3B3(−), 7D4, and 4C3 within the pericellular milieu of the superficial zone. Interestingly, although versican was absent from the pericellular matrix, this lectican frequently exhibited significant overlap with the CS SM epitope recognized by MAb 7D4 at the articular surface (Figure 4B). Of the three CS SM MAbs, 4C3 in particular co-localized prominently with aggrecan (Figure 4A) and perlecan (Figure 4C) within the pericellular matrix of the superficial zone, suggesting that either PG might carry the CS SM epitope recognized by this MAb. The labeling patterns for 7D4, and to a lesser extent 3B3, also displayed some degree of overlap with both aggrecan and perlecan (Figures 4A and 4C, respectively); however, there appeared to be fewer chondrins positive for these CS-PG epitope combinations.
Figure 4.
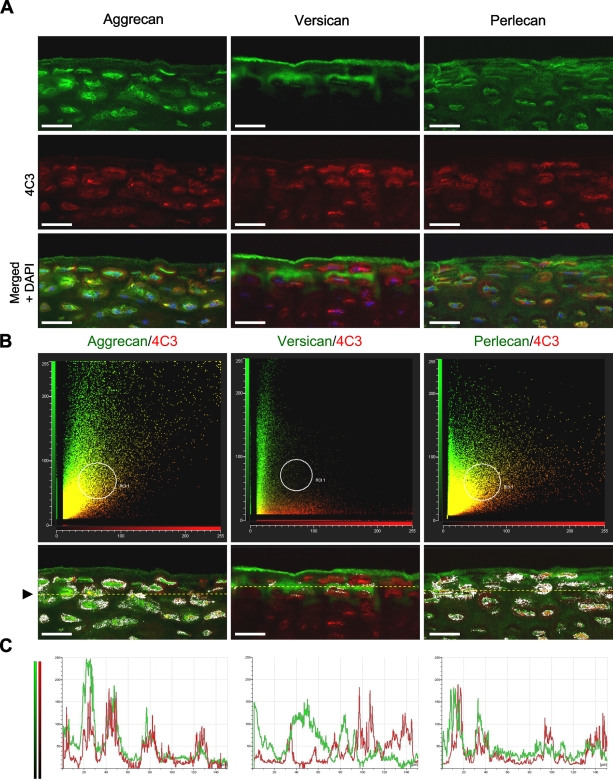
Dual labeling of the superficial zone of immature bovine articular cartilage with MAbs toward novel CS SMs [3B3(−), 7D4, and 4C3] and aggrecan (A); versican (B), and perlecan core proteins (C). CS SMs are shown in red (Alexa 594), and PG core proteins are shown in green (Alexa 488). Cell nuclei are depicted in blue (left side only). Regions of red-green co-localization are highlighted by a white overlay mask (right side only). Within the pericellular matrix, all three CS SM epitopes display some degree of overlap with both aggrecan and perlecan, but not versican, core proteins, with the 4C3 CS SM epitope showing the greatest co-localization of the three CS epitopes. Although versican occupies a distinct (interterritorial) matrix compartment, it shares some degree of overlap with the 7D4 CS SM epitope, as a thin band of IHC label at the articular surface (arrowheads). Bar = 25 μm.
To eliminate the possibility that fluorescence outside the focal plane of interest was contributing to the observed overlap in fluorescent signals, tissue sections dual labeled for aggrecan and 4C3 were “optically sectioned” by confocal microscopy. Also, to reduce the risk of spectral bleed-through, green (Alexa 488) and red (Alexa 594) fluorochromes were scanned sequentially using scan parameters optimized for the narrow excitation and emission bands of each probe. Confocal evaluation confirmed potential association of the 4C3 CS SM epitope with both aggrecan and perlecan, but not versican, specifically within the pericellular matrix of the superficial zone (Figure 5). Nonetheless, although the distributions of these PGs were similar to 4C3, they were not identical (Figure 5A), suggesting that other CS-PGs might also carry the 4C3 epitope. A comparison of scatter plots (cytofluorograms) that showed the frequency distributions of fluorescent intensities from green (Alexa 488) and red (Alexa 594) fluorochromes confirmed the similarities observed in the IHC labeling patterns, described above (Figure 5B). The convergent distributions of red and green pixels, made manifest as a yellow diagonal flare in the cytofluorograms, indicated that both aggrecan and perlecan, but not versican (which showed a divergent distribution with 4C3), had potential association with this CS SM epitope. Furthermore, analysis of the masked cytofluorogram revealed prominent red/green co-localization, specifically within the pericellular matrix compartment. A comparison of pixel intensity profiles of green and red fluorochromes along arbitrary line segments through the superficial zone (Figure 5C) also showed similarities in the fluorescent signals of both aggrecan and perlecan with 4C3, whereas versican had an almost reciprocal distribution.
Figure 5.
Confocal co-localization analysis of the 4C3 CS SM epitope with aggrecan, versican, and perlecan core proteins. (A) Confocal images showing labeling patterns associated with each PG core protein (green) and MAb 4C3 (red). (B) Cytofluorograms showing the frequency distributions of fluorescent intensities from green and red fluorochromes. The yellow diagonal flares denote regions of co-localization. The low-intensity overlapping signal (yellow pixels) evident within the versican/4C3 cytofluorogram represents weak background and electronic noise. Co-localized pixels, as defined within region of interest 1 (area defined by white circle), are represented as a white overlay mask in the underlying cartilage images. Arrowhead demarcates position of arbitrary line segment (yellow dotted line) made through the superficial zone to establish pixel intensity profiles of red and green fluorochromes. (C) Plots comparing the pixel intensity profiles of green and red fluorochromes across line segment (yellow dotted line denoted by arrowhead; above). Note similarity in intensity profiles for both aggrecan and 4C3 and also for perlecan and 4C3. Versican, in contrast, has an almost reciprocal distribution. Bar = 25 μm.
Flow Cytometric Analysis of CS SM Epitopes
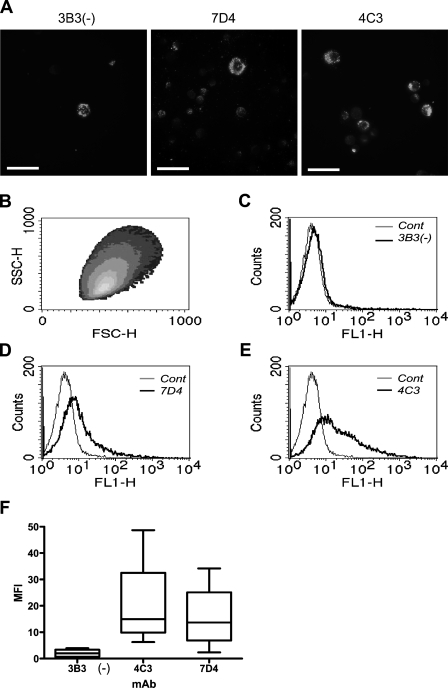
The results obtained from flow cytometric analysis of isolated chondrocytes immunolabeled with MAbs 3B3(−), 7D4, and 4C3 closely reflected the IHC labeling patterns observed in histological sections with these antibodies (Figure 6). Superficial zone chondrocytes showed very little staining with MAb 3B3(−) [mean MFI: 2.05 ± 0.83 (SEM); Figures 6A, 6C, and 6F]; however, these cells showed greater density of MAb binding when labeled with MAb 7D4 (MFI: 15.97 ± 6.69; Figures 6A, 6D, and 6F), with MAb 4C3 showing the greatest labeling (MFI: 21.20 ± 9.40; Figures 6A, 6E, and 6F).
Figure 6.
Isolation and flow cytometric analysis of surface-isolated chondrocytes. (A) Suspensions of chondrocytes from the superficial zone IHC labeled with MAbs 3B3(−), 7D4, and 4C3. Bar = 25 μm. (B) Density scatter plot of gated population. (C–E) Histograms showing labeling of cells for each antibody. (F) Box and whiskers plot of immunoreactivity (mean fluorescence intensity) to each antibody (n=4). Box shows the 25% and 75% percentile and median (horizontal line) values. Bars show upper and lower extremes.
Discussion
In this study, we used MAbs [3B3(−), 4C3, and 7D4] that recognize distinct SM epitopes in CS GAG chains to identify subpopulations of cells within the superficial zone of articular cartilage, which, we believe, designate early stages of stem/progenitor cell differentiation. Differences observed in the IHC labeling patterns of each antibody were subtle yet distinct. The CS epitope recognized by MAb 3B3(−) was expressed pericellularly by only a small subset of chondrocytes within the superficial zone; the CS epitope recognized by MAb 7D4 occurred both at the articular surface and pericellularly to a depth of two to three cells; and the epitope detected by MAb 4C3, although having a similar distribution to 7D4, extended to a slightly greater depth within the tissue. The IHC labeling patterns thus indicated that superficial zone cells differentially modify the composition of their GAG chains (i.e., they differentially regulate the enzymes that modify sugar residues within their GAG chains) (Tiedemann et al. 2005) on their PGs in a highly specific, spatially distinct manner. Furthermore, on this basis, there are at least three metabolically distinct subpopulations of chondrocytes resident within the superficial zone, which has previously been identified as an important stem cell environment in articular cartilage (Dowthwaite et al. 2004).
Novel CS epitopes recognized by MAbs 3B3(−) and 7D4 are widely synonymous with both naturally acquired and experimentally induced OA. In such cases, they are associated with clusters of proliferating chondrocytes and are believed to represent a cellular repair response (Carney et al. 1992; Ratcliffe et al. 1993; Visco et al. 1993; Roberts et al. 1994; Caterson et al. 1995; Slater et al. 1995). However, unusual CS SM epitopes are also expressed at many zones of growth and differentiation during embryonic development and in normal tissue homeostasis (Caterson et al. 1990). Novel CS sulphation sequences occur in the functionally distinct layers of skin (Sorrell et al. 1990); they are associated with the growth plate in developing long bones (Caterson et al. 1990; Gibson et al. 1996) and occur at important growth zones in the developing intervertebral disc (Hayes et al. 2001a). During lymphopoeisis, CS chains are differentially modified at sites of B-cell differentiation and maturation (Sorrell et al. 1988; Caterson et al. 1990), and in the brain, CS sulfation plays an important role in neurite outgrowth, synaptic plasticity, and neurological development (Rhodes and Fawcett 2004).
Recent studies have established the importance of CS chains on PGs in the binding of growth factors, cytokines, chemokines, enzymes, and adhesion molecules (Deepa et al. 2002; Kawashima et al. 2002; Bao et al. 2004; Nandini et al. 2004; Rapp et al. 2005; Tiedemann et al. 2005). CS is composed of repeating disaccharide units of glucuronic acid and N-acetylgalactosamine. The hydroxyl groups on these disaccharide units can be differentially sulfated on the 2-position of the glucuronosyl residue and the 4- or 6-positions of the N-acetyl galactosamine, thus producing enormous structural heterogeneity within the molecule. Because of their high sulfate and carboxyl group content, CS chains have a strong negative charge and thus an inherent ability to attract positively charged matrix molecules (e.g., growth factors, cytokines) through electrostatic interaction. However, CS-containing PGs can also interact with a wide variety of matrix molecules in a highly specific manner that is dependent on the precise carbohydrate composition of their CS chains (Deepa et al. 2002; Kawashima et al. 2002; Nandini et al. 2004; Tiedemann et al. 2005). In binding biologically active molecules through unique sulfation sequence motifs, GAG chains on PGs thus have the potential to directly influence many cell behaviors including cell proliferation, differentiation, migration, and matrix secretion in a highly specific way (Rapp et al. 2005). For example, Johnson et al. (2007) have recently showed dynamic changes in the sulphation pattern of cell-associated HS GAG chains, from low to high sulphation isoforms, during the transition of embryonic stem cells to differentiated cell types. These changes, mediated by “early” (N-sulfotransferases) and “late” (6O- and 3O-sulfotransferases) sulfotransferases, were shown in their study to result in an improved ability of the HS chains to bind fibroblast growth factor 2 (FGF2), which is an important growth factor involved in pluripotency and cell differentiation. In addition, recent work by Tiedemann et al. (2005) has also shown that soluble growth factors such as transforming growth factor (TGF)β1 can themselves regulate the detailed polysaccharide structure of CS GAG chains—and thus their emergent biological properties—by controlling the relevant sulfotransferase enzymes involved in their biosynthesis.
In the superficial zone of articular cartilage, the CS GAG chains of both ECM and cell-associated PGs may interact with a wide range of soluble signaling molecules (e.g., growth factors, cytokines) within the ECM that surrounds stem/progenitor cells (Figure 7). The superficial zone is known to be an important signaling center that contains members of the TGF-β, insulin-like growth factor (IGF), and FGF families of growth and differentiation factors (Archer et al. 1994; Hayes et al. 2001b; Vincent and Saklatvala 2006; Vincent et al. 2007). Once bound, these growth factors may either be sequestered within the ECM, thus protecting them from proteolytic degradation, or alternatively, activate specific receptors on the cell surface, thereby initiating signal transduction pathways regulating stem cell behavior. Similar signaling mechanisms may occur at the cartilage bone interface, where novel CS SMs are associated with cells at the mineralization front [i.e., the 3B3(−) and 4C3 motifs], and also with cells surrounding sites of vascular invasion in the calcified zone (i.e., the 7D4 motif), suggesting that these CS SMs may also play important roles in bone formation. This is supported by a recent study (Ling et al. 2006), which has shown that sulphated CS GAGs mediate the effects of FGF2 on the osteogenic potential of osteoprogenitor cells. Whether the CS SM [3B3(−) and 4C3]–positive cells detected at the mineralization zone in this study represent osteoprogenitors remains uncertain; however, the highly specific distribution of 7D4-positive cells around invading blood vessels suggests strongly that these cells are microvascular pericytes (Canfield et al. 2000), which are known to possess multipotential stem cell activity (Canfield et al. 2000; Farrington-Rock et al. 2004).
Figure 7.
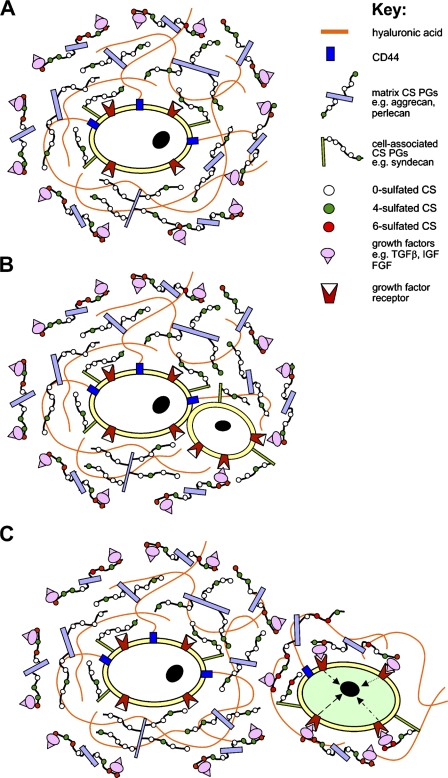
A hypothetical model showing the proposed role of differential CS sulfation of matrix and cell-associated PGs in forming a stem cell niche and thereby regulating the proliferation/differentiation state of stem/progenitor cells. (A) Stem cells are protected from the influence of growth factors by a shield of hyaluronic acid and proteoglycans containing non-sulphated or minimally sulphated CS glycosaminoglycans that prevent their binding with cell surface receptors. (B) During stem/progenitor cell division, one of the daughter cells is translocated outside the pericellular shield of non- and minimally sulphated CS proteoglycans, where it becomes susceptible to binding of growth factors. (C) This daughter cell is now completely removed from the stem cell niche, and growth factor–receptor binding leads to cell differentiation/proliferation (green cell). The parent/daughter cell, meanwhile, remains within the low sulphated CS-PG–shielded niche.
Previous studies have indicated that the epitopes recognized by MAbs 3B3(−) and 7D4 recognize non- and low-sulphated isoforms of CS, respectively (Couchman et al. 1984; Caterson et al. 1995; Ong-Chai 1999). Thus, the presence of ECM and cell-associated PGs carrying these lesser-sulphated GAGs, along with hyaluronan bound to CD44 receptors, around stem/progenitor cells may form a physical and biochemical barrier that prevents growth factor presentation and receptor binding (i.e., we suggest that within this SCN stem/progenitor cells are buffered from the influence of growth factors and other soluble signaling molecules by a shield of non-sulphated or minimally 4-sulphated CS-PGs and hyaluronic acid; Figure 7A). When cells are translocated out of this niche, as occurs during cell division (or, alternatively, in response to insult; for example, during repair/regenerative responses), the daughter cell becomes exposed to growth factors that are bound to more extensively sulphated PGs, whereas the parent cell remains protected within the SCN (Figure 7B). The subsequent interaction of the daughter cell with growth factors outside of the SCN results in activation of intracellular signaling pathways that drive cellular behaviors such as proliferation and differentiation (Figure 7C). Data supporting this hypothesis, that the SCN is composed of PGs with non- or undersulphated GAG chains and hyaluronan, has been reported in several recent publications (Matsumoto et al. 2006; Johnson et al. 2007).
The principal CS-containing PGs in articular cartilage include members of three PG families: the lecticans (hyalecticans), the small leucine-rich PGs (SLRPS), and HS-PG2, perlecan. Members of these families are distributed extensively throughout the ECM, bind a variety of both soluble and insoluble ligands, and can influence a wide variety of cell behaviors (Kresse and Schönherr 2001; Kinsella et al. 2004). Given the large amounts of matrix PG present in articular cartilage, the task of identifying the precise PG core proteins to which each CS SM is associated is particularly difficult, because these motifs are situated within the microenvironment of a minor population of cells within the superficial zone. We have thus compared the IHC distributions of the above PGs with each CS SM epitope [i.e., 3B3(−), 7D4, and 4C3) to identify potential associations between these molecules specifically within this location. Intriguingly, we have shown that the IHC labeling patterns of all three CS antibodies, but particularly MAb 4C3, have spatial similarities with the distributions observed of aggrecan and perlecan (i.e., co-localizing prominently within the pericellular milieu of the superficial zone). The overlapping distributions suggest that these CS motifs, but particularly 4C3, may be associated with the CS GAG chains of both aggrecan and perlecan and that novel CS sulfation may endow functional specialization to these PGs within the pericellular environment of the superficial zone. It is important to stress, however, that these SMs may also be associated with a range of other CS-substituted PGs in the SCN that include decorin and biglycan, as well as other HS-PGs that are associated with the cell surface (e.g., members of the syndecan family). Syndecan 3, for instance, has been shown to modulate the activity of bone morphogenetic protein during cartilage limb differentiation (Fisher et al. 2006) and, significantly, both syndecans 1 and 3 are upregulated in the chondrocyte clusters that are associated with OA (Pfander et al. 2001; Salminen-Mankonen et al. 2005). Indeed, our data suggest that the CS SMs [3B3(−), 7D4, and 4C3] are present on a variety of ECM and cell-associated PGs within the SCN; this is reflected in our co-localization analyses, which, although revealing close similarities in the staining patterns of certain CS SM-PG core protein combinations, clearly do not show mutual exclusivity. It is conceivable, however, that the SMs present on the CS GAG chains may project some way away from the antibody binding site on the PG core proteins and, in this sense, one might not expect a perfect correlation. It remains, nonetheless, that versican seems to lack all three CS SMs, at least pericellularly, because this PG occupies a distinct ECM compartment, occurring exclusively within the interterritorial matrix.
In the developing growth plate, perlecan and aggrecan are generally considered the principal candidate PGs involved in growth factor binding (Govindraj et al. 2002). In this tissue, the HS chains of perlecan, in particular, act as low-affinity coreceptors for the FGFs and ensure correct positioning of the FGFs to their cognate receptors (Knox and Whitelock, 2006; Melrose et al. 2006). As FGF signaling regulates chondrocyte proliferation in the growth plate (Govindraj et al. 2002; Knox and Whitelock 2006), thereby affecting long bone growth, these interactions must be tightly controlled. Recent evidence has shown that this control is provided by CS substitutions made to the perlecan core protein (Govindraj et al. 2002; Smith et al. 2007). Specifically, CS chains on perlecan prevent the HS chains from delivering FGF2 to their cognate receptors, possibly through a mechanism involving steric hindrance (Smith et al. 2007). The addition of CS GAG chains to perlecan thus determines its role as a matrix sink for FGF2 in this tissue context. The CS chains of perlecan have also been shown to bind collagen (Kvist et al. 2006), thus potentially enhancing the pool of perlecan-bound growth factor within the ECM.
Finally, our flow cytometric data revealed that MAbs 4C3 and 7D4 could be used to identify and potentially separate viable cells from the superficial zone with MAb 4C3 showing the greatest staining. Interestingly, the data showed very little staining of isolated chondrocytes with MAb 3B3(−). Thus, the trend closely reflected the IHC labeling patterns observed in histological sections with these antibodies. The cell surface/pericellular localization of the CS chains, recognized by each of the MAbs, suggests that greater yields of labeled cells might be obtainable by FACS using an isolation procedure that would preserve the pericellular matrix compartment intact (i.e., isolation of whole chondrins rather than chondrocytes). Sequential digestion with collagenase and dispase, for example, has been shown to release chondrins from their surrounding ECM in cartilage; however, this method destroys the CS epitope recognized by MAb 7D4 (Lee et al. 1997), making it inappropriate in the current context. Other physical isolation methods such as microaspiration (Alexopoulos et al. 2003) or homogenization and serial dilution (Poole 1990) thus may be of benefit in future studies of this sort.
In summary, this study provides evidence that the superficial zone of immature, bovine articular cartilage contains at least three metabolically distinct subpopulations of cells, unique from the chondrocytes of subjacent cartilaginous zones. These cell populations are identifiable using MAbs 3B3(−), 4C3, and 7D4, which recognize distinct novel SM epitopes in the native CS chains; the latter two MAbs show potential for sorting by FACS. The unique distributions of novel CS GAG chains specifically within the microenvironment of distinct superficial zone subpopulations seems to designate early stages of progenitor cell differentiation and is consistent with these molecules playing a functional role in regulating aspects of chondrogenesis. The overlapping distributions of these molecules with aggrecan and perlecan suggest that novel CS sulfation may contribute to some of the functions attributed to these lecticans, in particular, within the superficial zone of articular cartilage. However, it is likely that these SMs are also present on the CS chains of other ECM and cell-associated PGs. Ongoing work in our laboratory is aimed at using these CS SM antibodies as tools in the identification, isolation, and characterization of stem/progenitor cell populations in a variety of musculoskeletal tissues. These studies will lead to a greater understanding of the role novel CS sulfation plays in tissue development and regeneration in health and disease.
Acknowledgments
This work was supported by Biotechnology and Biological Sciences Research Council (BBSRC) (BBS/B/15414) and Arthritis Research Campaign funding.
References
- Alexopoulos LG, Haider MA, Vail TP, Guilak F (2003) Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng 125:323–333 [DOI] [PubMed] [Google Scholar]
- Alsalameh S, Amin R, Gemba T, Lotz M (2004) Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 50:1522–1532 [DOI] [PubMed] [Google Scholar]
- Archer CW, Morrison H, Pitsillides AA (1994) Cellular aspects of the development of diarthroidal joints and articular cartilage. J Anat 184:447–456 [PMC free article] [PubMed] [Google Scholar]
- Asher R, Perides G, Vanderhaeghen JJ, Bignami A (1991) Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res 28:410–421 [DOI] [PubMed] [Google Scholar]
- Bao X, Nishimura S, Mikami T, Yamada S, Itoh N, Sugahara K (2004) Chondroitin sulfate/dermatan sulphate hybrid chains from embryonic pig brain, which contain a higher proportion of L-iduronic acid than those from adult pig brain, exhibit neuritogenic and growth factor binding activities. J Biol Chem 279:9765–9776 [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895 [DOI] [PubMed] [Google Scholar]
- Canfield AE, Doherty MJ, Wood AC, Farrington C, Ashton B, Begum N, Harvey B, et al. (2000) Role of pericytes in vascular calcification: a review. Z Kardiol 89(suppl 2):20–27 [DOI] [PubMed] [Google Scholar]
- Carney SL, Billingham ME, Caterson B, Ratcliffe A, Bayliss MT, Hardingham TE, Muir H (1992) Changes in proteoglycan turnover in experimental canine osteoarthritic cartilage. Matrix 12:137–147 [DOI] [PubMed] [Google Scholar]
- Caterson B, Hughes CE, Roughley P, Mort JS (1995) Anabolic and catabolic markers of proteoglycan metabolism in osteoarthritis. Acta Orthop Scand Suppl 266:121–124 [PubMed] [Google Scholar]
- Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A, et al. (1990) Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci 97:411–417 [DOI] [PubMed] [Google Scholar]
- Couchman JR, Caterson B, Christner JE, Baker JR (1984) Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307:650–652 [DOI] [PubMed] [Google Scholar]
- Deepa SS, Umehara Y, Higashiyama S, Itoh N, Sugahara K (2002) Specific molecular interactions of oversulfated chondroitin sulphate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J Biol Chem 277:43707–43716 [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, et al. (2004) The surface of articular cartilage contains a progenitor cell population. J Cell Sci 117:889–897 [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE (2004) Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 110:2226–2232 [DOI] [PubMed] [Google Scholar]
- Fickert S, Fiedler J, Brenner RE (2004) Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther 6:R422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA (2006) Heparan sulphate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol 25:27–39 [DOI] [PubMed] [Google Scholar]
- Gibson G, Lin DL, Francki K, Caterson B, Foster B (1996) Type X collagen is colocalized with a proteoglycan epitope to form distinct morphological structures in bovine growth cartilage. Bone 19:307–315 [DOI] [PubMed] [Google Scholar]
- Govindraj P, West L, Koob TJ, Neame P, Doege K, Hassell JR (2002) Isolation and identification of the major heparin sulphate proteoglycans in the developing bovine rib growth plate. J Biol Chem 277:19461–19469 [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR (2001a) Extracellular matrix in development of the intervertebral disc. Matrix Biol 20:107–121 [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Dowthwaite GP, Webster SV, Archer CW (2003) The distribution of Notch receptors and their ligands during articular cartilage development. J Anat 202:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Hall A, Brown L, Tubo R, Caterson B (2007) Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J Histochem Cytochem 55:853–866 [DOI] [PubMed] [Google Scholar]
- Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW (2001b) The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 203:469–479 [DOI] [PubMed] [Google Scholar]
- Hiraoka K, Grogan S, Olee T, Lotz M (2006) Mesenchymal progenitor cells in adult human articular cartilage. Biorheology 43:447–454 [PubMed] [Google Scholar]
- Johnson CE, Crawford BE, Stavridis M, Ten Dam G, Wat AL, Rushton G, Ward CM, et al. (2007) Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells 25:1913–1923 [DOI] [PubMed] [Google Scholar]
- Kawashima H, Atarashi K, Hirose M, Hirose J, Yamada S, Sugahara K, Miyasaka M (2002) Oversulfated chondroitin/dermatan sulfates containing GlcAβ1/IdoAα1-3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J Biol Chem 277:12921–12930 [DOI] [PubMed] [Google Scholar]
- Kinsella MG, Bressler SL, Wight TN (2004) The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr 14:203–234 [DOI] [PubMed] [Google Scholar]
- Knox SM, Whitelock JM (2006) Perlecan: how does one molecule do so many things? Cell Mol Life Sci 63:2435–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresse H, Schönherr E (2001) Proteoglycans of the extracellular matrix and growth control. J Cell Physiol 189:266–274 [DOI] [PubMed] [Google Scholar]
- Kvist AJ, Johnson AE, Mörgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszódi A, et al. (2006) Chondroitin sulphate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem 281:33127–33139 [DOI] [PubMed] [Google Scholar]
- Lee GM, Poole CA, Kelley SS, Chang J, Caterson B (1997) Isolated chondrins: a viable alternative for studies of chondrocyte metabolism in vitro. Osteoarthritis Cartilage 5:261–274 [DOI] [PubMed] [Google Scholar]
- Ling L, Murali S, Dombrowski C, Haupt LM, Stein GS, van Wijnen AJ, Nurcombe V, et al. (2006) Sulfated glycosaminglycans mediate the effects of FGF2 on the osteogenic potential of rat calvarial osteoprogenitor cells. J Cell Physiol 209:811–825 [DOI] [PubMed] [Google Scholar]
- Martin JM, Smith M, Al-Rubeai M (2005) Cryopreservation and in vitro expansion of chondroprogenitor cells isolated from the superficial zone of articular cartilage. Biotechnol Prog 21:168–177 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kamiya N, Suwan K, Atsumi F, Shimizu K, Shinomura T, Yamada Y, et al. (2006) Identification and characterization of versican/PG-M aggregates in cartilage. J Biol Chem 281:18257–18263 [DOI] [PubMed] [Google Scholar]
- Melero-Martin JM, Dowling MA, Smith M, Al-Rubeai M (2006a) Expansion of chondroprogenitor cells on macroporous microcarriers as an alternative to conventional monolayer systems. Biomaterials 27:2970–2979 [DOI] [PubMed] [Google Scholar]
- Melero-Martin JM, Dowling MA, Smith M, Al-Rubeai M (2006b) Optimal in-vitro expansion of chondroprogenitor cells in monolayer culture. Biotechnol Bioeng 93:519–533 [DOI] [PubMed] [Google Scholar]
- Melrose J, Roughley P, Knox S, Smith S, Lord M, Whitelock J (2006) The structure, location, and function of perlecan, a prominent pericellular proteoglycan of fetal, postnatal, and mature hyaline cartilages. J Biol Chem 281:36905–36914 [DOI] [PubMed] [Google Scholar]
- Nandini CD, Mikami T, Ohta M, Itoh N, Akiyama-Nambu F, Sugahara K (2004) Structural and functional characterization of oversulfated chondroitin sulfate/dermatan sulfate hybrid chains from the notochord of hagfish. Neuritogenic and binding activities for growth factors and neurotrophic factors. J Biol Chem 279:50799–50809 [DOI] [PubMed] [Google Scholar]
- Ong-Chai S (1999) Investigation of the Epitope in Chondroitin Sulphate Chains Recognised by Monoclonal Antibody 7D4. PhD Dissertation. School of Biological Sciences, University of Manchester, Manchester, UK
- Pfander D, Swoboda B, Kirsch T (2001) Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritc chondrocytes. Am J Pathol 159:1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S (2001) Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res 391:S26–33 [DOI] [PubMed] [Google Scholar]
- Poole AR, Webber C, Pidoux I, Choi H, Rosenberg LC (1986) Localization of a dermatan sulphate proteoglycan (DS-PGII) in cartilage and the presence of an immunologically related species in other tissues. J Histochem Cytochem 34:619–625 [DOI] [PubMed] [Google Scholar]
- Poole CA (1990) Chondrins isolated from articular cartilage: methods and applications. In Maroudas A, Kuettner KE, eds. Methods in Cartilage Research. London, Academic Press, 78–83
- Rapp A, Brandl N, Huettinger M (2005) Evaluation of chondroitin sulfate bioactivity in hippocampal neurones and the astrocyte cell line U373: influence of position of sulfate groups and charge density. Basic Clin Pharmacol Toxicol 96:37–43 [DOI] [PubMed] [Google Scholar]
- Ratcliffe A, Shurety W, Caterson B (1993) The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum 36:543–551 [DOI] [PubMed] [Google Scholar]
- Redman SN, Bateman N, Haughton LM, Dowthwaite GP, Williams AS, Archer CW (2006) Evidence of a chondroprogenitor population in human osteoarthritic cartilage. Eur Cell Mater 12(suppl 1):69 [Google Scholar]
- Rees SG, Flannery CR, Little CB, Hughes CE, Caterson B, Dent CM (2000) Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J 350:181–188 [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Fawcett JW (2004) Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat 204:33–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K, Khan IM, Archer CW (2006) Identifying progenitor cells within articular cartilage. Eur Cell Mater 12(suppl 1):70 [Google Scholar]
- Roberts S, Caterson B, Evans H, Eisenstein SM (1994) Proteoglycan components of the intervertebral disc and cartilage endplate: an immunolocalization study of animal and human tissues. Histochem J 26:402–411 [DOI] [PubMed] [Google Scholar]
- Salminen-Mankonen H, Säämänen AM, Jalkanen M, Vuorio E, Pirilä L (2005) Syndecan-1 expression is upregulated in degenerating articular cartilage in a transgenic mouse model for osteoarthritis. Scand J Rheumatol 34:469–474 [DOI] [PubMed] [Google Scholar]
- Slater RR Jr, Bayliss MT, Lachiewicz PF, Visco DM, Caterson B (1995) Monoclonal antibodies that detect biochemical markers of arthritis in humans. Arthritis Rheum 38:655–659 [DOI] [PubMed] [Google Scholar]
- Smith SM, West LA, Govindraj P, Zhang X, Ornitz DM, Hassell JR (2007) Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors. Matrix Biol 26:175–184 [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Lintala AM, Mahmoodian F, Caterson B (1988) Epitope-specific changes in chondroitin sulfate/dermatan sulfate proteoglycans as markers in the lymphopoietic and granulopoietic compartments of developing bursae of Fabricius. J Immunol 140:4263–4270 [PubMed] [Google Scholar]
- Sorrell JM, Mahmoodian F, Schafer IA, Davis B, Caterson B (1990) Identification of monoclonal antibodies that recognize novel epitopes in native chondroitin/dermatan sulfate glycosaminoglycan chains: their use in mapping functionally distinct domains of human skin. J Histochem Cytochem 38:393–402 [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Olander B, Eklund E, Todorova L, Bengtsson M, Maccarana M, Westergren-Thorsson G, et al. (2005) Regulation of the chondroitin/dermatan fine structure by transforming growth factor-β1 through effects on polymer-modifying enzymes. Glycobiology 15:1277–1285 [DOI] [PubMed] [Google Scholar]
- Vincent T, Saklatvala J (2006) Basic fibroblast growth factor: an extracellular mechanotransducer in articular cartilage? Biochem Soc Trans 34:456–457 [DOI] [PubMed] [Google Scholar]
- Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J (2007) FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage 15:752–763 [DOI] [PubMed] [Google Scholar]
- Visco DM, Johnstone B, Hill MA, Jolly GA, Caterson B (1993) Immunohistochemical analysis of 3-B-3(-) and 7-D-4 epitope expression in canine osteoarthritis. Arthritis Rheum 36:1718–1725 [DOI] [PubMed] [Google Scholar]