Abstract
In vitro and in vivo experimental studies suggest that the transcription factor NF-κB plays a role in tubulointerstitial injury. We investigated possible cellular and molecular mechanisms involving NF-κB activation in the progression of tubulointerstitial lesions in human lupus nephritis (LN). Paraffin-embedded renal biopsies from 50 patients with LN and six control patients with minimal change disease (MCD) were examined by Southwestern histochemistry for in situ detection of active NF-κB and AP-1. Immunohistochemistry was performed to examine the expression of NF-κB, AP-1, and NF-κB regulatory proteins (IκB-α, p-IκB-α, and IKK-α proteins), as well as NF-κB and AP-1 downstream target proinflammatory molecules (ICAM-1, TNF-α, IL-1β, IL-6, and GM-CSF) and NF-κB upstream signaling molecules (CD40 and CD40L). We observed extensive upregulation of activated NF-κB in renal tubular cells and interstitial cells, in parallel with overactivation of transcription factor AP-1 in LN, as compared with normal controls and MCD. Tubular expression of activated NF-κB correlated well with the degree of tubulointerstitial histopathological indices and/or renal function. Tubulointerstitial IKK-α expression was specifically upregulated in LN. IκB-α and p-IκB-α were detected only in interstitial cells in LN. Tubulointerstitial expression levels of NF-κB and AP-1 downstream inflammatory molecules and NF-κB upstream signaling molecules CD40 and CD40L were markedly enhanced in LN as compared with MCD or normal controls and were associated with tubulointerstitial histopathological indices and/or renal function. The results suggest that altered IKK-α expression and NF-κB activation along with AP-1 overexpression may play a pathogenic role in tubulointerstitial injury in human LN mediated through a network of downstream proinflammatory molecules. (J Histochem Cytochem 56:517–529, 2008)
Keywords: nuclear factor-κB, AP-1, inhibitor κB-α, inhibitor IκB kinase-α, lupus nephritis, tubulointerstitial lesion, proinflammatory molecules, adhesion molecules, CD40/CD40L
The extent of tubulointerstitial injury characterized by tubular lesions and recruitment of macrophages is believed to be an important predictor of renal function in immune-mediated glomerulonephritides (GN) such as lupus nephritis (LN) (Yamamoto et al. 1993). Mounting evidence from both in vitro and in vivo studies suggest that renal tubular epithelial cells (TECs) play a central role in initiating and amplifying tubulointerstitial inflammation via cross-talk with inflammatory cells by production of a variety of inflammatory mediators in LN (Kelley et al. 1993; Kuroiwa and Lee 1998). Emerging evidence demonstrates the involvement of adhesion molecules ICAM-1, CD40/CD40L signaling molecules, and proinflammatory cytokines TNF-α, IL-1β, IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF) in tubulointerstitial injury (Fukatsu et al. 1993; Dal Canton 1995; Naito et al. 1996; Kuroiwa et al. 2000). However, the signaling mechanisms that underlie the interactions between TECs and interstitial infiltrates are poorly defined and remain to be clarified.
The transcription factor nuclear factor-κB (NF-κB) is a key regulator of the expression of numerous proteins involved in the inflammatory response (Li and Verma 2002). Many inflammatory molecules are involved in the progression of tubulointerstitial injury (Daha and van Kooten 2000) such as the adhesion molecules (e.g., ICAM-1/VCAM-1) and proinflammatory cytokines (e.g., TNF-α, IL-1, IL-6, and GM-CSF), which are known to be regulated independently and/or coregulated by transcription factor NF-κB and other transcription factors such as activated protein (AP-1) (Schreck and Baeuerle 1990; Sung et al. 1991; Newell et al. 1994; Thomas et al. 1997; Roebuck et al. 1999; Berendji-Grun et al. 2001; Guijarro and Egido 2001; Udalova and Kwiatkowski 2001). The classic form of NF-κB is a heterodimer of a p65 and p50 subunit in the kidney (Guijarro and Egido 2001). In the cytoplasm, NF-κB remains inactive through binding to its endogenous inhibitor known as inhibitor κB-α (IκB-α). A variety of stimuli can lead to the dissociation of this complex resulting in the release of active NF-κB. Degradation of IκB-α occurs through a mechanism by which it is phosphorylated by IκB kinase (IKK). The released NF-κB translocates to the nucleus where it may activate the transcription of downstream target genes (e.g., TNF-α and IL-1β), usually in collaboration with other transcription factors such as AP-1 (Ghosh et al. 1998).
NF-κB activation in renal tubular cells has been implicated in tubulointerstitial injury in proteinuria-induced rat models (Rangan et al. 1999; Gomez-Garre et al. 2001; Takase et al. 2003). In experimental proteinuric nephropathy characterized by tubulointerstitial injury, blocking NF-κB and AP-1 activation attenuated tubulointerstitial injury (Rangan et al. 1999; Takase et al. 2003). Gene transfer of truncated IκB-α into the tubulointerstitium markedly attenuated proteinuria-induced tubulointerstitial injury by specifically inhibiting tubular NF-κB activation along with tubular expression of NF-κB-dependent inflammatory mediators (Takase et al. 2003). Upregulation of activated tubular NF-κB has been suggested to play a role in tubulointerstitial injury, in parallel with upregulation of tubular expression of adhesion molecules, chemokines, or cytokines in human IgA nephropathy, membranous nephropathy (MN), minimal change disease (MCD), and diabetic nephropathy (Mezzano et al. 2001,2004; Ashizawa et al. 2003). However, scarce data are available on the role of NF-κB activation in tubulointerstitial inflammation of LN.
To investigate the possible cellular mechanisms underlying tubulointerstitial inflammation and injury in human LN, we studied in situ expression of activated NF-κB and AP-1 as well as the NF-κB regulatory protein IκB-α, the phosphorylated form of IκB-α (p-IκB-α) and IKK-α using Southwestern histochemistry (SWH) and immunohistochemistry (IHC) in renal biopsies from patients with LN. Expression of an adhesion molecule (ICAM-1) of signaling molecules (CD40 and CD40L) and of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and GM-CSF) involved in the NF-κB signaling pathway were also assessed by IHC.
Materials and Methods
Subjects
The Ethics Committee of the National University Hospital of Singapore (NUH) approved the use of human renal biopsy tissue for this study. Renal biopsies were obtained from 46 patients with LN during the period from 1997–2000, either by percutaneous renal biopsy at NUH or through referral from local nephrologists to the Department of Pathology of the NUH for definitive diagnosis. Strict adherence to the Declaration of Helsinki and the requirements for informed consent was observed in all cases. All patients fulfilled the criteria of the American Rheumatism Association for SLE diagnosis (Tan et al. 1982) and were not receiving immunosuppressive treatment before diagnostic renal biopsy. Tissue samples from six patients with MCD served as disease controls, whereas six samples of normal kidney tissue obtained from nephrectomies performed for treatment of renal cell carcinoma served as normal controls. Data on age, proteinuria, and serum creatinine at the time of biopsy were available for the majority of the patients. Clinical, demographic, and histological data are summarized in Table 1 .
Table 1.
Clinical and histopathological data of patients participating in the study
| Diagnosis and classification* | Number (female: male) | Age (years) | Proteinuria (g/day) | SCr (μmol/liter) | TIL (score) | Int mac (cells/mm2) |
|---|---|---|---|---|---|---|
| III | 2 (2:0) | 29.5 ± 0.71 | 1.55 ± 0.07 | 88.4** | 2 ± 2.82 | 27.73 ± 25.77 |
| IV | 35 (30:5) | 31.23 ± 12.26 | 3.87 ± 2.78 | 164.97 ± 172.46 | 1.55 ± 1.59 | 26.76 ± 23.00 |
| IV-S | 5 (4:1) | 33.6 ± 10.45 | 2.48 ± 1.12 | 60.19 ± 1.99 | 1 ± 1.44 | 14.99 ± 24.69 |
| IV-G | 30 (26:4) | 30.83 ± 12.89 | 4.11 ± 2.34 | 183.3 ± 181.12 | 1.65 ± 1.60 | 28.57 ± 22.69 |
| V | 9 (8:1) | 33.77 ± 14.39 | 3.49 ± 3.36 | 87.73 ± 28.16 | 1.89 ± 1.29 | 15.11 ± 19.28 |
| Total LN | 46 (45:6) | 31.67 ± 12.31 | 3.69 ± 2.82 | 145.68 ± 152.97 | 1.65 ± 1.74 | 24.72 ± 22.38 |
| MCD | 6 (5:1) | 30.83 ± 12.38 | 6.36 ± 3.59 | 93.83 ± 5.70 | 0.5 ± 0.93 | 4.33 ± 3.89 |
ISN/RPS classification of lupus nephritis (LN) in 2003.
Data from one patient are unavailable.
Data are expressed as mean ± SD. To convert serum creatinine from μmol/liter to mg/dl, divide by 88.4. Assessment of histopathological scores is described in Materials and Methods. SCr, serum creatinine; TIL, tubular interstitial lesion; Int mac, CD68-positive interstitial macrophages.
Biopsy samples were processed for routine analysis by light microscopy, immunofluorescence analysis, and/or electron microscopy for the diagnosis of LN as described previously (Khan and Sinniah 1995). Biopsies diagnosed as LN were classified according to the criteria defined by the International Society of Nephrology/Renal Pathology Society in 2003 (Weening et al. 2004). LN disease groups consisted of two subjects with focal LN (class III), 35 subjects with diffuse LN (class IV), and nine subjects with membranous LN (class V). The class IV group was comprised of five subjects with diffuse segmental LN (class IV-S) and 30 subjects with diffuse global LN (class IV-G).
Immunohistochemistry
The monoclonal mouse anti-RelA/p65 antibody (Chemicon International; Temecula, CA), which is directed against an epitope of p65 containing a nuclear localization signal, is specific for the detection of activated NF-κB (Kaltschmidt et al. 1995; Mezzano et al. 2001). Polyclonal goat anti-p50 and rabbit anti-c-jun and anti-c-fos antibodies (Santa Cruz Biotechnology; Santa Cruz, CA) were also applied on paraffin renal tissues previously by other groups (Mezzano et al. 2001). Polyclonal rabbit antibodies against human TNF-α, IL-1β (Rockland Immunochemicals; Gilbertsville, PA), and IL-6 (Pierce Biotechnology; Rockford, IL) and monoclonal mouse antibody against ICAM-1 (Santa Cruz Biotechnology) were used in our previous studies (Qiu et al. 2004; Zheng et al. 2006a). Other primary antibodies used were polyclonal rabbit anti-IKK-α, monoclonal mouse anti-IκB-α and -phosphorylated IκB-α (serine 32), and polyclonal goat anti-CD40L antibodies (Santa Cruz Biotechnology); polyclonal rabbit anti-GM-CSF antibody (Rockland Immunochemicals); monoclonal mouse anti-CD40 antibody (Serotect; Oxford, UK); and monoclonal mouse anti-CD68KP1 antibody (Dako; Carpinteria, CA). Specificity of other antibodies was confirmed by their reactivity with specific human targets by immunoprecipitation and/or Western blot analysis according to the manufacturer's description.
IHC was conducted on 4-μm-thick paraformaldehyde-fixed paraffin sections. Except for CD68, slides were subjected to microwave antigen retrieval with wet heat at 98C for 10 min at 150 W (Milestone Microwave Systems; Bergamo, Italy). Citrate buffer (100 mM, pH 6.0) was used with anti-p65, anti-p50, and anti-CD40L; 0.01 mol/liter, pH 6.0, citrate buffer was used with antibodies against c-jun, c-fos, IκB-α, p-IκB-α, IKK-α, CD40, TNF-α, IL-1β, IL-6, GM-CSF, and ICAM-1 before peroxidase blocking. Slides were predigested with protease for CD68 immunostaining. A two-step EnVision+ System peroxidase kit (Dako) as described previously (Zheng et al. 2006a) was used for the detection of p65, IKK-α, IκB-α, p-IκB-α, CD40, TNF-α, IL-1β, IL-6, GM-CSF, and ICAM-1. For anti-p50 and -CD40L, a three-step Dako LSAB+ Peroxidase Kit was used (Zheng et al. 2006a). DAB was used for colorimetric detection followed by mild counterstaining with PAS reagents and Gill's hematoxylin. Specificity of primary or secondary antibodies was verified by the replacement of primary antibodies with equal concentrations of non-immune sera or by omitting primary antibodies. Positive control was tonsillitis tissue for detection of these molecules.
SWH
SWH was performed as described previously (Hernandez-Presa et al. 1999). In brief, digoxigenin (DIG)-labeled double-stranded synthetic DNA with the consensus sequence of NF-κB (sense: 5′-AGTTGAGGGGACTTTCCCAGGC-3′) or with the consensus sequence of AP-1 (sense: 5′-CGCTTGATGAGTCAGCCGGAA-3′) (Gibco-BRL, LifeTechnology; Gaithersburg, MD) was used as a probe (DIG oligonucleotide 3′-end labeling kit; Roche Diagnostics, Indianapolis, IN).
Four-μM-thick paraffin-embedded kidney sections were dewaxed and rehydrated. Preparations were incubated with levamisole (Sigma; St Louis, MO) and postfixed with 0.2% paraformaldehyde for 30 min at 28C. Following pretreatment with pepsin A (433 U/mg; Sigma) in 1 N HCl for 30 min, sections were incubated with 0.1 mg/ml DNase I in HEPES–BSA for 30 min at 30C. Labeled probes diluted to 100 pmol/liter in HEPES–BSA containing 0.5 μg/ml poly(dI-dC) (Roche Diagnostics) were applied to each slide overnight at 37C. After incubation in blocking solution (0.01X SSC, 0.01% sodium dodecyl sulfate, 0.03% Tween 20, 0.1 mol/liter maleic acid, 0.15 mol/liter NaCl, pH 7.5), anti-DIG antibody conjugated with alkaline phosphatase (1:250 in blocking solution; Roche) was added overnight at 4C. Color reaction was developed using nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP; Roche) according to the manufacturer's instructions. Slides were counterstained with PAS reagent minus hematoxylin.
The following conditions were carried out as negative controls: (1) absence of probe; (2) DIG-labeled mutant NF-κB (sense: 5′-AGTTGAGGCTCCTTTCCCAGGC-3′) and AP-1 (sense: 5′-CGCTTGATGAGTTGGCCGGAA-3′) probes; and (3) competition assays with a 200-fold excess of unlabeled NF-κB or AP-1 followed by incubation with the labeled probe.
Semi-quantitative Histological Assessment
Histological evaluation was performed using light microscopy by two independent pathologists (LZ and RS) who were blinded to the clinical and demographic characteristics of the patients. The agreed-upon score was adopted for the final data set. In cases of disagreement in scoring, the process was repeated, and the final score was determined by consensus of both observers.
An eyepiece net with a regular array of 100 square lattices was used. Two methods were used to evaluate positive signals for the studied molecules in tubulointerstitum according to cellular staining patterns. For clear-cut individual cell staining including tubular cell nuclei staining for activated NF-κB or AP-1 by SWH and their subunits (p65/p50, c-jun/c-fos) by IHC, as well as interstitial cell staining for various biomarkers, the number of positively staining cells was determined in a sequence of 10–70 consecutive visual fields of ×40 high-power fields (0.0625 mm2 per square lattice) per renal biopsy. The only field adjustments were made to avoid glomeruli and large vessels. Results were expressed as the mean number of positive cells/mm2. For contiguous tubular cytoplasmic staining for IKK-α, CD40, CD40L, ICAM-1, IL-1β, TNF-α, IL-6, and GM-CSF, staining areas were evaluated by a seven-point scoring system as described previously by Rui-Mei et al. (1998). Briefly, the intensity of staining was graded as follows: 0 = absent staining, 1 = mild staining, 2 = moderate staining, and 3 = marked staining. Extent of staining was determined as follows: 0 (nil), 1 (<25%), 2 (25–50%), 3 (50–75%), and 4 (>75% of tubules stained). The grades were added together to obtain the final staining scores, ranging from 0 (negative staining) to 7 (maximal staining; marked intensity and >75% of the tubules stained).
Tubulointerstitial lesion (TIL) score was assessed as the measure of the severity of chronic tubulointerstitial injury including tubular lesions (TL) and interstitial fibrosis (IF) (Yamamoto et al. 1993). Morphological variables were graded on a semiquantitative scale of 0 to 3+ as follows: 0 (nil), 1+ (<25%), 2+ (25–50%), and 3+ (>50% of tubules) (Daniel et al. 2000). A total TIL score (0–6) was computed by summing the scores of each component.
Statistical Methods
Statistical analyses were performed using SigmaStat 3.1 programs (SPSS Inc.; Chicago, IL). Clinical and histopathological data (Table 1) were expressed as the mean ± SD. Results of semiquantitative data were expressed as the mean ± SE. Statistical significance was determined by one-way ANOVA followed by Bonferroni's test for multiple comparisons among various groups. For non-parametric data, one-way ANOVA on ranks followed by Dunn's test was used for multiple comparison testing. Correlation analyses were performed by calculating Pearson's correlation coefficients for parametric data and Spearman's correlation coefficients for non-parametric data; p values <0.05 were considered significant.
Results
Tubular Profiles
NF-κB Overactivation Concurrent With Upregulation of IKK-α in Tubular Epithelial Cells of Human LN
Tubular epithelial cell (TEC) nuclear staining for NF-κB by SWH was sparsely detected in normal kidneys (Figure 1A ). Consistent with SWH results, expression of NF-κB subunits p65 and p50 was barely detectable in nuclei of TECs but was readily detectable in the cytoplasm in normal control samples (Figures 1B and 1C). In contrast, enhanced nuclear and cytoplasmic staining for NF-κB, p65, and p50 was noted in both intact and damaged cortical tubules in a patient with LN (Figures 1I–1K). Tubular expression levels of p65 and p50 were positively correlated in LN samples (Pearson's coefficient r = 0.57, p<0.001). Although tubular expression of activated NF-κB, p65, and p50 was also upregulated in MCD as compared with normal controls (Figures 1E–1G), the average number of TEC nuclei positive for NF-κB and p50 in MCD was significantly less than that observed in LN (Figure 2A ).
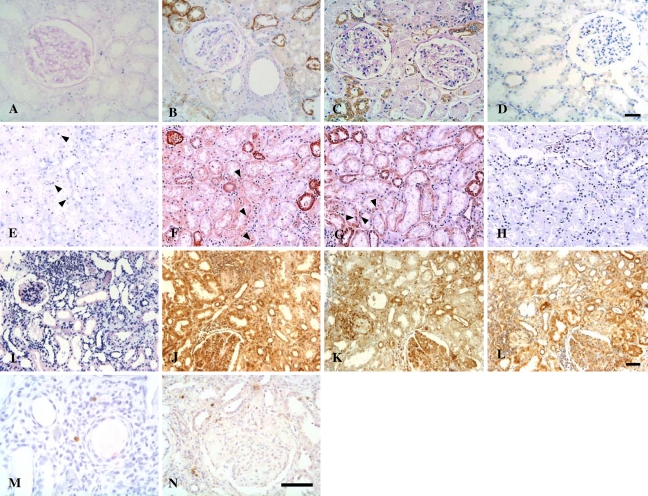
Figure 1.
In situ detection of activated NF-κB, IKK-α, IκB-α, and p-IκB-α in renal tissues. (A–D) Normal control. Nuclear staining of NF-κB (A), p65 (B), and p50 (C) and expression of IKK-α (D) were sparsely detected in normal kidney tissues; some tubules of normal kidneys demonstrated cytoplasmic expression of p65 and p50. (E–H) Renal sections from a patient with minimal change disease (MCD). Some tubular cell nuclei were positive for NF-κB (E), p65 (F), and p50 (G) (arrowheads); faint staining of IKK-α was scattered in some distal tubules from a patient with MCD (H). (I–L) Renal sections from a patient with class IV-G lupus nephritis (LN). Pronounced nuclear staining of NF-κB (I), p65 (J), and p50 (K), concurrent with overexpression of IKK-α (L), was observed in the glomeruli, the damaged tubular cells and the interstitial cells of a patient with class IV LN. IκB-α (M) and p-IκB-α (N) staining was observed in some interstitial cells in patients with class IV-G LN. Southwestern histochemistry (SWH) (dark blue nuclei in A,E,I); immunohistochemistry (brown nuclei or cytoplasm). Bar (A–D; E–L; M,N) = 50 μm.
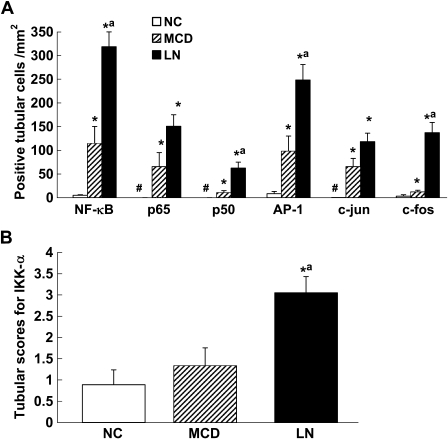
Figure 2.
Semiquantitative analysis of tubular expression of activated transcription factors (NF-κB and AP-1), IKK-α, and cytokines among comparison groups. (A) Quantification of tubular expression of activated NF-κB and AP-1 and their subunits (NF-κB: p65 and p50; AP-1: c-jun and c-fos). (B) Tubular expression of IKK-α. *p<0.05 vs NC; ap<0.05 vs MCD; #no marker expression is detectable. NC, normal control; MCD, minimal change disease; LN, lupus nephritis.
Normal kidneys and MCD both demonstrated faint immunoreactivity for IKK-α in renal TECs (Figures 1D and 1H). In contrast, pronounced upregulation of IKK-α (Figure 1L) was observed in renal TECs from the same LN sample shown previously in Figures 1I–1K, as confirmed by semiquantitative analysis (Figure 2B). Notably, expression of IκB-α and p-IκB-α was undetectable in cortical tubules in various comparison groups including LN (Figures 1M and 1N).
Enhanced Parallel Expression of Activated AP-1 With NF-κB in Human LN
In normal controls, nuclear staining of AP-1 and its subunits c-jun and c-fos was occasionally detected in some TECs (Figures 3A –3C). Nuclear staining of AP-1 and its subunits c-jun and c-fos was prominent in renal TECs from the same LN sample shown previously in Figures 1I–1L, in a pattern similar to that of activated NF-κB (Figures 3G–3I). Increased tubular expression of AP-1 and its subunits was also found in MCD (Figures 3D–3F) but was significantly lower than in LN (Figure 2A). Moreover, tubular activation of NF-κB and AP-1 was strongly correlated in LN patients (Pearson's coefficient r = 0.732, p<0.001), whereas no significant correlation was found in the control MCD patients (Spearman's coefficient r = 0.429, p = 0.397).
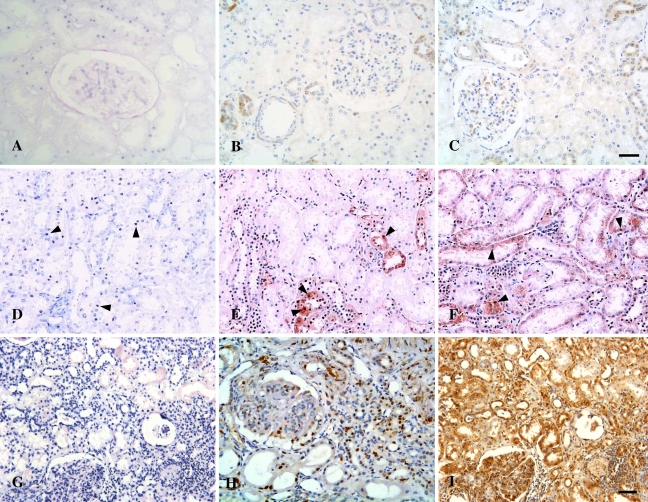
Figure 3.
Microphotographs of tubulointerstitial expression of activated AP-1 in patients with LN and normal controls. (A–C) Normal controls. Occasional nuclear staining signals for AP-1 (A), c-jun (B), and c-fos (C) were observed in one case of normal kidneys. (D–F) Renal sections from a patient with MCD. AP-1 (D), c-jun (E), and c-fos (F) were detected in some nuclei of tubular cells (arrowheads) in sections from the patient shown in Figures 1E–1H. (G–I) Renal sections from a patient with class IV-G LN. Striking nuclear expression of AP-1 (G), c-jun (H), and c-fos (I) were localized to damaged tubular cells and interstitial infiltrates in the patient shown in Figures 1I–1L. SWH (dark blue nuclei in A,D,G); IHC (brown nuclei). Bar (A–C, H; D–G, I) = 50 μm.
Enhanced Tubular Expression of Signaling Molecules CD40/CD40L and NF-κB-regulated Molecules (TNF-α, IL-1β, ICAM-1, IL-6, GM-CSF) in Human LN
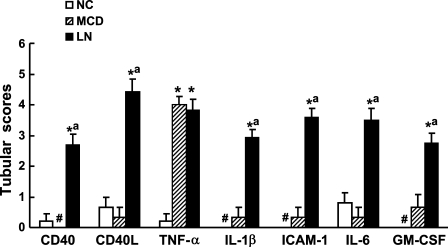
Normal controls and MCD patients showed absent or faint immunoreactivity for CD40 and CD40L in distal tubules (Figures 4A , 4B, 4E, and 4F). Increased expression of CD40 and CD40L was mainly localized in damaged renal tubules in LN as compared with normal controls and MCD (Figures 4I and 4J and Figure 5 ). Tubular expression of NF-κB-regulated molecules ICAM-1, IL-1β, IL-6, and GM-CSF was enhanced and most pronounced in damaged tubules of LN patients (Figures 4D, 4H, and 4L and Figure 6 ) as compared with normal controls and patients with MCD (Figure 5). In contrast to sparse staining of TNF-α in normal controls (Figure 4C), TNF-α expression was pronounced in both intact and damaged tubules of LN patients (Figure 4K) but did not differ from that of MCD patients (Figure 4G and Figure 5). Immunostaining of ICAM-1 and TNF-α was localized predominantly on the apical surface of cortical tubules, whereas CD40 was expressed mainly along the basal membrane of tubules, and tubular expression of other molecules was predominantly observed to be cytoplasmic or a mixed pattern (Figure 4 and Figure 6).
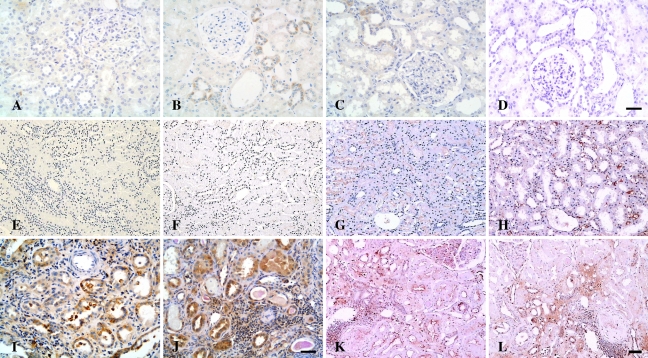
Figure 4.
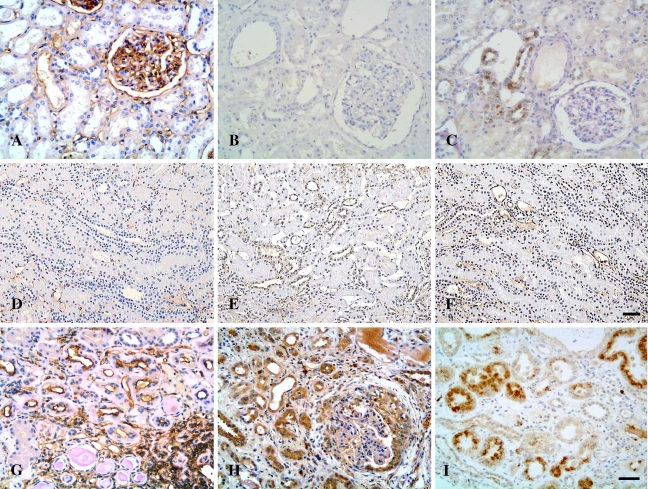
Microphotographs of immunostaining for CD40, CD40L, TNF-α, and IL-1β in controls and patients with LN. (A–D) Normal controls. Weak immunoreactivity with CD40 (A), CD40L (B), and TNF-α (C) was detected in renal tubules of normal kidneys, whereas IL-1β expression was absent in the normal kidney tissue (D). (E–H) MCD. Expression of CD40 (E) and CD40L (F) was barely observed in the tubulointerstitium of patients with MCD, whereas TNF-α expression (G) was localized to the apical surface of proximal tubules, and expression of IL-1β (H) was present in the peritubular capillaries of a patient with MCD. (I–L) LN. Pronounced overexpression of CD40 (I), CD40L (J), TNF-α (K), and IL-1β (L) was observed in damaged tubules and interstitial infiltrates in patients with class IV-G LN. Bar (A–D; E–H,K,L; I,J) = 50 μm.
Figure 5.
Semiquantitative analysis of tubular expression CD40, CD40L, TNF-α, IL-1β, ICAM-1, IL-6, and GM-CSF. *p<0.05 vs NC; ap<0.05 vs MCD; #no marker expression is detectable. NC, normal control; MCD, minimal change disease; LN, lupus nephritis.
Figure 6.
Microphotographs of tubulointerstitial expression of ICAM-1, GM-CSF and IL-6 in controls and patients with LN. (A–C) Normal controls. ICAM-1 (A) was constitutively expressed in the glomerular capillary walls, the peritubular capillaries, and the walls of blood vessels in normal kidney, whereas expression of IL-6 (B) was occasionally seen in the distal tubules of normal kidneys; expression of GM-CSF (C) was undetectable in normal controls. (D–F) MCD. Expression of ICAM-1 (D) was evident along the peritubular capillaries, whereas weak immunoreactivity with IL-6 (E) and GM-CSF (F) was detected in some distal tubules in renal sections from patients with MCD. (G–I) LN. Pronounced immunostaining of ICAM-1 (G), IL-6 (H), and GM-CSF (I) was demonstrated in damaged tubules and interstitial infiltrates in renal sections of patients with class IV-G LN. Bar (A–C, G–I; D;F)= 50 μm.
Interstitial Cells
We observed increased numbers of interstitial cells that were immunopositive for activated transcription factors (NF-κB and AP-1) and their corresponding subunits in LN as compared with MCD and normal controls (Figure 1 and Figure 3; Table 2 ). IKK-α was concomitantly expressed with NF-κB in interstitial infiltrates in patients with LN (Figure 1L; Table 2). In contrast to their absence in tubular cells, IκB-α and p-IκB-α staining was readily detected in interstitial cells (Figures 1M and 1N; Table 2).
Table 2.
Interstitial expression of NF-κB, AP-1, their subunits, and NF-κB modulators in patients with LN
| Markers (cells/mm2) | NC | MCD | LN |
|---|---|---|---|
| NF-κB1 | 0.12 ± 0.05 | 0.60 ± 0.49 | 78.32 ± 14.19b,d |
| p652 | 0 ± 0 | 0.07 ± 0.07 | 21.08 ± 4.88b,d |
| p502 | 0 ± 0 | 1.13 ± 0.98 | 74.06 ± 10.48b,d |
| AP-11 | 0.04 ± 0.03 | 0 ± 0 | 52.11 ± 10.95b,c |
| c-jun2 | 0.02 ± 0.02 | 0 ± 0 | 6.52 ± 1.12b,d |
| c-fos2 | 0.02 ± 0.02 | 0 ± 0 | 10.94 ± 1.92b,d |
| IκB-α | 0 ± 0 | 0 ± 0 | 0.28 ± 0.13 |
| p-IκB-α | 0 ± 0 | 0 ± 0 | 2.30 ± 0.53a,c |
| IKK-α | 0.49 ± 0.11 | 0 ± 0 | 1.11 ± 0.75a,c |
Results obtained by in situ Southwestern histochemistry (SWH).
Results obtained by immunohistochemistry.
p<0.05 vs NC.
p<0.01 vs NC.
p<0.05 vs MCD.
p<0.01 vs MCD.
Results are expressed as the mean ± SEM. Abbreviations as in Table 1.
CD40- and CD40L-positive interstitial infiltrates were frequently seen in patients with LN (9.35 ± 2.46 and 15.83 ± 5.30, respectively) (Figures 4I and 4J). Tubular expression of CD40 positively correlated with the number of interstitial cells that stained immunopositive for CD40L (Spearman's coefficient r = 0.63, p=0.001). Additionally, there were increased numbers of interstitial cells positive for ICAM-1 (37.98 ± 5.39), TNF-α (6.64 ± 1.49), IL-1β (28.50 ± 4.96), IL-6 (7.85 ± 2.33), and GM-CSF (14.19 ± 3.38) in LN patients in comparison with MCD patients (ICAM-1: 0.51 ± 0.32; other markers: 0 ± 0) and normal controls (IL-1β: 3.63 ± 1.02; ICAM-1: 0.19 ± 0.16; other markers: 0 ± 0) (Figure 4 and Figure 6).
Correlation Analyses
Clinicopathological Correlation Analysis
The average number of tubular cell nuclei that stained positively for p50 was weakly correlated with TIL scores, whereas a positive correlation was observed between tubular activation of p65 and p50 and the serum creatinine level (Table 3 ). TIL scores and/or the serum creatinine level were also positively correlated with tubular expression of CD40, IL-1β, ICAM-1, and GM-CSF with statistical significance or reaching marginal significance (Table 4 ). Positive correlations between tubular expression of CD40, ICAM-1, IL-6, and GM-CSF and interstitial infiltration of macrophages were observed (Table 4). There was a weak correlation between tubular activation of ICAM-1 and GM-CSF and the magnitude of proteinuria (Table 4). No significant correlations were observed between tubular expression of AP-1 or its subunits (c-jun and c-fos) and clinicopathological indices (data not shown).
Table 3.
Clinicopathological correlation analysis of tubulointerstitial expression of activated NF-κB in patients with LN
|
*NF-κB (cells/mm2)
|
p65 (cells/mm2)
|
p50 (cells/mm2)
|
|||||
|---|---|---|---|---|---|---|---|
| Clinical and pathological parameters | CC | p | CC | p | CC | p | |
| Tubule | Int mac (cells/mm2) | 0.052 | 0.761 | 0.165 | 0.308 | 0.146 | 0.376 |
| TIL (scores) | −0.108 | 0.476 | −0.093 | 0.527 | 0.294a | 0.042 | |
| SCr (μmol/liter) | 0.039 | 0.830 | 0.512a | 0.001 | 0.384a | 0.023 | |
| Proteinuria (g/day) | −0.143 | 0.379 | 0.098 | 0.530 | −0.055 | 0.724 | |
| Interstitium | Int mac (cells/mm2) | 0.486b | 0.002 | 0.347a | 0.031 | 0.400a | 0.032 |
| TIL (scores) | 0.436b | <0.002 | 0.064 | 0.786 | 0.247 | 0.087 | |
| SCr (μmol/liter) | 0.349 | 0.055 | 0.363a | 0.032 | 0.369a | 0.032 | |
| Proteinuria (g/day) | 0.226 | 0.166 | 0.080 | 0.619 | 0.085 | 0.591 | |
In situ SWH staining score.
Correlation was significant at the 0.05 level.
Correlation was significant at the 0.01 level.
Immunostaining scores were analyzed unless otherwise specified. CC, correlation coefficients; other abbreviations as in Table 1.
Table 4.
Clinicopathological correlation analysis of tubulointerstitial expression of inflammatory mediators in patients with LN
| Int mac
|
TIL
|
Scr
|
Proteinuria
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Markers | CC | p | CC | p | CC | p | CC | p | |
| Tubule | CD40 | 0.472b | 0.002 | 0.311a | 0.047 | 0.400a | 0.019 | 0.280 | 0.080 |
| CD40L | 0.386 | 0.052 | 0.111 | 0.585 | 0.167 | 0.482 | 0.012 | 0.951 | |
| TNF-α | 0.244 | 0.220 | 0.150 | 0.425 | 0.085 | 0.707 | −0.037 | 0.874 | |
| IL-1β | 0.276 | 0.098 | 0.309 | 0.052 | 0.397a | 0.020 | 0.266 | 0.167 | |
| ICAM-1 | 0.506b | 0.001 | 0.364a | 0.014 | 0.616b | <0.001 | 0.331a | 0.030 | |
| IL-6 | 0.414b | 0.009 | 0.211 | 0.184 | 0.559b | 0.001 | 0.110 | 0.505 | |
| GM-CSF | 0.620b | <0.001 | 0.453b | 0.003 | 0.817b | 0.001 | 0.330a | 0.040 | |
| Interstitium | CD40 | 0.420b | 0.007 | 0.292 | 0.064 | 0.274 | 0.116 | 0.061 | 0.707 |
| CD40L | 0.348 | 0.082 | 0.175 | 0.398 | 0.284 | 0.224 | 0.307 | 0.127 | |
| TNF-α | 0.008 | 0.971 | 0.083 | 0.686 | 0.434 | 0.056 | 0.237 | 0.235 | |
| IL-1β | −0.002 | 0.993 | 0.029 | 0.862 | 0.256 | 0.150 | 0.279 | 0.090 | |
| ICAM-1 | 0.470b | 0.002 | 0.391b | 0.008 | 0.640b | <0.001 | 0.123 | 0.434 | |
| IL-6 | −0.056 | 0.731 | −0.125 | 0.434 | 0.304 | 0.080 | 0.032 | 0.874 | |
| GM-CSF | 0.302 | 0.065 | 0.069 | 0.676 | 0.462b | 0.007 | 0.420a | 0.010 | |
Correlation was significant at the 0.05 level.
Correlation was significant at the 0.01 level.
CC, correlation coefficients; other abbreviations as in Table 1.
Interstitial expression of activated NF-κB, p65, and p50 positively correlated with histopathological indices and serum creatinine level (Table 3), whereas interstitial expression of AP-1 positively correlated with interstitial infiltration of macrophages (Spearman's coefficient r = 0.419, p=0.030). Moreover, interstitial expression of CD40 positively correlated with tubulointerstitial histological indices, whereas interstitial expression of ICAM-1 was positively correlated with both histological indices and serum creatinine level (Table 4). Interstitial expression of GM-CSF positively correlated with proteinuria and serum creatinine level; a trend toward a positive correlation with interstitial infiltration of macrophages was also observed, which nearly reached statistical significance (Table 4). A trend toward a positive correlation between the number of interstitial cells expressing TNF-α and the level of serum creatinine was also observed, which did not reach statistical significance (Table 4).
Correlation Analysis Between Tubulointerstitial Expression of Activated NF-κB and IKK-α in LN.
We observed positive correlations between tubular expression of IKK-α and both activated NF-κB and p50 (Spearman's coefficient r = 0.384, p=0.033; Spearman's coefficient r = 0.359, p=0.047), whereas marginal correlations were observed between interstitial expression of NF-κB and IKK-α (Pearson's coefficient r = 0.360, p=0.050).
Discussion
The major novel finding of our study is that activated transcription factor NF-κB is extensively upregulated in the renal tubular cells of LN patients as compared with normal controls and MCD patients. Expression pattern of p65 and p50 was similar to that of NF-κB detected by SWH in the tubulointerstitium of the LN group. A significant statistical association was found between nuclear immunopositive staining in tubular cells for p50 and p65, which comprises the two components of the “classic” NF-κB heterodimer. In addition to the “classic” NF-κB heterodimer, other subunits such as c-rel, p52, or RelB have also been reported to participate in transcriptional upregulation of inflammatory mediators in renal tubular cells (Morrissey and Klahr 1997). Expression of multiple distinct forms of NF-κB heterodimers is consistent with our finding that the number of SWH-stained tubular cell nuclei (overexpressing activated NF-κB) is overwhelmingly higher than that of p65- or p50-stained tubular cell nuclei in LN samples. Tubular activation of p65 and p50 positively correlated with the degree of renal function and/or tubulointerstitial histopathological indices, indicating a pathogenic role for the “classic form” of NF-κB in the progression of tubulointerstitial lesions. Notably, such a clinicopathological correlation was not seen for SWH-stained tubular cell nuclei. This finding is likely attributable to the lesser or indirect role of “non-classic” forms of NF-κB in the progression of tubulointerstitial lesions in LN samples.
In the present study, tubular overexpression of NF-κB downstream inflammatory mediators ICAM-1, IL-1β, IL-6, and GM-CSF was found to be positively correlated with the degree of renal function and/or tubulointerstitial histolopathological indices in LN samples, consistent with our proposal that NF-κB is a key mediator of tubulointerstitial injury. The proinflammatory role of NF-κB activation in renal tubular cells has been implicated in tubulointerstitial injury in the proteinuria-induced rat model through transcriptional activation of NF-κB-dependent inflammatory mediators (Rangan et al. 1999; Gomez-Garre et al. 2001; Takase et al. 2003). NF-κB augments expression of adhesion molecules such as ICAM-1 and VCAM-1 in renal tubular cells (Oertli et al. 1998; Tu et al. 2001). Proinflammatory cytokines such as TNF-α, IL-1, IL-6, and GM-CSF are also regulated by NF-κB in tubular epithelial cells or glomerular epithelial cells (Guijarro and Egido 2001; de Haij et al. 2002; Drumm et al. 2002; Greiber et al. 2002; Viedt et al. 2002). These inflammatory molecules have been implicated as critical mediators in the progression of tubulointerstitial lesions (Healy and Brady 1998). However, associations between NF-κB/TNF-α/IL-6 and tubulointerstitial lesion scores were not observed in this study. This is likely due to the existence of pleiotrophic pathways mediated by these molecules in renal inflammation. In our previous report, associations between NF-κB and proliferation in renal tubular cells suggested a pro-proliferative role for NF-κB in tubulointerstitial inflammation (Zheng et al. 2006b). An anti-apoptotic role for TNF-α has been reported in opossum kidney cells, proposed to be mediated by actin redistribution that involves NF-κB activation (Papakonstanti and Stournaras 2004). An anti-inflammatory role of IL-6 has also been suggested, apart from a pro-inflammatory role (Xing et al. 1998).
In patients with MCD, only tubular overexpression of TNF-α was associated with NF-κB activation in tubulointerstitial regions exhibiting minor degrees of injury. The role of TNF-α and NF-κB activation produced by tubular cells in patients with MCD remains unknown. Cellular response may be a secondary response to albuminuria itself, rather than playing a causative role in the pathogenesis of MCD (Cho et al. 2003).
Cooperation among NF-κB and other transcription factors such as AP-1 is required for effective induction of ICAM-1, GM-CSF, TNF-α, and IL-6 (Tsuboi et al. 1994; Dendorfer 1996; Sakiri et al. 1998; Viedt et al. 2002; Blaber et al. 2003). Cross-coupling between NF-κB and AP-1 resulting in a synergistic increase in activity at both AP-1 and NF-κB consensus DNA binding sites has been previously described (Adcock 1997). In experimental and human GN, AP-1 activation together with NF-κB overactivation has been found in tubular cells (Mezzano et al. 2001; Ruiz-Ortega et al. 2001). In the present study, tubular activation of AP-1 and its subunits in LN patients also exceeded that of normal control and MCD patients and significantly correlated with tubular NF-κB activation in LN. Results suggest that AP-1 activation may contribute to tubulointerstitial injury in cooperation with NF-κB in LN and that the differential expression of activated AP-1 in renal tubules between the LN and MCD groups may account, at least in part, for the observed difference in tubular expression of inflammatory mediators between groups.
We investigated the possible regulatory mechanisms of NF-κB activation in the kidney tissue of LN patients. IκB and IKK have been suggested to be involved in the regulation of NF-κB activation in renal tubular cells in vitro (Wang et al. 2000; Tu et al. 2001). In the present study, IKK-α expression was augmented in tubular cells in parallel with tubular activation of NF-κB in LN samples, suggesting the presence of an IKK–NF-κB pathway in renal tubular cells of LN. It has been proposed that receptor-mediated signaling by molecules such as CD40L and TNF-α may trigger activation of IKK, leading to phosphorylation of IκB, which then dissociates from NF-κB and is rapidly degraded, resulting in translocation of unbound NF-κB to nuclei (Hsing et al. 1997; Tu et al. 2001). Induction of NF-κB activation and downstream target genes by CD40/CD40L signaling has been documented in renal tubular cells (Woltman et al. 2000). In addition to TNF-α, elevated expression of CD40 and CD40L was observed in tubular cells and interstitial leukocytes and was associated with the severity of tubulointerstitial injury in LN in the current study. It is likely that IKK-α mediates CD40/CD40L signaling-induced NF-κB activation.
We were unable to detect expression of IκB-α and phosphorylated IκB-α (at Ser32) in renal tubules of the various comparison groups in the present study. Whether this is attributable to poor sensitivity of the monoclonal antibodies used or to technical factors remains unclear. It is possible that IκB-α, which is known to be degraded rapidly, is expressed at a level beyond the limit of sensitivity for the immunohistochemical detection methods used in the present study. Alternatively, other isoforms of IκB such as IκB-β may play an essential role in NF-κB activation in renal tubular cells. Recent studies indicate that the majority of p50/p65 complexes are regulated not only by IκB-α but also by IκB-β (Ghosh et al. 1998). IκB-α is thought to maintain the transient effect of inducing agents on the transcription of NF-κB responsive genes, whereas persistent activation of NF-κB is regulated by IκB-β in the nucleus (Ghosh et al. 1998). Thus, it is possible that persistent NF-κB activation in renal tubular cells is dependent on the IκB-β pathway in LN. This hypothesis deserves further investigation.
NF-κB and AP-1 activation also correlated well with interstitial infiltration of macrophages in LN samples. Recent studies suggest that NF-κB signaling is involved in activation of macrophages (Kluth et al. 2004). We observed IKK-α expression in interstitial cells in parallel with NF-κB activation in LN samples. Expression of IκB-α and, to a lesser extent, phosphorylated IκB-α was also detected in interstitial cells in LN, suggesting that the IKK-induced IκB-α phosphorylation pathway may contribute to NF-κB activation in interstitial cells. Interstitial expression of ICAM-1, CD40/CD40L molecules, and proinflammatory cytokines and their positive correlation with the severity of tubulointerstitial injury and/or renal function further support the hypothesis that renal tubular cells and interstitial infiltrates such as macrophages are involved in an interplay through a network of inflammatory mediators, which is crucial for the progression of tubulointerstitial injury in LN (Kuroiwa et al. 2000).
In conclusion, results of our study suggest that complex interactions between tubular cells and interstitial leukocytes through a network of inflammatory mediators may be integrated at the level of gene activation by the upregulation of transcription factors such as NF-κB and AP-1 in human LN. The IKK-induced NF-κB activation pathway is likely to be involved in both initiation and maintenance of tubulointerstitial injury by promoting production of proinflammatory mediators.
Acknowledgments
This work was funded by the Academic Research Fund Grant R-172-000-109-112 (to RS and SI-HH) and intramural funding from the Department of Medicine, National University of Singapore.
We thank Susan Lisa Nasr for assistance with the preparation of this manuscript. This work was undertaken in the Special Histopathology Laboratory in the Department of Pathology. We thank Dr. Koh Dow Rhoon for valuable advice and Dr. Thian Chai Lee (Johor Specialist Center, Malaysia) for assistance in providing the clinical data on the biopsy cases included in the present study.
References
- Adcock IM (1997) Transcription factors as activators of gene transcription: AP-1 and NF-κ B. Monaldi Arch Chest Dis 52:178–186 [PubMed] [Google Scholar]
- Ashizawa M, Miyazaki M, Abe K, Furusu A, Isomoto H, Harada T, Ozono Y, et al. (2003) Detection of nuclear factor-κB in IgA nephropathy using Southwestern histochemistry. Am J Kidney Dis 42:76–86 [DOI] [PubMed] [Google Scholar]
- Berendji-Grun D, Kolb-Bachofen V, Kroncke KD (2001) Nitric oxide inhibits endothelial IL-1[β]-induced ICAM-1 gene expression at the transcriptional level decreasing Sp1 and AP-1 activity. Mol Med 7:748–754 [PMC free article] [PubMed] [Google Scholar]
- Blaber R, Stylianou E, Clayton A, Steadman R (2003) Selective regulation of ICAM-1 and RANTES gene expression after ICAM-1 ligation on human renal fibroblasts. J Am Soc Nephrol 14:116–127 [DOI] [PubMed] [Google Scholar]
- Cho MH, Lee HS, Choe BH, Kwon SH, Chung KY, Koo JH, Ko CW (2003) Interleukin-8 and tumor necrosis factor-α are increased in minimal change disease but do not alter albumin permeability. Am J Nephrol 23:260–266 [DOI] [PubMed] [Google Scholar]
- Daha MR, van Kooten C (2000) Is the proximal tubular cell a proinflammatory cell? Nephrol Dial Transplant 15:41–43 [DOI] [PubMed] [Google Scholar]
- Dal Canton A (1995) Adhesion molecules in renal disease. Kidney Int 48:1687–1696 [DOI] [PubMed] [Google Scholar]
- Daniel L, Saingra Y, Giorgi R, Bouvier C, Pellissier JF, Berland Y (2000) Tubular lesions determine prognosis of IgA nephropathy. Am J Kidney Dis 35:13–20 [DOI] [PubMed] [Google Scholar]
- de Haij S, Woltman AM, Bakker AC, Daha MR, van Kooten C (2002) Production of inflammatory mediators by renal epithelial cells is insensitive to glucocorticoids. Br J Pharmacol 137:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendorfer U (1996) Molecular biology of cytokines. Artif Organs 20:437–444 [DOI] [PubMed] [Google Scholar]
- Drumm K, Bauer B, Freudinger R, Gekle M (2002) Albumin induces NF-κB expression in human proximal tubule-derived cells (IHKE-1). Cell Physiol Biochem 12:187–196 [DOI] [PubMed] [Google Scholar]
- Fukatsu A, Matsuo S, Yuzawa Y, Miyai H, Futenma A, Kato K (1993) Expression of interleukin 6 and major histocompatibility complex molecules in tubular epithelial cells of diseased human kidneys. Lab Invest 69:58–67 [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260 [DOI] [PubMed] [Google Scholar]
- Gomez-Garre D, Largo R, Tejera N, Fortes J, Manzarbeitia F, Egido J (2001) Activation of NF-κB in tubular epithelial cells of rats with intense proteinuria: role of angiotensin II and endothelin-1. Hypertension 37:1171–1178 [DOI] [PubMed] [Google Scholar]
- Greiber S, Muller B, Daemisch P, Pavenstadt H (2002) Reactive oxygen species alter gene expression in podocytes: induction of granulocyte macrophage-colony-stimulating factor. J Am Soc Nephrol 13:86–95 [DOI] [PubMed] [Google Scholar]
- Guijarro C, Egido J (2001) Transcription factor-κB (NF-κB) and renal disease. Kidney Int 59:415–424 [DOI] [PubMed] [Google Scholar]
- Healy E, Brady HR (1998) Role of tubule epithelial cells in the pathogenesis of tubulointerstitial fibrosis induced by glomerular disease. Curr Opin Nephrol Hypertens 7:525–530 [DOI] [PubMed] [Google Scholar]
- Hernandez-Presa MA, Gomez-Guerrero C, Egido J (1999) In situ non-radioactive detection of nuclear factors in paraffin sections by Southwestern histochemistry. Kidney Int 55:209–214 [DOI] [PubMed] [Google Scholar]
- Hsing Y, Hostager BS, Bishop GA (1997) Characterization of CD40 signaling determinants regulating nuclear factor-κ B activation in B lymphocytes. J Immunol 159:4898–4906 [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Henkel T, Stockinger H, Baeuerle PA (1995) Selective recognition of the activated form of transcription factor NF-κ B by a monoclonal antibody. Biol Chem Hoppe Seyler 376:9–16 [DOI] [PubMed] [Google Scholar]
- Kelley VR, Diaz-Gallo C, Jevnikar AM, Singer GG (1993) Renal tubular epithelial and T cell interactions in autoimmune renal disease. Kidney Int Suppl 39:108–115 [PubMed] [Google Scholar]
- Khan TN, Sinniah R (1995) Role of complement in renal tubular damage. Histopathology 26:351–356 [DOI] [PubMed] [Google Scholar]
- Kluth DC, Erwig LP, Rees AJ (2004) Multiple facets of macrophages in renal injury. Kidney Int 66:542–557 [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Lee EG (1998) Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus 7:597–603 [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Schlimgen R, Illei GG, McInnes IB, Boumpas DT (2000) Distinct T cell/renal tubular epithelial cell interactions define differential chemokine production: implications for tubulointerstitial injury in chronic glomerulonephritides. J Immunol 164:3323–3329 [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM (2002) NF-κB regulation in the immune system. Nat Rev Immunol 2:725–734 [DOI] [PubMed] [Google Scholar]
- Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, et al. (2004) NF-κB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant 19:2505–2512 [DOI] [PubMed] [Google Scholar]
- Mezzano SA, Barria M, Droguett MA, Burgos ME, Ardiles LG, Flores C, Egido J (2001) Tubular NF-κB and AP-1 activation in human proteinuric renal disease. Kidney Int 60:1366–1377 [DOI] [PubMed] [Google Scholar]
- Morrissey JJ, Klahr S (1997) Rapid communication. Enalapril decreases nuclear factor κ B activation in the kidney with ureteral obstruction. Kidney Int 52:926–933 [DOI] [PubMed] [Google Scholar]
- Naito T, Yokoyama H, Moore KJ, Dranoff G, Mulligan RC, Kelley VR (1996) Macrophage growth factors introduced into the kidney initiate renal injury. Mol Med 2:297–312 [PMC free article] [PubMed] [Google Scholar]
- Newell CL, Deisseroth AB, Lopez-Berestein G (1994) Interaction of nuclear proteins with an AP-1/CRE-like promoter sequence in the human TNF-α gene. J Leukoc Biol 56:27–35 [DOI] [PubMed] [Google Scholar]
- Oertli B, Beck-Schimmer B, Fan X, Wuthrich RP (1998) Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-κB and activating protein-1. J Immunol 161:3431–3437 [PubMed] [Google Scholar]
- Papakonstanti EA, Stournaras C (2004) Tumor necrosis factor-α promotes survival of opossum kidney cells via Cdc42-induced phospholipase C-γ1 activation and actin filament redistribution. Mol Biol Cell 15:1273–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu LQ, Sinniah R, I-Hong Hsu S (2004) Downregulation of Bcl-2 by podocytes is associated with progressive glomerular injury and clinical indices of poor renal prognosis in human IgA nephropathy. J Am Soc Nephrol 15:79–90 [DOI] [PubMed] [Google Scholar]
- Rangan GK, Wang Y, Tay YC, Harris DC (1999) Inhibition of nuclear factor-κB activation reduces cortical tubulointerstitial injury in proteinuric rats. Kidney Int 56:118–134 [DOI] [PubMed] [Google Scholar]
- Roebuck KA, Carpenter LR, Lakshminarayanan V, Page SM, Moy JN, Thomas LL (1999) Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-κB. J Leukoc Biol 65:291–298 [DOI] [PubMed] [Google Scholar]
- Rui-Mei L, Kara AU, Sinniah R (1998) Dysregulation of cytokine expression in tubulointerstitial nephritis associated with murine malaria. Kidney Int 53:845–852 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J (2001) Systemic infusion of angiotensin II into normal rats activates nuclear factor-κB and AP-1 in the kidney: role of AT1 and AT2 receptors. Am J Pathol 158:1743–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiri R, Ramegowda B, Tesh VL (1998) Shiga toxin type 1 activates tumor necrosis factor-α gene transcription and nuclear translocation of the transcriptional activators nuclear factor-κB and activator protein-1. Blood 92:558–566 [PubMed] [Google Scholar]
- Schreck R, Baeuerle PA (1990) NF-κB as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol Cell Biol 10:1281–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SJ, Walters JA, Hudson J, Gimble JM (1991) Tumor necrosis factor-α mRNA accumulation in human myelomonocytic cell lines. Role of transcriptional regulation by DNA sequence motifs and mRNA stabilization. J Immunol 147:2047–2054 [PubMed] [Google Scholar]
- Takase O, Hirahashi J, Takayanagi A, Chikaraishi A, Marumo T, Ozawa Y, Hayashi M, et al. (2003) Gene transfer of truncated IκBα prevents tubulointerstitial injury. Kidney Int 63:501–513 [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, et al. (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277 [DOI] [PubMed] [Google Scholar]
- Thomas RS, Tymms MJ, McKinlay LH, Shannon MF, Seth A, Kola I (1997) ETS1, NFκB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene 14:2845–2855 [DOI] [PubMed] [Google Scholar]
- Tsuboi A, Muramatsu M, Tsutsumi A, Arai K, Arai N (1994) Calcineurin activates transcription from the GM-CSF promoter in synergy with either protein kinase C or NF-κB/AP-1 in T cells. Biochem Biophys Res Commun 199:1064–1072 [DOI] [PubMed] [Google Scholar]
- Tu Z, Kelley VR, Collins T, Lee FS (2001) IκB kinase is critical for TNF-α-induced VCAM1 gene expression in renal tubular epithelial cells. J Immunol 166:6839–6846 [DOI] [PubMed] [Google Scholar]
- Udalova IA, Kwiatkowski D (2001) Interaction of AP-1 with a cluster of NF-κB binding elements in the human TNF promoter region. Biochem Biophys Res Commun 289:25–33 [DOI] [PubMed] [Google Scholar]
- Viedt C, Dechend R, Fei J, Hansch GM, Kreuzer J, Orth SR (2002) MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-κB and activating protein-1. J Am Soc Nephrol 13:1534–1547 [DOI] [PubMed] [Google Scholar]
- Wang Y, Rangan GK, Goodwin B, Tay YC, Harris DC (2000) Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-κB dependent. Kidney Int 57:2011–2022 [DOI] [PubMed] [Google Scholar]
- Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, et al. (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15:241–250 [DOI] [PubMed] [Google Scholar]
- Woltman AM, de Haij S, Boonstra JG, Gobin SJ, Daha MR, van Kooten C (2000) Interleukin-17 and CD40-ligand synergistically enhance cytokine and chemokine production by renal epithelial cells. J Am Soc Nephrol 11:2044–2055 [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X-F, Achong MA (1998) IL-6 is an anti-inflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nagase M, Hishida A, Honda N (1993) Interstitial inflammatory and chronic tubulointerstitial lesions in lupus nephritis: comparison with those in IgA nephropathy. Lupus 2:261–268 [DOI] [PubMed] [Google Scholar]
- Zheng L, Sinniah R, Hsu SI (2006a) In situ glomerular expression of activated NF-κB in human lupus nephritis and other non-proliferative proteinuric glomerulonephritides. Virchows Arch 448:172–183 [DOI] [PubMed] [Google Scholar]
- Zheng L, Sinniah R, I-Hong Hsu S (2006b) Renal cell apoptosis and proliferation may be linked to nuclear factor-κB activation and expression of inducible nitric oxide synthase in patients with lupus nephritis. Hum Pathol 37:637–647 [DOI] [PubMed] [Google Scholar]