Abstract
Current evidence suggests that leptin reduces food intake in part by enhancing the hindbrain neuronal response to meal-related gastrointestinal signals, including cholecystokinin (CCK), but the phenotypes of the relevant cells are not known. To identify neurons that participate in this interaction in the rat nucleus of the solitary tract (NTS), we induced c-Fos gene expression in NTS neurons with leptin and CCK. We focused on NTS catecholamine neurons because these cells have been implicated in the feeding response to CCK. Hindbrain sections from rats that received CCK with or without leptin pretreatment were immunostained for c-Fos and tyrosine hydroxylase (TH) by a double immunofluorescence procedure. Leptin pretreatment increased the number of NTS cells expressing c-Fos-like immunoreactivity (cFLI) 3-fold relative to CCK alone, but the number of TH-positive cells with cFLI was increased 6-fold. Next, cells detected by immunofluorescence for TH were collected by laser capture microdissection and pooled for real-time quantitative PCR of c-Fos mRNA. Here, neither le0ptin nor CCK alone affected the relative amount of mRNA in the TH cell–enriched samples, but leptin plus CCK substantially increased c-Fos mRNA content. These histochemical findings identify hindbrain catecholamine cells as potential mediators of the interaction between leptin and CCK. (J Histochem Cytochem 56:285–293, 2008)
Keywords: leptin, cholecystokinin, catecholamine neurons, food intake, satiety, c-Fos, energy homeostasis
The control of meal size is considered to reside primarily in the caudal brainstem. Signals arising from the gastrointestinal tract in response to ingested nutrients, such as the gut peptide cholecystokinin (CCK), directly or indirectly activate cells in the hindbrain that promote meal termination (Moran et al. 2001; Strader and Woods 2005). By activating its receptors on vagal afferent fibers, CCK stimulates neurons in hindbrain regions including the dorsal vagal complex, composed of the area postrema, dorsal motor nucleus of the vagus nerve, and the nucleus of the solitary tract (NTS). The recruitment of cells in this pathway is considered to be physiologically important for CCK-induced anorexia (Edwards et al. 1986; Moran et al. 1997). The adiposity hormone leptin is well known to play a key role in energy homeostasis through its actions on neuronal leptin receptors that reduce food intake while increasing energy expenditure (Morton et al. 2006). Several studies have reported that administration of leptin decreases meal size, an effect compatible with a model in which leptin reduces food intake through its interaction with meal-related signals that promote satiation (Kahler et al. 1998; Flynn and Plata-Salaman 1999). Indeed, in rodents, either central or peripheral leptin pretreatment enhances CCK-induced anorexia (Barrachina et al. 1997; Emond et al. 1999), whereas the feeding response to CCK is blunted in rodents lacking functional leptin receptors (Morton et al. 2005). Although most research on leptin action has focused on hypothalamic mediation, this synergistic interaction between leptin and CCK seems to take place, at least in part, in the caudal brainstem. Leptin pretreatment enhances not only the satiety response to CCK but also the activation of c-Fos gene transcription and translation in response to CCK in the NTS (Emond et al. 1999; Blevins et al. 2004). A major gap in our understanding of leptin's CNS anorexic action and role in food intake behavior is the lack of information about the specific hindbrain neurons and circuits that integrate leptin signaling to the brainstem with satiety signals such as CCK.
In this study, we used histochemical techniques to test the hypothesis that hindbrain catecholamine neurons are among several NTS neuronal populations with the potential to mediate the interaction between leptin and CCK, because previous studies have implicated these cells in the feeding response to CCK. Neurons in A2/C2 catecholamine groups of the hindbrain express c-Fos in response to CCK treatment and make up the largest group of CCK-responsive forebrain-projecting cells (Rinaman et al. 1993,1995). Moreover, Rinaman (2003) showed that selective lesion of NTS catecholamine cells severely attenuates CCK-induced anorexia. We therefore hypothesized that these NTS catecholamine cells are involved in the integration of leptin and CCK signaling in the brain. To evaluate this hypothesis, we asked whether induction of c-Fos, a protein transcription factor that is widely accepted to represent a marker of neuronal activation, is enhanced in the population of NTS cells that express tyrosine hydroxylase (TH), the rate-limiting enzyme for catecholamine synthesis, when peripheral CCK administration is preceded by a third-ICV injection of leptin. This is a widely used protocol for identifying NTS neurons that have the capability of responding to CCK satiety signals and leptin signaling to the CNS by both peripheral and ICV routes, and we adopted it here to approach the hypothesis question experimentally using double-label immunohistochemistry techniques to colocalize c-Fos protein in NTS TH cells, as well as with laser capture microdissection (LCM) to obtain TH-positive NTS cells for quantitative PCR analysis of c-Fos mRNA levels under the same conditions.
Materials and Methods
Animals
Naïve adult male Wistar rats (Charles River; Wilmington, MA) were individually housed in Plexiglass cages in a temperature-controlled room under a 12:12-hr light:dark cycle. Rats were given ad libitum access to water and standard rat chow (Purina 5001; Purina, St. Louis, MO) except where otherwise noted. All procedures conformed to institutional standards of animal care and use as specified in the National Research Council's Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care Committees at the Veterans Administration Puget Sound Health Care System and University of Washington.
Surgery
Each rat received a guide cannula (Plastics One, 26-G; Roanoke, VA), implanted 1.0 mm above the third ventricle under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia administered by IP injection or 2–4% isofluorane in 1 liter oxygen/min inhaled continuously during surgery, as previously described (Blevins et al. 2004). Stereotaxic coordinates for ICV cannula placement were 6.8 mm anterior to the interaural line, −7.0 mm ventral to the dura mater, and on the midline. The cannula was cemented to four jeweler's screws attached to the skull and closed with an obturator. Ceftriaxone (25 mg/kg) (Roche Laboratories; Nutley, NJ) and buprenorphine hydrochloride (0.3 mg/kg, IM; Rickett Colman Pharmaceuticals, Richmond, VA) were administered at completion of surgery. Rats recovered for at least 5 days while daily food intake and body weight were recorded. Correct cannula placement was verified after recovery from surgery by observation of angiotensin-II–induced drinking behavior (Fitzsimons 1998).
Drugs
Recombinant mouse leptin (National Hormone and Peptide Program; Torrence, CA) was dissolved in PBS (pH 7.9) and injected ICV at a dose (3.5 μg delivered in a volume of 2.5 μl) selected on the basis of previous work in which this dose of leptin has been shown to enhance the satiety effect of CCK but have no independent effect on c-Fos induction in the NTS (Emond et al. 1999; Blevins et al. 2004). CCK octapeptide (Peninsula Laboratories; San Carlos, CA) was dissolved in 0.1% BSA in saline and delivered through IP injection at a dose of 2.75 nmol/kg in a volume of 1 ml/kg. Based on our previous experience, this dose induces a detectable but submaximal induction of c-Fos-like immunoreactivity (cFLI) in NTS cells.
Procedures for Immunofluorescence Colocalization of TH and cFLI
Experimental Design
Subjects in this study represent a subset from a larger experiment on the pharmacology and behavioral effects of the interaction between leptin and CCK (Blevins et al. 2004). Rats (310–410 g) were fasted before injections on the day of the experiment. Saline (2 μl) was delivered to the third ventricle 30–45 min before third-ICV leptin or vehicle injections. Sixty min later, immediately before the onset of the dark cycle, CCK was injected IP. Ninety min after CCK treatment, rats were deeply anesthetized and transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA). Brains were removed and postfixed overnight in 4% PFA and transferred to 25% sucrose in PBS for 24 hr. The brains were frozen in isopentane on dry ice. Coronal cryostat sections (10 μm) through the NTS were slide-mounted and stored at −80C until selection for immunohistochemical staining.
Double Label Immunofluorescence
Anatomically matched sections through the NTS at the level of the area postrema were selected for staining. Slide-mounted sections were first blocked for 60 min at room temperature with buffer containing 5% normal goat serum, 5% normal donkey serum, and 0.1% BSA in 10 mM PBS and then incubated overnight at 4C with a “cocktail” of the primary antibodies, 1:5000 rabbit polyclonal anti-c-Fos (Calbiochem; San Diego, CA), and 1:1000 mouse monoclonal anti-TH (MAB318; Chemicon International, Temecula, CA) diluted in 0.1% BSA in 10 mM PBS. Slides were rinsed with PBS and incubated for 60 min at room temperature with the secondary antibodies, 1:200 goat anti-mouse IgG-Alexa-488 (Molecular Probes; Eugene, OR) and donkey 1:200 anti-rabbit IgG-Cy-3 (Jackson Immunoresearch; West Grove, PA), diluted in 0.1% BSA in 10 mM PBS. After rinsing with PBS, slides were coverslipped with an antifading glycerol-based mounting media containing Hoechst 33258 (Sigma-Aldrich; St. Louis, MO), used here to identify cell nuclei and to aid in anatomic localization. Control sections incubated with normal rabbit serum and nonspecific monoclonal antibodies did not show staining of NTS cells. Likewise, controls performed to show the possibility of inappropriate cross-reactivity between the primary and secondary antibodies of the different species showed negative staining. This protocol and these antibodies have been used in previous studies on central nervous system immunostaining for TH and c-Fos (Blevins et al. 2003,2004; Figlewicz et al. 2003).
Quantitative Analysis of Immunostaining
Slides were examined using a ×10 objective on a Nikon Eclipse E600 fluorescence microscope (Melville, NY) with the appropriate filter sets, and digital RGB images were acquired with a Diagnostic Images (Sterling Heights, MI) SPOT RT Color camera and SPOT software. NIH Image software (Bethesda, MD) was used to count c-Fos-positive cells and TH-positive cells. cFLI and TH double-labeled cells were counted manually. TH-positive cells were counted only if the cell nucleus was observable within the plane of section because such cells could be clearly identified as c-Fos positive or negative. Labeled catecholamine neurons in the C2/A2 groups of the NTS were counted bilaterally at the level of the area postrema on two sections per rat, and a mean value was taken for each subject. Group means are an average of the individual subject means.
Statistical Analysis
The numbers of c-Fos-positive, TH-positive, and double-labeled NTS cells in rats that had been injected with vehicle vs leptin before CCK were compared through independent samples two-tailed Student's t-test. Group differences in percentage of c-Fos-positive cells colocalized with TH-like immunoreactivity and the percentage of TH-positive colocalized with cFLI were also assessed by independent samples two-tailed Student's t-test.
Procedures for Laser Capture of NTS TH Cells and PCR Analysis
Subjects
Rats (mean body weight = 385 g) were fasted on the day of the experiment starting 6 hr before the onset of the dark cycle. Third-ICV injections of leptin or vehicle were administered 60 min before the dark cycle. Rats were injected IP with vehicle or CCK 1 hr later, immediately before the dark cycle. The four treatment groups in the study were as follows: vehicle ICV/vehicle IP (n=4); leptin ICV/vehicle (n=5); vehicle ICV/CCK IP (n=5); and leptin ICV/CCK IP (n=5). Sixty min after IP leptin injections, rats were deeply anesthetized with ketamine/xylazine and decapitated. Brains were rapidly removed and frozen at −37C in isopentane. Coronal cryostat sections (10 μm) through the NTS at the level of the area postrema were collected for rapid immunostaining and subsequent LCM.
LCM of Immunostained Neurons
Slide-mounted cryotat sections of unfixed rat hindbrains were removed from −70C storage and allowed to thaw before fixation in ice-cold 100% MeOH for 3 min. The slides were briefly dipped in cold 10 mM PBS and incubated for 3 min with 1:25 mouse anti-TH monoclonal antibody diluted in PBS. This was followed by four brief rinses in cold PBS. The samples were incubated for 3 min in 1:50 secondary goat anti-mouse-Cy3 antibody (115-165-068; Jackson ImmunoResearch) diluted in PBS. Slides were washed four times in cold PBS and dehydrated (1 min each in 75%, 95%, and 2× 1 min in 100% EtOH), followed by 5 min in xylene. After air-drying for 10 min, brain sections were visualized on the Arcturus AutoPix Fluorescent Laser Capture Microdissection System (Molecular Devices Corporation; Sunnyvale, CA). TH-positive cells were picked by LCM and collected on the Arcturus plastic sample caps. Approximately 150–200 TH-positive neurons from six coronal sections of NTS at the level of the area postrema were collected and pooled on a single cap for each animal. RNA was extracted, purified, and treated with DNase, using the Arcturus PicoPure RNA isolation kit. The RNA was used as a template to generate cDNA using the Applied Biosystems High Capacity cDNA Archive kit (4322171; Applied Biosystems, Foster City, CA). Levels of mRNA for TH, c-Fos, and GAPDH (internal control) were measured by quantitative real-time PCR on an Applied Biosystems Prism 7000 Sequence Detection System.
The primers and probes for GAPDH and c-Fos mRNA were designed with the aid of Primer Express version 2.0.0 software (Applied Biosystems). GAPDH mRNA was measured using 1 μM forward (5′-GCCAGCCTCGTCTCATAGACA-3′) and 1 μM reverse (5′-GTCCGATACGGCCAAATCC-3′) primers in conjunction with 0.4 μM TaqMan probe primer (VIC-5′-ATGGTGAAGGTCGGTGTG-3′). c-Fos mRNA was measured with 1 μM forward (GAGCCCTCCTCTGACTCACTGA) and 1 μM reverse (TGCCTTCTCTGACTGCTCACA) primers in conjunction with 0.4 μM Taqman probe primer (TGCCTTCTCTGACTGCTCACA). The TH mRNA TaqMan probe and primers were obtained from Applied Biosystems (4331182 reference Rn00562500_m1). All measurements were made using the Applied Biosystems Taqman Universal PCR Master Mix and the ABI Prism 7000 Sequence Detection System. The samples were incubated first at 50C for 2 min and then at 95C for 10 min and were subjected to 40 cycles of alternating 95C for 15 sec, followed by 60C for 1 min. Relative amounts of TH and c-Fos mRNA were calculated using the Comparative CT method that generates relative TH and c-Fos mRNA levels adjusted for the GAPDH endogenous control mRNA. The data points for each animal represent the average of triplicate measurements.
Statistical Analysis
TH mRNA levels in TH-positive vs TH-negative tissue samples taken from a subset of experimental subjects (n=4) were compared using a one-tailed paired samples t-test. The effects of leptin and CCK on c-Fos mRNA levels in NTS TH cells were assessed by between-subject two-way ANOVA (ICV treatment × IP treatment). Post hoc comparisons were done by Tukey's honestly significant difference test.
Results
Immunofluorescence Colocalization of TH and cFLI
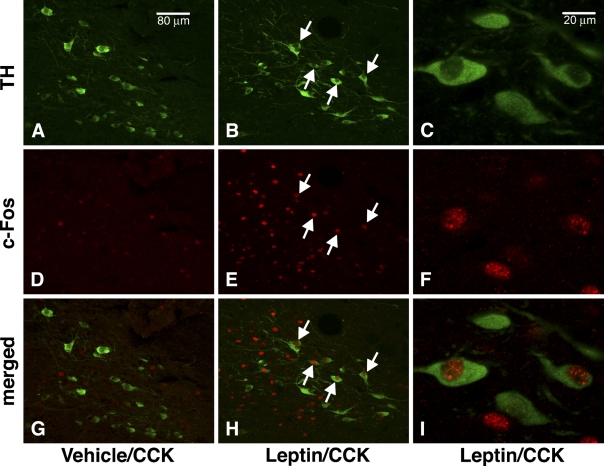
The immunofluorescence procedure revealed neurons containing cFLI and TH in the NTS, the known location of A2/C2 catecholamine cell groups. The cFLI staining was concentrated in neuronal nuclei, whereas TH immunofluorescence was distributed in the neuronal perikarya and fibers, which were the expected locations of the respective proteins (Figure 1). This different morphological distribution of cFLI and TH facilitated the visual identification of double-labeled cells. As expected, there was no visual difference in the numbers and intensity of immunostained TH-positive neurons among the treatment groups (Figures 1A and 1B). CCK administration in the absence of leptin pretreatment under the conditions of this protocol had a minimal effect on numbers of cFLI in the NTS (Figure 1D). In contrast, animals that received ICV leptin pretreatment showed a robust NTS c-Fos response to CCK (Figure 1E), similar to previous reports (Emond et al. 1999; Blevins et al. 2004).
Figure 1.
Fluorescence immunostaining and confocal microscopy of cholecystokinin (CCK)-induced Fos expression in A2/C2 catecholamine (TH-positive) neurons in the nucleus of the solitary tract (NTS) after pretreatment with vehicle (A,D,G) or leptin (B,C,E,F,H,I). Images were acquired with our Leica SP1 confocal microscope. TH-positive catecholamine neurons, identified by green Alexa-488 cytoplasmic fluorescence, are visible in the NTS (A–C). In D–F, c-Fos-immunoreactive cell nuclei are identified in the same field as A–C by red Cy3 nuclear fluorescence. Note relative abundance of c-Fos-positive cells in the NTS after leptin pretreatment (E) compared with vehicle (D). Colocalization of TH- and c-Fos-like immunoreactivity is shown by merging images of TH- and c-Fos-positive cells (G–I). Arrows indicate several c-Fos-positive/TH-positive cells. TH-positive cells that expressed Fos were scarce in the NTS of rats pretreated with vehicle (G). C,F, and I show TH- and c-Fos-positive cells at higher magnification. Bars: A,B,D,E,G,H = 80 μm; C,F,I = 20 μm.
We next determined whether the effect of leptin pretreatment to enhance the cFLI response to CCK occurred preferentially in NTS TH-positive neurons. In the absence of ICV leptin pretreatment, few TH-positive cells in the NTS contained cFLI, as shown by the paucity of immunostained neuronal nuclei (Figure 1G). By contrast, after ICV leptin pretreatment, many TH-positive cells contained nuclei immunostained for cFLI (Figure 1H).
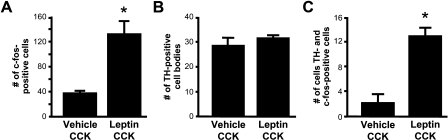
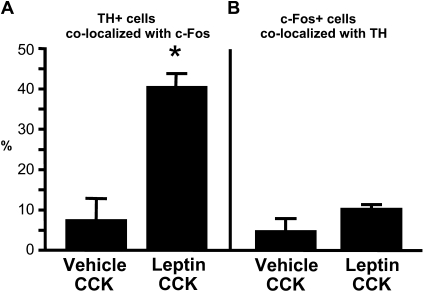
Quantitative analysis of the NTS indicated that ICV leptin pretreatment strongly increased the number of c-Fos-positive cells in the NTS of rats after a subthreshold peripheral dose of CCK administration compared with this dose of CCK given after an ICV vehicle injection [t(7) = 4.41; p<0.01; Figure 2A]. There was no difference in the numbers of TH-positive NTS neurons among the treatment groups [t(7) = 1.11; Figure 2B]. However, ICV leptin pretreatment resulted in a 6-fold increase in the number of TH-positive neurons that contained nuclear cFLI staining [t(7) = 6.21; p<0.001; Figure 2C]. Leptin pretreatment also markedly increased the percentage of the TH cell population that expressed c-Fos in response to CCK from 7% to 40% [t(7) = 6.00; p<0.001; Figure 3A]. The population of NTS neurons that expressed cFLI in response to either CCK alone or to leptin and CCK in combination was not limited to catecholaminergic neurons. On the contrary, TH-positive neurons made up only 5% of all cFLI-positive cells in the NTS of rats in the vehicle/CCK group, and the stimulatory effect of leptin on the percentage of NTS cFLI-positive cells that were also TH-positive did not achieve statistical significance [t(7) = 2.00; p = 0.08; Figure 3B].
Figure 2.
Effect of leptin pretreatment on c-Fos response to CCK in NTS cells. Leptin pretreatment increases the number of c-Fos-positive cells in the NTS after IP CCK injection, *p<0.01 (A). The number of TH-positive cell bodies in the NTS is not affected by leptin injection (B). A significantly greater number of TH-positive cells coexpressed c-Fos after leptin and CCK treatment compared with vehicle and CCK treatment, *p<0.01 (C).
Figure 3.
Percentage colocalization of TH- and c-Fos-like immunoreactivity. The percentage of all NTS TH-positive cells that are also c-Fos-positive after CCK injection is significantly increased by leptin pretreatment, *p<0.001 (A). The percentage of all NTS c-Fos-positive cells that are also TH-positive is small, even after leptin pretreatment (B).
Laser Capture of NTS TH Cells and PCR Analysis
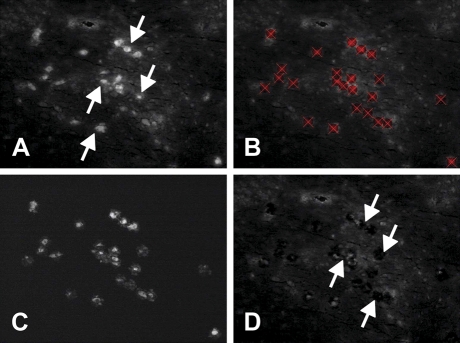
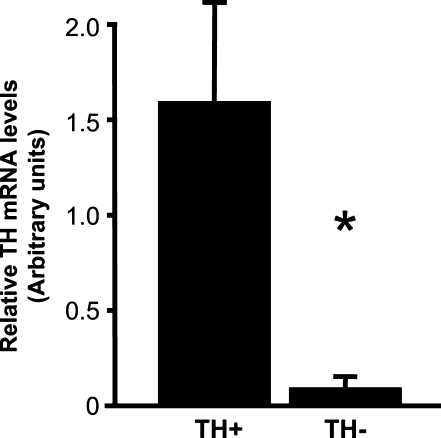
Our rapid immunostaining procedure before LCM enabled the immunocytochemical identification of TH-positive neurons in the NTS (Figure 4A). TH-positive cells were marked for picking by laser capture (Figure 4B), and after they were picked (150–200 cells/rat), the marked cells could be visually detected by fluorescence after they had been transferred to the Arcturus plastic sample caps (Figure 4C). Examination of the NTS section after cell picking revealed holes where the labeled cells had been removed (Figure 4D). The success of this immuno-laser capture procedure for obtaining enriched samples of TH-positive neurons was confirmed by real-time quantitative PCR of these RNA extracts, which showed substantially higher levels of TH mRNA in laser-captured samples of TH-immunoreactive cells compared with samples of nearby cells that were negative for TH immunoreactivity [t(3) = 2.81; p<0.05; Figure 5].
Figure 4.
Laser capture microdissection (LCM) method. (A) Coronal section through NTS showing fluorescence (white), indicating the presence of TH immunoreactivity (white arrows identify several TH cells). (B) TH-positive cells (red X) targeted for laser capture. (C) Tissue that has been removed from the slide and collected onto a plastic cap. This material is homogenized, and RNA is isolated for RT-PCR. (D) White arrows indicate examples of holes left in the tissue section after the cells seen in A–C were removed by the laser.
Figure 5.
Relative TH mRNA levels in a TH-enriched NTS cell sample vs a TH-negative sample, *p<0.05.
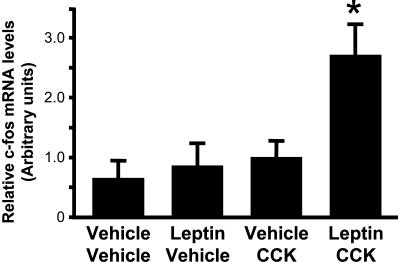
Analysis of the extracted RNA from the different treatment groups showed that neither the leptin/vehicle (i.e., leptin alone) nor the vehicle/CCK (i.e., CCK alone) groups showed a significant increase in c-Fos mRNA in TH cell–enriched NTS samples compared with the vehicle/vehicle group. In animals that received ICV leptin treatment before IP CCK, however, the level of c-Fos mRNA in the TH cell–enriched samples was increased ∼2.5-fold [interaction between leptin and CCK: F(1,15) = 4.32; p<0.05; Figure 6]. The combination of the ICV leptin and peripheral CCK treatments resulted in significantly increased levels of c-Fos mRNA in NTS TH cells compared with either leptin or CCK alone (p<0.01).
Figure 6.
Relative c-Fos mRNA levels in TH-enriched NTS cell sample. Leptin pretreatment significantly enhances the c-Fos response to CCK, *p<0.01, relative to all other treatment groups. mRNA content was measured by RT-PCR on samples taken from the NTS of rats in each of four treatment groups using LCM.
Discussion
The significant new finding of this study was that A2/C2 catecholamine neurons in the rat NTS are preferentially activated, as measured by expression of c-Fos mRNA and immunoreactive cFLI protein, during the synergistic interaction of peripheral CCK and ICV leptin on food intake. This finding was based in part on histochemical techniques that identified cFLI immunostaining in NTS neurons that contained immunoreactive TH. In addition, we used the technique of immuno-LCM to collect enriched populations of NTS TH-positive neurons for real-time quantitative PCR. The latter studies confirmed the visual and quantitative findings of the immunostaining studies, showing that the synergistic interaction between the actions of these peptides also increases mRNA levels for c-Fos in this population of A2/C2 catecholamine neurons.
Growing evidence suggests that negative feedback adiposity signals, such as leptin, reduce food intake, at least in part by enhancing the satiety response to meal-related signals, including CCK. To better understand the basis for this interaction, we studied the hypothesis that catecholamine neurons in the NTS integrate the effects of leptin and CCK. Using a combination of LCM and real-time quantitative PCR, we report that third-ICV leptin pretreatment markedly enhances the effect of IP CCK administration to increase expression of the gene encoding c-Fos, a marker of neuronal activation, in NTS catecholaminergic neurons. Furthermore, our histochemical results showed that when leptin was given before CCK, the activation of TH neurons (relative to animals that received CCK without leptin pretreatment) was twice as great as was observed in unselected NTS neurons (i.e., 6-fold vs 3-fold). This result establishes TH neurons as selective targets of the interaction between leptin and CCK in the NTS. It is also clear from our immunohistochemical results, however, that other non-TH-positive cell populations within the NTS are involved in the integration of leptin and CCK signaling, and it will be important to identify their phenotypes in the future.
The use of LCM to analyze c-Fos mRNA expression in catecholamine neurons of the NTS is a novel aspect of this study. This strategy allowed us to ask whether NTS catecholamine cells are involved in the leptin-induced potentiation of the c-Fos response to CCK in the NTS using a combination of rapid immunostaining with LCM. This technique has been shown to be a valid method for obtaining mRNA from an immunohistochemically defined subpopulation of cells selected from heterogeneous tissue, and the protocol that we used here has also been validated (Fend et al. 1999). In the present study, our selection of TH-positive neurons by LCM probably included a small amount of cytoplasm from adjacent cells, such as non-TH neurons and glial or other cell types. Nevertheless, the samples of NTS catecholamine neurons captured on the basis of TH-like immunoreactivity were substantially enriched in TH mRNA compared with control samples of TH-negative NTS tissue. This control indicated that our immunostaining/LCM procedure is an effective method for obtaining a TH neuron–enriched sample of NTS tissue for RT-PCR analysis. Moreover, this same population of NTS TH-positive neurons was highly enriched with c-Fos mRNA after leptin pretreatment and CCK administration, a finding confirmed by our immunocytochemical observations of increased cFLI in NTS TH-positive cells after leptin plus CCK treatment.
The specific mechanisms through which leptin enhances the c-Fos response to CCK in NTS TH cells are not yet clear, but the available data support the hypothesis that leptin potentiates the stimulatory effect of CCK on these neurons through actions in both the hindbrain and the forebrain. Recent electrophysiological studies have shown that the majority of TH neurons in the mouse NTS receive direct stimulatory input from vagal afferent fibers (Appleyard et al. 2005). If this is also true in the rat, the TH-positive cells that we identified in this analysis are likely to be among the primary recipients of CCK-induced vagal afferent excitation. Leptin also has the potential to act directly on these NTS catecholamine cells. Hay-Schmidt et al. (2001) reported that every identifiable catecholamine cell in the A2 and C2 groups of the NTS coexpressed the leptin receptor, and although the antibody used in that study was unable to discriminate between the various leptin receptor isoforms, the signaling form of the leptin receptor (Ob-Rb) is expressed at high density in the NTS (Grill et al. 2002; Hosoi et al. 2002) and is therefore likely to be found on some, if not all, of these TH-positive neurons. Alternatively, or in addition, other cell types within the NTS may be directly affected by leptin and indirectly affect the activity of a downstream TH neuron through synaptic contact.
It is widely accepted that leptin acts within the hypothalamus to reduce food intake, and previous evidence indicates that it can enhance the response of unselected NTS neurons to CCK through descending projections from the forebrain. Our laboratory has published evidence that the NTS c-Fos response to CCK can be modulated by leptin action restricted to the hypothalamus (Morton et al. 2005). Genetically obese fak/fak rats, which lack functional leptin receptors, are less sensitive to the feeding and NTS neuronal effects of CCK than wild-type controls. Adenoviral gene therapy to restore functional leptin expression exclusively in the mediobasal hypothalamus enhances both the feeding and NTS c-Fos response to CCK in these rats, showing the existence of feeding-relevant leptin-sensitive pathways for communication between forebrain and hindbrain. In addition, recent studies from our group have identified a leptin-sensitive oxytocinergic projection from the paraventricular nucleus of the hypothalamus to the NTS that seems to modulate CCK responsiveness (Blevins et al. 2003,2004). This or other leptin-sensitive descending projections from the hypothalamus may be pathways through which leptin action in the forebrain affects the ability of CCK to activate NTS catecholamine cells. The question of whether descending projections from the forebrain contribute to the effect of leptin to enhance the stimulatory effect of CCK specifically among catecholaminergic neurons in the NTS awaits further study.
Although our findings do not address the mechanism by which TH neurons could mediate the synergistic effects of leptin and CCK on food intake, they nevertheless represent an important contribution to understanding the basis for this interaction. Rinaman (2003) used an immuno-lesioning strategy to show that NTS catecholamine neurons are needed for CCK-induced satiety and, although this hypothesis remains untested, a leptin-induced augmentation of CCK's stimulatory effect on these cells could conceivably contribute to the enhanced satiety response. The present identification of NTS TH neurons as candidate mediators of this effect provides a compelling rationale for studies that critically test this hypothesis.
A priority for future work is to elucidate the mechanisms through which activation of NTS neurons leads to inhibition of food intake. In addition to regulating the activity of parasympathetic motor neurons supplying the gastrointestinal tract, NTS neurons project widely throughout the brain and innervate areas involved in diverse aspects of food intake control. For example, several hypothalamic subnuclei (e.g., the paraventricular nucleus and lateral hypothalamus) involved in food intake and autonomic regulation receive projections from NTS neurons. Areas involved in perception of food reward (e.g., nucleus accumbens), and nuclei involved in the perception of and response to aversive stimuli (e.g., parabrachial nucleus, amygdala) are also innervated by the NTS, as are nuclei that control oromotor aspects of ingestion (i.e., the motor nuclei of the facial, trigeminal, and hypoglossal nerves) (Sawchenko 1983). Experimental strategies in which TH neurons that supply these different brain areas are selectively inhibited or ablated may shed light on the neurocircuitry linking the activity of NTS neurons to food intake inhibition.
Acknowledgments
This material is based on work supported by the Office of Research and Development, Medical Research Service, Veterans Health Administration of the Department of Veterans Affairs. D.G.B. is the recipient of a Department of Veterans Affairs Senior Research Career Scientist Award at the Department of Veterans Affairs Puget Sound Health Care System. This work was also supported by a National Institutes of Health (NIH) individual National Research Service Award fellowship (to DLW), NIH Grants DK-52989, DK-12829, DK-68340, and NS-32273 (to MWS), and the Diabetes and Endocrinology Research Center (DERC) and Clinical Nutrition Research Unit of the University of Washington.
The authors thank David Caldwell, Jenny Kam, Loan Nguyen, and the DERC Cellular and Molecular Imaging Core at the University of Washington for expert technical assistance.
References
- Appleyard SM, Kobayashi K, Okano H, Low MJ, Andresen MC (2005) Opioids Inhibit Sensory Afferent Activation of Tyrosine Hydroxylase Neurons in the Nucleus of the Solitary Tract. Washington, DC, Society for Neuroscience
- Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y (1997) Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 94:10455–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG (2003) Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993:30–41 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG (2004) Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–96 [DOI] [PubMed] [Google Scholar]
- Edwards GL, Ladenheim EE, Ritter RC (1986) Dorsomedial hindbrain participation in cholecystokinin-induced satiety. Am J Physiol 251:R971–977 [DOI] [PubMed] [Google Scholar]
- Emond M, Schwartz GJ, Ladenheim EE, Moran TH (1999) Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol 276:R1545–1549 [DOI] [PubMed] [Google Scholar]
- Fend F, Emmert-Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, Raffeld M (1999) Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol 154:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG (2003) Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115 [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT (1998) Angiotensin, thirst, and sodium appetite. Physiol Rev 78:583–686 [DOI] [PubMed] [Google Scholar]
- Flynn MC, Plata-Salaman CR (1999) Leptin (OB protein) and meal size. Nutrition 15:508–509 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG (2002) Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143:239–246 [DOI] [PubMed] [Google Scholar]
- Hay-Schmidt A, Helboe L, Larsen PJ (2001) Leptin receptor immunoreactivity is present in ascending serotonergic and catecholaminergic neurons of the rat. Neuroendocrinology 73:215–226 [DOI] [PubMed] [Google Scholar]
- Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y (2002) Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology 143:3498–3504 [DOI] [PubMed] [Google Scholar]
- Kahler A, Geary N, Eckel LA, Campfield LA, Smith FJ, Langhans W (1998) Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am J Physiol 275:R180–185 [DOI] [PubMed] [Google Scholar]
- Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ (1997) Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol 272:R1245–1251 [DOI] [PubMed] [Google Scholar]
- Moran TH, Ladenheim EE, Schwartz GJ (2001) Within-meal gut feedback signaling. Int J Obes Relat Metab Disord 25(suppl 5):S39–41 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, et al. (2005) Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Rinaman L (2003) Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci 23:10084–10092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG (1995) Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol 360:246–256 [DOI] [PubMed] [Google Scholar]
- Rinaman L, Verbalis JG, Stricker EM, Hoffman GE (1993) Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J Comp Neurol 338:475–490 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE (1983) Central connections of the sensory and motor nuclei of the vagus nerve. J Auton Nerv Syst 9:13–26 [DOI] [PubMed] [Google Scholar]
- Strader AD, Woods SC (2005) Gastrointestinal hormones and food intake. Gastroenterology 128:175–191 [DOI] [PubMed] [Google Scholar]