Abstract
In early postnatal mouse skin, the NG2 proteoglycan is expressed in the subcutis, the dermis, the outer root sheath of hair follicles, and the basal keratinocyte layer of the epidermis. With further development, NG2 is most prominently expressed by stem cells in the hair follicle bulge region, as also observed in adult human skin. During telogen and anagen phases of the adult hair cycle, NG2 is also found in stem cell populations that reside in dermal papillae and the outer root sheaths of hair follicles. Ablation of NG2 produces alterations in both the epidermis and subcutis layers of neonatal skin. Compared with wild type, the NG2 null epidermis does not achieve its full thickness due to reduced proliferation of basal keratinocytes that serve as the stem cell population in this layer. Thickening of the subcutis is also delayed in NG2 null skin due to deficiencies in the adipocyte population. (J Histochem Cytochem 56:295–303, 2008)
Keywords: NG2 proteoglycan, skin, hair follicle, stem cell, keratinocyte, adipocyte
Normal cells express an array of receptors and signaling molecules that mediate appropriate interactions with the extracellular environment. Because of changes in the tissue environment and changes in cell function over the course of development, the panel of molecules present in mature cells often differs to some extent from that found in undifferentiated, totipotent stem cells or in immature progenitor cells that have made an initial commitment to a particular lineage. For example, stem cells are thought to reside in specialized environmental niches that help to preserve the “stemness” of the population (Watt and Hogan 2000; Spradling et al. 2001). Receptors/transducers needed for cellular interaction with the niche environment are often lost as stem cells or partially committed progenitors mature along developmental lineage pathways.
The developmental expression pattern of the NG2 chondroitin sulfate proteoglycan, also known as the melanoma chondroitin sulfate proteoglycan, makes it useful as a marker of progenitor cells in a variety of tissues. NG2 is expressed by immature progenitors in several types of normal tissues and in most cases is downregulated with progenitor differentiation. Immature cells that express NG2 include oligodendrocyte progenitors in the central nervous system (Nishiyama et al. 1996; Stallcup 2002; Lin et al. 2006), chondroblasts and osteoblasts in skeletal tissue (Nishiyama et al. 1991; Fukushi et al. 2003), myofibroblasts and smooth muscle cells in the intestine and macrovasculature (Grako and Stallcup 1995; Ozerdem et al 2001; Terada et al. 2006), and nascent pericytes in microvasculature (Ozerdem et al. 2001,2002).
Adding further support to the NG2/progenitor cell connection, two recent reports document the expression of the proteoglycan by stem cells associated with human interfollicular epidermis and hair follicles (Legg et al. 2003; Ghali et al. 2004). As with other types of progenitor cells, a possible role for NG2 in epidermal stem cell migration is noted in these studies. On the basis of these reports, we were spurred to investigate the pattern of NG2 expression in wild-type (WT) mouse skin and to use the NG2 null mouse to determine the consequences of NG2 ablation during skin development. Our results show that NG2 expression in mouse skin mimics in many respects the pattern seen in human skin. Moreover, NG2 ablation has effects on specific layers of the developing skin, namely, epidermis and subcutis.
Materials and Methods
Mice
C57Bl/6 WT and NG2 null mice (Grako et al. 1999) were maintained as separate colonies in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Burnham vivarium. All experimental work was carried out according to Office of Laboratory Animal Welfare guidelines, subsequent to approval by the Burnham Institutional Animal Care and Use Committee.
Antibodies and Reagents
Rabbit and guinea pig antibodies against NG2 have been described previously (Ozerdem et al. 2001,2002). Antibodies against CK-5, CK-10, CK-15, and Ki67 were obtained from Abcam, Inc. (Cambridge, MA). Anti-FABP-4 was from R&D Systems (Minneapolis, MN), and anti-BrdU was from Serotec (Oxford, UK). Secondary antibodies were obtained from Molecular Probes (Eugene, OR). BrdU was purchased from Sigma-Aldrich (St Louis, MO).
Histology and Immunocytochemistry
Dorsal skin samples were dissected from the caudal area of mice ranging in age from late embryogenesis to adulthood. These specimens were fixed overnight at 4C in 4% paraformaldehyde. Tissue was embedded in paraffin and cut into 5-μm sections for immunohistochemistry or embedded in optimal cutting temperature compound, frozen, and cut into 10-μm sections for immunofluorescence. Immunofluorescence labeling was performed as previously described (Fukushi et al. 2003). Immunoperoxidase labeling was performed using a Dako Envision kit (Dako; Carpinteria, CA) according to the manufacturer's instructions.
For BrdU labeling, pregnant females at day 17 of gestation were injected IP with 80 μg BrdU per gram of body weight. Pups from these females were taken at various ages postnatally for determination of BrdU incorporation via immunolabeling with BrdU antibody (Ozerdem and Stallcup 2004).
For Oil Red O staining, cryosections were washed with PBS, incubated for 30 min at room temperature in a 0.5% solution of Oil Red O in 60% isopropanol, and counterstained with hematoxylin.
In Situ Hybridization
A 1-kb fragment corresponding to mouse NG2 base pairs 5458–6358 (AN2) (Schneider et al. 2001) was amplified from mouse RNA by RT-PCR and inserted into the pBluescript II KS(+) vector (Stratagene; La Jolla, CA). Digoxigenin-labeled sense and anti-sense cRNA probes were generated by T7- or T3-primed RNA polymerase reactions, respectively (Roche Molecular Biochemicals; Mannhein, Germany). RNA in situ hybridization was performed on 4% paraformaldehyde-fixed paraffin sections using a biotinylated tyramine protocol (Kerstens et al. 1995).
Results and Discussion
NG2 Expression During Mouse Skin Development
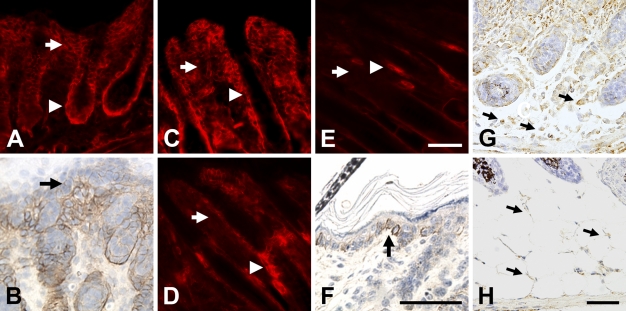
In early postnatal mouse skin, there is NG2 expression associated with blood vessels (data not shown), consistent with our previous reports of the expression of the proteoglycan by microvascular pericytes (Ozerdem et al. 2001,2002). In addition, the skin also exhibits extensive non-vascular expression of NG2, which we investigated in more depth as a function of developmental age. The proteoglycan was not detected in prenatal skin but was first evident at a low level on the day of birth. At postnatal day 1 (Figure 1A), NG2 is expressed throughout the dermis (arrow) and even more strongly in the outer root sheath of hair follicles (arrowhead). Significantly, superficial to the strongly labeled dermis, NG2 is also found in lesser quantities in the basal layer of keratinocytes (Figure 1B, arrow), the progenitor population of the epidermis (Jones et al. 1995; Jensen et al. 1999). At day 3 (Figure 1C), NG2 expression is still strong in the outer layer of hair follicles (arrowhead) but is becoming weaker in the dermis (arrow). At 7 days of age (Figure 1D), NG2 has almost vanished from the dermis (arrow) and is strongly expressed mainly in the bulge region of the hair follicle (arrowhead). This situation is maintained at postnatal day 10 and beyond (Figure 1E), with NG2 expression detectable primarily in the hair follicle bulge region (arrowhead).
Figure 1.
NG2 expression during early postnatal skin development. On postnatal day 1 (A), NG2 expression is strong in both the dermis (arrow) and outer root sheath of the hair follicle (arrowhead). Superficial to the dermis, immunoperoxidase staining reveals weaker expression of NG2 in the basal layer of keratinocytes (arrow in B). At day 3 (C), NG2 immunoreactivity remains strong in the hair follicle outer root sheath (arrowhead) but begins to diminish in the dermis (arrow). By days 7 (D) and 10 (E), NG2 has virtually disappeared from the dermis (arrow) and is mainly seen in the hair follicle bulge region (arrowheads). Immunoperoxidase labeling (F) reveals NG2 expression by basal keratinocytes at day 10 (arrow). In situ hybridization was used at postnatal days 1 (G) and 7 (H) to detect NG2 transcripts in adipocytes located in the subcutis (arrows); i.e., beneath the dermis and hair follicles. Bar = 50 μm.
In the absence of NG2 expression in the dermis, the low proteoglycan level present in basal keratinocytes is more easily seen at day 10 (Figure 1F, arrow) and into adulthood. Expression of NG2 in the interfollicular epidermis is a hallmark of adult human skin (Legg et al. 2003; Ghali et al. 2004). The more prominent labeling of clusters of NG2-positive basal keratinocytes seen in adult human epidermis may be due to the presence of more extensive areas of interfollicular epidermis in the human. In the mouse, hair follicles are more densely packed than in the human, leaving less space for clusters of NG2-positive basal keratinocytes. NG2 expression in the dermis was not noted in the two studies of human skin. This may be due to the fact that these studies did not examine neonatal skin (Legg et al. 2003; Ghali et al. 2004), the stage at which we have detected strong NG2 expression in the mouse dermis.
Although not readily detected in our immunofluorescence analysis, developing adipocytes in the subcutis also express NG2, as shown at postnatal days 1 and 7 by the use of in situ hybridization (Figures 1G and 1H). Identification of these cells as adipocytes was confirmed by their location in the subcutis, their morphology, and their expression of fatty acid-binding protein (FABP)-4, also known as aP2 (Hunt et al. 1986; Taylor-Jones et al. 2002; Damon et al. 2006) (data not shown). The finding of NG2 transcripts in adipocytes adds these cells to the list of cell types that express the proteoglycan. It is of interest that adipocytes exhibit a high degree of developmental plasticity and share lineage relationships with chondroblasts, osteoblasts, and pericytes (Schor and Canfield 1998; Sims 2000), all of which are cell types previously shown to express NG2.
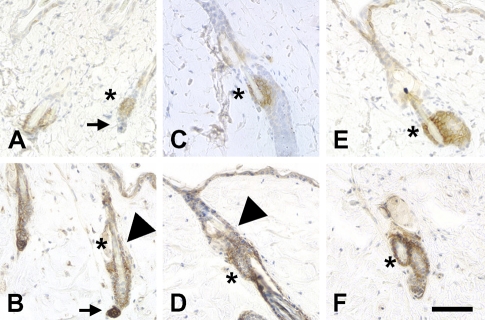
Along with the basal layer of keratinocytes, the bulge region of hair follicles is of particular interest as a stem cell niche. The bulge region not only contributes to hair development but also serves as a reservoir of progenitor cells for skin development and regeneration (Cotsarelis et al. 1990; Reynolds and Jahoda 1993; Taylor et al. 2000; Oshima et al. 2001). Prominent expression of NG2 in the hair follicle bulge region has been reported in studies on human skin (Legg et al. 2003; Ghali et al. 2004). In the adult mouse, we confirmed NG2 expression in this niche by comparing NG2 labeling with that of cytokeratin (CK)-15, which is expressed by stem cells in the bulge region (Lyle et al. 1998). We also used CK-15 expression as a tool for studying changes in NG2 expression as a function of the mouse hair cycle. Phases of the adult hair cycle were determined according to Muller-Rover et al. (2001). During the telogen (quiescence) and anagen (active growth) phases (Figures 2A–2D), NG2 expression is strong not only in the CK-15-positive bulge region (asterisks in all panels) but also in dermal papillae (marked with arrow in telogen phase, Figures 2A and 2B) and in the outer root sheath of hair follicles (arrowheads). In contrast, during the regressive catagen phase of the cycle (Figures 2E and 2F), NG2 expression is restricted to CK-15-positive stem cells in the bulge region. Thus, NG2 expression by bulge region stem cells is a constant throughout the hair cycle. The NG2 expression pattern in stable (telogen) or growing (anagen) hair follicles is also suggestive. The outer root sheath of the hair follicle is contiguous with the interfollicular epidermis, sharing some of the same properties as a stem cell niche (Sonnenberg et al. 1991; Commo et al. 2000; Panteleyev et al. 2001). Dermal papillae, in addition to providing inductive signals that orchestrate follicle growth (Jahoda et al. 1984; Reynolds and Jahoda 1991), also serve as a source of mesenchymal stem cells for renewal of adipocytes, chondrocytes, osteoblasts, hematopoietic cells, and even neural cells (Fernandes et al. 2004; Shi et al. 2004; Hoogduijn et al. 2006).
Figure 2.
NG2 expression by stem cells as a function of the hair cycle. Cytokeratin (CK)-15 immunoreactivity (A,C,E) was used to mark stem cells in the bulge region during telogen (A,B), anagen (C,D), and catagen (E,F) phases of the hair cycle. NG2 expression (B,D,F) is always seen in the bulge region (asterisks), but during telogen and anagen it is also seen in dermal papillae (arrows in telogen A,B) and the outer root sheath of hair follicles (arrowheads). Bar = 100 μm.
Skin Development in the NG2 Null Mouse
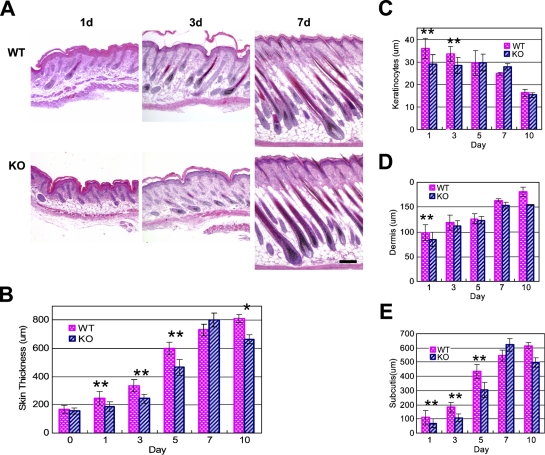
As an initial means of determining a functional role for NG2 in skin development, we compared the morphological features of WT and NG2 null skin (Figure 3A) and quantified the thickness of individual skin layers. Although overall skin thickness increases during the first week postnatally in both WT and NG2 null mice (Figure 3B), thickening of NG2 null skin lags behind that of WT skin during postnatal days 1–5. This analysis was extended to specific layers of the developing skin: the epidermis (Figure 3C), the dermis (Figure 3D), and the subcutis (Figure 3E). Whereas no consistent differences were detected in the dermis or in hair follicle development, changes in both the epidermis and subcutis were evident in the NG2 null mouse. In the WT mouse, the epidermis reaches its maximum thickness at birth and then steadily decreases in width over the course of the first week postnatally. In contrast, the NG2 null epidermis does not attain the full thickness seen in the WT. Instead, the NG2 null epidermis maintains a constant, reduced thickness during postnatal week 1, undergoing a decrease only at 10 days postnatally. The converse situation is seen in the subcutis. The increasing thickness of this layer during week 1 accounts for much of the overall increase in skin thickness seen in the WT mouse. The thickness of the NG2 null subcutis lags behind that of the WT during the first 5 postnatal days.
Figure 3.
Skin development in the NG2 null mouse. Images of hematoxylin- and eosin-stained skin sections (transverse) were prepared from wild-type (WT) and NG2 null mice during the first 10 days postnatally (A) and used to measure the overall skin thickness (B) and the thickness of the epidermis (C), dermis (D), and subcutis (E). At least six mice were used at each time point to establish the average values shown in the panels. Results were analyzed using the two-tailed Student's t-test. Statistically significant differences between WT and NG2 null samples are designated as follows: **p<0.01; *p<0.05. Bar = 200 μm.
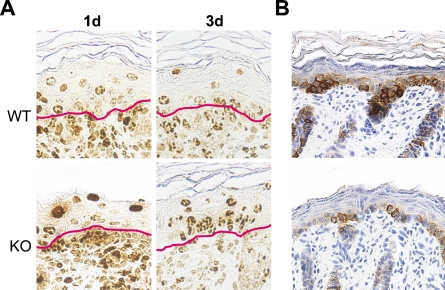
Because we have previously shown that NG2 ablation results in a significant decrease in the proliferation of microvascular pericytes (Ozerdem and Stallcup 2004), it seemed likely that decreased cell proliferation may also be a factor in altering skin development in the NG2 null mouse. Evidence for an effect of NG2 ablation on cell proliferation in the keratinocyte layer was obtained via combined use of BrdU and Ki67 labeling and immunolabeling for CK-5, which is expressed by basal layer keratinocytes (Fuchs and Green 1980; Moll et al. 1982). Dividing cells in mouse embryos were tagged by injection of BrdU into pregnant mice on the 17th day of gestation. At this stage, BrdU-labeled cells are abundant in the dermis but are seen much less frequently in the overlying epidermis. BrdU labeling in the epidermis is initially detected in the basal layer of keratinocytes, the progenitor population that expresses NG2. With increasing developmental age, these basal cells progressively differentiate, move upward, and are detected at suprabasal levels on postnatal days 1 and 3 (Watt and Green 1982; Fuchs and Byrne 1994; Lavker and Sun 2000) (Figure 4A).
Figure 4.
Decreased keratinocyte production in the NG2 null mouse. (A) Following BrdU administration at 17 days of gestation, BrdU-labeled cells in the skin were visualized at days 1 and 3 by staining with anti-BrdU antibody. Red lines in each panel mark the dermis/epidermis boundary. (B) Immunoperoxidase labeling for CK-5 illustrates the deficiency in basal keratinocytes in NG2 null skin.
At day 1, the BrdU label has become diluted in suprabasal WT keratinocytes due to continued proliferation of these cells. In contrast, the BrdU label remains strong in many suprabasal NG2 null keratinocytes at day 1, suggesting a failure of these cells to proliferate to the same extent as WT cells. Table 1 semiquantitatively compares the dilution of the BrdU label in WT and NG2 null keratinocytes, revealing the retention of label by the NG2 null population at day 1. By day 3, dilution of the label is also apparent in NG2 null keratinocytes, showing that these cells also proliferate, albeit with a slower time course. Impaired proliferation of NG2 null keratinocytes is also reflected by the reduced number of Ki67-labeled basal keratinocytes at day 1 (Table 2). As seen in the BrdU study, proliferation of NG2 null keratinocytes recovers to the level of WT keratinocytes by day 3. These findings are consistent with the observation that the epidermis of the NG2 null mouse is reduced in thickness at postnatal days 1 and 3 (Figure 3C).
Table 1.
Keratinocyte proliferation—dilution of BrdU label in suprabasal keratinocytes
| WT (%)
|
KO (%)
|
|||||
|---|---|---|---|---|---|---|
| Intensity | ++ | + | +/− | ++ | + | +/− |
| P1 | 7.6 | 92.4 | 0.0 | 58.1 | 41.9 | 0.0 |
| P3 | 5.0 | 60.3 | 34.7 | 13.6 | 61.4 | 25.0 |
Following BrdU injection into pregnant females at 17 days of gestation, the intensity of BrdU labeling was determined at postnatal days 1 and 3 (P1 and P3) for keratinocytes in the suprabasal layer of the wild-type (WT) and NG2 knockout (KO) epidermis. Cells with strong uniform nuclear labeling were scored as ++. Cells with diminished, patchy nuclear labeling were scored as + or +/−, depending on the label intensity (see Figure 4A for examples). Percentages of cells were determined in each of the three labeling categories. At P1, >90% of WT keratinocytes exhibit dilution of the BrdU label compared to only 40% of KO keratinocytes. By P3, these values have become more similar for WT and KO samples.
Table 2.
Keratinocyte proliferation—Ki67 labeling of basal keratinocytes
| Intensity | WT (%) | KO (%) |
|---|---|---|
| P1 | 30.4 | 11.3 |
| P3 | 28.0 | 25.9 |
Using Ki67-immunostained sections counterstained with hematoxylin, we counted the total number of basal keratinocytes and the number of Ki67-labeled basal keratinocytes. These numbers were used to calculate the percent of Ki67-positive basal keratinocytes at P1 and P3 in samples of WT and NG2 null skin. In P1 NG2 KO mice, proliferative basal keratinocytes were clearly reduced in number compared to their WT counterparts. By P3, proliferation in KO specimens recovers to nearly the WT level.
The slowly cycling, label-retaining characteristics of NG2 null keratinocytes resemble those of epidermal stem cells. An intriguing idea is that acquisition of NG2 in the WT mouse may mark a developmental transition from slowly cycling epidermal stem cells to more actively proliferating, partially committed keratinocyte progenitors. This would be consistent with our previous observations on NG2 expression in other developing tissues. For example, NG2 is not expressed by multipotent stem cells in germinal zones of the central nervous system or limb bud but is expressed by oligodendrocyte progenitors and chondroblasts, respectively, that arise from stem cells in these zones (Stallcup et al. 1983; Nishiyama et al. 1991,1996). Our BrdU/Ki67 data for keratinocyte proliferation suggest that stem cells in the NG2 null mouse may have greater difficulty than WT cells in making the transition to more rapidly cycling progenitors.
In parallel with the BrdU/Ki67 studies, we detected a large deficiency in the number of CK-5-positive basal layer keratinocytes in the postnatal day 1 NG2 null mouse (Figure 4B), consistent with a reduction in the ability of these cells to proliferate in the absence of NG2. Significantly, although there were fewer CK-5-positive keratinocytes in the NG2 null skin at postnatal day 1, at later stages of development these cells expressed the differentiation marker CK-10 (Rugg and Leigh 2004) on the same developmental schedule as in WT mice (data not shown). Together with the BrdU/Ki67 data, these suggest that NG2 ablation affects keratinocyte proliferation more significantly than keratinocyte differentiation.
In future studies it will be of great interest to determine if NG2 ablation is accompanied by changes in the quantity or distribution of β1 integrins and/or extracellular matrix components such as types V and VI collagen, each of which has been shown to be important NG2 ligands. Skin-associated stem cells are normally characterized by high levels of β1 integrin expression (Jones and Watt 1993; Legg et al. 2003). Because NG2 binds to β1 integrins and activates their signaling to promote both cell motility and proliferation (Iida et al. 1995; Eisenmann et al. 1999; Fukushi et al. 2004; Makagiansar et al. 2004,2007), loss of NG2/integrin interactions may have profound effects on the dynamics of the epidermal stem cell niche. Similarly, NG2 represents an important cell surface receptor for collagens V and VI (Burg et al. 1997; Tillet et al. 1997,2002; Petrini et al. 2005). Loss of NG2 may therefore perturb cell/matrix interactions within the niche.
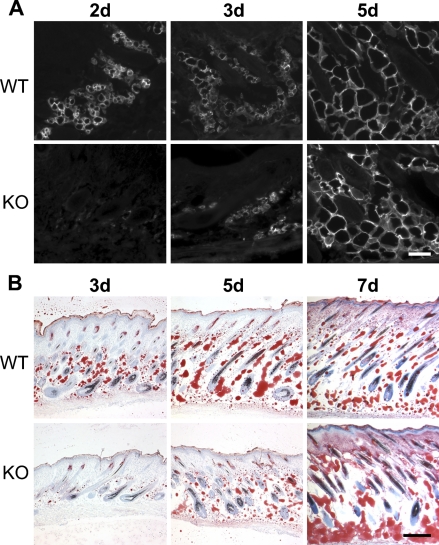
Expression of NG2 by adipocytes (Figure 1) provides a possible explanation for the deficiency in subcutis development observed in the NG2 null mouse. We used expression of aP2/FABP-4 as a marker for adipocyte development. At postnatal day 1, aP2/FABP-4 levels are low in the subcutis of both WT and NG2 null mice (not shown). However, adipocyte expression of aP2/FABP-4 increases dramatically in postnatal day 2 WT mice and remains high through day 5 (Figure 5A). Significant aP2/FABP-4 expression in NG2 null mice is not seen until postnatal day 5. This deficit in adipocyte function is also seen in the deposition of lipid, as detected by staining with Oil Red O (Figure 5B). Whereas significant lipid deposition can be detected at postnatal day 3 in the WT mouse, deposition in the knockout (KO) mouse lags behind until postnatal day 7. Even at this time point, lipid distribution in the NG2 null mouse appears extremely disorganized compared with that seen in the WT animal. We cannot determine from the aP2/FABP-4 and Oil Red O data whether NG2 null skin contains fewer adipocyte progenitors than WT skin or whether adipocyte maturation is retarded by NG2 ablation. This distinction will require the use of additional markers for preadipocytes and mature adipocytes.
Figure 5.
Altered adipocyte development in the NG2 null mouse. (A) Sections of WT and NG2 null skin at days 2, 3, and 5 were immunostained with antibody against aP2/FABP-4. Expression of aP2/FABP-4 is delayed in NG2 null adipocytes. (B) Staining with Oil Red O was used to examine lipid deposition in WT and NG2 null skin at days 3, 5, and 7. Delay in lipid deposition mirrors the delay in FABP-4 expression shown in A. Bars: A = 100 μm; B = 200 μm.
It has been reported that the aP2/FABP-4 null mouse does not exhibit any developmental deficiencies under normal laboratory conditions, possibly due to the redundant function of FABP-5 (Hotamisligel et al. 1996). It therefore follows that the ablation of NG2 does not produce developmental deficiencies in adipocytes by affecting aP2/FABP-4 function. Instead, the delayed expression of aP2/FABP-4 in NG2 null mice must reflect the effect of NG2 ablation on a more basic aspect of the adipocyte differentiation program. Our evidence points to reduced cell proliferation as one factor that affects both adipocytes and basal keratinocytes in the NG2 null mouse. In this respect, the ability of NG2 to enhance FGF- and PDGF-mediated signaling may provide at least a partial explanation for the deficiencies observed in the NG2 null mouse. Aortic smooth muscle cells derived from the NG2 null mouse fail to proliferate normally in response to PDGF-AA and FGF2 (Goretzki et al. 1999; Grako et al. 1999; unpublished data). Experimental corneal angiogenesis in response to FGF2 is greatly reduced in the NG2 null mouse due to reduced proliferation and recruitment of pericytes to developing vasculature (Ozerdem and Stallcup 2004). Various members of the large FGF family, along with their cognate receptors, play important roles in the proliferation and differentiation of adipocytes (Yamasaki et al. 1999; Hutley et al. 2004; Newell et al. 2006; Zaragosi et al. 2006). Similarly, specific FGF isoforms are known to promote keratinocyte proliferation during both normal skin development and wound healing (Petiot et al. 2003; Beer et al. 2005; Komi-Kuramochi et al. 2005; Braun et al. 2006; Nagy et al. 2006). Along with certain FGF isoforms, PDGF family members can also be potent adipocyte mitogens (Schmidt et al. 1990; Hua et al. 2004). In addition, PDGF promotes re-epithelialization during wound healing (Cheng et al. 2007). Additional work will be required to identify a precise role for NG2 in promoting growth factor signaling in the context of the keratinocytes and adipocytes.
Surprisingly, development of the NG2 null dermis appears unaffected by NG2 ablation, even though the dermis represents a major site of NG2 expression in newborn WT skin. Similarly, in spite of strong NG2 expression in the WT hair follicle sheath and bulge region, we did not find defects in hair development in NG2 null mice. It is possible that the existence of compensatory mechanisms partially masks the loss of NG2 in early postnatal skin and hair follicles. It is noteworthy that, although we have not detected significant differences in developmental vascularization in WT and NG2 null mice, vascularization in response to a pathological challenge is significantly reduced in the adult KO animal (Ozerdem and Stallcup 2004). It is our hope that future studies on wound healing and hair regrowth will reveal deficiencies in these processes in adult NG2 null mice. Such studies will enhance our knowledge of NG2 as a functional player in the biology of progenitor cells that reside in adult tissues.
Acknowledgments
This work was supported by NIH Grants R01 CA-095287 and P01 HD-025938 (to WBS) and NIH Grants R01 NS-032717 and R01 NS041332 (to YY).
We are indebted to Dr. Minoru Fukuda (Burnham Institute for Medical Research; La Jolla, CA) for use of his brightfield microscope, Jennifer Meerloo (Burnham Institute for Medical Research) for assistance with image analysis, and Regina Kapono (Burnham Institute for Medical Research) for help with the manuscript.
References
- Beer H, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, Werner S (2005) The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10, and FGF-22 and regulates FGF activity: implications for epithelial repair. Oncogene 24:5269–5277 [DOI] [PubMed] [Google Scholar]
- Braun S, Krampert M, Bodó E, Kümin A, Born-Berclaz, Paus R, Werner S (2006) Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV radiation, chemotherapeutic or toxic agents. J Cell Sci 119:4841–4849 [DOI] [PubMed] [Google Scholar]
- Burg M, Nishiyama A, Stallcup WB (1997) A central segment of the NG2 proteoglycan is critical for the ability of glioma cells to bind and migrate toward type VI collagen. Exp Cell Res 235:254–264 [DOI] [PubMed] [Google Scholar]
- Cheng B, Liu H, Fu X, Sun T, Sheng Z (2007) Recombinant human platelet-derived growth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats. J Dermatol Sci 45:193–201. Published online January 31, 2007 (DOI: 10.1016/J.Jdermsci.2006.11.014) [DOI] [PubMed] [Google Scholar]
- Commo S, Gaillard O, Bernard B (2000) The human hair follicle contains two distinct K19 positive compartments in the outer root sheath: a unifying hypothesis for stem cell reservoir. Differentiation 66:157–164 [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun T, Lavker R (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61:1329–1337 [DOI] [PubMed] [Google Scholar]
- Damon M, Louveau I, Lefaucheur L, Lebret B, Vincent A, Leroy P, Sanchez M, et al. (2006) Number of intramuscular adipocytes and fatty acid binding protein-4 content are significant indicators of intramuscular fat level in crossbred Large White × Duroc pigs. J Anim Sci 84:1083–1092 [DOI] [PubMed] [Google Scholar]
- Eisenmann K, McCarthy J, Simpson M, Keely P, Guan JL, Tachibana K, Lim L, et al. (1999) Melanoma chondroitin sulphate proteoglycan regulates cell spreading through cdc42, Ack-1, and p130cas. Nat Cell Biol 1:507–513 [DOI] [PubMed] [Google Scholar]
- Fernandes K, McKenzie I, Mill P, Smith K, Akhavan M, Barnabe-Heider F, Biernaskie J, et al. (2004) A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 6:1082–1093 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Byrne C (1994) The epidermis: rising to the surface. Curr Opin Genet Dev 4:725–736 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Green H (1980) Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19:1033–1042 [DOI] [PubMed] [Google Scholar]
- Fukushi J, Inatani M, Yamaguchi Y, Stallcup WB (2003) Expression of NG2 proteoglycan during endochondral and intramembranous ossification. Dev Dyn 228:143–148 [DOI] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar I, Stallcup WB (2004) NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and α3β1 integrin. Mol Biol Cell 15:3580–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali L, Wong ST, Tidman N, Quinn A, Philpott M, Leigh I (2004) Epidermal and hair follicle progenitor cells express melanoma-associated chondroitin sulfate proteoglycan core protein. J Invest Dermatol 122:433–442 [DOI] [PubMed] [Google Scholar]
- Goretzki L, Burg M, Grako K, Stallcup WB (1999) High affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem 274:16831–16837 [DOI] [PubMed] [Google Scholar]
- Grako K, Ochiya T, Barritt D, Nishiyama A, Stallcup WB (1999) PDGF α-receptor is unresponsive to PDFG-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci 112:905–915 [DOI] [PubMed] [Google Scholar]
- Grako K, Stallcup WB (1995) Participation of NG2 proteoglycan in rat aortic smooth muscle cell responsesto platelet-derived growth factor. Exp Cell Res 221:231–240 [DOI] [PubMed] [Google Scholar]
- Hoogduijn M, Gorjup E, Genever P (2006) Comparative characteristics of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev 15:49–60 [DOI] [PubMed] [Google Scholar]
- Hotamisligel G, Johnson R, Distel R, Ellis R, Papaioannou V, Spiegelman B (1996) Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adiocyte fatty acid binding protein. Science 274:1377–1379 [DOI] [PubMed] [Google Scholar]
- Hua K, Deng J, Harp J (2004) Interleukin-4 inhibits platelet-derived growth factor-induced preadipocyte proliferation. Cytokine 25:61–67 [DOI] [PubMed] [Google Scholar]
- Hunt C, Ro J, Dobson D, Min H, Spiegelman B (1986) Adipocyte P2 gene: developmental expression and homology of 5′-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci USA 83:3786–3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutley L, Shurety W, Newell F, McGeary R, Pelton N, Grant J, Herington A, et al. (2004) Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes 53:3097–3106 [DOI] [PubMed] [Google Scholar]
- Iida J, Meijne A, Spiro R, Roos E, Furcht L, McCarthy J (1995) Spreading and focal contact formation of human melanoma cells in response to stimulation of both melanoma-associated proteoglycan (NG2) and α4β1 integrin. Cancer Res 55:2177–2185 [PubMed] [Google Scholar]
- Jahoda C, Horne K, Oliver R (1984) Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311:560–562 [DOI] [PubMed] [Google Scholar]
- Jensen U, Lowell S, Watt FM (1999) The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labeling and lineage analysis. Development 126:2409–2418 [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM (1995) Stem cell patterning and fate in human epidermis. Cell 80:83–93 [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM (1993) Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73:713–724 [DOI] [PubMed] [Google Scholar]
- Kerstens H, Poddighe P, Hanselaar A (1995) A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem 43:347–352 [DOI] [PubMed] [Google Scholar]
- Komi-Kuramochi A, Kawano M, Oda Y, Asada M, Suzuki M, Oki J, Imamura T (2005) Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. J Endocrinol 186:273–289 [DOI] [PubMed] [Google Scholar]
- Lavker R, Sun T (2000) Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA 97:13473–13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg J, Jensen U, Broad S, Leigh I, Watt F (2003) Role of melanoma chondroitin sulfate proteoglycan in patterning stem cells in human interfollicular epidermis. Development 130:6049–6063 [DOI] [PubMed] [Google Scholar]
- Lin T, Xiang Z, Cui L, Stallcup WB, Reeves SA (2006) New mouse oligodendrocyte precursor (mOP) cells for studies on oligodendrocyte maturation and function. J Neurosci Methods 157:187–194 [DOI] [PubMed] [Google Scholar]
- Lyle S, Christofidue-Solomidou M, Liu Y, Elder D, Albelda S, Cotsarelis G (1998) The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci 111:3179–3188 [DOI] [PubMed] [Google Scholar]
- Makagiansar I, Williams S, Dahlin-Huppe K, Fukushi J, Mustelin T, Stallcup WB (2004) Phosphorylation of NG2 proteoglycan by protein kinase C-α regulates polarized membrane distribution and cell motility. J Biol Chem 279:55262–55270 [DOI] [PubMed] [Google Scholar]
- Makagiansar I, Williams S, Mustelin T, Stallcup WB (2007) Differential phosphorylation of NG2 proteoglycan by ERK and PKCα helps balance cell proliferation and migration. J Cell Biol 178:155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Franke W, Schiller D, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors, and cultured cells. Cell 31:11–24 [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay I, Stenn K, et al. (2001) A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 117:3–15 [DOI] [PubMed] [Google Scholar]
- Nagy N, Bata-Csorgo Z, Kopasz N, Szeg C, Pivarcsi A, Koreck A, Dobozy A, et al. (2006) The expression of keratinocyte growth factor receptor (FGFR2-IIIb) correlates with the high proliferative rate of HaCaT keratinocytes. Exp Dermatol 15:596–605 [DOI] [PubMed] [Google Scholar]
- Newell F, Su H, Tornqvist H, Whitehead J, Prins J, Hutley L (2006) Characterization of the transcriptional and functional effects of fibroblast growth factor-1 on human preadipocyte differentiation. FASEB J 20:E2133–2145 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Dahlin K, Stallcup WB (1991) The expression of NG2 proteoglycan in developing rat limb. Development 111:933–944 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin X, Giese N, Heldin CH, Stallcup WB (1996) Co-localization of NG2 proteoglycan and PDGF alpha receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43:299–314 [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y (2001) Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104:233–245 [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako K, Dahlin-Huppe K, Monosov E, Stallcup WB (2001) The NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222:218–227 [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Monosov E, Stallcup WB (2002) NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res 63:129–134 [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB (2004) Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis 7:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleyev A, Jahoda C, Christiano A (2001) Hair follicle predetermination. J Cell Sci 114:3419–3431 [DOI] [PubMed] [Google Scholar]
- Petiot A, Conti F, Grose R, Revest J, Hodivala-Dilke K, Dickson C (2003) A crucial role for Fgfr2-IIIb signaling in epidermal development and hair follicle patterning. Development 130:5493–5501 [DOI] [PubMed] [Google Scholar]
- Petrini S, Tessa A, Stallcup WB, Sabatelli P, Pescatori M, Giusti B, Carrozzo R, et al. (2005) Altered expression of the MCSP/NG2 chondroitin sulfate proteoglycan in collagen VI deficiency. Mol Cell Neurosci 30:408–417 [DOI] [PubMed] [Google Scholar]
- Reynolds A, Jahoda C (1991) Inductive properties of hair follicle cells. Ann NY Acad Sci 642:226–242 [DOI] [PubMed] [Google Scholar]
- Reynolds A, Jahoda C (1993) Hair fiber progenitor cells: developmental status and interactive potential. Dev Biol 4:241–250 [Google Scholar]
- Rugg EL, Leigh IM (2004) The keratins and their disorders. Am J Med Genet C Semin Med Genet 131C:4–11 [DOI] [PubMed] [Google Scholar]
- Schmidt W, Poll-Jordan G, Loffler G (1990) Adipocyte conversion of 3T3–L1 cells in a serum-free culture system depends on epidermal growth factor, insulin-like growth factor 1, corticosterone, and cyclic AMP. J Biol Chem 265:15489–15495 [PubMed] [Google Scholar]
- Schneider S, Bosse F, D'Urso D, Muller H, Sereda M, Nave K, Niehaus A, et al. (2001) The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci 21:920–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor A, Canfield D (1998) Osteogenic potential of vascular pericytes in marrow stromal cultures. In Beresford J, Owen M, eds. Marrow Stromal Cell Culture. Cambridge, UK, Cambridge University Press, 128–148
- Shi C, Mai Y, Cheng T (2004) Identification of hematopoietic cell populations from the dermal papillae of human hair follicles. Transplant Proc 36:3208–3211 [DOI] [PubMed] [Google Scholar]
- Sims D (2000) Diversity within pericytes. Clin Exp Pharmacol Physiol 27:842–846 [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, Kennel SJ, et al. (1991) Integrin α6β4 complex is located in hemi-desmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol 113:907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414:98–104 [DOI] [PubMed] [Google Scholar]
- Stallcup WB (2002) The NG2 proteoglycan: past insights and future prospects. J Neurocytol 31:423–435 [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L, Levine JM (1983) Cell-surface molecules that characterize different stages in the development of cerebellar interneurons. Cold Spring Harbor Symp Quant Biol 48:761–774 [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer M, Jensen P, Sun T, Lavker R (2000) Involvement of follicular stem cells forming not only the follicle but also the epidermis. Cell 102:451–461 [DOI] [PubMed] [Google Scholar]
- Taylor-Jones J, McGehee R, Rando T, Lecka-Czernik B, Lipschitz D, Peterson C (2002) Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev 123:649–661 [DOI] [PubMed] [Google Scholar]
- Terada N, Ohno N, Murata S, Katoh R, Stallcup WB, Ohno S (2006) Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem Cell Biol 126:483–490 [DOI] [PubMed] [Google Scholar]
- Tillet E, Gential B, Garrone R, Stallcup WB (2002) NG2 proteoglycan mediates β1 integrin-independent cell adhesion and spreading on collagen VI. J Cell Biochem 86:726–736 [DOI] [PubMed] [Google Scholar]
- Tillet E, Ruggerio F, Nishiyama A, Stallcup WB (1997) The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central non-globular domain of its core protein. J Biol Chem 272:10769–10776 [DOI] [PubMed] [Google Scholar]
- Watt F, Green H (1982) Stratification and terminal differentiation of cultured epidermal cells. Nature 295:434–436 [DOI] [PubMed] [Google Scholar]
- Watt F, Hogan B (2000) Out of Eden: stem cells and their niches. Science 287:1427–1430 [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Emoto H, Konishi M, Mikami T, Ohuchi H, Nakao K, Itoh N (1999) FGF-10 is a growth factor for preadipocyes in white adipose tissue. Biochem Biophys Res Commun 258:109–112 [DOI] [PubMed] [Google Scholar]
- Zaragosi L, Ailhaud G, Dani C (2006) Autocrine fibroblast growth factor-2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells 24:2412–2419 [DOI] [PubMed] [Google Scholar]