Abstract
Histo-blood group ABH antigens are widely distributed in human tissues. The epitopes of ABH antigens are carried by at least four different peripheral core isotypes of internal carbohydrate backbones (type 1–4). Each type of ABH antigen is expressed tissue specifically, and aberrant expression of ABH antigens is often observed during oncogenesis. We immunohistochemically examined the expression of A type 3 antigens in wounded and diseased skin tissues (A and AB blood groups). In uninjured skin, the expression of A type 3 antigens was restricted to the eccrine sweat gland. In addition to the sweat glands, A type 3 antigens were found in vascular endothelial cells of the wound sites. The extent of A type 3 antigens expression related to postinfliction intervals. A significantly higher expression rate of A type 3 antigens in endothelial cells was also observed in diseased skin, suggesting that inflammation might induce A type 3 antigen expression in endothelial cells. Double-color immunofluorescence staining of the specimens showed that von Willebrand factor (vWF) was a core-protein of A type 3 determinants aberrantly expressed in endothelial cells in inflamed tissues, suggesting that aberrant expression of A type 3 antigens is involved in stabilization of vWF in inflammation. (J Histochem Cytochem 56:223–231, 2008)
Keywords: histo-blood group A type 3 antigen, vascular endothelial cell, wound healing, inflammation, von Willebrand factor
Histo-blood group ABH determinants are well characterized as oligo-saccharides and carried on various glycopeptides and glycolipids, which are widely distributed in human tissues (Oriol 1995; Ravn and Dabelsteen 2000). The epitope of the H antigen, the precursor of A, B, Ley, and Leb antigens of ABO and Lewis histo-blood groups, is carried by at least four different peripheral core isotypes of internal carbohydrate backbones (type 1, Galβ1-3GlcNAcβ1-R; type 2, Galβ1-4GlcNAcβ1-R; type 3, Galβ1-3GalNAcα1-3Galβ1-R; type 4, Galβ1-3GalNAcβ1-3Galα1-R) (Clausen and Hakomori 1989). These chains are built by sequential addition of saccharide units on the luminal side of the Golgi apparatus by specific glycosyltransferases (Nakajima et al. 1988). Each type of ABH antigen is expressed tissue specifically. It is well known that type 1 antigens are expressed mainly in tissues of endodermal origin, and type 2 antigens are expressed in various tissues of both ecto- and endodermal origin. The expression of type 3 and 4 antigens is less well characterized but also expressed in tissues of ecto- and endodermal origin (Clausen and Hakomori 1989), which were identified as two forms: glycolipids as in repetitive A structures (Clausen et al. 1986a) and O-linked mucin-type structures (Donald 1981; Dabelsteen et al. 1990).
Aberrant expression of ABH antigens is often observed in the oncogenesis of various organs (Cooper et al. 1991; Dabelsteen and Gao 2005); however, there is little knowledge about the alteration of ABH antigen expression in other diseased tissues (Dabelsteen et al. 1990). In inflamed tissues such as infected, diseased, or wounded tissues, various cytokines affect gene expression patterns in vascular endothelial cells. Normal vascular endothelial cells specifically express type 2 antigens on their cell surface (Bryne et al. 1993). The expression of ABH blood group antigens in vascular endothelial cells also can be altered in such inflammatory tissues. Indeed, recent reports showed the aberrant expression of Ley/H antigens in vascular endothelial cells in inflammatory sites (Koch et al. 1994). Furthermore, the expression of these antigens seemed to be involved in inflammatory processes, suggesting that ABH blood group antigens played some roles in inflammation.
In this study, we immunohistochemically examined A type 3 antigen expression in vascular endothelial cells in wounded and diseased skin tissues and showed the inflammation-specific expression of A type 3 antigens on von Willebrand factor (vWF) in vascular endothelial cells.
Materials and Methods
Antibodies
Histo-blood group A type 3–specific monoclonal antibody (AR-1, IgM) was produced by immunizing mice with blood group A erythrocyte membranes. The specificity of AR-1 was determined by TLC immunostaining of glycosphingolipids extracted from blood group A erythrocyte membranes, which was confirmed by comparing with authentic anti-A type 3 monoclonal antibody TH-1 (Clausen et al. 1985). Histo-blood group H type 3/4-specific monoclonal antibody (MBr1, IgM) (Bremer et al. 1984) was purchased from Alexis Biochemicals (Lausen, Switzerland). Rabbit anti-human vWF polyclonal antibody was obtained from Dako Cytomation Japan (Kyoto, Japan). FITC-conjugated donkey anti-rabbit IgG polyclonal antibody (Jackson ImmunoResearch Laboratories; West Grove, PA) and Cyanine dye 3 (Cy3)-conjugated goat anti-mouse IgM polyclonal antibody (Chemicon International; Temecula, CA) were used for double-color immunofluorescence staining.
Human Skin Wound Specimens
At forensic autopsy (Institute for Legal Medicine; University of Munich, Munich, Germany), open skin wound samples were obtained from cadavers with blood groups of A or AB type. Briefly, a total of 30 human skin wounds (stab wound, n=9; laceration, n=4; incised wound, n=11; surgical wound, n=6) with different postinfliction intervals ranging from a few minutes to 21 days were used in this study. Their individual ages ranged from 20 to 80 years (mean age: 47.9 years), and the postmortem interval was <3 days in each case. None of the cases had suffered from severe malnutrition, malignant diseases, or metabolic disorders, and no substances such as cytostatic agents or glucocorticoids, which may possibly influence wound healing, were administered during medical treatment. According to postinfliction intervals, the wound specimens were classified into three groups as follows: Group I, 0–12 hr (n=14); Group II, 1–4 days (n=11); Group III, 7–21 days (n=5). Uninjured skin from the same individuals was also taken as a control.
Human Diseased Skin Tissues
Diseased skin tissue specimens (atopic dermatitis, n=4; eczema, n=6, psoriasis vulgaris, n=5; erythema nodosum, n=3; drug eruption, n=2; leukocyte-elastic vasculitis, n=1) were obtained by biopsy from the patients (blood groups A and AB) with informed consent for diagnosis in Wakayama Medical University Hospital.
Immunohistochemistry
The specimens were fixed in 4% formaldehyde solution with PBS (pH 7.2) and embedded in paraffin, followed by sectioning at a thickness of 4 μm. The sections were dewaxed, rehydrated, and immersed in 3% H2O2 for 5 min at room temperature to inactivate endogenous peroxidase activity and incubated with 5% skimmed milk in 10 mM Tris-HCl (pH 7.4) containing 0.5 M NaCl and 0.05% Tween-20 for 20 min. The sections were incubated with AR-1 diluted at 1:20 at room temperature in a moist chamber for 1 hr. Thereafter, these sections were incubated with anti-mouse immunoglobulins conjugated to horseradish peroxidase (HRP)-labeled polymer (Envision+; Dako Cytomation) at room temperature in a moist chamber for 30 min, and positive reactions were visualized with 4-chroroaminoethylcarbasol. Hematoxylin was applied to the sections for counterstaining.
Immunostaining for vWF was performed with rabbit anti-human vWF (1:500) and anti-rabbit immunoglobulins conjugated to HRP-labeled polymer (Envision+; Dako Cytomation) as described above.
Double-color Immunofluorescence Staining
A double-color immunofluorescence analysis was also performed to determine the types of A type 3–expressing cells in wounded skin samples, as described previously (Ishida et al. 2004). Briefly, dewaxed and rehydrated sections were incubated with PBS containing 1% normal donkey serum, normal goat serum, and 1% BSA to reduce nonspecific reactions. Thereafter, the sections were further incubated in pairs of AR-1 (1:40) and anti-vWF (1:400) antibody at 4C overnight. After incubation with Cy3-conjugated anti-mouse IgM polyclonal antibody (1:400) and FITC-conjugated anti-rabbit IgG polyclonal antibody (1:50) at room temperature for 1 hr, the sections were preserved under coverslips in Vectashield mounting medium (Vector Laboratories; Burlingame, CA), and images were obtained using a LAM5Pascal Exciter (Carl Zeiss Japan; Tokyo, Japan) laser scanning confocal microscope.
Morphometrical Analysis
Morphometrical analysis was performed for semiquantitative evaluation of the immunohistochemical findings by two different investigators without prior knowledge. Briefly, in each section, 10 microscopic fields (magnification, ×400) were randomly selected, and the ratio of the number of A type 3–positive vascular endothelial cells was calculated in each microscopic field. The adjacent sections were stained with rabbit anti-human vWF antibody to identify vascular endothelial cells. The average ratio of the 10 selected microscopic fields was evaluated as A type 3 expression score in each wound specimen.
Statistical Analysis
Results are expressed as the mean ± SEM. In each group of 30 human skin wounds, one-factor ANOVA was used to determine whether differences existed among the group means, followed by the Scheffe F test to identify significantly different means.
Results
Localization of A Type 3 and H Type 3/4 Antigens in Normal Skin Tissue
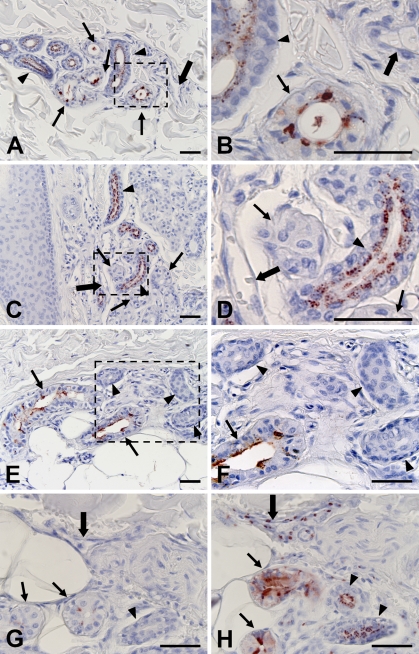
In normal skin, A type 3 antigens defined by AR-1 were localized in the cytoplasm of dark cells and inner layer cells of ducts in eccrine sweat glands in specimens from secretors, individuals expressing secretor (Se) gene–encoded FUT II, and secreting blood group antigens in saliva (Figures 1A and 1B). On the other hand, only duct cells of eccrine sweat glands expressed A type 3 antigens in specimens from non-secretors, individuals not expressing Se gene–encoded FUT II, and individuals not secreting blood group antigens in saliva (Figures 1C and 1D). In addition to eccrine sweat glands, the cytoplasm of vascular endothelial cells in the dermis near the epidermis was occasionally stained by AR-1 (data not shown). Vascular endothelial cells in the subcutaneous tissue did not express A type 3 antigens. In contrast to A type 3 antigens, H type 3/4 antigens defined by MBr1 were localized in the cytoplasm of dark cells of eccrine sweat glands but not in duct cells (Figures 1E and 1F). The expression of H type 3/4 antigens depended on the secretor status but was irrespective of the ABO blood group. Furthermore, H type 3/4 antigens were not detected in any vascular endothelial cells. Absorption of AR-1 with blood group A red cells completely abolished their reactivity to the sweat glands and vascular endothelial cells (Figure 1G). Moreover, absorption of AR-1 with blood group O red cells had no effect on their reactivity (Figure 1H), indicating that AR-1 reacted specifically to blood group A antigens.
Figure 1.
Localization of histo-blood group A type 3 antigens reactive to AR-1, and H type 3/4 antigens reactive to MBr1 in normal skin. A type 3 antigens are localized in dark cells (arrows) and duct epithelial cells (arrowheads) in eccrine sweat glands in specimen from A blood group secretor (A,B). Only duct epithelial cells of eccrine sweat glands were reactive to AR-1 in specimens from non-secretors (C,D). Histo-blood group H type 3/4 antigens were detected in dark cells but not duct cells of eccrine sweat glands in specimens from O blood group secretors (E,F). Most vascular endothelial cells did not express type 3 antigens (large arrows). Areas indicated by boxes in A, C, and E are depicted at higher magnification in B, D, and F, respectively. Two sections from a wound specimen (Group II) were stained with AR-1 absorbed with blood group A (G) and O red cells (H), respectively. Absorption with blood group A red cells abolished reactivity of AR-1 (G). Bar = 50 μm.
Enhanced Expression of A Type 3 Antigens in Wounded Skin Tissue
In Group I (0–12 hr), very few vascular endothelial cells were scattered as positive for AR-1, which was essentially identical to normal skin tissues (data not shown). In contrast to Group I, the ratio of A type 3 antigen–positive vascular endothelial cells was remarkably elevated in Group II (1–4 days) and Group III (7–21 days) (Figures 2B and 2E), which was confirmed by vWF expression in adjacent sections (Figures 2A and 2D). Furthermore, the extent of antigen expression also significantly increased in these specimens. Secretor status did not affect the expression of A type 3 antigen in vascular endothelial cells. A type 3 antigens were detected in the cytoplasm but not on the cell surface of endothelial cells with a granular pattern (Figures 2C and 2F).
Figure 2.
Immunohistochemical staining of the skin of a 1-day-old wound (Group II). Vascular endothelial cells in the dermis (A) and subcutaneous region (D) of 1-day-old wound specimens were identified by immunostaining with anti-human von Willebrand factor (vWF) antibody. A type 3 antigens were strongly detected in vascular endothelial cells in the adjacent sections (B,E). Areas indicated by boxes in B and E are depicted at higher magnification in C and F, respectively. Bar = 50 μm.
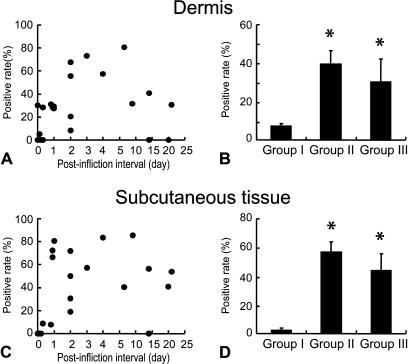
The ratio of A type 3 antigen–positive cells in vascular endothelial cells defined by vWF expression was highest in Group II, in which the majority of endothelial cells were positive (Figures 3A–3D). In wound specimens with postinfliction intervals of 7–21 days (Group III), the A type 3 antigen–positive cell ratio decreased gradually, although compared with Group II, it remained high, suggesting that the expression level of A type 3 antigens in vascular endothelial cells is in accordance with the stage of the wound healing process (Figure 3).
Figure 3.
Ratio of A type 3–positive vascular endothelial cells in the dermis (A) and subcutaneous tissue (C) in relation to postinfliction intervals. Vascular endothelial cells were identified by immunostaining of adjacent sections with anti-human vWF antibody as shown in Figure 2. Mean value and SE of A type 3–positive vascular endothelial cells in the dermis (B) and subcutaneous tissues (D) in each wound group. *Significant difference from Group I was observed statistically (p<0.05).
Enhanced Expression of A Type 3 Antigens in Diseased Skin Tissues
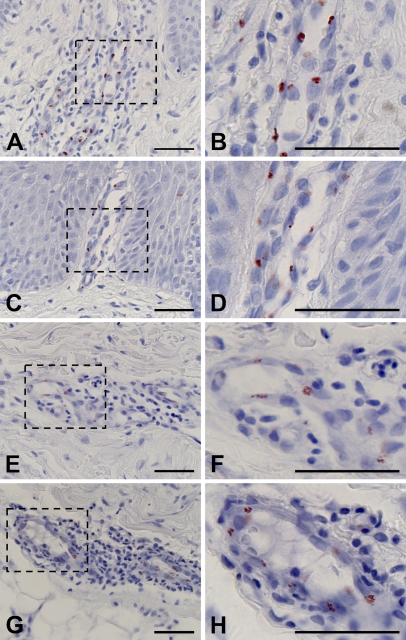
The expression of A type 3 antigens was examined in biopsy specimens of diseased skin such as atopic dermatitis (Figures 4A and 4B), eczema (Figures 4C and 4D), psoriasis vulgaris (Figures 4E and 4F), erythema nodosum (Figures 4G and 4H), drug eruption (data not shown), and leukocyte-elastic vasculitis (data not shown) tissues from blood group A or AB patients. Aberrant expression of A type 3 antigens was observed to various extents in all specimens. In atopic dermatitis (Figures 4A and 4B) and eczema (Figures 4C and 4D), the expression of A type 3 antigens was remarkably enhanced in endothelial cells of dilated capillaries in the papillae. A type 3 antigens were also highly expressed in vascular endothelial cells in the reticular layer of dermis in which inflammatory changes such as perivascular lymphocytic infiltrate, vascular proliferation with thickening of the endothelium, and edema were observed (Figures 4A–4H). These observations suggested that phenotypic alteration of endothelial cells in inflammation induced the expression of A type 3 antigens.
Figure 4.
Immunohistochemical staining of diseased skin tissues. A type 3 antigens were detected in the vascular endothelial cells in the dermis of atopic dermatitis (A), eczema (C), psoriasis (E), and erythema nodosum (G) specimens.Areas indicated by boxes in A, C, E, and G are depicted at higher magnification in B, D, F, and H, respectively. Bar = 50 μm.
Identification of Core Protein Carrying A Type 3 Antigens
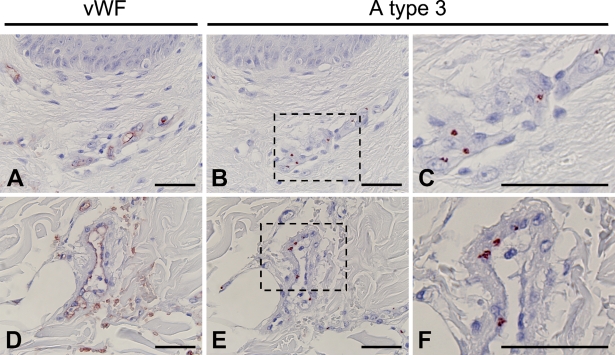
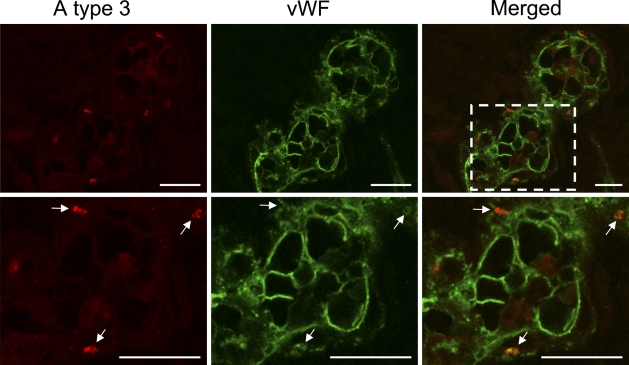
Current evidence suggests that ABH determinants are added to N-linked oligosaccharide chains of vWF in the post-Golgi of endothelial cells before secretion and that there may be significant heterogeneity in the amount of ABH antigen expressed on vWF secreted by endothelial cells (Matsui et al. 1992,1999; Brown et al. 2002). Therefore, it is likely that aberrantly expressed A type 3 antigens in endothelial cells are carried on vWF. To address this possibility, we performed double-color immunofluorescence analysis using a combination of monoclonal antibody AR-1 and anti-human vWF antibody. We found that AR-1 reactive A type 3 antigens were localized in the cytoplasm of cells stained with vWF, confirming that A type 3–positive cells were vascular endothelial cells (Figure 5). Furthermore, the images obtained by using a laser scanning confocal microscope showed that the intracellular spots detected by AR-1 were merged to the cytoplasmic regions reactive to anti-human vWF antibody, which strongly suggested that vWF was a core-protein of A type 3 determinants aberrantly expressed in endothelial cells in inflammation. However, core protein of A type 3 determinants in endothelial cells remains to be studied.
Figure 5.
Double-color immunofluorescence analysis of wounded skin tissues using a combination of AR-1 and antibody to vWF. A type 3 antigens (red) located in cells stained with anti-vWF (green) in 4-day-old wounded skin. Area indicated by box in merged panel is depicted at higher magnification in bottom panels. All AR-1–reactive spots were found in the regions reactive to anti-human vWF antibody. As shown in bottom panels, the discrete spots (white arrows) reactive to AR1 were completely merged to anti-human vWF-reactive spots. Bar = 20 μm.
Discussion
In normal tissues, A type 3 antigens have been found as glycolipids in erythrocytes (Clausen et al. 1986a) and as O-linked mucin-type structures in saliva and ovarian cyst fluid (Donald 1981) from blood group A individuals. In addition to these tissues, the expression of type 3 antigens was detected immunologically in the stomach mucosa, large intestine mucosa, pancreas, biliary ducts, gall bladder, sweat glands, bronchial epithelium, kidney, and posterior root ganglia but not in vascular endothelial cells (Pendu et al. 1986). Herein, we described aberrant expression of A type 3 antigens in vascular endothelial cells in wounded and diseased skin tissues.
The A type 3 antigen structure on glycolipids is constructed by adding A determinant to the terminal GalNAc of the A type 2 structure; therefore, it is designated repetitive A. The A type 3 antigen structure on mucins is constructed by adding fucose and GalNAc to the terminal galactose of T antigen (Macartney 1986; Nakayama et al. 1987; Sotozono et al. 1994). At present, we cannot define which structure of A type 3 antigen is expressed in vascular endothelial cells; however, monoclonal antibody MBr1 specific for H type 3/4 on mucins (Bremer et al. 1984; Clausen et al. 1986a,b; Adobati et al. 1997) reacted to dark cells in eccrine sweat glands but not to duct cells and vascular endothelial cells, suggesting that A type 3 antigens in eccrine sweat glands are carried on mucins and those in the ducts of eccrine glands and vascular endothelial cells are carried on other than mucins. In this study, we showed Se gene–dependent expression of A type 3 antigens in dark cells of sweat glands and their Se gene–independent expression in duct cells of sweat glands and vascular endothelial cells (Figure 1). Similar Se gene–dependent expression of A/H type 3 chain in oral epithelium and salivary glands (Mandel et al. 1991; Liu et al. 1999) and secretor status–independent expression of type 3 chain A/H structures in erythrocyte membranes have been reported (Clausen et al. 1985,1987).
In wounded and diseased tissues, vascular endothelial cells seem to be affected by various immune modulators, especially cytokines that induce the gene expression of proinflammatory molecules such as adhesion molecules in endothelial cells (Matsunaga et al. 1998; Cumberbatch et al. 2002; Takamiya et al. 2002; Hayashi et al. 2004), suggesting that the expression of A type 3 antigens is induced by cytokines. In normal and wounded skin samples with short postinfliction intervals (Group I), A type 3 antigens could be occasionally detected in vascular endothelial cells in the dermis near the epidermis but not subcutaneously. It is known that keratinocytes in the epidermis constitutively produce IL-1α (Gahring et al. 1985; Hauser et al. 1986) that may occasionally induce the expression of A type 3 antigens in vascular endothelial cells in normal skin.
The expression level of A type 3 antigens in wounded skin depended on postinfliction intervals, which is consistent with the fact that various cytokines are generated dependently of postinfliction intervals at wound sites. Immunohistochemical examination of human skin wounds revealed that the expression of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α was induced quickly and peaked within 90 min after injury in the epidermis, subepidermis, blood vessels, and sweat glands (Grellner 2002). On the other hand, the induction of IL-1α expression was relatively slow and persisted for several weeks after injury (Kondo et al. 1999). A substantial amount of A type 3 antigens was expressed even in 3-week-old skin wounds, which was similar to the expression pattern of IL-1α in human skin wounds. Furthermore, IL-1α is known to induce the expression of adhesion molecules in vascular endothelial cells (Chang et al. 2002), suggesting that IL-1α is involved in A type 3 expression in endothelial cells at wound sites. On the other hand, a significant increase of IL-1α accompanied with an increase in IL-1 receptor antagonist was observed in psoriatic skin (Bonifati et al. 1997; Debets et al. 1997; Terui et al. 1998); therefore, IL-1α also may be a candidate of inducers of the expression of A type 3 antigens in psoriasis.
Dabelsteen et al. (1990) reported that the glycosylation pattern in epidermis changed in psoriasis and mucosal wound. Type 2 and 3 antigens (H and T) were expressed at earlier stages of cell maturation, and Tn and sialyl-Tn antigens, precursors of T antigen, were aberrantly expressed in psoriatic epithelium. In contrast to our results, decreased expression of A antigens concomitant with increase of H and Ley antigens expression was observed in migrating epithelial cells in mucosal wounds (Dabelsteen et al. 1998). Alterations of cytokines and growth factors in diseased and wounded tissues may induce these changes. It seems that inflammatory cytokines affect both endothelial cells and epithelial cells in wound sites; however, the action of cytokines depends on target cells. For instance, IL-1 and IL-2 show quite different effects on subpopulations of thymocytes (Conlon et al. 1982).
Similar aberrant expression of blood group type 2 antigens, Ley/H, in vascular endothelial cells in inflammatory disease was reported, in which Ley/H antigen expression was rapidly induced by immune modulators, cytokines, lipopolysaccharide, phorbol-12-myristate-13-acetate, calcium ionophore, thrombin, histamine, and growth factors (Koch et al. 1994; Halloran et al. 2000). Enhanced expression of Ley/H antigens was observed in diseased skin tissues such as psoriatic skin, which was quite similar to the A type 3 antigen expression pattern. Interestingly, not only proinflammatory cytokines, TNF-α and IL-1α, but also an anti-inflammatory cytokine, IL-4, induced Ley/H antigen expression in human dermal microvascular endothelial cells (Halloran et al. 2000); therefore, it is likely that various immune modulators also induce A type 3 antigen expression, which enables endothelial cells to express A type 3 antigens persistently in wound sites.
It was shown that Ley/H antigens acted as an endothelial-selective, cytokine-inducible, angiogenic mediator, suggesting that Ley/H antigens play roles in the wound healing process. Furthermore, H type 2 antigens mediate leukocyte-endothelial adhesion through intracellular adhesion molecule, suggesting that they are involved in the progression of inflammation (Zhu et al. 2003). On the other hand, A type 3 determinants seemed to be carried on vWF in endothelial cells in inflamed tissues (Figure 5), suggesting that aberrantly expressed A type 3 antigens play a role in blood coagulation. Although a core protein of A type 3 determinants in endothelial cells must be further studied, our findings are supported by current evidence that ABH determinants are added to the N-linked oligosaccharide chains of vWF in endothelial cells before secretion (Matsui et al. 1992,1999), and there is a difference in the intracellular addition of A-antigen to vWF by endothelium from different vascular beds (Brown et al. 2002). It is well established that the ABO blood group exerts a major quantitative effect on plasma vWF levels, with significantly lower levels in blood group O individuals (Gill et al. 1987; Shima et al. 1995; Jenkins and O'Donnell 2006). Furthermore, accumulating evidence suggest that reduction in the number of sugars on the oligosaccharide chains of vWF is clearly associated with an increased susceptibility to cleavage by ADAMTS13 (Bowen 2003; O'Donnell et al. 2005). On the other hand, plasma vWF level is elevated in inflammation (Zezos et al. 2005), which is controlled, at least in part, by cytokines and chemokines (Bernardo et al. 2004). Therefore, aberrant expression of A type 3 antigens on vWF in endothelial cells in inflamed tissue may contribute to stabilization of plasma vWF in inflammation.
Acknowledgments
The authors thank Dr. Henrik Clausen (Department of Medical Biochemistry and Genetics, the Faculty of Health Sciences, University of Copenhagen, Denmark) for providing monoclonal antibodies, TH-1, and HH14 and invaluable comments on the manuscript and Dr. Toshikazu Kondo for valuable discussion and encouragement.
References
- Adobati E, Panza L, Russo G, Colnaghi MI, Canevari S (1997) In vitro mimicry of CaMBr1 tumor-associated antigen by synthetic oligosaccharides. Glycobiology 7:173–178 [DOI] [PubMed] [Google Scholar]
- Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF (2004) Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 104:100–106 [DOI] [PubMed] [Google Scholar]
- Bonifati C, Carducci M, Mussi A, D'auria L, Ameglio F (1997) IL-1 α, IL-1β and psoriasis: conflicting results in the literature. Opposite behaviour of the two cytokines in lesional or non-lesional extracts of whole skin. J Biol Regul Homeost Agents 11:133–136 [PubMed] [Google Scholar]
- Bowen DJ (2003) An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost 1:33–40 [DOI] [PubMed] [Google Scholar]
- Bremer EG, Levery SB, Sonnino S, Ghidoni R, Canevari S, Kannagi R, Hakomori S (1984) Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem 259:14773–14777 [PubMed] [Google Scholar]
- Brown SA, Collins PW, Bowen DJ (2002) Heterogeneous detection of A-antigen on von Willebrand factor derived from platelets, endothelial cells and plasma. Thromb Haemost 87:990–996 [PubMed] [Google Scholar]
- Bryne M, Lilleholt S, Thrane PS, Koppang HS, Dabelsteen E (1993) Distribution of blood-group-related carbohydrate antigens on oral endothelial cells. Histochem J 25:339–347 [DOI] [PubMed] [Google Scholar]
- Chang CH, Hang Y, Issekutz AC, Griffith M, Lin KH, Anderson R (2002) Interleukin-1α released from epithelial cells after adenovirus type 37 infection activates intercellular adhesion molecule 1 expression on human vescular endothelial cells. J Virol 76:427–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen H, Hakomori S (1989) ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang 56:1–20 [DOI] [PubMed] [Google Scholar]
- Clausen H, Holmes E, Hakomori S (1986a) Novel blood group H glycolipid antigens exclusively expressed in blood group A and AB erythrocytes (type 3 chain H). II. Differential conversion of different H substrates by A1 and A2 enzymes, and type 3 chain H expression in relation to secretor status. J Biol Chem 261:1388–1392 [PubMed] [Google Scholar]
- Clausen H, Levery SB, Kannagi R, Hakomori S (1986b) Novel blood group H glycolipid antigens exclusively expressed in blood group A and AB erythrocytes (type 3 chain H). I. Isolation and chemical characterization. J Biol Chem 261:1380–1387 [PubMed] [Google Scholar]
- Clausen H, Levery SB, Nudelman ED, Stroud M, Salyan ME, Hakomori S (1987) Isolation and characterization of novel glycolipids with blood group A-related structures: galactosyl-A and sialosylgalactosyl-A. J Biol Chem 262:14228–14234 [PubMed] [Google Scholar]
- Clausen H, Levery SB, Nudelman E, Tsuchiya S, Hakomori S (1985) Repetitive A epitope (type 3 chain A) defined by blood group A1-specific monoclonal antibody TH-1: chemical basis of qualitative A1 and A2 distinction. Proc Natl Acad Sci USA 82:1199–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon PJ, Henney CS, Gillis S (1982) Cytokine-dependent thymocyte responses: characterization of IL 1 and IL 2 target subpopulations and mechanism of action. J Immunol 128:797–801 [PubMed] [Google Scholar]
- Cooper HS, Malecha MJ, Bass C, Fagel PL, Steplewski Z (1991) Expression of blood group antigens H-2, Le(y), and sialylated-Le(a) in human colorectal carcinoma. An immunohistochemical study using double-labeling techniques. Am J Pathol 138:103–110 [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Groves RW, Antonopoulos C, Kimber I (2002) Differential regulation of epidermal langerhans cell migration by interleukins (IL)-1α and IL-1β during irritant- and allergen-induced cutaneous immune responses. Toxicol Appl Pharmacol 182:126–135 [DOI] [PubMed] [Google Scholar]
- Dabelsteen E, Broby-Johansen U, Jeppe-Jensen D, Mandel U (1990) Cell surface glycosylation patterns in psoriasis. APMIS 98:221–228 [DOI] [PubMed] [Google Scholar]
- Dabelsteen E, Gao S (2005) ABO blood-group antigens in oral cancer. J Dent Res 84:21–28 [DOI] [PubMed] [Google Scholar]
- Dabelsteen E, Grøn B, Mandel U, Mackenzie I (1998) Altered expression of epithelial cell surface glycoconjugates and intermediate filaments at the margins of mucosal wounds. J Invest Dermatol 111:592–597 [DOI] [PubMed] [Google Scholar]
- Debets R, Hegmans JP, Croughs P, Troost RJ, Prins JB, Benner R, Prens EP (1997) The IL-1 system in psoriatic skin: IL-1 antagonist sphere of influence in lesional psoriatic epidermis. J Immunol 158:2955–2963 [PubMed] [Google Scholar]
- Donald ASR (1981) A-active trisaccharide isolated from A1 and A2 blood-group-specific glycoproteins. Eur J Biochem 120:243–249 [DOI] [PubMed] [Google Scholar]
- Gahring LC, Buckley A, Daynes RA (1985) Presence of epidermal-derived thymocyte activating factor/interleukin 1 in normal human stratum corneum. J Clin Invest 76:1585–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ Jr, Montgomery RR (1987) The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood 69:1691–1695 [PubMed] [Google Scholar]
- Grellner W (2002) Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in human skin wounds. Forensic Sci Int 130:90–96 [DOI] [PubMed] [Google Scholar]
- Halloran MM, Carley WW, Polverini PJ, Haskell CJ, Phan S, Anderson BJ, Woods JM, et al. (2000) Ley/H: an endothelial-selective, cytokine-inducible, angiogenic mediator. J Immunol 164:4868–4877 [DOI] [PubMed] [Google Scholar]
- Hauser C, Saurat JH, Schmitt A, Jaunin F, Dayer JM (1986) Interleukin 1 is present in normal human epidermis. J Immunol 136:3317–3321 [PubMed] [Google Scholar]
- Hayashi T, Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2004) Forensic application of VEGF expression to skin wound age determination. Int J Legal Med 118:320–325 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Tsuneyama K, Lu P, Takayasu T, Mukaida N (2004) The pathogenic roles of tumor necrosis factor receptor p55 in acetaminophen-induced liver injury in mice. J Leukoc Biol 75:59–67 [DOI] [PubMed] [Google Scholar]
- Jenkins PV, O'Donnell JS (2006) ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion 46:1836–1844 [DOI] [PubMed] [Google Scholar]
- Koch AE, Nickoloff BJ, Holgersson J, Seed B, Haines GK, Burrows JC, Leibovich SJ (1994) 4A11, a monoclonal antibody recognizing a novel antigen expressed on aberrant vascular endothelium. Upregulation in an in vivo model of contact dermatitis. Am J Pathol 144:244–259 [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Ohshima T, Eisenmenger W (1999) Immunohistochemical and morphometrical study on the temporal expression of interleukin-1α (IL-1α) in human skin wounds for forensic wound age determination. Int J Legal Med 112:249–252 [DOI] [PubMed] [Google Scholar]
- Liu Y, Fujitani N, Koda Y, Soejima M, Kimura H (1999) Presence of H type 3/4 chains of ABO histo-blood group system in serous cells of human submandibular gland and regulation of their expression by the secretor gene (FUT2). J Histochem Cytochem 47:889–894 [DOI] [PubMed] [Google Scholar]
- Macartney JC (1986) Lectin histochemistry of galactose and N-acetyl-galactosamine glycoconjugates in normal gastric mucosa and gastric cancer and the relationship with ABO and secretor status. J Pathol 150:135–144 [DOI] [PubMed] [Google Scholar]
- Mandel U, Petersen OW, Sørensen H, Vedtofte P, Hakomori S, Clausen H, Dabelsteen E (1991) Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J Invest Dermatol 97:713–721 [DOI] [PubMed] [Google Scholar]
- Matsui T, Shimoyama T, Matsumoto M, Fujimura Y, Takemoto Y, Sako M, Hamako J, et al. (1999) ABO blood group antigens on human plasma von Willebrand factor after ABO-mismatched bone marrow transplantation. Blood 94:2895–2900 [PubMed] [Google Scholar]
- Matsui T, Titani K, Mizuochi T (1992) Structures of the asparagine-linked oligosaccharide chains of human von Willebrand factor. Occurrence of blood group A, B, and H(O) structures. J Biol Chem 267:8723–8731 [PubMed] [Google Scholar]
- Matsunaga T, Katayama I, Yokozeki H, Nishioka K (1998) Epidermal cytokine mRNA expression induced by hapten differs from that induced by primary irritant in human skin organ culture system. J Dermatol 25:421–428 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Ito N, Nishi K, Okamura Y, Hirota T (1988) Cytochemical localization of blood group substances in human salivary glands using lectin-gold complexes. J Histochem Cytochem 36:337–348 [DOI] [PubMed] [Google Scholar]
- Nakayama J, Ota M, Honda T, Katsuyama T (1987) Histochemical demonstration of sugar residues by lectin and immunocytochemical techniques for blood group antigens in human colon. Histochem J 19:454–464 [DOI] [PubMed] [Google Scholar]
- O'Donnell JS, McKinnon TA, Crawley JT, Lane DA, Laffan MA (2005) Bombay phenotype is associated with reduced plasma-VWF levels and an increased susceptibility to ADAMTS13 proteolysis. Blood 106:1988–1991 [DOI] [PubMed] [Google Scholar]
- Oriol R (1995) ABO, Hh, Lewis, and secretion serology, genetics and tissue distribution. In Cartron J-P, Rouger P, eds. Blood Cell Biochemistry, vol. 6, Molecular basis of human blood group antigens. New York, Plenum Press, 37–73
- Pendu JLE, Lambert F, Samuelsson B, Breimer ME, Seitz RC, Urdaniz MP, Suesa N, et al. (1986) Monoclonal antibodies specific for type 3 and type 4 chain-based blood group determinants: relationship to the A1 and A2 subgroups. Glycoconj J 3:255–271 [Google Scholar]
- Ravn V, Dabelsteen E (2000) Tissue distribution of histo-blood group antigens. APMIS 108:1–28 [DOI] [PubMed] [Google Scholar]
- Shima M, Fujimura Y, Nishiyama T, Tsujiuchi T, Narita N, Matsui T, Titani K, et al. (1995) ABO blood group genotype and plasma von Willebrand factor in normal individuals. Vox Sang 68:236–240 [DOI] [PubMed] [Google Scholar]
- Sotozono MA, Okada Y, Tsuji T (1994) The Thomsen-Friedenreich antigen-related carbohydrate antigens in human gastric intestinal metaplasia and cancer. J Histochem Cytochem 42:1575–1584 [DOI] [PubMed] [Google Scholar]
- Takamiya M, Saigusa K, Aoki Y (2002) Immunohistochemical study of basic fibroblast growth factor and vascular endothelial growth factor expression for age determination of cutaneous wounds. Am J Forensic Med Pathol 23:264–267 [DOI] [PubMed] [Google Scholar]
- Terui T, Hirao T, Sato Y, Uesugi T, Honda M, Iguchi M, Matsumura N, et al. (1998) An increased ratio of interleukin-1 receptor antagonist to interleukin-1α in inflammatory skin diseases. Exp Dermatol 7:327–334 [DOI] [PubMed] [Google Scholar]
- Zezos P, Papaioannou G, Nikolaidis N, Vasiliadis T, Giouleme O, Evgenidis N (2005) Elevated plasma von Willebrand factor levels in patients with active ulcerative colitis reflect endothelial perturbation due to systemic inflammation. World J Gastroenterol 11:7639–7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Amin MA, Kim MJ, Katschke KJ Jr, Park CC, Koch AE (2003) A novel function for a glucose analog of blood group H antigen as a mediator of leukocyte-endothelial adhesion via intracellular adhesion molecule 1. J Biol Chem 278:21869–21877 [DOI] [PubMed] [Google Scholar]