Abstract
Experimental data suggest that the endogenous cannabinoid system is involved in gastric function in different animal species. In most of them, CB1 receptors have been localized on vagal terminals innervating the external wall of the stomach. We aimed at studying the putative presence and distribution of these receptors in the human gastric mucosa. To this end, we first performed Western blotting, RT-PCR, in situ hybridization, and immunohistochemical analysis of CB1 protein distribution in biopsy samples of healthy individuals. To determine the precise cell populations expressing CB1 receptors, we performed double immunofluorescence plus confocal microscopy analysis of the same samples. Our results show that CB1 receptors are present in the gastric epithelium of the mucosa. Specifically, they are expressed by a subpopulation of mucosal cells, the acid-secreting parietal cells, as shown by double immunohistochemical staining and by their differential abundance in subregions of the gastric mucosa. These results reinforce the notion of a prominent role for the endocannabinoid system in the gastric function in humans and postulate the use of cannabinoid CB1 receptors in parietal cells as new therapeutic targets for the regulation of gastric acid production. (J Histochem Cytochem 56:511–516, 2008)
Keywords: cannabinoids, receptor, acid secretion, parietal cell
Cannabinoid CB1 receptors belong to the superfamily of G protein–coupled membrane receptors, and their activation leads to inhibition of adenylate cyclase activity, thus decreasing the cytoplasmic levels of cAMP, and to a decrease in calcium (Ca2+) entry into cells through the inhibition of Ca2+ channels of the L, N, and T types (for a review, see Howlett et al. 2002).
CB1-mediated effects are being studied in a number of paradigms, mainly at the vascular and central nervous system (CNS) levels. However, growing evidence supports the idea that the endocannabinoid system (ECS) (constituted by receptors, endogenous ligands, and enzymes responsible for their synthesis and degradation) plays a prominent role in other physiological systems. Among them, gastrointestinal function is one of the most relevant, because cannabinoids have been long known to modify several digestive functions, such as gastric emptying, appetite, and intestinal motility (Pertwee 2001; Coutts and Izzo 2004). These actions are exerted through receptors located in the CNS (i.e., hypothalamic nuclei) and in the periphery (Izzo et al. 2001; Pertwee 2001).
The distribution and pathophysiological actions of CB1 receptors in the digestive tract have been studied mostly in rodents, although there are also data from pigs, guinea pigs, and humans (Kulkarni-Narla and Brown 2000; Coutts et al. 2002). Most of this research has been focused on the role of the ECS at the enteric level, providing valuable information on the anti-inflammatory, antisecretory, and anticontractile effects of exogenous and endogenous cannabinoids (for a recent review, see Coutts and Izzo 2004). However, few data exist at the gastric level. Adami et al. (2002) have shown that, in the rat, CB1 receptors are present in pre- and postganglionic cholinergic neural elements innervating smooth muscle, mucosal, and submucosal blood vessels. These authors reported an inhibitory role for these receptors in 2-deoxy-glucose and pentagastrin-induced gastric acid secretion (Adami et al. 2002). Furthermore, this group has very recently reported that peripherally located CB1 receptors are responsible for this antisecretory role in the rat, with a higher influence than that from those located in the CNS (Adami et al. 2004).
More recently, Burdyga et al. (2004) provided functional evidence on the possible influence of these receptors in appetite control in the rat and in humans at the gastric and intestinal levels. These authors indicated that CB1 receptors selectively located in vagal terminals are upregulated as a consequence of the fasting state. Furthermore, cholecystokinin (a well-known mediator of satiety) was shown to inhibit this increase in CB1 receptor expression both in the rat and human nodose ganglia. Interestingly, these authors did not report on the distribution of CB1 receptors in the human mucosa, because their study was restricted to neural elements of the gastric wall. Taken together, their results are highly suggestive of a critical role of CB1 receptors in the peripheral control of the appetite stimulus, because changes in CB1 expression may modulate vagal nerve function, and expand previous data on the possible role of the ECS at the intestinal level in the rat (Gomez et al. 2002).
We focused on the study of the location of CB1 receptors in the human gastric mucosa to identify the precise cellular elements that contain these receptors. Our results indicate that CB1 receptors are present in parietal cells of the human gastric mucosa and can be postulated as possible pharmacological targets for the modulation of acid secretion.
Materials and Methods
Subjects and Samples
Twenty patients (8 men and 12 women of middle age) with dyspeptic symptoms referred to our endoscopy unit for upper gastrointestinal endoscopic examination were invited to participate in this study. Two biopsy samples from the antral mucosa and two samples from the corporal mucosa were obtained for histopathological study by using a disposable radial jaw forceps (Boston Scientific Iberica; Barcelona, Spain). Only samples from patients with a normal endoscopic examination, normal histopathological study, and absence of colonization by Helicobacter pylori were included in this study. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was reviewed and approved by the local ethics committee. Written information about the nature and purpose of this study was provided to all participants, and written consent was obtained from all of them. Gastric tissue from each patient was separated and processed for freezing (Western blot, in situ hybridization, and RT-PCR) or for fixation (immunohistochemistry and immunofluorescence). Fixation was done by immersion in 4% buffered formaldehyde; tissues were paraffin embedded and cut on a Leica microtome (Leica; Wetzlar, Germany).
Immunohistochemistry
Tissue sections (3 μm thick) were washed in 50 mM potassium PBS (KPBS), and endogenous peroxidase was blocked by incubation in peroxidase-blocking solution (Dako; Copenhagen, Denmark) for 30 min at room temperature. The antibody used was the polyclonal CB1 receptor (1:2000; ABR, Golden, CO) at 4C overnight. After incubation with the primary antibody, the avidin-biotin-peroxidase complex method (Vector Elite; Burlingame, CA) was used, and a visible reaction product was produced by treating the sections with DAB (Dako). Controls for the immunohistochemistry included preabsorption and coincubation of the antibody with the corresponding immunogenic protein (fusion protein against amino acids 1–100 of human-CB1 at 5 μg/ml) and incubation in the absence of primary antibody. Sections were sealed with coverslips, and observations and photography were carried out using a Nikon 90i microscope and DXM1200F camera (Nikon; Haarlem, The Netherlands).
Immunofluorescence and Confocal Microscopy
To obtain complete identification of the cellular type exhibiting CB1 receptor immunostaining, double-labeling studies were performed. Tissue sections were washed in TBS and incubated with the corresponding primary antibodies: polyclonal CB1 receptor (1:2000; ABR), monoclonal H+/K+-ATPase antibody (1:1000; Calbiochem, Merck Chemicals, Darmstadt, Germany), monoclonal chromogranin A (1:50; Dako), and monoclonal synaptophysin (1:50; Dako) at 4C overnight. Sections were washed in TBS and incubated with Alexa 546 anti-mouse antibody conjugate and Alexa 488 anti-rabbit antibody conjugate (10 μg/ml; Molecular Probes, Eugene, OR) at 37C for 2 hr. Sections were mounted in aqueous medium with Vectashield (Vector). Visualization of the samples was performed with a confocal Nikon Eclipse C1 coupled to a Nikon 90i upright microscope.
Western Blotting
The protocol used was basically as previously described (Benito et al. 2003), with slight modifications. Biopsy samples of the gastric mucosa were homogenized in M-PER mammalian protein extraction reagent (1 g of tissue/10 ml; Pierce Chemical, Rockford, IL). Protein extract (20 μg) was reduced, denatured, and separated by electrophoresis. After separation, the proteins in the gel were transferred to polyvinylidene fluoride membrane (Millipore; Bedford, MA). Remaining binding sites on the membrane were blocked by overnight incubation in PBS–Tween (PBST) containing 4% nonfat dried milk at 4C. Primary antibody incubation was carried out at in a 1:500 dilution in PBST containing 4% nonfat dried milk for 2.5 hr at 30C. In some experiments, the antibody was preincubated with 8 μg/ml of the same immunizing peptide used for the generation of the antibody. After washing with PBST, the nitrocellulose membrane was incubated with an alkaline phosphatase (AP)-conjugated goat anti-rabbit secondary antibody (Sigma; Barcelona, Spain) in a dilution of 1:2000 in PBST containing 4% nonfat dried milk for 2 hr at 30C. The nitrocellulose membrane was washed extensively with PBST, followed by PBS. Finally, the immune complex was visualized by incubating in the presence of nitroblue tetrazolium (NBT)-5-bromo-4-chloro-3-indolyl-phosphate (BCIP) chromogen (PerkinElmer Life Sciences; Boston, MA).
RT and Real-time PCR
Frozen tissues were homogenized in RNATidy reagent (Applichem; Darmstadt, Germany), and after RNA extraction, samples were treated with DNAase (Roche Diagnostics; Barcelona, Spain) to eliminate possible DNA contamination. In each case, 1 μg of total RNA was transcribed into cDNA using the First Strand cDNA Synthesis kit for RT-PCR (AMV; Roche Diagnostics). The reaction mixture was kept frozen at –80C until enzymatic amplification. Quantitative PCR was performed in a LightCycler (Roche Diagnostics) with LightCycler FastStart DNA Master Sybr GreenI (Roche Diagnostics), 2 μl of RT reaction, and 0.5 μM of the specific primers for CB1 receptor. Initial denaturation at 95C for 10 min was followed by 40 cycles with denaturation at 95C for 10 s, annealing at 65C for 5 s, and elongation at 72C for 8 s. The following primers were used for the RT-PCR analysis: forward, 5′-CAAGCCCGCATGGACATTAGGTTA-3′; reverse, 5′-TCCGAGTCCCCCATGCTGTTATC-3′. The length of the resulting RT-PCR fragment was 198 bp. To verify the specificity of the technique, we also included some samples of mRNA extracted from human cerebella (kindly provided by the Banco de Tejidos para Investigación Neurológica de Madrid), which is known to exhibit high expression levels of CB1 receptors.
In Situ Hybridization
CB1 mRNA detection was performed using digoxigenin (DIG)-labeled antisense riboprobes on paraffin-embedded tissue sections from the same cases used in the immunohistochemical studies. RNA probes were constructed from linearized cDNA (kindly supplied by Dr. M. Parmentier, Insititute de Recherche Interdisciplinaire, Faculté de Médecine, Brussels, Belgium) encoding the human CB1 receptor. Control slides consisted of serial sections hybridized with DIG-labeled sense probe.
The protocol used here has been described elsewhere (Hermann et al. 2002). Briefly, the slides with human tissue sections were rehydrated and washed in PBS containing 136 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4. Endogenous peroxidase was quenched by incubation for 15 min in 1% H2O2 in 100% methanol; slides were rinsed twice in PBS and treated with proteinase K (20 μg/ml; Roche Diagnostics) in 50 mM Tris-HCL and 5 mM EDTA (pH 8.0) for 10 min at 37C. Acetylation was performed by incubation in 0.1 M triethanolamine (pH 8.0) with 0.25% acetic anhydride for 10 min at room temperature. After washes in PBS, tissues were dehydrated in graded series of ethanol (50%, 70%, 80%, 95%, and 100%) and air-dried. Hybridization was carried out using an hybridization buffer [50% deionized formamide, 20 mM Tris-HCl (pH 8.0), 0.3 M NaCl, 5 mM EDTA (pH 8.0), 10% dextran sulfate, 1× Denhardt solution, 0.5 mg/ml tRNA (Roche Diagnostics), 0.2 mg/ml salmon sperm DNA, 200 mM dithiothreitol, and 0.5 μg/ml DIG-labeled probe]. Before applying to the tissue, the hybridization cocktail was denatured for 2 min at 95C. Slides were incubated overnight at 50C in a humidified chamber.
After hybridization, sections were washed in 2× SSC (1× SSC contains 150 mM NaCl and 15 mM sodium citrate, pH 7.4) briefly at room temperature. Afterward, four high-stringency washes were carried out at 60C with 5× SSC and 0.05% Tween-20 and then with 50% formamide, 2× SSC, and 0.05% Tween-20, with 50% formamide, 1× SSC, and 0.05% Tween-20, and finally with 0.1× SSC and 0.05% Tween-20. Afterward, intracellular alkaline phosphatase was destroyed by incubation with 15 mM iodoacetamide in 0.5 M NaCl, 10 mM Tris-HCl (pH 8.0), 5 mM EDTA, and 0.05% Tween-20. Detection of the digoxigenin label was performed using a rabbit anti-digoxigenin antibody coupled to horseradish peroxidase (1:1000 dilution in blocking buffer; TSA-Plus DNP-AP System, PerkinElmer Life Sciences) followed by Tyramide signal amplification (TSA-Plus DNP-AP System; PerkinElmer Life Sciences). A visible reaction product was visualized by incubation with BCIP and NBT for 15 min. Slides were washed, mounted (Faramount; Dako), and sealed with nail polish.
Results
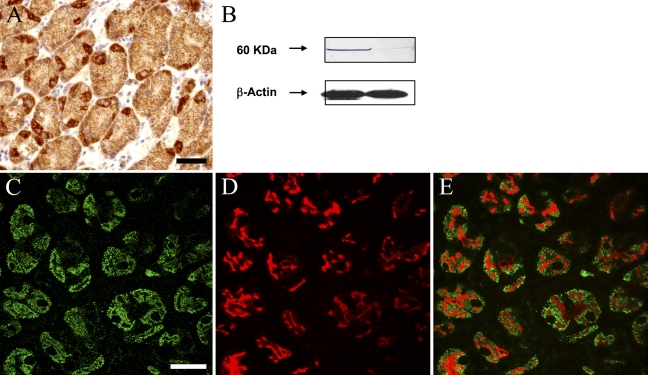
Several technical approaches were used to verify the presence of CB1 receptors on parietal cells of the human stomach. First, immunohistochemical staining showed a clear, well-defined pattern of staining, coincident with selective cells within gastric glands, with disposition and typical morphology of parietal cells (Figure 1A ). The signal was limited to cytoplasmic areas of these cells, leaving the round-shaped, large-sized cell nuclei devoid of any staining (Figure 1). The absence of primary antibody and the preabsorption and coincubation with the immunizing peptide completely prevented any staining (data not shown).
Figure 1.
Cannabinoid CB1 receptors are present in parietal cells of the human gastric mucosa. (A) Immunohistochemical localization of CB1 receptors in selective cells of human gastric glands. Note that only a small number of glandular cells show strong immunoreactivity for CB1 receptors. These cells exhibit a large-sized, centered nucleus, and the staining is restricted to cytoplasmic and membrane regions. (B) Representative Western blot of a donor in which gastric samples from corporal (left lane) and antral mucosa (right lane) were used. Note the intense 60-kDa band on the samples from corporal mucosa (a region known to contain high amounts of acid-secreting parietal cells) compared with the almost absent band on samples from the antral region (known to be devoid of this type cell). (C,D) Confocal images showing immunofluorescent colocalization of CB1 immunoreactivity (C) and H+/K+ ATPase immunoreactivity (D) in human biopsies of gastric mucosa. As expected, CB1 receptors exhibit a membrane and cytoplasmic distribution, whereas the transporter is limited to cytoplasmic vesicles. Note the match between cells expressing both phenotypic markers (merged image, E) that indicate that parietal cells are the only cell type of the gastric mucosa expressing cannabinoid CB1 receptors. Markers for other cell populations of the human gastric mucosa showed no colocalization with CB1 receptors (data not shown). Bars: A = 100 μm; C–E = 50 μm.
Second, Western blots from human biopsies of the gastric mucosa showed a single band of ∼60 kDa, consistent with the expected CB1 protein molecular mass (Figure 1B). It is important to note that CB1 protein levels were significantly higher in biopsy samples from the body of the stomach (a region enriched with acid-secreting parietal cells) compared with those in samples from the antral region (where almost no parietal cells are present; Figure 1B). The presence of the immunizing peptide completely abolished the signal, thus verifying the specificity of the antibody (data not shown).
Third, because glands belonging to the gastric mucosa are composed of diverse cell types, we sought to define the specific CB1-positive cell type. To that end, we performed double immunohistochemical stainings with specific markers for the main cellular types of the human gastric mucosa: H+/K+-ATPase for parietal cells and chromogranin A and synaptophysin for endocrine cells. From our observations, it can be concluded that only acid-secreting parietal cells express CB1 receptors, because CB1 immunoreactivity was sharply limited to H+/K+-ATPase–positive cells (Figures 1D and 1E). Interestingly, CB1 receptors exhibited an homogeneous distribution throughout the cell cytoplasm, whereas the transporter was limited to vesicles. This distribution is characteristic of acid-secreting cells in a resting state, because the H+/K+-ATPase is sequestered in a population of cytoplasmic vesicles called tubulovesicles that fuse with the apical secretory membrane on activation of acid secretion (Carmosino et al. 2000; Yao and Forte 2003). No colocalization was observed for the other markers (data not shown). Taken together, these data indicate that acid-secreting parietal cells of the human gastric mucosa are the only cell type that express cannabinoid receptors of the CB1 type.
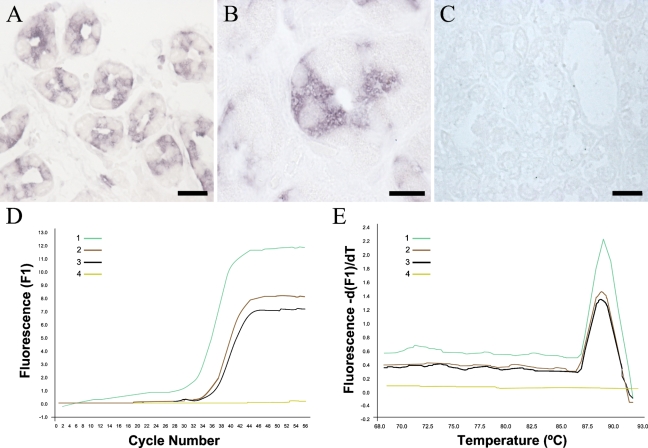
Finally, CB1 mRNA was detected in specific cells of gastric glands (Figure 2 ) by in situ hybridization and by RT followed by PCR amplification. Thus, discrete cells of gastric glands showed a strong signal for CB1 mRNA in density and distribution similar to those of parietal cells (Figures 2A and 2B). Serial sections hybridized with a DIG-labeled sense probe showed no labeling, thus corroborating the specificity of the signal (Figure 2C). In addition, RT-PCR showed CB1 mRNA in biopsies of human gastric epithelia at lower levels than those obtained in human cerebellum (Figure 2D). Melting curves showed the specificity of the amplified fragment corresponded to the human CB1 receptor (Figure 2E).
Figure 2.
CB1 mRNA is present in epithelial cells of the human gastric mucosa. (A,B) Hybridization with the antisense probe for CB1 mRNA followed by Tyramide signal amplification showed a strong signal in selective cells of the glandular epithelium, with morphological features coincident with the CB1-positive cells shown in Figure 1. Note the selective cytoplasmic distribution of the labeling, with nuclei lacking any staining. (C) Hybridization with the sense probe produced no significant staining. (D) Amplification curves of CB1 mRNA by RT-PCR in human cerebellum (Sample 1, positive control), human biopsies of gastric epithelium (Samples 2 and 3), and water (Sample 4, negative control). (E) Melting curves of the same samples as in D. Bars: A,C = 100 μm; B = 50 μm.
Discussion
The inhibitory action of cannabinoids on gastric acid secretion has been known for many years (for a review, see Pertwee 2001). Thus far, this effect has been attributed to receptors localized in vagal nerve terminals innervating the external gastric wall and has been observed in different animal species, including humans. Our data suggest that CB1 receptors located in acid-secreting parietal cells could also account for this effect and that the ECS might play a more relevant role in human gastric pathophysiology than previously thought.
Acid secretion is subjected to strict regulation at different levels, including specific brain nuclei, vagal terminals, and mucosal cells (Schubert 2003). Our data expand our current knowledge on CB1 receptor distribution to that already known in CNS structures and cholinergic nerve terminals and suggest that they might therefore participate in the control of acid secretion at each of those levels. Furthermore, they allow us to postulate the use of cannabinoid agonists and/or antagonists as new therapeutic agents for acid-related gastric diseases (Pertwee 2001). Unfortunately, to our knowledge, no data on the status of CB1 receptors in human gastric pathology have been reported thus far.
Gastric acid production is a well-known physiological process that plays a key role in food digestion and that, if disarranged, leads to a variety of damaging events that may ultimately affect the integrity of the gastric wall (Schubert 2003). Acid production and release are carried out by a specific cell type of the mucosa, the parietal cell, and are known to be triggered by several stimuli. Histamine is, by far, the most powerful stimulant of acid secretion because it binds and activates histamine H2 receptors located on parietal cells. Acetylcholine and gastrin, acting through muscarinic M3 and CCK2 receptors, respectively, are also important mediators of this process. Interestingly, whereas H2 effect is mediated through the increase of cAMP levels, M3 and CCK2 potentiate acid secretion by modifying Ca2+ intracellular levels (Olbe et al. 2003). As mentioned in the Introduction, CB1 activation is known to decrease both intracellular cAMP and Ca2+ levels; thus, it seems reasonable to speculate that CB1 agonists may inhibit acid secretion by directly acting on parietal cells through the dampening of these signal transduction pathways.
It is important to note that current treatment of gastric ulcers and other acid-related diseases such as esophageal reflux are based on acid secretion inhibition by H2 receptors antagonists or by proton pump inhibitors (Olbe et al. 2003). In both cases, parietal cells are the cellular substrate for these therapeutic effects. With the exception of somatostatin receptor 2 (Allen et al. 2002), no other membrane receptor capable of directly inhibiting parietal cell acid secretion has been described.
Finally, the inhibitory effect of cannabinoids on gastric motility is well known. This effect is probably caused by the activation of CB1 receptors located on nerve terminals innervating the digestive tract (Coutts and Izzo 2004). The data reported herein open the possibility to dissociate cannabinoid effects on gastric secretion and gastric motility through the study of already available compounds or the development of new cannabinoid agonists and/or antagonists with local, restricted effects on parietal cells.
Acknowledgments
The technical support of Montserrat Díaz-Meco and the assistant work of Julia Molina are gratefully acknowledged. The authors declare no competing interests.
References
- Adami M, Frati P, Bertini S, Kulkarni-Narla A, Brown DR, de Caro G, Coruzzi G, et al. (2002) Gastric antisecretory role and immunohistochemical localization of cannabinoid receptors in the rat stomach. Br J Pharmacol 135:1598–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami M, Zamfirova R, Sotirov E, Tashev R, Dobrinova Y, Todorov S, Coruzzi G (2004) Gastric antisecretory effects of synthetic cannabinoids after central or peripheral administration in the rat. Brain Res Bull 64:357–361 [DOI] [PubMed] [Google Scholar]
- Allen JP, Canty AJ, Schulz S, Humphrey PP, Emson PC, Young HM (2002) Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J Comp Neurol 454:329–340 [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J (2003) Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci 23:11136–11141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ (2004) Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 24:2708–2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmosino M, Procino G, Casavola V, Svelto M, Valenti G (2000) The cultured human gastric cells HGT-1 express the principal transporters involved in acid secretion. Pflugers Arch 440:871–880 [DOI] [PubMed] [Google Scholar]
- Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S (2002) Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol 448:410–422 [DOI] [PubMed] [Google Scholar]
- Coutts AA, Izzo AA (2004) The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol 4:572–579 [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, et al. (2002) A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22:9612–9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B (2002) Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109:451–460 [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202 [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Capasso F (2001) The gastrointestinal pharmacology of cannabinoids. Curr Opin Pharmacol 1:597–603 [DOI] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Brown DR (2000) Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res 302:73–80 [DOI] [PubMed] [Google Scholar]
- Olbe L, Carlsson E, Lindberg P (2003) A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov 2:132–139 [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2001) Cannabinoids and the gastrointestinal tract. Gut 48:859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert ML (2003) Gastric secretion. Curr Opin Gastroenterol 19:519–525 [DOI] [PubMed] [Google Scholar]
- Yao X, Forte JG (2003) Cell biology of acid secretion by the parietal cell. Annu Rev Physiol 65:103–131 [DOI] [PubMed] [Google Scholar]