Abstract
The N-myc downstream-regulated gene (NDRG) family consists of four proteins: NDRG1, NDRG2, NDRG3, and NDRG4 in mammals. NDRG1 has been thoroughly studied as an intracellular protein associated with stress response, cell growth, and differentiation. A nonsense mutation in the NDRG1 gene causes hereditary motor and sensory neuropathy, Charcot–Marie–Tooth disease type 4D. We previously generated Ndrg1-deficient mice and found that they exhibited peripheral nerve degeneration caused by severe demyelination, but that the complicated motor abilities were retained. These results implied that other NDRG family proteins may compensate for the NDRG1 deficiency in the central nervous system. In this study we raised specific antibodies against each member of the NDRG protein family and examined their cellular expression patterns in the mouse brain. In the cerebrum, NDRG1 and NDRG2 were localized in oligodendrocytes and astrocytes, respectively, whereas NDRG3 and NDRG4 were ubiquitous. In the cerebellum, NDRG1 and NDRG4 were localized in Purkinje cells and NDRG2 in Bergmann glial cells. NDRG3 was detected in the nuclei in most cells. These expression patterns demonstrated the cell type-specific and ubiquitous localization of the NDRG family proteins. Each NDRG may play a partially redundant role in specific cells in the brain. (J Histochem Cytochem 56:175–182, 2008)
Keywords: NDRG1, NDRG2, NDRG3, NDRG4, brain, Charcot–Marie–Tooth disease, oligodendrocyte, astrocyte, immunohistochemistry
The N-myc downstream-regulated gene (NDRG) family consists of four proteins: NDRG1, NDRG2, NDRG3, and NDRG4 in mammals. They are intracellular proteins of 340 to 394 amino acids and share 53 to 65% sequence identities to each other (Okuda and Kondoh 1999; Zhou et al. 2001; Qu et al. 2002). Among this family of proteins, NDRG1 has been thoroughly studied as an inducible protein by a number of stress and pathological conditions (Kovacevic and Richardson 2006). NDRG1 was first identified as a stress stimuli-induced gene (Kokame et al. 1996; Zhou et al. 1998). It was also reported as a downregulated gene in tumors (van Belzen et al. 1997), regulated by p53 (Kurdistani et al. 1998; Stein et al. 2004), associated with the differentiation and malignant states of cancers (Piquemal et al. 1999; Xu et al. 1999; Guan et al. 2000; Bandyopadhyay et al. 2003), and regulated by N-myc (Shimono et al. 1999).
NDRG1 is the gene responsible for Charcot–Marie–Tooth disease type 4D (CMT4D), also called hereditary motor and sensory neuropathy-Lom (Kalaydjieva et al. 1996,2000). Patients exhibit early-onset peripheral neuropathy, which progresses in adulthood to severe disability characterized by muscle weakness, sensory loss, and neural deafness. Diseases caused by deficiency of NDRG2, NDRG3, or NDRG4 have not yet been identified. Although studies about the NDRG family proteins have been accumulating, their molecular functions are still elusive.
To clarify the physiological roles of NDRG1, we generated Ndrg1-deficient mice and analyzed their phenotypes (Okuda et al. 2004). They exhibited a progressive demyelinating disorder of the peripheral nerves. Sporadic demyelination began by 5 weeks of age in the sciatic nerve, whereas the proliferation of Schwann cells and initial myelination were normal for 2 weeks after birth. In wild-type mice, NDRG1 was abundantly expressed in the cytoplasm of Schwann cells rather than in the myelin sheaths. Therefore, NDRG1 deficiency leads to autonomous dysfunction of Schwann cells, resulting in demyelination in peripheral nerves. This suggests that NDRG1 is essential for the maintenance of myelin sheaths in peripheral nerves. In contrast to muscle weakness caused by peripheral nerve degeneration, the complicated motor abilities in Ndrg1-deficient mice were relatively retained. These results suggest that functional compensation for NDRG1 deficiency may exist in the central nervous system (CNS).
Transcripts of NDRG1, NDRG2, NDRG3, and NDRG4 have been detected in the brain (Zhou et al. 2001; Okuda et al. 2004). In the present study we raised specific antibodies against each member of NDRGs and examined their expression patterns in the mouse CNS by immunohistochemical analysis. We found the cell type-specific and ubiquitous localization of NDRGs.
Materials and Methods
Animals
Adult male mice (C57BL/6 Cr Slc) aged 8 to 12 weeks (Japan SLC Inc.; Hamamatsu, Japan) were used in this study. Adult or 4-week-old Ndrg1-deficient male mice (Okuda et al. 2004) were also used. Mice were bred at a controlled temperature (22C) and lighting (lights on at 8 am and off at 8 pm) for at least 1 week. Animal experiments were conducted in accordance with the guidelines for the care and use of experimental animals of the National Cardiovascular Center in Japan.
Antibodies and Vectors
Rabbit polyclonal anti-human NDRG1 and anti-human NDRG4 antibodies were raised against bacterial recombinant glutathione S-transferase-fusion proteins of NDRG1 and NDRG4, respectively, and purified by the fusion protein-immobilized affinity column chromatography (Agarwala et al. 2000; Zhou et al. 2001). Rabbit polyclonal anti-NDRG2 and anti-NDRG3 antibodies were raised against the following synthetic peptides conjugated with keyhole limpet hemocyanin and purified by peptide-immobilized affinity column chromatography: Q351SSESGTLPSGPPGH365 for mouse NDRG2 and F343SRSVTSNQSDGTQE357 for mouse NDRG3. The mouse monoclonal anti-2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) antibody (clone 11-5B) was purchased from Sigma-Aldrich (St Louis, MO). Mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody and mouse monoclonal anti-neuronal nuclei (NeuN) antibody were purchased from Chemicon (Temecula, CA).
Expression vectors for the green fluorescent protein (GFP)-fusion proteins of mouse NDRG1, NDRG2, NDRG3, and NDRG4 were constructed with the pEGFP-N1 vector (Clontech; Mountain View, CA). COS-1 cells were transfected with these vectors using the FuGENE6 transfection reagent (Roche Diagnostics; Indianapolis, IN). Two days later, cells were collected and lysed with lysis buffer (10 mM Tris–HCl, 2 mM EDTA, 50 mM dithiothreitol, 2% SDS, 6% glycerol, pH 6.8).
Western Blotting Analysis
The excised whole brain was homogenized in the lysis buffer. Protein lysates were subjected to SDS-PAGE (10–20% gradient gel; Daiichi Pure Chemicals, Tokyo, Japan) and transferred to a polyvinylidene difluoride membrane (Bio-Rad; Hercules, CA). An equal amount of loading of the protein samples was confirmed by using the RC DC protein assay kit (Bio-Rad). After blocking with 3% skim milk in PBS with 0.05% Tween-20, the membrane was incubated with 0.5-μg/ml antibodies and then with a 1:1000 dilution of peroxidase-conjugated goat anti-rabbit IgG (Zymed; South San Francisco, CA). Chemiluminescent signals were developed using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences; Wellesley, MA) and detected by an image analyzer LAS-1000plus (Fuji Film; Tokyo, Japan).
Histological Analyses
Mice (8–12 weeks old) were anesthetized with Nembutal (Abbott Laboratories; North Chicago, IL) and perfused with ice-cold 4% paraformaldehyde in PBS. Brain was then excised and fixed in 4% paraformaldehyde in PBS overnight at 4C. For light microscopy, specimens were embedded in paraffin by standard procedures. Four-μm-thick paraffin sections were stained with hematoxylin–eosin or luxol–fast blue (Klüver-Barrera staining). Slides were examined with the Axioplan 2 microscope (Carl Zeiss; Oberkochen, Germany). For immunofluorescence microscopy, 4% paraformaldehyde-fixed brain specimens were washed with PBS at 4C, immersed in a 10–20% sucrose concentration ascending series in PBS overnight at 4C, and embedded in optimal cutting temperature compound (OCT; Sakura Finetek Torrance, CA) on dry ice. Five-μm-thick frozen sections were cut by a cryostat microtome and washed with PBS. After blocking with 10% normal goat serum for 30 min at room temperature, sections were incubated with a 1 μg/ml solution of each anti-NDRG antibody, a 1:200 dilution of anti-CNPase, a 1:200 dilution of anti-GFAP, and a 1:200 dilution of anti-NeuN overnight at 4C. They were then incubated with a 1:200 dilution of AlexaFluor 488-conjugated anti-rabbit IgG antibody and/or a 1:200 dilution of AlexaFluor 546-conjugated anti-mouse IgG antibody (Invitrogen; Carlsbad, CA) for 1 hr at room temperature. Fluorescence was detected with the Axiovert 200 microscope and photographed with the AxioCam (Carl Zeiss). For negative controls of immunostaining, normal rabbit IgG was used as a primary antibody instead of as a specific antibody.
Results
Raising Specific Antibodies Against the NDRG Family Proteins
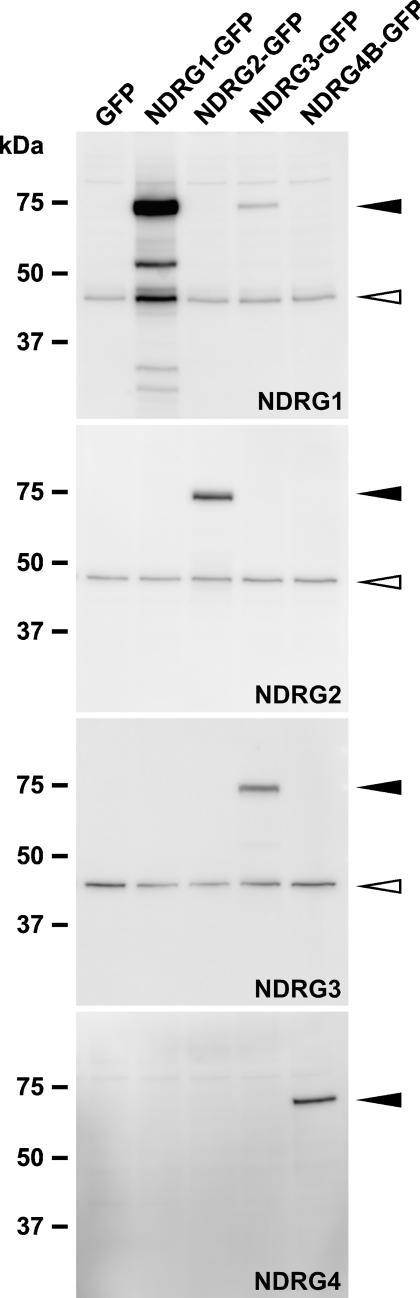
We previously raised antibodies against human NDRG1 and NDRG4 (Agarwala et al. 2000; Zhou et al. 2001). Western blotting analysis showed that anti-NDRG1 and anti-NDRG4 specifically reacted with recombinant mouse NDRG1-GFP and NDRG4-GFP, respectively, and that anti-NDRG1 faintly cross-reacted with recombinant mouse NDRG3-GFP (Figure 1). In addition to these antibodies, in the present study we raised antibodies against mouse NDRG2 and NDRG3. They specifically reacted with recombinant mouse NDRG2-GFP and NDRG3-GFP, respectively, without any cross-reactions (Figure 1).
Figure 1.
Antibody specificity against NDRG family proteins. Lysates of COS-1 cells transfected with green fluorescent protein (GFP) or mouse NDRG–GFP vectors were analyzed by Western blotting. Each antibody specifically reacted with respective NDRG–GFP fusion proteins (closed arrowheads). Bands indicated by open arrowheads were endogenous NDRG proteins. Each lane contained 5 μg of total protein.
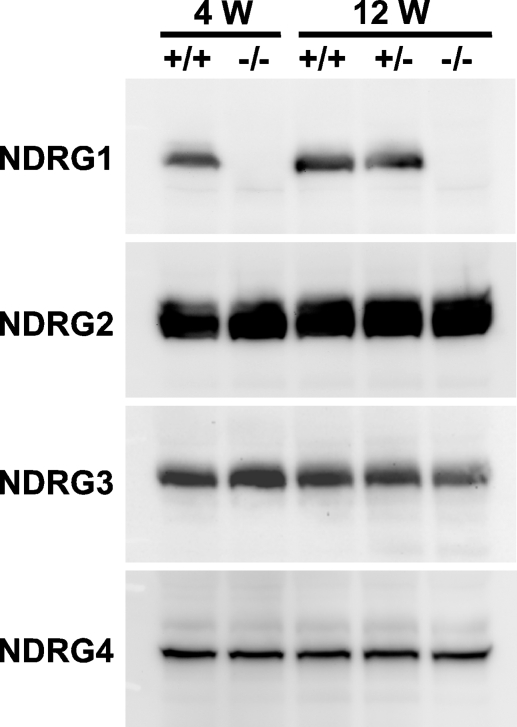
Using these antibodies, we detected all the NDRG family proteins in the brain of wild-type mice aged 4 and 12 weeks (Figure 2). Expression amounts of NDRG2, NDRG3, and NDRG4 proteins were not affected in the brain of Ndrg1-deficient mice, suggesting that the loss of NDRG1 did not affect expression levels of the other NDRGs (Figure 2).
Figure 2.
Expression of NDRG family proteins in the mouse brain. Brain lysates were prepared from the wild-type and Ndrg1-deficient mice aged 4 or 12 weeks and analyzed by Western blotting. Each lane contained 5 μg of total protein. +/+, wild-type mice; −/−, homozygous Ndrg1-deficient mice; +/−, heterozygous Ndrg1-deficient mice.
Histological Assessment of the Brain of Ndrg1-deficient Mice
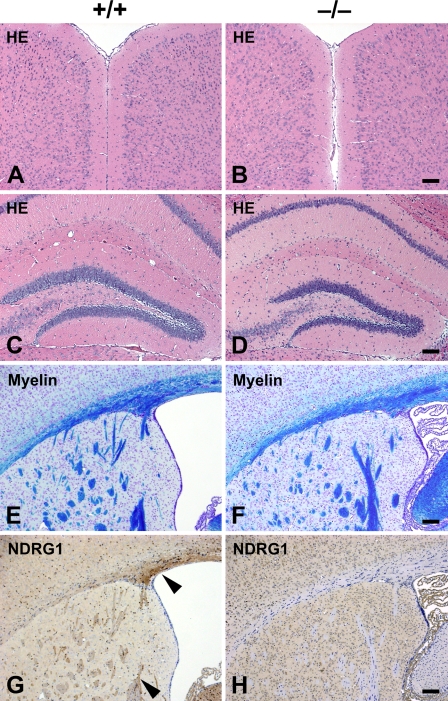
We previously reported the phenotypes of Ndrg1-deficient mice (Okuda et al. 2004). They exhibited muscle weakness caused by peripheral nerve degeneration, but their complicated motor abilities were relatively retained. These phenotypes suggest that CNS can withstand the loss of NDRG1. According to the analysis of conventional histological sections, no abnormalities were observed in the brain of Ndrg1-deficient mice (Figure 3). Organization of the cerebral cortices and other laminated regions seemed normal (Figures 3A and 3B). The structure of the hippocampus in the Ndrg1-deficient mice was not different from the wild-type one (Figures 3C and 3D). Luxol–fast blue staining for the myelin sheaths revealed that myelination was not affected in the brain of Ndrg1-deficient mice (Figures 3E and 3F). Immunohistochemical analysis of the wild-type mouse brain using anti-NDRG1 antibody exhibited specific staining in the axon bundles of the corpus callosum, corpus striatum, and fimbria hippocampus (Figure 3G). These NDRG1-staining signals were not detected in the Ndrg1-deficient mouse brain (Figure 3H).
Figure 3.
Histological assessment of the brain of adult Ndrg1-deficient mice. Transverse sections of cerebral neocortex (A,B) and hippocampus (C,D) of adult wild-type (+/+; A,C) and adult Ndrg1-deficient (−/−; B,D) mice were compared. There were no significant differences in the structure of the brain regardless of genotype. Transverse sections of the forebrain at the level of the corpus callosum (E,F) and lateral ventricle (G,H) of adult wild-type (E,G) and adult Ndrg1-deficient (F,H) mice were compared. Normal myelination was observed in wild-type (E) and Ndrg1-deficient mice (F). NDRG1 was detected in the axon bundles of the corpus callosum and corpus striatum in wild-type mice (arrowheads in G). NDRG1 expression was not detected in the Ndrg1-deficient mice (H). HE, hematoxylin–eosin staining; myelin, luxol–fast blue staining; NDRG1, anti-NDRG1 immunostaining. Bar = 100 μm.
Expression Patterns of NDRGs in the Brain
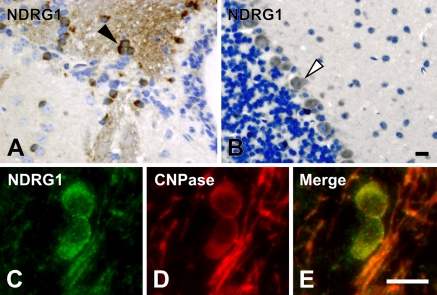
To investigate the expression characteristics of NDRGs in the brain, we performed immunohistochemical analysis using the antibodies specific for each NDRG. First, NDRG1 was strongly detected in the cytoplasm of oligodendrocytes in the cerebrum (Figure 4A). Specific expression of NDRG1 in the oligodendrocytes was confirmed by double staining for NDRG1 and CNPase. CNPase is a marker for oligodendrocytes (Figures 4C–4E). Although the cytoplasm was a principal site of NDRG1 localization, a fibrous staining pattern was also detected, suggesting that NDRG1 was partially localized in the processes of oligodendrocytes. In addition to the oligodendrocyte localization, weaker staining of NDRG1 was detected in Purkinje cells of the cerebellum (Figure 4B). NDRG1 was also strongly expressed in ependymal cells in the cerebrum (data not shown).
Figure 4.
Localization of NDRG1 in the brain. NDRG1 was detected in the cytoplasm of the oligodendrocyte in the cerebrum (arrowhead in A) and in Purkinje cells in the cerebellum (open arrowhead in B). Expression of NDRG1 (C) was colocalized with an oligodendrocyte-specific marker CNPase (D). Merged image is shown in E. Bar = 10 μm.
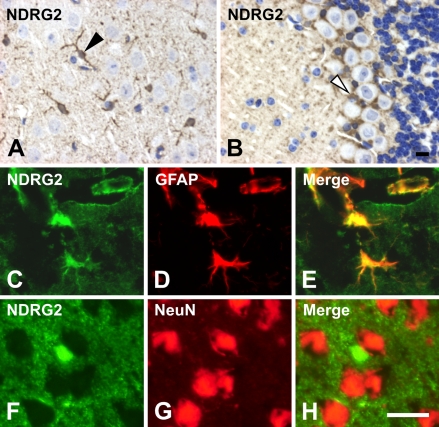
NDRG2 was strongly detected in the astrocytes of the cerebrum (Figure 5A), which was confirmed by double staining for NDRG2 and GFAP (Figures 5C–5E). GFAP is a commonly used marker for astrocytes. In the cerebellum, NDRG2 was also detected in Bergmann glial cells (Figure 5B). In both the cerebrum and cerebellum, NDRG2 was moderately expressed in most cells except neurons in the cerebral cortex and in Purkinje cells in the cerebellum (Figures 5A and 5B). Double staining for NDRG2 and NeuN indicated that NeuN-positive cells were NDRG2 negative in the cerebrum (Figures 5F–5H). NeuN is a marker for neurons. NDRG2 was less expressed in oligodendrocytes (data not shown). In the presence of immunogen peptides, anti-NDRG2 antibody did not give any signals (data not shown).
Figure 5.
Localization of NDRG2 in the brain. NDRG2 was detected in the astrocytes in the cerebrum (arrowhead in A) and in Bergmann glial cells in the cerebellum (open arrowhead in B). Expression of NDRG2 (C,F) was colocalized with an astrocyte-specific marker GFAP (D), but not with a neuron marker NeuN (G). Merged images are shown in E and H, respectively. Bar = 10 μm.
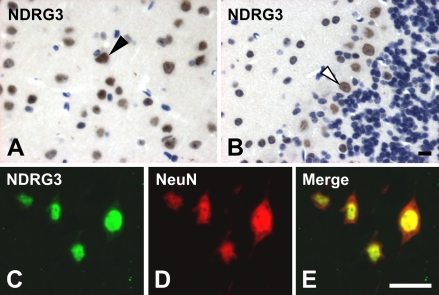
In contrast to the cytoplasmic localization of NDRG1 and NDRG2, expression of NDRG3 was observed in the nuclei of most cells in the cerebrum (Figure 6A). Nuclear localization of NDRG3 was also observed in other tissues (data not shown). Expression of NDRG3 was relatively strong in the neurons because most of the strong NDRG3-positive cells were NeuN-positive neurons (Figures 6C–6E). Significant expression of NDRG3 was also seen in the nuclei of Purkinje cells in the cerebellum, with less expression in the granule cells (Figure 6B). In the presence of immunogen peptides, anti-NDRG3 antibody did not give any signals (data not shown).
Figure 6.
Localization of NDRG3 in the brain. NDRG3 was detected in the nucleus of most cells in the cerebrum, but especially strongly in the neurons (arrowhead in A) and nucleus of Purkinje cells in the cerebellum (open arrowhead in B). Expression of NDRG3 (C) was colocalized with a neuron marker NeuN (D). Merged images are shown in E. Bar = 10 μm.
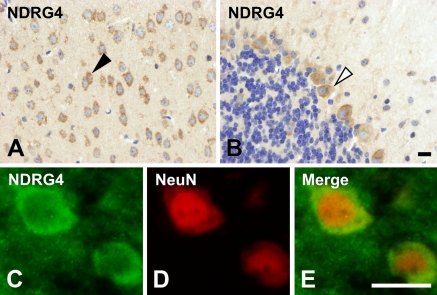
Expression of NDRG4 was detected in most brain cells, especially in the neurons of the cerebrum (Figure 7A, arrowhead) and Purkinje cells of the cerebellum (Figure 7B, open arrowhead). NDRG4-positive cells corresponded to cells expressing a neuron marker NeuN (Figures 7C–7E). NDRG4 was dominantly localized in the cytoplasm of these cells. Cytoplasmic localization of NDRG4 in Purkinje cells (Figure 7B) was similar to that of NDRG1 (Figure 4B). NDRG4 was less expressed in granule cells (Figure 7B) like NDRG1 (Figure 4B).
Figure 7.
Localization of NDRG4 in the brain. NDRG4 was detected in most cells in the cerebrum, but especially strongly in the neurons (arrowhead in A) and Purkinje cells in the cerebellum (open arrowhead in B). Strong expression of NDRG4 (C) was colocalized with a neuron marker NeuN (D). Merged images are shown in E. Bar = 10 μm.
Discussion
Although NDRG1 is essential for structural and functional maintenance of myelin sheaths in the peripheral nervous system (PNS) (Okuda et al. 2004), the morphology of the brain was not affected by the loss of NDRG1 (Figure 3). To understand the tissue- and cell-specific roles of NDRGs, the localization of each NDRG should be clarified in detail. In the present study we developed specific antibodies and examined the expression patterns of NDRGs in the mouse CNS. The expression patterns of each NDRG are summarized in Table 1.
Table 1.
Summary of major expression cells of NDRG family proteins in the brain
| NDRG1 | NDRG2 | NDRG3 | NDRG4 | |
|---|---|---|---|---|
| Cerebrum | Oligodendrocytes | Astrocytes | Most cells (nucleus) | Most cells |
| Ependymal cells | ||||
| Cerebellum | Purkinje cells | Bergmann glia | Purkinje cells (nucleus) | Purkinje cells |
| Most cells (nucleus) |
Antibody specificity was examined by Western blotting analysis using recombinant NDRG–GFP fusion proteins. Each antibody specifically reacted with the corresponding protein, although the anti-NDRG1 antibody exhibited a weak cross-reaction to NDRG3 (Figure 1). This may be caused by a relatively high identity of NDRG3 to NDRG1 in amino acid sequence compared with NDRG2 and NDRG4. Immunohistochemical analysis, however, could fortunately discriminate NDRG1 from NDRG3: anti-NDRG1 showed a cytoplasmic staining pattern in particular cells, whereas anti-NDRG3 reacted with the nuclei in most cells of the brain.
We demonstrated here that NDRG1 was mainly localized in the oligodendrocytes (Figure 4). Another group (Wakisaka et al. 2003), however, has reported immunohistochemical data inconsistent with the present study. That report demonstrated that the localization of NDRG1 is changed from hippocampal neurons to astrocytes during postnatal development in the rat brain. Although the inconsistency may be caused by the difference in animal species or developmental process, the possibility of unexpected cross-reactions of their antibody to other NDRGs (probably NDRG2) cannot be ruled out. In fact, our observation of NDRG1 localization in oligodendrocytes is consistent with that of another report (Berger et al. 2004).
The oligodendrocyte is a glial cell engaged in the formation of myelin sheaths in the CNS, whereas the Schwann cell expressing NDRG1 plays an analogous role in the PNS. NDRG1, therefore, may contribute to cellular processes in the development or maintenance of myelin sheaths. Although the loss of NDRG1 in Schwann cells led to demyelination in the sciatic nerves (Okuda et al. 2004), the loss in oligodendrocytes had no effect in the brain (Figure 3). These observations suggested that other NDRGs may compensate for the NDRG1 deficiency in oligodendrocytes but cannot do so in Schwann cells. In fact, all NDRGs except NDRG1 were less expressed in the sciatic nerve than in the brain (Okuda et al. 2004).
NDRG2 was localized to the astrocytes in the cerebrum and to Bergmann glial cells in the cerebellum (Figure 5). NDRG3 was expressed in most cells in the cerebrum and cerebellum, and the subcellular localization of NDRG3 was restricted in the nucleus (Figure 6). These marked differences from NDRG1 in the cellular and subcellular localization suggested that NDRG2 and NDRG3 may not have a redundant function of NDRG1. In fact, NDRG2 and NDRG3 were unable to compensate for the NDRG1 deficiency in sciatic nerves despite their expression in the tissue (Okuda et al. 2004).
In contrast to NDRG2 and NDRG3, NDRG4 may be a likely candidate of compensators for the NDRG1 deficiency in the brain. NDRG4 was abundantly expressed in the brain, especially in the neurons and Purkinje cells (Figure 7), the latter of which were also rich in NDRG1. NDRG1 was originally identified as a gene upregulated with homocysteine treatment (Kokame et al. 1996), and expression of NDRG4 is also induced by homocysteine (Nishimoto et al. 2003). These similarities between NDRG1 and NDRG4 may signify their functional similarity. Failure in compensation for the loss of NDRG1 in the Ndrg1-deficient PNS can be explained by the fact that there is little expression of NDRG4 in the sciatic nerves (Okuda et al. 2004). Further analysis, however, will be needed to clarify the functional specificity and redundancy of NDRGs in the CNS and also in other physiological systems. Developing and analyzing knockout mice for NDRG2, NDRG3, and NDRG4 would be the most effective approach.
Acknowledgments
This work was supported in part by Grants-in-Aid from the Ministry of Health, Labor, and Welfare of Japan; the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO).
References
- Agarwala KL, Kokame K, Kato H, Miyata T (2000) Phosphorylation of RTP, an ER stress-responsive cytoplasmic protein. Biochem Biophys Res Commun 272:641–647 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, Saito K, et al. (2003) The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res 63:1731–1736 [PubMed] [Google Scholar]
- Berger P, Sirkowski EE, Scherer SS, Suter U (2004) Expression analysis of the N-Myc downstream-regulated gene 1 indicates that myelinating Schwann cells are the primary disease target in hereditary motor and sensory neuropathy-Lom. Neurobiol Dis 17:290–299 [DOI] [PubMed] [Google Scholar]
- Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM, Pardee AB (2000) Drg-1 as a differentiation-related, putative metastatic suppressor gene in human colon cancer. Cancer Res 60:749–755 [PubMed] [Google Scholar]
- Kalaydjieva L, Gresham D, Gooding R, Heather L, Baas F, de Jonge R, Blechschmidt K, et al. (2000) N-myc downstream-regulated gene 1 is mutated in hereditary motor and sensory neuropathy-Lom. Am J Hum Genet 67:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjieva L, Hallmayer J, Chandler D, Savov A, Nikolova A, Angelicheva D, King RH, et al. (1996) Gene mapping in Gypsies identifies a novel demyelinating neuropathy on chromosome 8q24. Nat Genet 14:214–217 [DOI] [PubMed] [Google Scholar]
- Kokame K, Kato H, Miyata T (1996) Homocysteine-respondent genes in vascular endothelial cells identified by differential display analysis. GRP78/BiP and novel genes. J Biol Chem 271:29659–29665 [DOI] [PubMed] [Google Scholar]
- Kovacevic Z, Richardson DR (2006) The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis 27:2355–2366 [DOI] [PubMed] [Google Scholar]
- Kurdistani SK, Arizti P, Reimer CL, Sugrue MM, Aaronson SA, Lee SW (1998) Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res 58:4439–4444 [PubMed] [Google Scholar]
- Nishimoto S, Tawara J, Toyoda H, Kitamura K, Komurasaki T (2003) A novel homocysteine-responsive gene, smap8, modulates mitogenesis in rat vascular smooth muscle cells. Eur J Biochem 270:2521–2531 [DOI] [PubMed] [Google Scholar]
- Okuda T, Higashi Y, Kokame K, Tanaka C, Kondoh H, Miyata T (2004) Ndrg1-deficient mice exhibit a progressive demyelinating disorder of peripheral nerves. Mol Cell Biol 24:3949–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Kondoh H (1999) Identification of new genes Ndr2 and Ndr3 which are related to Ndr1/RTP/Drg1 but show distinct tissue specificity and response to N-myc. Biochem Biophys Res Commun 266:208–215 [DOI] [PubMed] [Google Scholar]
- Piquemal D, Joulia D, Balaguer P, Basset A, Marti J, Commes T (1999) Differential expression of the RTP/Drg1/Ndr1 gene product in proliferating and growth arrested cells. Biochim Biophys Acta 1450:364–373 [DOI] [PubMed] [Google Scholar]
- Qu X, Zhai Y, Wei H, Zhang C, Xing G, Yu Y, He F (2002) Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol Cell Biochem 229:35–44 [DOI] [PubMed] [Google Scholar]
- Shimono A, Okuda T, Kondoh H (1999) N-myc-dependent repression of Ndr1, a gene identified by direct subtraction of whole mouse embryo cDNAs between wild type and N-myc mutant. Mech Dev 83:39–52 [DOI] [PubMed] [Google Scholar]
- Stein S, Thomas EK, Herzog B, Westfall MD, Rocheleau JV, Jackson RS 2nd, Wang M, et al. (2004) NDRG1 is necessary for p53-dependent apoptosis. J Biol Chem 279:48930–48940 [DOI] [PubMed] [Google Scholar]
- van Belzen N, Dinjens WN, Diesveld MP, Groen NA, van der Made AC, Nozawa Y, Vlietstra R, et al. (1997) A novel gene which is up-regulated during colon epithelial cell differentiation and down-regulated in colorectal neoplasms. Lab Invest 77:85–92 [PubMed] [Google Scholar]
- Wakisaka Y, Furuta A, Masuda K, Morikawa W, Kuwano M, Iwaki T (2003) Cellular distribution of NDRG1 protein in the rat kidney and brain during normal postnatal development. J Histochem Cytochem 51:1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Lin L, Rote NS (1999) Identification of a stress-induced protein during human trophoblast differentiation by differential display analysis. Biol Reprod 61:681–686 [DOI] [PubMed] [Google Scholar]
- Zhou D, Salnikow K, Costa M (1998) Cap43, a novel gene specifically induced by Ni2+ compounds. Cancer Res 58:2182–2189 [PubMed] [Google Scholar]
- Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T (2001) Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics 73:86–97 [DOI] [PubMed] [Google Scholar]