Abstract
Glycodelin (Gd) is a major reproductive glycoprotein and a mediator for immunomodulatory effects directed to cellular, humoral, and innate immunity. Human pregnancy depends on a diversity of physiological processes including modulation of the maternal immunosystem. We evaluated the expression of Gd protein and mRNA in first trimester decidual tissue of normal pregnancies and spontaneous abortion and hydatidiform moles. Furthermore, in vitro experiments on endometrial cancer cells to analyze the effect of human chorionic gonadotropin (hCG) on Gd regulation were performed. In decidual tissue of abortion patients, Gd expression was significantly decreased compared with normal gestation, which was confirmed by in situ hybridization. In mole pregnancy, an upregulation of Gd in the first 8 weeks of pregnancy was present. Gd is a main product of decidual tissue in the first trimester of human pregnancy. Reduced Gd expression in abortive pregnancy could lead to an increased activation of the maternal immunosystem, thus causing rejection of the developing fetus. Moreover, Gd expression in endometrial cancer cells in vitro could be stimulated by addition of hCG. Therefore, we speculate that hCG could be one of the factors regulating Gd expression because hCG is downregulated in women with abortion and upregulated in mole pregnancy. In addition, we found a positive feedback loop in Gd and hCG expression in human pregnancy. (J Histochem Cytochem 56:477–485, 2008)
Keywords: glycodelin, decidua, abortion, hydatidiform mole, in situ hybridization, immunocytochemistry, immunohistochemistry
Glycodelin (Gd), previously known as placental protein 14, is a glycoprotein with varying oligosaccharides and has been predominantly localized in organs of the genital tract in rats, baboons, and humans (Joshi et al. 1981; Mazurkiewicz et al. 1981; Keil et al. 1999). Several Gd isoforms with differences in glycosylation have been described: Gd-A (endometrium/decidua, amniotic fluid, and maternal serum) (Julkunen et al. 1985; Dell et al. 1995; Koistinen et al. 2003), Gd-S (seminal vesicles and seminal plasma) (Morris et al. 1996), Gd-F (follicular fluid and oviduct) (Tse et al. 2002), and Gd-C (cumulus oophorus) (Chiu et al. 2007).
During the normal human ovulatory cycle, Gd is upregulated in the luteal phase, being highest between 8 and 10 days after ovulation (Mylonas et al. 2006). In maternal serum and amniotic fluid of normal human pregnancy, Gd levels are highest between 10 and 16 weeks of gestation (Julkunen et al. 1985). There is evidence that maternal Gd serum levels serve as a biochemical parameter in the differential diagnosis between intact and incomplete abortion (Foth and Romer 2003).
Recently, we were able to show that Gd is an efficient inhibitor of E-selectin–mediated cell adhesion. Because of its high concentration in the decidua and its potential ability to block selectin-mediated cell adhesion, Gd may play an important role at the fetomaternal interphase, thus promoting the establishment of fetal immunotolerance (Jeschke et al. 2003b). Moreover, we were able to show that human chorionic gonadotropin (hCG) protein and mRNA release in term placental trophoblast cells were significantly stimulated by Gd-A, whereas human placental lactogen release was inhibited (Reimer et al. 2000; Jeschke et al. 2003a). Our results further indicated paracrine effects of Gd-A in reproductive tissues and at the feto-maternal trophoblast interface (Jeschke et al. 2005a,b). These data support the concept that Gd-A is a modulator of trophoblast function.
Because Gd is a major secretory decidual product during pregnancy and plays a role in establishment of fetal immunotolerance, this protein may provide an important marker of decidual development in normal pregnancy, abortion, and hydatidiform mole.
Furthermore, a relationship between serum levels of Gd and enhanced probability of abortion has been suggested in former studies (Tomczak et al. 1996).
Therefore, we studied decidual tissue of normal, abortive, and mole pregnancy to assess possible differences in the expression of Gd with the use of specific monoclonal and polyclonal antibodies and Gd mRNA expression by in situ hybridization methods. Additionally, in vitro experiments were performed to clarify the role of hCG in the regulation of Gd in human first trimester pregnancy.
Materials and Methods
Tissue Samples
Decidual tissues were obtained from 16 women with first trimester abortion, 16 control women with termination of normal pregnancy, and 16 mole pregnancies. All women were treated in the Department of Obstetrics and Gynecology, Maistrasse, Ludwig-Maximilians University (LMU), Munich, Germany. The Human Investigation Review Board of the LMU Munich approved the study.
Immunohistochemistry
Immunohistochemistry on paraffin sections (3 μm) of the different decidual tissue specimens was done by incubating the slides in methanol/H2O2 (30 min) to inhibit endogenous peroxidase activity, followed by washing in PBS (5 min) and treating with goat serum (30 min, 22C), UV-Block (45 min, 22C), and human AB serum (30 min, 22C) to reduce nonspecific background staining.
Incubation with the primary antibodies (Table 1 ) was done overnight at 4C. Specific binding of the antibodies to Gd-A was analyzed by Western blot analysis (Bergemann et al. 2003; Jeschke et al. 2003b). Monoclonal and polyclonal peptide antibodies were used to avoid glycosylation-dependent staining differences as previously described (Mylonas et al. 2006).
Table 1.
Antibodies used for immunohistochemistry/cytochemistry
| Antibody | Clone | Isotype | Dilution | Source |
|---|---|---|---|---|
| Glycodelin | IgG rabbit | 2.5 μg/ml | Zytomed | |
| Glycodelin A | 6F2 | IgG mouse | 1 μg/ml | Zytomed |
| LH/hCG receptor | IgG rabbit | 1:100 | Dianova; Hamburg, Germany |
LH, luteinizing hormone; hCG, human chorionic gonadotropin.
Sections were incubated with the biotinylated secondary antibody (1 hr, 22C) and with avidin-conjugated peroxidase (45 min, room temperature) in case of monoclonal Gd-A antibodies.
The polyclonal antibody was investigated using the horseradish peroxidase-polymer system (Zytomed Systems; Berlin, Germany). Between each step, sections were washed with PBS (pH 7.4). Peroxidase staining reaction was done with diaminobenzidine/H2O2 (1 mg/ml; 5 min) and stopped in tap water (10 min). Sections were counterstained in hemalum (1 min) and coverslipped. In controls, the primary antibody was replaced with preimmune mouse serum.
The intensity and distribution patterns of the staining reaction was evaluated by two blinded, independent observers using the semiquantitative International Remmele Score as previously described (Remmele et al. 1986), and it was calculated by multiplication of the optical staining intensity (graded as 0 = no, 1 = weak, 2 = moderate, and 3 = strong staining) and the percentage of positive-stained cells (0 = no staining, 1 = <10% of the cells, 2 = 11–50% of the cells, 3 = 51–80% of the cells, and 4 = >81% of the cells).
Preparation of Riboprobes
A 227-bp fragment of the Gd cDNA (positions +41 to +268) was cloned into the EcoR1 restriction site of pBluescript SK− (Stratagene; Amsterdam, The Netherlands) and labeled with digoxigenin (DIG) by in vitro transcription using the DIG RNA labeling Kit (SP6/T7; Roche Biochemicals, Mannheim, Germany) as described previously (Keil et al. 1999). The antisense cRNA was used for the detection of Gd mRNA, whereas the sense cRNA probe served as a negative control.
In Situ Hybridization Analysis of Gd on Paraffin Sections
Non-radioactive in situ hybridization was performed on paraffin sections (4 μm) that had been fixed in 4% paraformaldehyde. Sections were rehydrated and permeabilized by pepsin digestion (750 μg/ml pepsin in 0.2 M HCl, 37C, 30 min).
Postfixation (paraformaldehyde 4%, 20 min, 4C) was followed by acetylation using 0.25% acetic anhydride in triethanolamine (0.1 M, pH 8.0, 15 min). After dehydration in ethanol (70%, 95%, and 100%), the sections were hybridized for 16 hr (56C) in a solution containing 50% formamide, 50% solution D (4 M guanidine thiocyanate, 25 mM sodium citrate, pH 7.0), 0.5% blocking reagent (Roche Biochemicals), 210 μg/ml t-RNA derived from E. coli MRE 600 (Roche Biochemicals), and 125 ng DIG-labeled cRNA probe.
After washing with decreased concentrations of SSC (20× SSC: 3 M NaCl, 0.3 M sodium citrate, pH 7.4), sections were incubated 1 hr with blocking reagent (Roche Biochemicals). Bound riboprobe was visualized by incubation with alkaline phosphatase–conjugated anti-DIG antibody (Roche Biochemicals) and subsequent substrate reaction using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue-tetrazolium chloride.
Computerized Analysis of Gd mRNA Expression
The level of Gd mRNA expression was determined in a blinded fashion in one run with identical staff, equipment, and chemicals. From each section, five digital pictures were taken at random of different places of the decidual tissue (200-fold magnification; 3CCD color camera, HV-C20M; Hitachi, Denshi, Japan, and Axiolab; Carl Zeiss, Jena, Germany). For standardization of the measurement in each picture, the optical density of white background color was attuned to 250.
For all sections, the mean optical density and the quantity of pixels having a positive reaction for Gd was assessed using KSRun software (imaging system KS400, release 3.0; Zeiss).
Cell Culture
Human endometrial cancer cells (HEC 1b) were obtained from ECACC (Salisbury, UK). The cells (passages between 3 and 7) were cultivated in cell culture medium (DMEM; Sigma, Taufkirchen, Germany) containing 10% FBS. All cell cultures were maintained in a humidified 5% CO2 atmosphere at 37C. The cells were incubated with hCG (Sigma) in different concentrations (0–2000 IU/ml) and cultivated for up to 72 hr on chamber slides (Nunc; Roskilde, Denmark).
Immunocytochemistry
Expression of Gd was analyzed using a specific monoclonal antibody (Table 1) and the ABC staining method (Vectastain Elite mouse-IgG-Kit; Vector, Burlingame, CA). Staining intensity was studied by using the semiquantitative Remmele score. In brief, HECs were cultivated in chamber slides for up to 72 hr, dried, wrapped, and stored at −80C.
After thawing, cells were briefly fixed with formalin (Merck; Darmstadt, Germany; 5% in PBS, 5 min). Slides were incubated in methanol/H2O2 (30 min) to inhibit endogenous peroxidase activity, washed in PBS (5 min), and treated with goat serum (20 min, room temperature) to reduce nonspecific background staining. Incubation with the primary antibody (Table 1) was done overnight at 4C.
Sections were incubated with the biotinylated secondary anti-mouse antibody (1 hr, room temperature) and avidin-biotinylated peroxidase (45 min, room temperature). Between each step, the sections were washed with PBS (pH 7.4) three times. Peroxidase staining reaction was done with diaminobenzidine/H2O2 (1 mg/ml; 5 min) and stopped in tap water (10 min). Sections were counterstained in hematoxylin (1 min) and coverslipped. In controls, the primary antibody was replaced with preimmune mouse serum.
The slides were finally embedded in mounting buffer and examined with a Zeiss Axiophot photomicroscope (Zeiss). Digital images were obtained with a digital camera system (Axiocam; Zeiss).
Statistics
The SPSS/PC software package version 15.0 (SPSS; Chicago, IL) was used for collection, processing, and statistical data analysis. Statistical analysis was performed using the non-parametric Wilcoxon test for matched pairs and non-parametrical Mann-Whitney U test for comparison of the means. p<0.05 was considered statistically significant.
Results
Gd Expression in Normal Pregnancy and Abortion
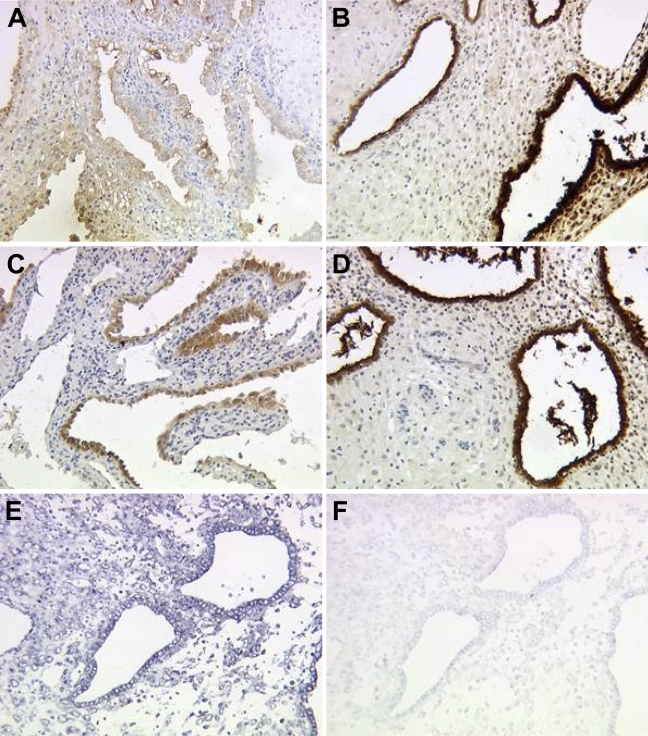
Gd protein and mRNA are mainly expressed in glandular epithelial cells of normal human first trimester decidua (Figures 1A and 1B, pAK; 1C and 1D, mAk; 1E and 1F, mRNA).
Figure 1.
Immunohistochemical staining of glycodelin (Gd) in normal first trimester decidua. Gd showed moderate expression in glandular epithelial tissue of the decidua in the 6th week of gestation (A,C), whereas Gd expression is strongly upregulated to the 12th week of gestation (B,D), determined with either polyclonal (A,B) or monoclonal antibodies (C,D) against Gd. Strong expression of glycodelin mRNA in normal glandular epithelial decidual tissue in the ninth week of gestation (E, antisense; F, sense), detected by in situ hybridization, confirmed results of immunohistochemistry.
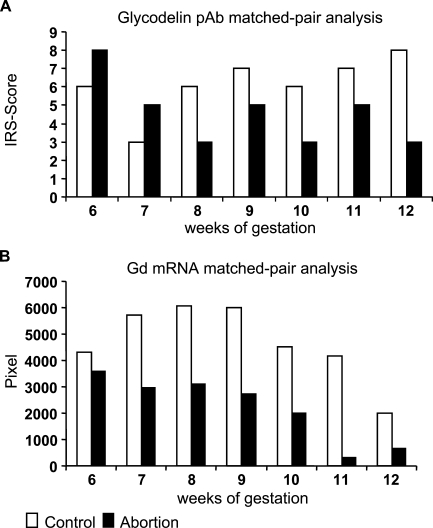
During normal gestation, we identified an increased expression of Gd in normal human first trimester glandular epithelial cells of the decidua from the 6th to 12th week of gestation, determined with both polyclonal Gd peptide antibody (Figure 2A ) and by in situ hybridization (Figure 2B).
Figure 2.
Staining intensity of Gd in normal and abortive decidual tissue was determined by the semiquantitative immunohistochemical International Remmele Score (IRS) on the different tissue slides. Data shown represent matched pair analyses ranging from the 6th to 12th week of gestation. Differences in Gd expression in normal and abortive glandular epithelial decidual tissue are statistically significant [p=0.016 for the polyclonal antibody, p=0.011 for the monoclonal antibody (A) and p=0.002 for in situ hybridization (B)].
Focusing on decidual tissue from abortion patients, we found significant lower expression of Gd compared with normal pregnancy analyzed with the polyclonal peptide antibody (p=0.016; Figure 2A) and with the monoclonal antibody against Gd-A (p=0.011; data not shown). Again, data were confirmed by in situ hybridization showing reduced Gd mRNA expression compared with normal pregnancy (p=0.002; Figure 2B).
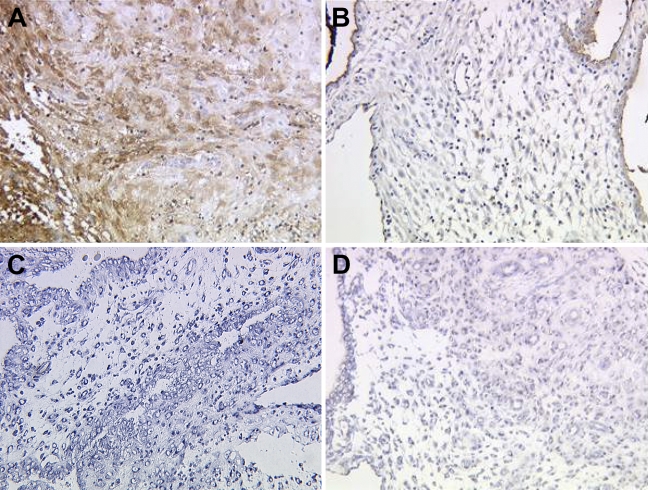
In normal human pregnancy, we also found an increased expression of Gd in decidual stroma cells in ongoing weeks of gestation on the protein and mRNA level (Figures 3A and 3C). In abortive tissue, Gd expression in decidual stromal cells is reduced on the protein (Figure 3B) and mRNA levels (Figure 3D).
Figure 3.
In decidual stromal tissue, Gd showed high expression in normal pregnancy (A, 12th week of gestation) and significantly reduced expression in abortive decidual stroma (B, 12th week of gestation) obtained with the polyclonal antibody. Strong expression of Gd mRNA in normal stromal decidual tissue in the seventh week of gestation (C, antisense) and low expression of Gd mRNA in abortive stromal decidual tissue (D, antisense), detected by in situ hybridization, confirmed results of immunohistochemistry.
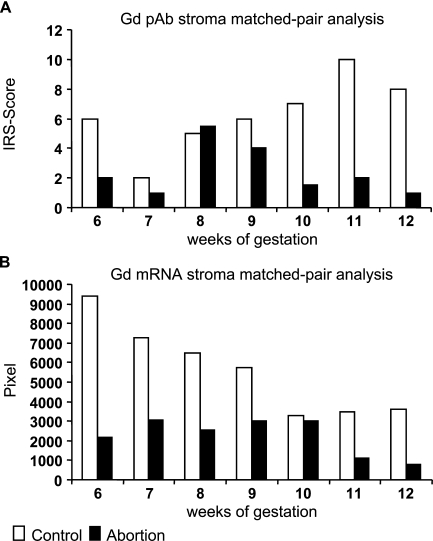
Reduced Gd expression compared with normal pregnancy (p=0.018) could be shown with the polyclonal peptide antibody from the 6th to the 12th week of pregnancy (Figure 4A ). With regard to Gd-A expression in decidual stroma, no significant differences were observed between normal pregnancy and abortion with the monoclonal antibody (data not shown). In addition, in situ hybridization experiments confirmed results obtained with the polyclonal antibody showing significant reduced Gd mRNA expression (p=0.026; Figure 4B).
Figure 4.
Staining intensity of Gd in normal and abortive decidual tissue was determined by the semiquantitative immunohistochemical IRS score on the different tissue slides. Data shown represent matched pair analyses ranging from the sixth week of gestation. Differences in Gd expression in normal and abortive decidual stromal tissue are statistically significant [p=0.018 for the polyclonal antibody (A) and p=0.026 for in situ hybridization (B)].
Gd Expression in Hydatidiform Moles
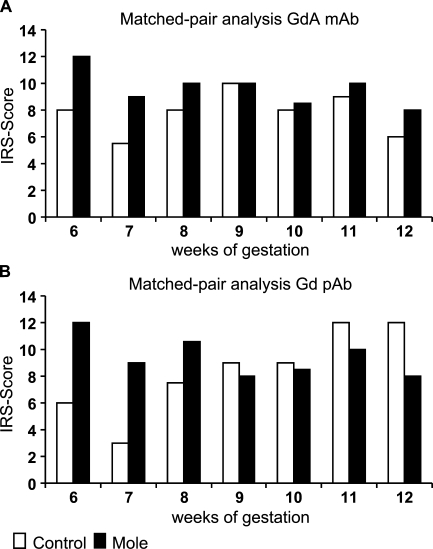
In contrast to normal pregnancy, the highest levels of Gd expression were already seen in the 6th week of mole pregnancy and remained at high levels until the 11th and 12th week of gestation in glandular epithelial cells of the decidua.
By studying Gd expression in mole pregnancies with a monoclonal and a polyclonal antibody, significant upregulation (p=0.018, in both cases) was seen in mole compared with normal human pregnancy (Figures 5A and 5B). No significant difference was seen between Gd mRNA expression in mole compared with normal human pregnancy by in situ hybridization.
Figure 5.
Staining intensity of Gd in normal and mole decidual tissue was determined by the semiquantitative immunohistochemical IRS score on the different tissue slides. Data shown represent matched pair analyses ranging from the 6th to 12th week of gestation. Stained with mAb (A) or pAb (B) against Gd.
In Vitro Stimulation Experiments on Gd Expression
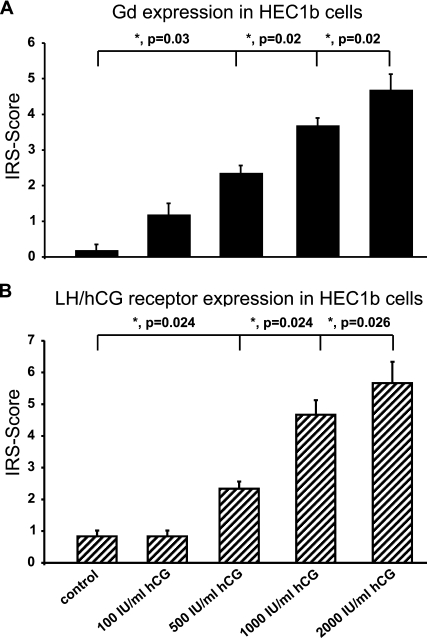
Endometrial carcinoma cells of the cell line HEC 1b showed only faint expression of Gd after cultivation for up to 72 hr. Addition of hCG (100, 500, 1000, and 2000 IU/ml) increased Gd expression in a concentration-dependent manner (Figure 6A ). HEC 1b cells stimulated with 500, 1000, and 2000 IU/ml hCG showed significant upregulation of Gd compared with unstimulated HEC cells (p=0.026, 0.024, and 0.024, respectively; Figure 6A).
Figure 6.
Endometrial carcinoma cells of the cell line HEC1b produced faint amounts of Gd. Production of Gd could be stimulated by addition of increasing amounts of human chorionic gonadotropin (hCG). A summary of the staining results is presented in A. Significant differences in Gd expression are marked with an asterisk. Cells of the cell line HEC1b showed mediated expression of luteinizing hormone (LH)/hCG receptor in unstimulated cells, whereas cells incubated with increasing amounts of hCG showed increased expression of LH/hCG receptor, and cells stimulated with 2000 IU/ml hCG showed very strong upregulation of LH/hCG receptor. A summary of the staining results is presented in B. Significant differences in LH/hCG receptor expression are marked with an asterisk.
In addition, upregulation of Gd in HEC cells was mediated by the gonadotropin receptor. Addition of hCG (100, 500, 1000, and 2000 IU/ml) increased gonadotropin receptor expression in a dose-dependent manner after cultivation for up to 72 hr (Figure 6B). HEC 1b cells stimulated with 500, 1000, and 2000 IU/ml hCG showed significant upregulation of luteinizing hormone (LH)/hCG receptor expression compared with unstimulated controls (p=0.024, 0.024, and 0.026, respectively; Figure 6B).
Discussion
Using monoclonal and polyclonal antibodies raised against Gd and in situ hybridization, we observed strong variations in Gd expression between normal abortive, and mole pregnancy.
Especially in abortive tissue, downregulation of Gd was obvious already from the 6th week of pregnancy, whereas in normal decidual tissue, the highest levels of Gd were found between the 11th and 12th week of gestation. Our results were confirmed on a protein and mRNA level. With regard to Gd expression in abortive serum samples, decreased concentrations were found compared with normal controls (Tomczak et al. 1996). Results obtained with the monoclonal or polyclonal antibody showed identical staining results except for decidual stromal tissue of the abortive placentas, possibly indicating differences in the glycosylation pattern of Gd (Mylonas et al. 2006). Expression of Gd was prominent in glandular epithelial tissue, whereas in stromal tissue, only weak expression of this glycoprotein was detected in the period between 6 and 12 weeks of gestation. Therefore, we speculate that the high amounts of Gd in the first trimester of pregnancy are mainly produced by glandular epithelial and not decidual stromal cells.
The immunosuppressive properties of Gd are important, because, even though the human fetus is a semi-allograft containing maternal and paternal antigens, it is not rejected by the maternal immunosystem (Seppala 2004).
T cells and natural killer (NK) cells are effectors of fetal rejection, and NK cells do have the ability to kill cells identified as non-self without any prior exposure. Both Gd and NK cells are abundant at the feto–maternal interface. Gd inhibits NK cell activity (Okamoto et al. 1991) and stimulates Th2-type cytokine production in monocytes (Miller et al. 1998), shown by an increased production of interleukin 6 (IL-6) and IL-10 after Gd exposure (Laird et al. 1993,1994). Gd directly inhibits T-cell activity (Rachmilewitz et al. 1999) on an early phase of activation by diminishing T-cell responses (at the time of T-cell receptor triggering) and inducing T-cell apoptosis (Rachmilewitz et al. 2001). The immunosuppressive effect of Gd might be dependent on the blocking of E-selectin–mediated cell adhesion (Dell et al. 1995). The fucosylated LacdiNAc structures are able to bind more effectively to E-selectin than sialylated Lewis X antigens (Grinnell et al. 1994).
Recently, it was shown that both Gd-A and serum Gd are very efficient inhibitors of the E-selectin–mediated cell adhesion in vitro, suggesting an important role in carcinogenesis and probably also in human pregnancy (Jeschke et al. 2003b).
In mole pregnancy, an upregulation of Gd was identified as early as in the sixth week of pregnancy. These high levels of Gd expression remained until the 12th week of pregnancy, identified by both antibodies. On the mRNA level, higher Gd expression was not detected from the 7th to 12th week in comparison to normal pregnancy, which might be because of stimulation effects of Gd mRNA in very early pregnancy.
Because abortion is accompanied by low levels of hCG in contrast to mole pregnancy with very high hCG levels, we speculated that hCG might be a modulator of Gd expression. This hypothesis was supported by our formal findings that hCG expression in trophoblast cells could be stimulated by Gd in vitro (Jeschke et al. 2003a,2005a), suggesting a regulative feedback loop between decidual Gd and trophoblast hCG. These speculations are supported by the findings that hCG application of in vitro fertilization-treated patients in the luteal phase of the menstrual cycle induces higher pregnancy rates (Fatemi et al. 2007), which is accompanied by higher Gd serum levels (Anthony et al. 1993).
Our in vitro studies clearly showed that addition of hCG to HEC 1b cells upregulated Gd expression in a concentration-dependent manner, further supporting our hypothesis. These cells were used as a model for endometrial glandular epithelial cells.
Further experiments will show if this loop is functional in primary glandular epithelial and stromal cells of the human endometrium. In addition, we were able to show that Gd upregulation in HEC 1b cells is mediated by the gonadotropin receptor in a concentration-dependent manner.
Therefore, trophoblast-derived hCG might be one of the factors that is essential for Gd regulation in human pregnancy.
In conclusion, Gd in decidual tissue seems to be a biomarker for disturbed pregnancy because it is significantly downregulated in abortive pregnancy and upregulated in premalignant conditions as in hydatidiform mole.
Especially in hydatidiform mole, hCG is significantly increased and serves as an indicator for the disease itself. Our study indicates a possible feedback mechanism between hCG and Gd expression.
Acknowledgments
B.T. was supported by the “Friedrich Baur-Stiftung,” “Förderung für Forschung und Lehre (FöFoLe),” and Hochschul-Wissenschafts-Programm, Ludwig-Maximilians-University, Munich, Germany. This work is part of the doctoral thesis of K.R.
We thank Susanne Kunze, Christina Kuhn (Department of Obstetrics and Gynecology–Maistrasse, Ludwig-Maximilians-University), and Dietlind Schulz [Department of Pathophysiology, Ernst-Moritz-Arndt University (EMAU) Greifswald] for technical assistance and Dr. R. Jarchow (Computing Center, EMAU Greifswald) for support.
References
- Anthony FW, Smith EM, Gadd SC, Masson GM, Chard T, Perry L (1993) Placental protein 14 secretion during in vitro fertilization cycles with and without human chorionic gonadotropin for luteal support. Fertil Steril 59:187–191 [DOI] [PubMed] [Google Scholar]
- Bergemann C, Reimer T, Muller H, Hosel A, Briese V, Friese K, Jeschke U (2003) Stimulation of hCG protein and mRNA levels in trophoblast tumour cells Jeg3 and BeWo by glycodelin A. Anticancer Res 23:1107–1113 [PubMed] [Google Scholar]
- Chiu PC, Chung MK, Koistinen R, Koistinen H, Seppala M, Ho PC, Ng EH, et al. (2007) Cumulus oophorus-associated glycodelin-C displaces sperm-bound glycodelin-A and -F and stimulates spermatozoa-zona pellucida binding. J Biol Chem 282:5378–5388 [DOI] [PubMed] [Google Scholar]
- Dell A, Morris HR, Easton RL, Panico M, Patankar M, Oehniger S, Koistinen R, et al. (1995) Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J Biol Chem 270:24116–24126 [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P (2007) An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update 13:581–590 [DOI] [PubMed] [Google Scholar]
- Foth D, Romer T (2003) Glycodelin serum levels in women with ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol 108:199–202 [DOI] [PubMed] [Google Scholar]
- Grinnell BW, Hermann RB, Yan SB (1994) Human protein C inhibits selectin-mediated cell adhesion: role of unique fucosylated oligosaccharide. Glycobiology 4:221–225 [DOI] [PubMed] [Google Scholar]
- Jeschke U, Karsten U, Reimer T, Richter DU, Bergemann C, Briese V, Mylonas I, et al. (2005a) Stimulation of hCG protein and mRNA in first trimester villous cytotrophoblast cells in vitro by glycodelin A. J Perinat Med 33:212–218 [DOI] [PubMed] [Google Scholar]
- Jeschke U, Richter DU, Reimer T, Bergemann C, Briese V, Karsten U, Mylonas I, et al. (2005b) Glycodelin A and differentiation of first trimester trophoblast cells in vitro. Arch Gynecol Obstet 272:151–159 [DOI] [PubMed] [Google Scholar]
- Jeschke U, Richter DU, Walzel H, Bergemann C, Mylonas I, Sharma S, Keil C, et al. (2003a) Stimulation of hCG and inhibition of hPL in isolated human trophoblast cells in vitro by glycodelin A. Arch Gynecol Obstet 268:162–167 [DOI] [PubMed] [Google Scholar]
- Jeschke U, Wang X, Briese V, Friese K, Stahn R (2003b) Glycodelin and amniotic fluid transferrin as inhibitors of E-selectin-mediated cell adhesion. Histochem Cell Biol 119:345–354 [DOI] [PubMed] [Google Scholar]
- Joshi SG, Szarowski DH, Bank JF (1981) Decidua-associated antigens in the baboon. Biol Reprod 25:591–598 [DOI] [PubMed] [Google Scholar]
- Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppala M (1985) Distribution of placental protein 14 in tissues and body fluids during pregnancy. Br J Obstet Gynaecol 92:1145–1151 [DOI] [PubMed] [Google Scholar]
- Keil C, Husen B, Giebel J, Rune G, Walther R (1999) Glycodelin mRNA is expressed in the genital tract of male and female rats (Rattus norvegicus). J Mol Endocrinol 23:57–66 [DOI] [PubMed] [Google Scholar]
- Koistinen H, Easton RL, Chiu PC, Chalabi S, Halttunen M, Dell A, Morris HR, et al. (2003) Differences in glycosylation and sperm-egg binding inhibition of pregnancy-related glycodelin. Biol Reprod 69:1545–1551 [DOI] [PubMed] [Google Scholar]
- Laird SM, Li TC, Bolton AE (1993) The production of placental protein 14 and interleukin 6 by human endometrial cells in culture. Hum Reprod 8:793–798 [DOI] [PubMed] [Google Scholar]
- Laird SM, Tuckerman E, Li TC, Bolton AE (1994) Stimulation of human endometrial epithelial cell interleukin 6 production by interleukin 1 and placental protein 14. Hum Reprod 9:1339–1343 [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz JE, Bank JF, Joshi SG (1981) Immunocytochemical localization of a progestagen-associated endometrial protein in the human decidua. J Clin Endocrinol Metab 52:1006–1008 [DOI] [PubMed] [Google Scholar]
- Miller RE, Fayen JD, Chakraborty S, Weber MC, Tykocinski ML (1998) A receptor for the lipocalin placental protein 14 on human monocytes. FEBS Lett 436:455–460 [DOI] [PubMed] [Google Scholar]
- Morris HR, Dell A, Easton RL, Panico M, Koistinen H, Koistinen R, Oehninger S, et al. (1996) Gender-specific glycosylation of human glycodelin affects its contraceptive activity. J Biol Chem 271:32159–32167 [DOI] [PubMed] [Google Scholar]
- Mylonas I, Jeschke U, Kunert-Keil C, Shabani N, Dian D, Bauerfeind I, Kuhn C, et al. (2006) Glycodelin A is expressed differentially in normal human endometrial tissue throughout the menstrual cycle as assessed by immunohistochemistry and in situ hybridization. Fertil Steril 86:1488–1497 [DOI] [PubMed] [Google Scholar]
- Okamoto N, Uchida A, Takakura K, Kariya Y, Kanzaki H, Riittinen L, Koistinen R, et al. (1991) Suppression by human placental protein 14 of natural killer cell activity. Am J Reprod Immunol 26:137–142 [DOI] [PubMed] [Google Scholar]
- Rachmilewitz J, Riely GJ, Huang JH, Chen A, Tykocinski ML (2001) A rheostatic mechanism for T-cell inhibition based on elevation of activation thresholds. Blood 98:3727–3732 [DOI] [PubMed] [Google Scholar]
- Rachmilewitz J, Riely GJ, Tykocinski ML (1999) Placental protein 14 functions as a direct T-cell inhibitor. Cell Immunol 191:26–33 [DOI] [PubMed] [Google Scholar]
- Reimer T, Koczan D, Briese V, Friese K, Richter D, Thiesen HJ, Jeschke U (2000) Absolute quantification of human chorionic gonadotropin-beta mRNA with TaqMan detection. 4. Mol Biotechnol 14:47–57 [DOI] [PubMed] [Google Scholar]
- Remmele W, Hildebrand U, Hienz HA, Klein PJ, Vierbuchen M, Behnken LJ, Heicke B, et al. (1986) Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A Pathol Anat Histopathol 409:127–147 [DOI] [PubMed] [Google Scholar]
- Seppala M (2004) Advances in uterine protein research: reproduction and cancer. Int J Gynaecol Obstet 85:105–118 [DOI] [PubMed] [Google Scholar]
- Tomczak S, Briese V, Kunkel S, Muller H (1996) Serum placental protein 14 (PP14) levels in patients with threatened abortion. Arch Gynecol Obstet 258:165–169 [DOI] [PubMed] [Google Scholar]
- Tse JY, Chiu PC, Lee KF, Seppala M, Koistinen H, Koistinen R, Yao YQ, et al. (2002) The synthesis and fate of glycodelin in human ovary during folliculogenesis. Mol Hum Reprod 8:142–148 [DOI] [PubMed] [Google Scholar]