Abstract
We have recently identified a novel collectin, CL-K1, that may play a role in innate immunity as a member of the collectin family. In this study using mice, we investigated the tissue distribution of CL-K1 for better understanding of its pathophysiological relevance. Real-time PCR analyses demonstrated that CL-K1 mRNA was expressed in all tissues tested. Immunohistochemical analyses demonstrated that CL-K1 was expressed in proximal tubules of kidney, in mucosa of the gastrointestinal tract, and in bronchial glands of bronchioles similar to the localization of SP-A and SP-D in these pulmonary structures. Immunohistochemistry also showed that CL-K1 was highly expressed in hepatocytes around the central veins in liver, which suggests that murine CL-K1 may be mainly produced in the liver and secreted into the blood stream as is human CL-K1. CL-K1 was especially detected in vascular smooth muscle in several types of tissues. In addition, it was also expressed in intestinal Paneth cells, in mesangial cells of kidney, in pancreatic islet D cells, and in neurons of the brain. It is of interest that this profile of CL-K1 expression is unique among the collectins. Together these histological findings may be useful for understanding the biological function of this novel collectin. (J Histochem Cytochem 56:243–252, 2008)
Keywords: CL-K1, Colec11, collectin, mannin-binding lectin, mouse, somatostatin, Paneth cells
Collectins are a family of proteins that contain two characteristic structures, a collagen-like region and a carbohydrate recognition domain (CRD) (Drickamer 1988). There are three classical collectins in humans: mannan-binding lectin (MBL) (Kawasaki et al. 1983; Sastry et al. 1991; Laursen et al. 1998; Laursen and Nielsen 2000) and surfactant proteins A and D (SP-A and SP-D) (Benson et al. 1985; Haagsman et al. 1987; Andersen et al. 1992). MBL, a plasma collectin synthesized in the liver (Sastry et al. 1991; Andersen et al. 1992; Hansen et al. 2002), can kill bacteria through activation of the complement pathway or by opsonization via collectin receptors (Kawasaki et al. 1989; Schweinle et al. 1989). SP-A and SP-D are mainly produced by alveolar type II cells and Clara cells in the lung (White et al. 1985; Lu et al. 1992; Madsen et al. 2000,2003; Paananen et al. 2001) and can mediate opsonization of bacteria and neutralization of viral growth. In addition, SP-A and SP-D associate directly with macrophages and stimulate phagocytosis or oxidant-dependent microbial clearance (Sano and Kuroki 2005). Thus, collectins play an important role in innate immunity.
Recently, cDNAs encoding three novel collectins, collectin liver 1 (CL-L1) (Ohtani et al. 1999), collectin placenta 1 (CL-P1) (Nakamura et al. 2001; Ohtani et al. 2001), and collectin kidney 1 (CL-K1) (Keshi et al. 2006) were isolated and characterized by our group as well as by other investigators. CL-L1 is mainly expressed in liver as a cytoplasmic protein. CL-P1 is a membrane-type collectin expressed in vascular endothelial cells, which binds to oxidized low-density lipoprotein (OxLDL) as a scavenger receptor. We have very recently demonstrated a novel human and murine collectin, CL-K1 (Keshi et al. 2006). According to the Mouse Genome Informatics database (http://www.informatics.jax.org/), CL-K1 was first cloned and deposited as RIKEN cDNA 1010001H16 in 2001. It was later assigned the name Colec11 (collectin subfamily member 11) in 2003. The Colec11 gene name is that used in the major databases including the Genome Expression Omnibus (http://www.ncbi.nlm.nih.gov/projects/geo/) that has 675 entries for expression data of this gene as determined by cDNA microarray. CL-K1 harbors a 25-amino acid signal sequence and is a secreted type of collectin present in human plasma. CL-K1 can also bind to microbial lipopolysaccharide (LPS) and lipoteichoic acid (LTA), suggesting that it may play an important role in innate immunity. However, little is known about the tissue distribution of CL-K1. In the present study we generated specific antibody against this collectin for use in immunohistochemistry (IHC) and determined the tissue distribution of CL-K1 in mice.
Materials and Methods
Animals and Tissues
Nine-week-old male C57Bl/6Ncrj mice (Charles River; Tokyo, Japan) were housed at 22C under 12 hr light/dark cycle (lights on at 7 am) conditions and were allowed access to food and water ad libitum. For histology and IHC, mice were anesthetized with 2.5% avertin and perfused through the left ventricle with 20 ml of ice-cold PBS and then with 4% paraformaldehyde in PBS at 4C for 20 min. Various tissues were then collected, and specimens were dehydrated and embedded in paraffin. For double staining of CD31 and CL-K1, various mouse tissues were fixed by IHV Zinc Fixative (BD Biosciences Pharmingen; San Diego, CA) at room temperature for 24 hr and embedded in paraffin. Five-μm-thick sections were stained for IHC with Mayer's hematoxylin. All experiments were carried out in accordance with the rules and guidelines of the Animal Experiment Committee of Asahikawa Medical College.
RNA Isolation and First-strand cDNA Synthesis
Total RNA was isolated from small pieces of mouse tissue (80–100 μg) using Trizol reagent (Invitrogen; Carlsbad, CA). RNA was reverse-transcribed using RETROscript (Ambion; Austin, TX). One μg of total RNA was mixed with 2 μl of random decamers and nuclease-free water in a total volume of 12 μl and heated at 80C for 3 min. The mixture was then chilled on ice and incubated with 2 μl of 10X RT buffer, 4 μl dNTP mix, 1 μl RNase inhibitor, and 1 μl reverse transcriptase at 44C for 60 min. Reaction mixtures were further incubated for 10 min at 92C. cDNA was stored at −30C until used for real-time PCR.
Analysis of mRNA Expression by Real-time PCR
Real-time PCR was performed with the Real Time PCR system (7500; Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. A Taqman probe primer set for CL-K1 (Mm01289834-m1) was purchased from Applied Biosystems.
Antibodies
Recombinant human CL-K1 including the neck and CRD domains (amino acids 107–271) together with six histidines was expressed in Escherichia coli GI724 using pPLH3 expression vector as described previously (Keshi et al. 2006). CL-K1-CRD-his protein was extracted and purified with Ni-NTA Agarose (Qiagen; Valencia, CA) according to the manufacturer's instructions. The N-terminal amino acid sequence of the purified recombinant protein was confirmed to be CL-K1-CRD-his. The purified recombinant protein was further characterized as CL-K1-CRD-his by SDS-PAGE and immunoblotting. New Zealand White rabbits were injected three times at 2-week intervals with 200 μg of the above fusion protein in incomplete Freund's adjuvant. After immunization, whole sera from rabbits were applied to HiTrap Protein G HP (Amersham Biosciences; Piscataway, NJ), and anti-CL-K1 rabbit IgG fractions were eluted with 0.1 M glycine–HCl buffer (pH 2.5). Furthermore, the anti-CL-K1 IgG was affinity purified using a CL-K1-CRD-his-conjugated antigen column, HiTrap NHS-activated HP (Amersham Biosciences), as described previously (Takeuchi et al. 1997). The IgG fraction, which passed through the CL-K1 antigen column, was used as the control IgG. Extent of purification was determined by ELISA as described.
ELISA
Microtiter plates were coated overnight at 4C with 10 μg/ml of various collectins, namely, CL-L1-CRD-his, CL-P1-CRD-his, CL-K1-CRD-his, mouse CL-K1-CRD-his, and MBL-CRD-his, in the coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 0.05% NaN3, pH 9.6). Plates were washed with TBS (Tris-buffered saline containing 20 mM Tris–HCl and 140 mM NaCl, pH 7.4)/TC (0.05% Tween 20 and 5 mM CaCl2) and incubated at 37C for 1 hr with various preparations of anti-CL-K1 antibodies containing the IgG fraction of the anti-CL-K1 serum, the affinity-purified anti-CL-K1 IgG, or the control IgG fraction. After washing, they were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Chemicon International; Temecula, CA) followed by color development using a TMB Peroxidase Substrate System (Kierkegaard and Perry Laboratories; Gaithersburg, MD). The reaction was stopped with 1 M phosphoric acid, and absorbance was measured at 450 nm.
Immunocytochemistry
CHO-K1 cells (ATCC; Rockville, MD) were stably transfected with human CL-K1 expression vectors as described previously (Keshi et al. 2006). Transfected cells (CHO/CL-K1) were plated in 14-mm wells of 35-mm plastic culture dishes (Matsunami Glass Industries; Tokyo, Japan) and cultured in Ham's F-12 medium containing 5% FBS. CHO/CL-K1 cells were fixed with 4% paraformaldehyde in PBS at 4C, permeabilized, and blocked in BlockAce (Dainippon Seiyaku; Osaka, Japan) for 1 hr at room temperature. Cells were then incubated with affinity-purified CL-K1 IgG or control IgG (1 μg/ml) overnight at 4C followed by treatment with anti-rabbit IgG-conjugated Alexa 488 and TO-PRO-3 (Molecular Probes; Eugene, OR). Fluorescent images were observed with a confocal laser-scanning microscope (CLSM, FV1000; Olympus Optical, Tokyo, Japan). All immunofluorescence images show fluorescence overlaid on phase contrast images.
IHC and Immunofluorescence Analyses
IHC staining was carried out with the avidin–biotin complex method and, for immunofluorescence, the indirect fluorescence staining method was used. Five-μm-thick tissue sections were cut and placed onto slides, and almost all sets of slides were processed together in the following steps. Slides were deparaffinized through a series of xylene and ethanol baths. Sections were blocked in BlockAce (Dainippon Seiyaku) for 1 hr at room temperature and then incubated in affinity-purified anti-CL-K1 IgG or control IgG (5 μg/ml) overnight at 4C. Each section was incubated with biotinylated guinea pig anti-rabbit IgG for 1 hr followed by incubation with avidin–biotin–alkaline phosphatase complex for 1 hr. Finally, the sections were treated with Alkaline Phosphatase Substrate Kit II (Vector Laboratories; Burlingame, CA). Endogenous alkaline phosphatase activity was blocked with Levamisol solution (Vector Laboratories). Sections were briefly counterstained with hematoxylin. In the case of immunofluorescence staining, secondary antibodies were incubated with Alexa Fluor 488 anti-rabbit IgG and TO-PRO-3 (Molecular Probes) for 1 hr. For double staining with somatostatin, glucagon, or insulin (Santa Cruz Biotechnology; Santa Cruz, CA) of stomach and pancreas, secondary antibody was used together with Alexa Fluor 594 anti-goat IgG. IHC and fluorescent images were examined with a CLSM. For double staining with mouse CD31 (BD Biosciences Pharmingen), secondary antibody was used together with Alexa Flour 594 anti-rat IgG.
Results
CL-K1 mRNA Expression in Murine Tissues
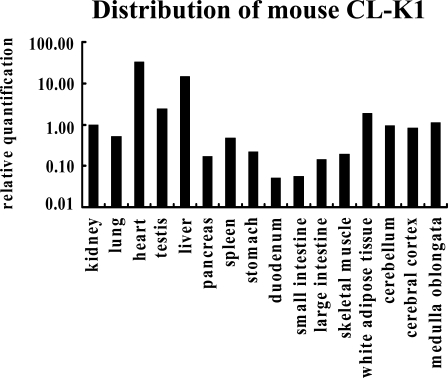
To investigate the tissue distribution of CL-K1 mRNA, real-time PCR analyses were performed with RNAs purified from a number of mouse tissues. We used specific primer pairs and a Taqman probe to detect the CL-K1 neck–CRD fragment. Data in Figure 1 show CL-K1 mRNA per 18S RNA and were further normalized to kidney defined as 1.0. Data of CL-K1 mRNA expression were normalized to kidney because we identified CL-K1 from kidney. Using real-time PCR analysis, CL-K1 mRNA was detectable in almost all organs tested (Figure 1). CL-K1 mRNA was found at the highest level in heart, and a relatively high expression of CL-K1 mRNA was detected in liver, testis, white adipose tissue, brain, and kidney.
Figure 1.
Estimation of the amount of CL-K1 mRNA in different tissues. Relative mRNA levels were measured by TaqMan RT-PCR. Data were normalized based on the value of 18S rRNA.
Characterization of the CL-K1 Affinity Antibody
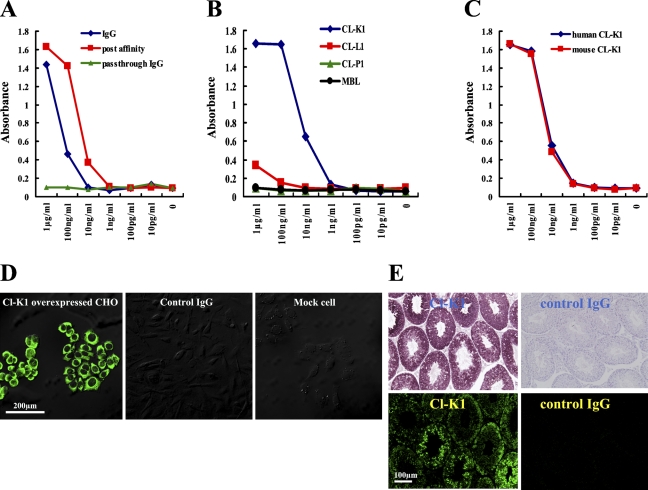
We first generated a specific antibody against CL-K1 for use in IHC. This antibody was raised against the CL-K1 neck–CRD region, which is highly conserved in humans, mice, and rats (Keshi et al. 2006). To increase antibody titer, we used a CL-K1 affinity column after IgG purification. As shown in Figures 2A and 2B, an ELISA for measuring titers of several antibodies against CL-K1 recombinant proteins revealed that CL-K1 antibodies were strongly reactive with the mouse CL-K1 protein. Because the affinity-purified antibody had an ∼10 times higher titer than the unpurified preparation, we used the former in the following experiments. Figure 2B indicates that the CL-K1 IgG reacted specifically with CL-K1 rather than with CL-L1, CL-P1, or MBL. Figure 2C shows that the affinity-purified antibody was capable of detecting mouse CL-K1 as well as human CL-K1. CL-K1 overexpressed CHO cells (CHO/CL-K1), or empty vector transfected CHO cells (mock) were stained with the affinity-purified CL-K1 or control antibody, respectively. As shown in Figure 2D (left and middle panels), the CL-K1 affinity-purified antibody could detect human CL-K1 protein in the cytoplasm of CHO/CL-K1 cells, whereas the control IgG could not. In addition, the CL-K1 antibody failed to react with anything in CHO/mock cells, as shown in the right panel of Figure 2D, clearly demonstrating the high specificity of the affinity-purified antibody.
Figure 2.
Specificity of our CL-K1 polyclonal antibody was analyzed by ELISA, immunocytochemistry, and immunohistochemistry (IHC). The anti-CL-K1 IgG fraction (IgG) was purified from rabbit serum. After IgG purification, the affinity antibody (postaffinity) was purified on an antigen column, and the pass-through IgG was used as the control IgG (pass-through IgG). (A) Results of ELISA analysis using anti-CL-K1 IgG, postaffinity antibody, or pass-through IgG. ELISA analyses of anti-CL-K1 antibodies against human CL-K1. (B) Results of ELISA analyses of anti-CL-K1 affinity antibody reactivity with other collectins, namely, CL-L1, CL-P1, and MBL. (C) Cross-reactivity between human and murine CL-K1 recombinant protein. (D) Immunofluorescence in CHO cells overexpressing CL-K1 (left and middle panels) as well as in empty vector expressed CHO cells (mock cells) (right panel). (E) IHC staining and immunofluorescence staining with affinity antibody or control IgG in murine testis.
CL-K1 Expression in Murine Tissues
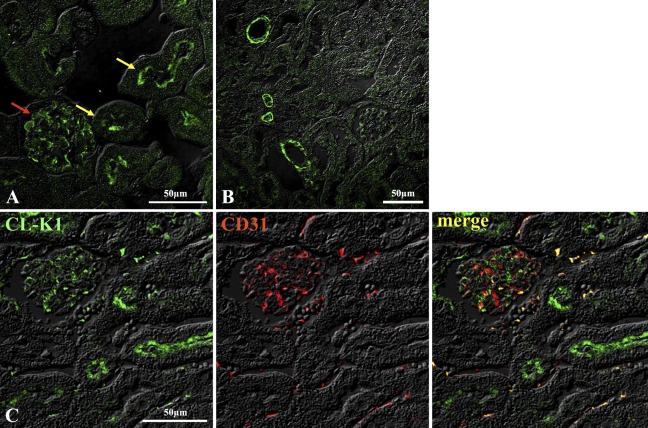
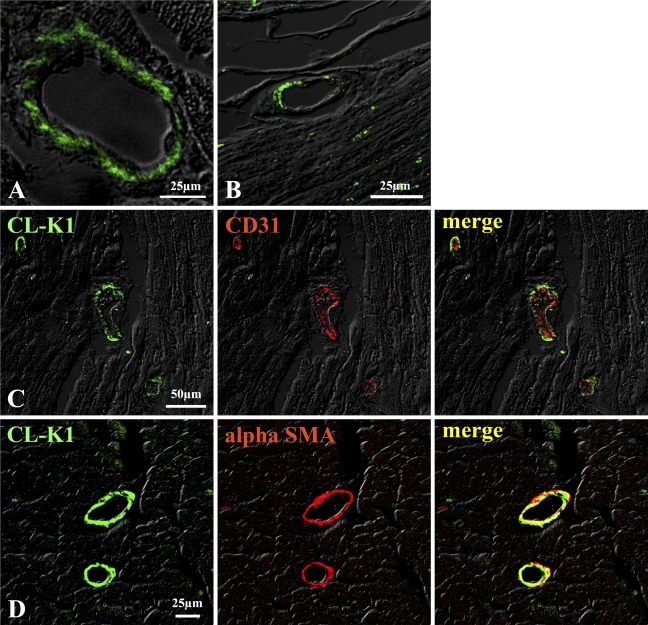
IHC and immunofluorescent analyses were performed in several murine tissues to investigate expression of the CL-K1 protein. Figure 2E shows that CL-K1 antibody could react with the testis, but the pass-through IgG used as a control could not detect any antigen in the testis, suggesting a specificity of the CL-K1 antibody. Using the CL-K1 affinity-purified IgG, IHC and immunofluorescent analyses were performed with tissues of murine kidney, lung, heart, testis, liver, pancreas, digestive organs (including the esophagus, stomach, small intestine, and large intestine), and brain. Figures 3A and 3B show results of immunofluorescence analysis of renal cortex demonstrating that CL-K1 was expressed in mesangial cells, podocyte, or microvascular endothelial cells of glomerulus (Figure 3A, red arrow) and in the brush border of proximal tubules (Figure 3A, yellow arrow). To further characterize the CL-K1 immunoreactive cells in the renal cortex, immunofluorescent analysis using both CL-K1 and antibody against CD31, a marker for endothelial cells, was performed. As demonstrated in Figure 3C, the merge image showed that endothelial cells do not express CL-K1, supporting the fact that CL-K1 may be expressed in the mesangial cells. Figure 3B shows that CL-K1 was also expressed in the vascular portion of the kidney. As shown in Figures 4A and 4B, CL-K1 was observed in the vascular portion of the heart and small intestine as well as in those of kidney. Furthermore, the double-immunofluorescence analyses presented in Figures 4C and 4D indicated that CL-K1 was expressed specifically in smooth muscle cells but not in endothelial cells. This indicates that vascular smooth muscle cells in all tissues are made up of primary cells expressing CL-K1.
Figure 3.
IHC of murine renal cortex (A) and vascular smooth muscle cells in kidney (B). CL-K1 protein was expressed in mesangial cells in glomerulus (red arrow in A) and in brush border of proximal tubules (yellow arrow in A). Double immunofluorescence staining (C) demonstrates that CL-K1 was not colocalized in microvascular endothelial cell.
Figure 4.
IHC of vascular cells in heart (A) and small intestine (B). CL-K1 expression was detected in vascular portion in heart (A) and small intestine (B). Double immunofluorescence staining (C,D) demonstrates that CL-K1 was colocalized in vascular smooth muscle cells but not in endothelial cells.
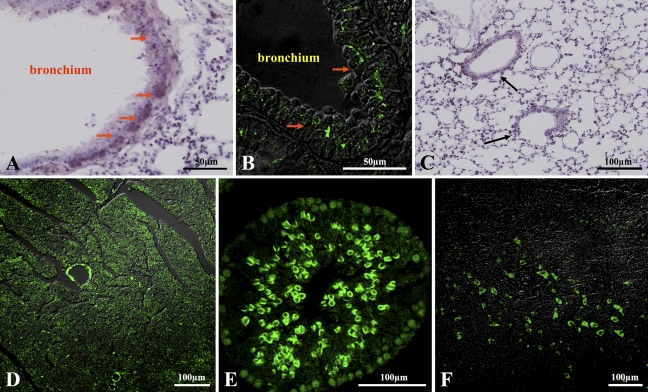
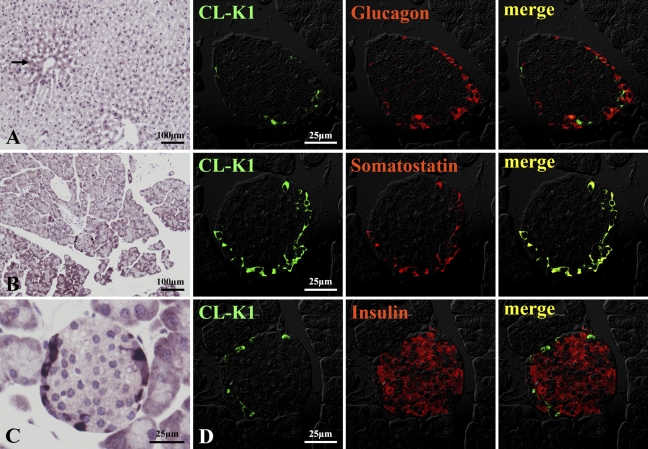
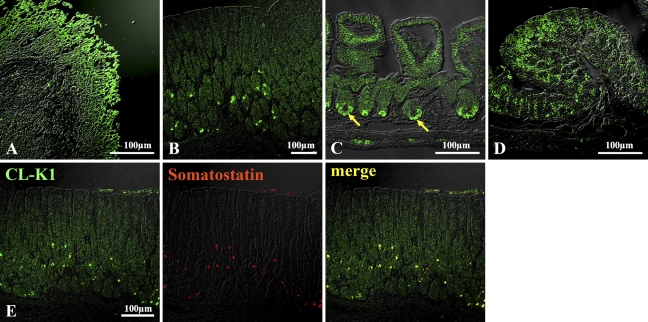
IHC localization of CL-K1 in lung, heart, testis, and brain is shown in Figure 5. CL-K1 expression was strong in bronchial glands of bronchium (Figures 5A–5C). CL-K1 was also expressed in bronchial glands of bronchioles (Figures 5A and 5B, red arrow) and respiratory bronchioles (Figure 5C, black arrow). Figure 5D indicates that CL-K1 was expressed in whole myocardium as well as in the vascular portion of this tissue, but not in endocardium. Figure 5E shows that CL-K1 was expressed in the cytoplasm of spermatocytes. In brain, CL-K1 was abundantly and ubiquitously expressed in neurons of the central nervous system (data not shown). Figure 5F indicates that representative neurons were stained in the medulla oblongata. IHC localization of CL-K1 in liver and pancreas is shown in Figure 6. CL-K1 was expressed in hepatocytes, especially around the central veins (Figure 6A, black arrow). Figures 6B and 6C show that CL-K1 was expressed in pancreatic acinar cells and islet cells. In the case of the islets, CL-K1 was especially expressed in the marginal cells. Double-immunofluorescence analyses presented in Figure 6D indicate that CL-K1 was expressed specifically in D cells that produce somatostatin but not in α- and β-cells, which produce glucagon and insulin, respectively. Figure 7 shows IHC localization of CL-K1 in murine digestive tract. CL-K1 was expressed in epithelial cells of all mucosa of the digestive tract including the esophagus (Figure 7A), stomach (Figures 7B and 7E), small intestine (Figure 7C), and large intestine (Figure 7D). CL-K1 was strongly stained on the surface of esophageal mucosa. In stomach, CL-K1 was expressed in whole mucosa of gastric glands. Double-immunofluorescence analyses revealed that CL-K1 in stomach was also specifically localized in D cells containing somatostatin. In small intestinal mucosa, CL-K1 was expressed in Paneth cells as well as in intestinal crypt (Figure 7C, yellow arrow). In the large intestine, CL-K1 was expressed in epithelial mucosa (Figure 7D).
Figure 5.
IHC localization of CL-K1 in murine lung, heart, testis, and brain. CL-K1 expression was especially strong in bronchial glands of bronchium (red arrow in A,B). In peripheral lung (C), CL-K1 was also expressed in respiratory bronchioles (black arrow). In heart and testis, CL-K1 was expressed in lamina elastica of coronary artery in myocardium (D) and in cytoplasm of spermatocytes (E). (F) Representative neurons stained with CL-K1 antibody in the reticular formation of the medulla oblongata.
Figure 6.
IHC localization of CL-K1 in liver and pancreas. In liver (A), CL-K1 was expressed in hepatocytes. A relatively high expression of CL-K1 was seen in hepatocytes around the central vein (black arrow). In pancreas (B), CL-K1 was expressed not only in acinar cells but also in islet cells (C). Double immunofluorescence staining (D) demonstrates that CL-K1 was colocalized in somatostatin-containing D cells but not in glucagon-containing α-cells or insulin-containing β-cells.
Figure 7.
IHC localization of CL-K1 in gastrointestinal tract. In esophagus (A), stomach (B), small intestine (C), and large intestine (D), CL-K1 was expressed in epithelium. In stomach, CL-K1 was colocalized with somatostatin in somatostatin-containing cells (E). In small intestine, CL-K1 was expressed in Paneth cells (yellow arrow in C). In large intestine, CL-K1 was expressed in epithelial mucosa (D).
Discussion
Collectins interact with glycoconjugated and lipid moieties present on the surface of microorganisms and allergens, as well as with receptors on host cells. Through these interactions, they play a crucial role in innate immunity. However, a single type of collectin cannot meet the requirements for all functions of innate immunity, and several collectins are required for host defense (van de Wetering et al. 2004). In our previous report we demonstrated that CL-K1 could bind to bacterial LPS and LTA. Thus, this novel collectin may be involved in host defense against microorganisms. With regard to the tissue distribution of human CL-K1, we have shown by RT-PCR that CL-K1 mRNA is expressed in most human tissues (Keshi et al. 2006). The present study using mice was carried out to determine the precise tissue distribution of CL-K1 protein expression to reach a better understanding of the biological functions of this novel collectin. For this purpose, we generated a new affinity-purified anti-CL-K1 antibody. This polyclonal antibody raised against the CL-K1 neck–CRD domain recognized full-length CL-K1 overexpressed in CHO cells. We have previously demonstrated by RT-PCR that CL-K1 mRNA expression is ubiquitous in human tissues (Keshi et al. 2006). In this study, we quantitatively evaluated the tissue expression of CL-K1 mRNA in mice using real-time PCR. Real-time PCR study demonstrated that CL-K1 mRNA was distributed in all organs. Among the murine tissues expressing CL-K1 mRNA (see Figure 1), a relatively high level of expression was observed in heart, liver, testis, kidney, and white adipose tissue. Results of immunostaining of these tissues clearly demonstrated that heart, liver, testis, and kidney express CL-K1 protein, in strong agreement with the observations of mRNA expression by real-time PCR. The major finding in the present study was that CL-K1 was expressed in proximal tubules in kidney, bronchial glands of bronchioles, and mucosa of gastrointestinal tract. CL-K1 is a secreted type of collectin and would be expected to be secreted into lumen of these various tissues. This expression pattern is similar to those of SP-A and SP-D in the bronchial glands of bronchioles (Madsen et al. 2000,2003). Sites of CL-K1 expression in kidney, lung, and gastrointestinal tract coincide with areas subject to microbial growth, suggesting that CL-K1 has an important role in defense against microorganisms invading the urinary tract, respiratory tract, and lumen of the digestive tract. In kidney, CL-K1 was identified in mesangial cells of glomeruli, in addition to the proximal tubules. We have reported in our recent publication that CL-K1 is made in the liver and may secrete into the blood stream (Keshi et al. 2006). In addition, molecular mass of CL-K1 is ∼37 kDa. One may speculate that collectin could be passively deposited in the mesangium. It is therefore speculated that CL-K1 immunoreactivity found in the mesangial cells may be passively deposited from systemic circulation, but at this time we are unable to rule out the possibility. However, the possibility may be low because native CL-K1 exists as an oligomer structure in the blood and its molecular mass is >100 kDa, as described in our recent publication (Keshi et al. 2006). These evidences indicate that CL-K1 immunoreactive products in the mesangial cells could not be passively deposited. Further studies such as in situ hybridization are needed to clarify whether CL-K1 is indeed produced by mesangial cells or other cells stained with the CL-K1 antibody. Recent studies on IgA glomerulonephritis have demonstrated that IgA2 harboring polysaccharide chains tend to be agglutinated with each other so that deposits of IgA2 accumulate in mesangial cells and activate the lectin pathway in glomeruli (Hisano et al. 2001,2005; Oortwijn et al. 2006). These experiments indicate that IgA2 with sugar chains are important in agglutination and adhesion in glomeruli. However, characterization of the ligands involved has not been carried out. Our findings suggest that CL-K1 may be involved in the triggering of glomerulonephritis because it would act as a ligand against polysaccharides with IgA. This concept will be further explored in a future study. On the other hand, results of the real-time PCR and IHC clearly demonstrated that CL-K1 mRNA was highly expressed in liver, and that CL-K1 protein expression was homogeneously localized in hepatocytes where it was especially high around the central veins. We have already shown that CL-K1 protein is secreted into human blood (Keshi et al. 2006). These results suggest that murine CL-K1 is mainly produced in hepatocytes in the liver and secreted into the blood stream, as is human CL-K1. In pancreas, CL-K1 was expressed in acinar cells and islet cells. According to immunostaining results, it is of interest that CL-K1 was strongly associated with somatostatin in the islets, but not with insulin or glucagon. Moreover, in gastric mucosa, cells producing CL-K1 corresponded to those producing somatostatin. Somatostatin is a peptide hormone known to regulate the endocrine system, affect neurotransmission, and inhibit release of a variety of secondary hormones. Recently, several reports have implicated somatostatin in innate immunity (Seboek et al. 2004; Zavros et al. 2004). These results also suggest that somatostatin may have a special relationship with CL-K1 in host defense mechanisms. In small intestine, CL-K1 was highly expressed in Paneth cells that contain epithelial granulocytes in the basement area of crypts. Defensins are secreted from Paneth cells and contribute to mucosal barrier function through their potent antimicrobial activities (Ouellette and Lualdi 1990; Ouellette et al. 1992a,b; Ayabe et al. 2000). The fact that CL-K1 was localized in Paneth cells indicates that this molecule would be advantageous in host defense because it would likely be secreted into the lumen together with defensins with which they would play a cooperative role as antimicrobial molecules. In the central nervous system, CL-K1 was mainly expressed in neurons of the brain. Because CL-K1 expression was localized in the cytoplasm and not in dendritic portion of the cell, it would not contribute to any specific neuronal network formation. The relatively high expression of CL-K1 mRNA observed in the central nervous system was in agreement with IHC observations. In lung, gastrointestinal tract, and testis, CL-K1 was expressed in the region exposed to outer environment, indicating that CL-K1 plays an important role in innate immunity systems as other collectins. On the other hand, CL-K1 expressed in heart, liver, and brain may play unexpected roles because the sites of CL-K1 expression are unlikely involved in host defense. We do not know the physiological relevance of CL-K1 expressed in heart, liver, and neurons in brain. Further studies are needed to clarify what kinds of biological actions CL-K1 possesses, in addition to its expected action as a collectin.
In conclusion, we determined the tissue distribution of CL-K1 protein in mice. These findings may be useful for understanding the biological significance of this novel collectin in future studies.
Acknowledgments
This work was supported by grants from the Grants-in-Aid for Scientific Research (19790464, 16390161, 19390227) from the Ministry of Education, Culture, Sports, Sciences, and Technology; Grant of Core Research for Evolution Science and Technology from the Japan Society for the Promotion of Sciences; and the Japan Health Sciences Foundation (KH21011) (to NW). This work was also supported by grants from Fuso Pharmaceutical Industries Ltd., the Fugaku Trust for Medical Research, the Smoking Research Foundation (to NW), the Akiyama Foundation (to KO), and the Takeda Science Foundation (to WM and NW).
References
- Andersen O, Friis P, Holm Nielsen E, Vilsgaard K, Leslie RG, Svehag SE (1992) Purification, subunit characterization and ultrastructure of three soluble bovine lectins: conglutinin, mannose-binding protein and the pentraxin serum amyloid P-component. Scand J Immunol 36:131–141 [DOI] [PubMed] [Google Scholar]
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ (2000) Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1:99–100 [DOI] [PubMed] [Google Scholar]
- Benson B, Hawgood S, Schilling J, Clements J, Damm D, Cordell B, White RT (1985) Structure of canine pulmonary surfactant apoprotein: cDNA and complete amino acid sequence. Proc Natl Acad Sci USA 82:6379–6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K (1988) Two distinct classes of carbohydrate recognition domains in animal lectins. J Biol Chem 263:9557–9560 [PubMed] [Google Scholar]
- Haagsman HP, Hawgood S, Sargeant T, Buckley D, White RT, Drickamer K, Benson BJ (1987) The major lung surfactant protein, SP28–36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem 262:13877–13880 [PubMed] [Google Scholar]
- Hansen S, Holm D, Moeller V, Vitved L, Bendixen C, Reid KB, Skjoedt K, et al. (2002) CL-46, a novel collectin highly expressed in bovine thymus and liver. J Immunol 169:5726–5734 [DOI] [PubMed] [Google Scholar]
- Hisano S, Matsushita M, Fujita T, Endo Y, Takebayashi S (2001) Mesangial IgA2 deposits and lectin pathway-mediated complement activation in IgA glomerulonephritis. Am J Kidney Dis 38:1082–1088 [DOI] [PubMed] [Google Scholar]
- Hisano S, Matsushita M, Fujita T, Iwasaki H (2005) Activation of the lectin complement pathway in Henoch-Schonlein purpura nephritis. Am J Kidney Dis 45:295–302 [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Kawasaki T, Yamashina I (1983) Isolation and characterization of a mannan-binding protein from human serum. J Biochem (Tokyo) 94:937–947 [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Kawasaki T, Yamashina I (1989) A serum lectin (mannan-binding protein) has complement-dependent bactericidal activity. J Biochem (Tokyo) 106:483–489 [DOI] [PubMed] [Google Scholar]
- Keshi H, Sakamoto T, Kawai T, Ohtani K, Katoh T, Jang SJ, Motomura W, et al. (2006) Identification and characterization of a novel human collectin CL-K1. Microbiol Immunol 50:1001–1013 [DOI] [PubMed] [Google Scholar]
- Laursen SB, Dalgaard TS, Thiel S, Lim BL, Jensen TV, Juul-Madsen HR, Takahashi A, et al. (1998) Cloning and sequencing of a cDNA encoding chicken mannan-binding lectin (MBL) and comparison with mammalian analogues. Immunology 93:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen SB, Nielsen OL (2000) Mannan-binding lectin (MBL) in chickens: molecular and functional aspects. Dev Comp Immunol 24:85–101 [DOI] [PubMed] [Google Scholar]
- Lu J, Willis AC, Reid KB (1992) Purification, characterization and cDNA cloning of human lung surfactant protein D. Biochem J 284:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U (2000) Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol 164:5866–5870 [DOI] [PubMed] [Google Scholar]
- Madsen J, Tornoe I, Nielsen O, Koch C, Steinhilber W, Holmskov U (2003) Expression and localization of lung surfactant protein A in human tissues. Am J Respir Cell Mol Biol 29:591–597 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Funakoshi H, Miyamoto K, Tokunaga F, Nakamura T (2001) Molecular cloning and functional characterization of a human scavenger receptor with C-type lectin (SRCL), a novel member of a scavenger receptor family. Biochem Biophys Res Commun 280:1028–1035 [DOI] [PubMed] [Google Scholar]
- Ohtani K, Suzuki Y, Eda S, Kawai T, Kase T, Keshi H, Sakai Y, et al. (2001) The membrane-type collectin CL-P1 is a scavenger receptor on vascular endothelial cells. J Biol Chem 276:44222–44228 [DOI] [PubMed] [Google Scholar]
- Ohtani K, Suzuki Y, Eda S, Kawai T, Kase T, Yamazaki H, Shimada T, et al. (1999) Molecular cloning of a novel human collectin from liver (CL-L1). J Biol Chem 274:13681–13689 [DOI] [PubMed] [Google Scholar]
- Oortwijn BD, Roos A, Royle L, van Gijlswijk-Janssen DJ, Faber-Krol MC, Eijgenraam JW, Dwek RA, et al. (2006) Differential glycosylation of polymeric and monomeric IgA: a possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol 18:3529–3539 [DOI] [PubMed] [Google Scholar]
- Ouellette AJ, Lualdi JC (1990) A novel mouse gene family coding for cationic, cysteine-rich peptides. Regulation in small intestine and cells of myeloid origin. J Biol Chem 15:9831–9837 [PubMed] [Google Scholar]
- Ouellette AJ, Miller SI, Henschen AH, Selsted ME (1992a) Purification and primary structure of murine cryptdin-1, a Paneth cell defensin. FEBS Lett 304:146–148 [DOI] [PubMed] [Google Scholar]
- Ouellette AJ, Miller SI, Henschen AH, Selsted ME (1992b) Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol 118:929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paananen R, Sormunen R, Glumoff V, van Eijk M, Hallman M (2001) Surfactant proteins A and D in Eustachian tube epithelium. Am J Physiol Lung Cell Mol Physiol 281:L660–667 [DOI] [PubMed] [Google Scholar]
- Sano H, Kuroki Y (2005) The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol 42:279–287 [DOI] [PubMed] [Google Scholar]
- Sastry K, Zahedi K, Lelias JM, Whitehead AS, Ezekowitz RA (1991) Molecular characterization of the mouse mannose-binding proteins. The mannose-binding protein A but not C is an acute phase reactant. J Immunol 147:692–697 [PubMed] [Google Scholar]
- Schweinle JE, Hitchcock PJ, Tenner AJ, Hammer CH, Frank MM, Joiner KA (1989) Human mannose-binding protein activates the alternative complement pathway and enhances serum bactericidal activity on a mannose-rich isolate of Salmonella. J Clin Invest 84:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seboek D, Linscheid P, Zulewski H, Langer I, Christ-Crain M, Keller U, Muller B (2004) Somatostatin is expressed and secreted by human adipose tissue upon infection and inflammation. J Clin Endocrinol Metab 89:4833–4839 [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y (1997) SAPAPs. A family of PSD-95/SAP90- associated proteins localized at postsynaptic density. J Biol Chem 272:11943–11951 [DOI] [PubMed] [Google Scholar]
- van de Wetering JK, van Golde LM, Batenburg JJ (2004) Collectins, players of the innate immune system. Eur J Biochem 271:1229–1249 [DOI] [PubMed] [Google Scholar]
- White RT, Damm D, Miller J, Spratt K, Schilling J, Hawgood S, Benson B, et al. (1985) Isolation and characterization of the human pulmonary surfactant apoprotein gene. Nature 317:361–363 [DOI] [PubMed] [Google Scholar]
- Zavros Y, Kao JY, Merchant JL (2004) Inflammation and cancer III. Somatostatin and the innate immune system. Am J Physiol Gastrointest Liver Physiol 286:G698–701 [DOI] [PubMed] [Google Scholar]