Abstract
Aquaporins (AQP) have important solute transport functions in many tissues including the epididymal efferent ducts (ED) and in the liver. We investigated the effect of neonatal exposure to diethylstilbestrol (DES) on AQP9 expressions in the ED and in the liver of rats. DES was administered from day 2 to day 20 postnatally at a dose of 4,8 μg/day, and AQP9 protein and mRNA were measured by immunoblotting and real-time PCR, respectively, along with immunohistochemistry. DES caused hepatic downregulation of AQP9 at both the protein and mRNA level; however, decreased AQP9 labeling was only observed in the periportal zone. In the ED, AQP9 protein expression was increased in the DES-treated animals by 300% that could be ascribed to a widening of the ED lumen, whereas no difference was observed in AQP9 mRNA expression. Immunohistochemical findings revealed that AQP9 expression was confined to the epithelial cells of the ED. In conclusion, neonatal DES exposure appears to upregulate AQP9 channels in the ED in male rats, whereas a downregulation in the hepatic expression was observed, particularly in the periacinous area.(J Histochem Cytochem 56:425–432, 2008)
Keywords: epididymal efferent ducts, estrogen, liver, aquaporin 9, diethylstilbestrol
Aquaporins (AQP) were discovered 15 years ago (Preston and Agre 1991), and since then at least 13 mammalian AQPs have been identified (Agre et al. 2002; Castle 2005). In humans, AQP9 is highly expressed in leukocytes and to a lesser extent in liver tissue (Ko et al. 1999; Tsukaguchi et al. 1999) and in the epididymis (Pastor-Soler et al. 2001). In rats, AQP9 has been observed in liver, testes, epididymis, epididymal efferent ducts (ED), and the brain (Elkjaer et al. 2000; Nicchia et al. 2001). Human and rat AQP9 are 295 amino acid-long proteins with a sequence homology of ∼75% (Elkjaer et al. 2000). In addition to water, AQP9 displays transport capacity for glycerol, urea, and other small solutes (lactate, carbamides, polyols, purines, and pyrimidines) as well as 5-fluorouracil (Tsukaguchi et al. 1998,1999; Badaut et al. 2001).
Hepatic AQP9 is localized on the sinusoidal surface of hepatocytes (Nicchia et al. 2001; Nihei et al. 2001; Carbrey et al. 2003), whereas the apical bile canaliculi are not stained by AQP9 antibodies. The physiological role and the regulation of AQP9 expression in the liver have not yet been elucidated. Previous findings indicate that estrogens are involved in the regulation of blood glucose concentrations by inhibiting gluconeogenesis and increasing glycogen storage in liver and muscle (Matute and Kalkhoff 1973; Ahmed-Sorour and Bailey 1981). AQP9 transports glycerol, a substrate for gluconeogenesis, and it could therefore be speculated whether estrogens regulate expression of AQP9 in the liver as part of regulating blood glucose. Indeed, the total level of AQP9 expression in the liver is higher in male than in female rats, also having a more localized periacinous expression (Nicchia et al. 2001). Nevertheless, in adult male rats, ethinyl estradiol at a dose sufficiently high to cause cholestasis had no effect on AQP9 expression (Carreras et al. 2007).
Exposure to endocrine disruptors (including estrogen-like chemicals) during perinatal life has been suspected to contribute to the apparent increases in incidences of testicular cancer, cryptorchidism, and hypospadia along with the decrease in semen quality, phrased as “testicular dysgenesis syndrome” (Sharpe and Skakkebaek 1993; Skakkebaek et al. 2001; Sharpe 2006). Sensitivity to estrogen-induced disturbances of the male reproductive tissues seems to be elevated during neonatal and fetal life compared with adulthood (Aksglaede et al. 2006). During neonatal life, proliferation of the male reproductive tract is relatively slow, and it is not until the increase in testosterone observed at puberty that growth accelerates (Sun and Flickinger 1979). Disruption of the estrogen–androgen balance upon excessive estrogen exposure induces abnormal expression patterns of ERα/β and androgen receptor (Atanassova et al. 2001; McKinnell et al. 2001), which may disturb correct growth and differentiation of the tissues.
Aquaporins expressed in the ED mediate water transport out of the lumen, decreasing the water content of the semen and thereby increasing sperm quality. Estrogens have been recognized to play a physiological role in the regulation of fluid transport in the male reproductive system, and this may be regulated by expression of aquaporins, possibly AQP9 (Oliveira et al. 2005). ED expresses AQP1, 9, and 10, and both AQP1 and AQP9 seem to be involved in estrogen-regulated water reabsorption (Fisher et al. 1998; Oliveira et al. 2005). Effects of estrogen and certain androgen metabolites are thought to be mediated via the estrogen receptors (ERα and β) that are highly expressed in the ED (Hess et al. 1997b; Picciarelli-Lima et al. 2006).
Structural and functional development of ED are susceptible to changes after estrogen exposure during early life, and this has been associated with downregulation of AQP1 expression (Fisher et al. 1998,1999). In contrast, estrogen exposure caused upregulation of AQP9 expression in adult rats. Thus, the effect of neonatal estrogen exposure on the regulation of AQP9 expression in the ED is of great interest. In this study we investigated mRNA and protein expression as well as immunolocalization of protein AQP9 in liver and ED from rats exposed postnatally to DES.
Materials and Methods
Animals and Treatment
Two-day-old male Spraque Dawley rats were obtained from Taconic Europe (Ry, Denmark). A total of four rat nurses nursed six to seven siblings each until the pups were sacrificed on postnatal day (PND) 20. Animals received a standard diet (Altromin Standard Diet #1314; Lage, Germany) and tap water ad libitum. They were housed in an environmentally controlled animal facility operating at 18–22C, 40–60% humidity, and a 12-hr light/dark cycle. Each litter received the same treatment, which was either 0.1 mg diethylstilbestrol (DES) or DES–placebo as pellets that released hormone with a constant rate during a period of 21 days (Innovative Research of America; Sarasota, FL). Pellets were placed SC on PND 2. The total amount of hormone released was ∼86 μg/rat. Animals were weighed on PND 2, 6, 9, 13, 17, and 20 to ensure an even weight gain in the exposed vs the unexposed animals. Institutional guidelines for animal welfare were followed, and the experiments were approved by the Danish Animal Experimental Inspectorate.
Immunoblot Analysis
Liver and ED (n=6) were collected immediately after the animals were sacrificed by cervical dislocation, and AQP9 protein level was measured by Western blotting. Approximately 0.8 g liver tissue was homogenized in 4 ml homogenizing buffer containing 300 mM sucrose, 25 mM imidazole, 1 mM EDTA, 0.1 mg/ml pefabloc, 4 μg/ml leupeptin, 184 μg/ml sodium orthovanadate, 1 mg/ml sodium fluoride, and 82 ng/ml okadeic acid (Sigma; St Louis, MO). Similarly, EDs (pooled from two animals within the same group) were homogenized in 500 μl homogenizing buffer. Protein concentration of the samples was assessed using Pierce BCA (Pierce Biotechnology; Rockford, IL), and all samples were adjusted to the same level. Sample buffer was added to a final concentration of 485 mM Tris–HCl, 8.7% glycerol, 104 mM SDS, 20 mM DTT, and 0.9 mM bromphenol blue. Samples were heated for 10 min at 90C (liver samples) or for 15 min at 60C (ED samples) and run on 12% polyacrylamide gels. Proteins were electrotransferred to PVDF blotting membranes (Millipore Corporation; Bedford, MA) and blocked for 1 hr in PBS-T (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1% Tween 20, pH 7.5). Membranes were washed and incubated overnight at 4C with anti-AQP9 antibody (Santa Cruz Biotechnology; Heidelberg, Germany). Blots were washed in PBS-T and incubated for 1 hr with horseradish peroxidase (HRP)-conjugated secondary antibody (Dako; Copenhagen, Denmark). After the final washing in PBS-T, proteins were visualized using the ECL Plus chemiluminescence system (Amersham; Buckinghamshire, UK). To confirm specificity of the primary antibody, some membranes were incubated with primary antibodies preabsorbed with the immunizing AQP9 peptide.
Immunohistochemistry
Animals (n=6) were anesthetized using 4% halothane followed by 1% halothane for maintenance in 1:1 N2O/O2. Liver was perfused in situ through the aorta using 4% formaldehyde in phosphate buffer, pH 7 (Bie and Berntsen; Rødovre, Denmark). A section of the liver was immersed in formaldehyde and then paraffin embedded. EDs were fixed by immersion in Bouin's solution (Sigma) and embedded in paraffin. All sections were treated similarly, and immunohistochemical procedures were similar for ER and AQP9. In short, tissue sections were dewaxed in xylene (Bie and Berntsen) and rehydrated through decreasing concentrations of ethanol (De Danske Spritfabrikker; Aalborg, Denmark). Antigen retrieval was done by microwave irradiation in 0.01 sodium citrate buffer, pH 6, and then blocked for endogenous peroxidase by incubation with 3% H2O2 for 10 min. Nonspecific binding was blocked using 1% BSA in PBS. Sections were incubated overnight at 4C with the respective primary antibody. The polyclonal AQP91-A antibody (Alpha Diagnostic International; San Antonio, TX) raised in rabbit was used for detection of AQP9. Antibody dilution used was 1:200 for AQP9.
After incubation with the primary antibody, tissue sections were washed and incubated with a HRP-labeled polymer conjugated with secondary antibodies (EnVision+ System; Dako, Carpinteria, CA). Visualization was done by adding 3,3′-diaminobenzidine (DAB), washing, and counterstaining with hematoxylin. Finally, tissue sections were dehydrated and mounted with Eukitt (Bie and Berntsen). No staining was detected when the AQP9 antibody preabsorbed with the synthetic AQP9 peptide was used as the first antibody (results not shown).
mRNA Quantification
Tissues for mRNA quantification (ED and liver, n=6) were immersed into liquid nitrogen immediately after sacrificing the animals. Total RNA was purified from ED and liver using Trizol Reagent (Invitrogen Ltd.; Leek, Belgium) in accordance with the manufacturer's protocol, and cDNA was prepared using Geneamp RNA PCR kit (Applied Biosystems; IJssel, The Netherlands).
Primers were designed to span the intron region between two exons and were thus cDNA specific. For 18S rRNA analysis, we used a commercially available probe and primer solution (TaqMan RRNA control reagents, VIC Probe; Applied Biosystems, Foster City, CA). For AQP9 the following nucleotides were used (all purchased from TIB MolBiol; Berlin, Germany): forward primer: 5′-ggT CTT Tgg CAT TTA TTA TgA T-3′, reverse primer: 5′-Agg AAC ATg gTA gAC ACC ACT Tg-3′, TaqMan probe: 5′-FAM-AgC TCC ATT CAT ATC CAC gCC Agg T-TAMRA-3′. AQP9 and 18S RNA levels were quantified in separate tubes. Final concentrations of probe and primers were 0.2 and 0.5 μM, respectively. PCR reactions were performed in triplicate in the LightCycler system (Roche Diagnostics; Hvidovre, Denmark) in 15-μl reactions. A total of 0.5 μl cDNA preparation was mixed with MgCl2 (final concentration 7 μM) and 1.5 μl LightCycler master mix.
For the PCR reaction the following protocol was used: activation of TAQ polymerase (95C for 10 min), 45 cycles of 95C for 2 sec, 60C for 50 sec followed by single fluorescence measurement and cooling to 40C for 30 sec. For each animal the individual level of initial target cDNA was expressed as the difference in Ct values (cycle number at detection threshold – crossing point) between the average of the triplicate of AQP9 and the average of the triplicate of 18S in the parallel samples. The relative amount of target mRNA normalized to 18S mRNA was calculated as 2–dCt.
Average standard deviation (SD) on triplicates was 8.8%. SD of repeated measurements of the same sample (the control) in separate experiments was 10.0%. PCR was quantitative over a range of 250-fold dilution.
Statistics
All data were tested for normal distribution and homogeneity of variance by Shapiro–Wilk's and Levenes test, respectively. Effects of the exposure were tested with Student's t-test for independent samples; p<0.05 was considered significant.
Results
BWs and relative and absolute liver and kidney weights were unaffected by DES treatment, whereas relative and absolute testis weights were significantly decreased after DES treatment (Table 1 ).
Table 1.
Effects of treatment with DES or placebo at PND 2–20 on body and relative organ weights
| Placebo n = 7 | DES n = 7 | |
|---|---|---|
| BW (g) | 59.0 (5.7) | 61.6 (4.6) |
| Organ weights as percentage of BWs | ||
| Liver | 4.01 (0.42) | 4.29 (0.14) |
| Kidney | 1.11 (0.11) | 1.18 (0.07) |
| Testis | 0.46 (0.03) | 0.18 (0.01)* |
p<0.05.
Organ weights are presented as percentages of body weights (BW). Data are mean ± SD. PND, postnatal day; DES, diethylstilbestrol.
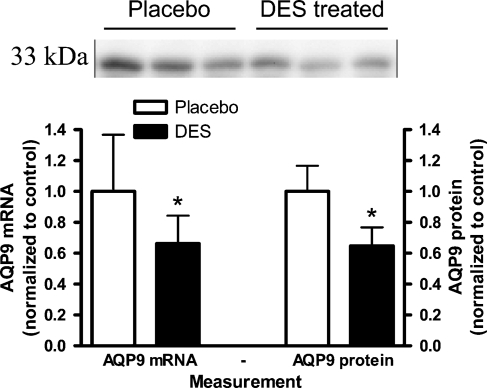
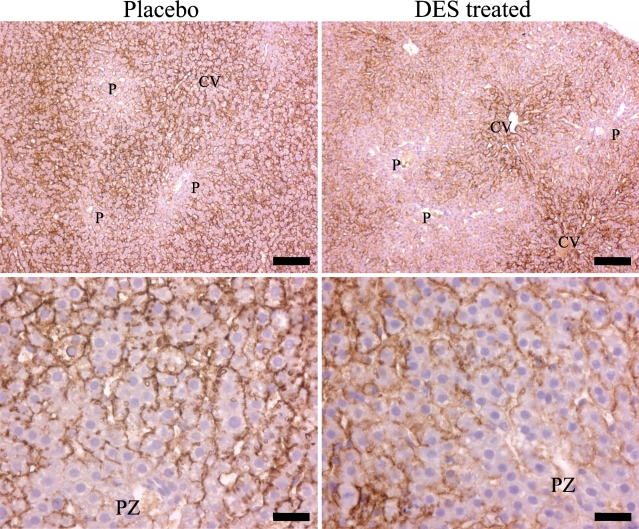
Hepatic AQP9 mRNA level normalized to 18S in the liver was significantly reduced in the DES-treated animals compared with the placebo group, p<0.05 (Figure 1 ). Immunoblotting revealed an ∼33-kDa band in liver (and ED). Hepatic AQP9 protein level was reduced in the DES group compared with the placebo group, p<0.001 (Figure 1). Immunostaining revealed intense AQP9 expression localized selectively to the basolateral membrane of the hepatocytes (Figure 2 ). Liver tissue from control animals revealed a homogeneous staining between the portal system and the central vein (CV), except for the hepatocytes in the periportal zone (PZ) that expressed less AQP9 protein (Figure 2, lower left panel). In the DES-exposed rats, PZ lacking staining had extended, whereas staining in the periacinous zone (closest to the CV) was similar to the control animals (Figure 2, lower right panel).
Figure 1.
Relative hepatic aquaporin (AQP) 9 mRNA and protein levels in control (open bars) and diethylstilbestrol (DES) -exposed (filled bars). Data are mean ± SD, n=7. Western blotting revealed a 33-kDa band with anti-AQP9 antibodies. *p<0.05.
Figure 2.
Immunostaining of AQP9 in the liver from rats neonatally exposed to DES (right upper and lower panels) or placebo (left upper and lower panels). Intense immunostaining was observed only on the sinusoidal membrane of the hepatocytes. CV represents the central vein (terminal hepatic vein) that drains the blood supplied from the portal system (P) with the portal vein and the hepatic artery. PZ represents the periportal zone. Bars: Upper panel = 100 μm; lower panel = 25 μm.
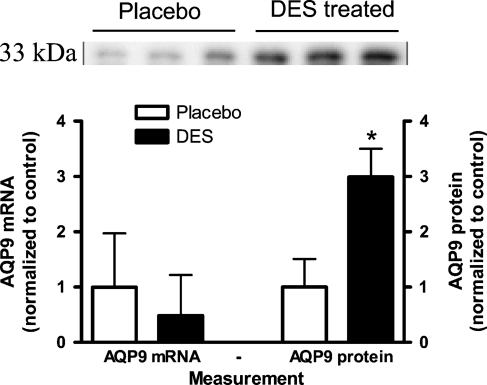
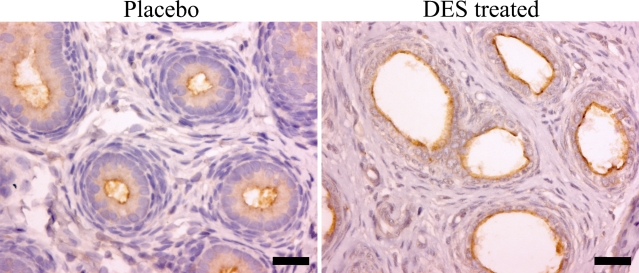
In the ED, AQP9 protein level was significantly increased by 300% in the DES-exposed group, p<0.01 (Figure 3 ). No labeling was detected using anti-AQP9 preabsorbed with the immunizing peptide. To ensure an even protein loading, actin labeling was performed, and no difference was found between the exposed and the control group (results not shown). In contrast to the AQP9 protein level, there was no change in AQP9 mRNA levels between groups (Figure 3). In Figure 4 the distribution of AQP9 in the ED is shown in control (left) and DES (right) -exposed animals. After DES treatment, ED were dilated with decreased epithelial cell height. AQP9 expression in the ED was confined to the epithelial cells; however, whether DES treatment had changed AQP9 expression was difficult to interpret from the immunohistochemical findings.
Figure 3.
Relative AQP9 mRNA and protein levels in control (open bars) and DES-exposed rats (filled bars) in the efferent ducts (ED). Data are mean ± SD, n=7. Western blotting revealed a 33-kDa band with anti-AQP9 antibodies. *p<0.05.
Figure 4.
Effects of DES on neonatal morphology and AQP9 staining of the epididymal ED. Representative patterns of immunostaining are shown for control rats (left panel) and rats treated with DES (right panel). DES-treated animals had enlargement of the ED lumen and reduced epithelial cell height. Bar = 25 μm.
Discussion
In this study we examined AQP9 expression in the liver and ED in rats after neonatal exposure to the synthetic estrogen, DES. Overall, we observed downregulation in the liver and upregulation in the ED. AQP9 expression in the liver was confined to the basolateral membrane of the hepatocytes in male rats as recognized by others (Elkjaer et al. 2000; Nihei et al. 2001; Huebert et al. 2002; Carbrey et al. 2003; Talbot et al. 2003). Interestingly, a recent study revealed that the tissue-specific expression seems to be sex linked: females had the strongest expression close to the CV (the periacinous zone), whereas males had a more homogeneous expression pattern in both the periacinous and PZs (Nicchia et al. 2001). Indeed, males had higher liver expression of AQP9 overall, at both the mRNA and protein level, compared with females. In the present study the decreased hepatic AQP9 expression of both mRNA and protein, as well as the decreased staining of AQP9 in the PZ observed after DES exposure, suggests a feminization of the treated animals compared with the controls. An estrogenic effect of DES is further supported by the lower testis weights.
In a recent study by Carreras et al. (2007), adult male Wistar rats were administered 5 mg ethinylestradiol/kg BW/day for 5 days to induce intrahepatic cholestasis. Treated animals had decreased BW and increased liver weight indicating a general toxic response, whereas ethinylestradiol-induced cholestasis did not seem to change the protein level or localization of AQP9. In comparison, the dose and timing used in this study were very different; we only exposed our rats to 4.8 μg DES/day, corresponding to ∼80 μg DES/kg BW/day for 18 days. We found no changes in either BW or liver weight, whereas both protein and mRNA AQP9 expression were downregulated, and the intrahepatic immunoexpression pattern had changed. Differences between findings of the two studies support the hypothesis that neonatal animals are more susceptible to estrogen-induced changes compared with adult animals.
Key results from AQP9 knockout mice revealed that these animals have increased plasma levels of glycerol and triglycerides compared with controls (Rojek et al. 2007). This provides evidence that AQP9 is important in hepatic glycerol metabolism.
Estrogen replacement in ovariectomized mice and virgin female rats has previously been reported to inhibit gluconeogenesis and increase glycogen storage in liver and muscle (Matute and Kalkhoff 1973; Ahmed-Sorour and Bailey 1981). Moreover, ovariectomized rats displayed increased basal plasma glucose levels that were normalized or even further decreased after estrogen replacement (Mandour et al. 1977; Ahmed-Sorour and Bailey 1980; Bailey and Ahmed-Sorour 1980). Further evidence for estrogen regulation of plasma glucose levels was found in male aromatase (converting androgens to estrogens) knockout mice that developed glucose intolerance and insulin resistance after 12 weeks (Takeda et al. 2003). In addition, ERα knockout mice had higher fasting blood glucose and plasma insulin compared with controls (Bryzgalova et al. 2006). Furthermore, DES, the synthetic estrogen used in this study, has previously been reported to inhibit glucagon release from the pancreas (Alonso-Magdalena et al. 2005). Glucagon plays at least two roles in the regulation of blood glucose by the liver: (1) activation of protein kinase and phosphorylase kinase leading to glucogen cleavage, thus increasing glucose availability and (2) glucagon regulates the expression of AQP7 in adipose tissue and AQP9 in hepatocytes. It has been proposed that glycerol is shuttled from adipocytes to the liver where it is a substrate for gluconeogenesis (Kuriyama et al. 2002). Accordingly, reduced glucagon release may be the mechanism whereby DES downregulates hepatic AQP9.
Specific downregulation of AQP9 in the PZ visualized by immunostaning may arise from a higher gluconeogenetic activity as previously shown in this particular tissue region (Jungermann and Kietzmann 2000). Together, estrogens regulate blood glucose levels at several points, and the downregulation of hepatic AQP9 in the PZ observed after DES exposure presumably results in inhibited glycerol influx with decreased gluconeogenesis.
ED has two principal functions, namely, sperm transport and fluid reabsorption. Approximately 90% of the luminal fluid from the rete testis is reabsorbed to concentrate sperm prior to its entering the epididymal lumen (Clulow et al. 1998). Recently, AQP9 knockout mice were bred and the homozygous AQP9−/− males were fertile with normal sperm motility and morphology (Rojek et al. 2007). Unfortunately, EDs were not examined and, even though fertility was normal, an upconcentration defect of the sperm (with diluted ED due to inhibited fluid reabsorption) may be possible. AQP1, also expressed in the ED, is susceptible to alterations in expression by neonatal estrogen treatment (Fisher et al. 1998,1999), and Fisher et al. (1998) found decreased AQP1 expression, epithelial cell height, and testis size particularly on PND 18 and 25 after neonatal DES exposure in rats. Our experiments were performed with comparable dose and time exposure to DES as used by Fisher et al. (1998) to investigate possible concurrent changes in AQP9 expression. Animals were sacrificed on PND 20, and it has previously been shown that rats aged 21 days have an AQP9 expression pattern similar to that of adult rats (Badran and Hermo 2002).
AQP9 seems to be of great importance in fluid transport regulated by ER in the reproductive system. In females, AQP9 immunoreactivity of the oviducts was lost after ovariectomy, whereas replacement with estradiol or estradiol and progesterone restored immunoreactivity and increased both protein and mRNA levels of AQP9 (Branes et al. 2005). In the ED of the male reproductive tract, we found a 300% increase in AQP9 protein expression in DES-treated animals compared with controls, whereas mRNA level was similar in the two groups. Anti-AQP9 staining was observed in the epithelial cells facing the tubuli lumen and no clear up- or downregulation was observed after DES treatment. Nonetheless, the dilated lumen of the DES-treated animals (as described by others) (Fisher et al. 1998) may increase the area where AQP9 is expressed, which supports the observed upregulated protein level. The markedly dilated ED are also observed in ERα knockout mice and are due to inhibited fluid reabsorption causing increased intratubular pressure (Eddy et al. 1996; Hess et al. 1997a). Discrepancy in protein and mRNA expression levels may be explained by a decreased turnover of AQP9 protein or by increased likelihood of the epitopes to be recognized by the AQP9 antibodies in the DES-treated animals compared with the control. Given the striking increase in protein expression and the large SD values in mRNA measurements, a true increase in AQP9 expression after neonatal DES exposure is likely. Supportive data from adult rats include that decreased AQP9 expression due to castration was alleviated by estradiol, whereas anti-estrogen treatment reduced AQP9 expression (Oliveira et al. 2005; Picciarelli-Lima et al. 2006). Concurrently, anti-estrogen exposure decreased ERα expression, supporting a direct regulation of AQP9 by estrogens (Oliveira et al. 2003). These findings, along with previous findings, indicate that the estrogenic regulation of AQP9 is important throughout life.
In conclusion, estrogens upregulate AQP9 channels in the ED after neonatal DES exposure; however, this may be attributed to a widening of the ED lumen caused by inhibited fluid reabsorption and increased intratubular pressure. In the liver, neonatal DES exposure caused a downregulation of AQP9 channels, particularly in the periacinous zone. Estrogens regulate blood glucose levels, and a downregulation in hepatic AQP9 may result in reduced glycerol influx, decreased gluconeogenesis, and decreased blood glucose level.
Acknowledgments
Financial support for this study was received from the A.P. Møller Foundation for the Advancement of Medical Science and the Torben and Alice Frimodts Foundation.
The authors thank Majken Dalgaard, Institute of Food Safety and Toxicology, Danish Veterinary and Food Administration, Denmark and Anne Andersen and Claus Bøgelund, National University Hospital, Denmark for help with immunohistochemical procedures. We also acknowledge Amer Mujezinovic, Iben Nielsen, Louise Frandsen, and Malene Torp, Department of Pharmacology, University of Copenhagen, Denmark for expert technical assistance.
References
- Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, et al. (2002) Aquaporin water channels—from atomic structure to clinical medicine. J Physiol 542:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed-Sorour H, Bailey CJ (1980) Role of ovarian hormones in the long-term control of glucose homeostasis. Interaction with insulin, glucagon and epinephrine. Horm Res 13:396–403 [DOI] [PubMed] [Google Scholar]
- Ahmed-Sorour H, Bailey CJ (1981) Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation and gluconeogenesis. Ann Nutr Metab 25:208–212 [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson AM (2006) The sensitivity of the child to sex steroids: possible impact of exogenous estrogens. Hum Reprod Update 12:341–349 [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A (2005) Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect 113:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Williams K, Turner KJ, Fisher JS, Saunders PT, Millar MR, et al. (2001) Age-, cell- and region-specific immunoexpression of estrogen receptor α (but not estrogen receptor β) during postnatal development of the epididymis and vas deferens of the rat and disruption of this pattern by neonatal treatment with diethylstilbestrol. Endocrinology 142:874–886 [DOI] [PubMed] [Google Scholar]
- Badaut J, Hirt L, Granziera C, Bogousslavsky J, Magistretti PJ, Regli L (2001) Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab 21:477–482 [DOI] [PubMed] [Google Scholar]
- Badran HH, Hermo LS (2002) Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J Androl 23:358–373 [PubMed] [Google Scholar]
- Bailey CJ, Ahmed-Sorour H (1980) Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia 19:475–481 [DOI] [PubMed] [Google Scholar]
- Branes MC, Morales B, Rios M, Villalon MJ (2005) Regulation of the immunoexpression of aquaporin 9 by ovarian hormones in the rat oviductal epithelium. Am J Physiol Cell Physiol 288:C1048–1057 [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, et al. (2006) Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49:588–597 [DOI] [PubMed] [Google Scholar]
- Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P (2003) Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA 100:2945–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras FI, Lehmann GL, Ferri D, Tioni MF, Calamita G, Marinelli RA (2007) Defective hepatocyte aquaporin-8 expression and reduced canalicular membrane water permeability in estrogen-induced cholestasis. Am J Physiol Gastrointest Liver Physiol 292:G905–912 [DOI] [PubMed] [Google Scholar]
- Castle NA (2005) Aquaporins as targets for drug discovery. Drug Discov Today 10:485–493 [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA, Man SY (1998) Fluid and electrolyte reabsorption in the ductuli efferentes testis. J Reprod Fertil Suppl 53:1–14 [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS (1996) Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137:4796–4805 [DOI] [PubMed] [Google Scholar]
- Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, Frokiaer J, et al. (2000) Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun 276:1118–1128 [DOI] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Brown D, Sharpe RM (1999) Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environ Health Perspect 107:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Fraser HM, Saunders PT, Brown D, Sharpe RM (1998) Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology 139:3935–3945 [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB (1997a) A role for oestrogens in the male reproductive system. Nature 390:509–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, et al. (1997b) Estrogen receptor (α and β) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl 18:602–611 [PubMed] [Google Scholar]
- Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF (2002) Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J Biol Chem 277:22710–22717 [DOI] [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T (2000) Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology 31:255–260 [DOI] [PubMed] [Google Scholar]
- Ko SB, Uchida S, Naruse S, Kuwahara M, Ishibashi K, Marumo F, Hayakawa T, et al. (1999) Cloning and functional expression of rAOP9L a new member of aquaporin family from rat liver. Biochem Mol Biol Int 47:309–318 [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, Maeda N, et al. (2002) Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 51:2915–2921 [DOI] [PubMed] [Google Scholar]
- Mandour T, Kissebah AH, Wynn V (1977) Mechanism of oestrogen and progesterone effects on lipid and carbohydrate metabolism: alteration in the insulin:glucagon molar ratio and hepatic enzyme activity. Eur J Clin Invest 7:181–187 [DOI] [PubMed] [Google Scholar]
- Matute ML, Kalkhoff RK (1973) Sex steroid influence on hepatic gluconeogenesis and glucogen formation. Endocrinology 92:762–768 [DOI] [PubMed] [Google Scholar]
- McKinnell C, Atanassova N, Williams K, Fisher JS, Walker M, Turner KJ, Saunders TK, et al. (2001) Suppression of androgen action and the induction of gross abnormalities of the reproductive tract in male rats treated neonatally with diethylstilbestrol. J Androl 22:323–338 [PubMed] [Google Scholar]
- Nicchia GP, Frigeri A, Nico B, Ribatti D, Svelto M (2001) Tissue distribution and membrane localization of aquaporin-9 water channel: evidence for sex-linked differences in liver. J Histochem Cytochem 49:1547–1556 [DOI] [PubMed] [Google Scholar]
- Nihei K, Koyama Y, Tani T, Yaoita E, Ohshiro K, Adhikary LP, Kurosaki I, et al. (2001) Immunolocalization of aquaporin-9 in rat hepatocytes and Leydig cells. Arch Histol Cytol 64:81–88 [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hermo L, Hess RA (2005) Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell 97:385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Nie R, Carnes K, Franca LR, Prins GS, Saunders PT, Hess RA (2003) The antiestrogen ICI 182,780 decreases the expression of estrogen receptor-alpha but has no effect on estrogen receptor-beta and androgen receptor in rat efferent ductules. Reprod Biol Endocrinol 1:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler N, Bagnis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, Breton S, et al. (2001) Aquaporin 9 expression along the male reproductive tract. Biol Reprod 65:384–393 [DOI] [PubMed] [Google Scholar]
- Picciarelli-Lima P, Oliveira AG, Reis AM, Kalapothakis E, Mahecha GA, Hess RA, Oliveira CA (2006) Effects of 3-beta-diol, an androgen metabolite with intrinsic estrogen-like effects, in modulating the aquaporin-9 expression in the rat efferent ductules. Reprod Biol Endocrinol 4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88:11110–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, et al. (2007) Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci USA 104:3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM (2006) Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab 20:91–110 [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE (1993) Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341:1392–1395 [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978 [DOI] [PubMed] [Google Scholar]
- Sun EL, Flickinger CJ (1979) Development of cell types and of regional differences in the postnatal rat epididymis. Am J Anat 154:27–55 [DOI] [PubMed] [Google Scholar]
- Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, et al. (2003) Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol 176:237–246 [DOI] [PubMed] [Google Scholar]
- Talbot NC, Garrett WM, Caperna TJ (2003) Analysis of the expression of aquaporin-1 and aquaporin-9 in pig liver tissue: comparison with rat liver tissue. Cells Tissues Organs 174:117–128 [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, van Hoek AN, et al. (1998) Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem 273:24737–24743 [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA (1999) Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol 277:F685–696 [DOI] [PubMed] [Google Scholar]