Abstract
Background
Brachial artery distensibility (BrachD) was measured in healthy children to identify associations with atherosclerotic risk factors.
Methods
969 black and white subjects 13-22 years were classified as lean (L) or overweight (O) and hyperinsulinemic (H-I) or normoinsulinemic (N-I). BP and BrachD were obtained with a DynaPulse Pathway instrument. ANOVA was performed looking for group mean differences. Correlations between BrachD and risk variables were examined. Determinates of BrachD were determined by backward elimination regression stratified by BMI-Insulin group.
Results
Decreased BrachD correlated with male gender, O, higher BP, HR, fasting glucose and log of fasting insulin after adjusting for pulse pressure (PP). BrachD was greatest in L/N-I with progressive decreases seen in L/H-I, O/N-I, and O/H-I subjects. Regression modeling found PP and HR were major determinates of BrachD. Glucose was significant for subjects with N-I regardless of adiposity. Excluding BP, glucose remained important in N-I subjects. Gender was significant for all. HR retained significance only in O subjects regardless of insulin level.
Conclusions
In healthy adolescents, hyperinsulinemia and obesity adversely affect brachial artery function with overweight contributing to a greater degree. In normoinsulinemic subjects, fasting glucose was inversely related to BrachD. Metabolic factors may play a role in vascular function in youth.
Keywords: Elasticity, Obesity, Insulin, Pediatrics
Introduction
Atherosclerotic vascular disease (AVD) is the major cause of mortality in the United States (http://www.cdc.gov/nccdphp/burdenbook2004).[1] Autopsy studies have demonstrated that AVD can begin in adolescence.[2, 3] Recent reports have focused on risk factor profiles associated with the development of AVD in adolescents and young adults.[2, 4] However, few studies have directly measured vascular function. Such studies would add substantially to our understanding of the development and progression of AVD especially in this young population in whom clinical manifestations of AVD are rare.
Brachial artery distensibility (BrachD), a reproducible,[5] validated non-invasive measure of arterial function,[6, 7] has been linked to the development and progression of AVD in adults. Stiffer brachial vessels are found in subjects with increased coronary artery calcium[8] and those with established congestive heart failure.[9] Decreased brachial artery distensibility is also found in adults with type 2 diabetes,[10] and is associated with CV risk factors such as obesity,[11] and hypertension.[5, 12, 13] In children and adolescents, studies have related decreased brachial flow mediated dilation, a measure of endothelial function, to hypercholesterolemia,[14] obesity[15, 16] and diabetes.[17] However, there have been very few studies relating risk factors for AVD to arterial stiffness in youth, and only one study using brachial artery distensibility as the primary endpoint. In this recent study, Whincup and coworkers demonstrated an inverse relationship between brachial distensibility and BP, adiposity, and fasting insulin in adolescents.[18] This important study suggests that conventional AVD risk factors are important in the first steps of vascular disease. However, there were several limitations to this study including the method of subject selection, lack of ethnic diversity, smaller sample size and less standardized data collection technique may limit the generalizability of these results. Therefore, we measured BrachD in a large population of healthy bi-racial adolescents and young adults to define the distribution of BrachD and identify associations between levels of BrachD and known risk factors for the development of AVD including overweight, increased blood pressure, elevated fasting plasma glucose, and hyperinsulinemia. Confirming and extending the observations of previous investigators may prove that arterial distensibility is a powerful marker of early CV compromise relating to obesity and the metabolic disturbances it induces.
Methods
Study Population
The study population consisted of 969 subjects aged 13-22 years (mean age = 17.8 years) who were part of the ongoing Princeton School District (PSD) study, a longitudinal, population based study of the natural history of obesity, insulin resistance and diabetes in a large urban-suburban school district in Cincinnati.[19] To enter PSD, subjects had to be in the 5th through 12th grades in 2001, have no known chronic disease, and be receiving no medication known to affect carbohydrate metabolism. The 969 subjects included in these analyses were a sample of the cohort that was examined in 2004. The age of the sample was older than the entire cohort (42% were 17 years or younger in the sample versus 82% in the entire cohort, p< 0.0001) since only high school students were examined during this time frame. There was no significant difference in race with 48% of the sample Caucasian versus 50% of the cohort. No gender differences were present either as the sample was 55% female while girls comprised 49% of the cohort. Ninety Five percent of the subjects had completed puberty at the time of the study. The current analyses include data that were collected approximately 4 years after the baseline examination. Pregnant females were excluded from the study. All subjects had fasting plasma glucose < 100 mg/dl (5.5 mmol/L).
The protocol was reviewed and approved by the Institutional Review Board at Cincinnati Children’s Hospital. Written informed consent was obtained from the participant if the subject was > 18 years of age or from the parent or guardian if the participant was < 18 years of age. Written assent was obtained from all participants > 11 years of age but < 18 years of age
Anthropometrics
After written informed consent was obtained, two measures of height were obtained with a portable stadiometer (RoadRod model; Quick Medical, North Bend, WA or Accustat, Genentech) by trained personnel. Weight was also measured twice and averaged using a digital scale (770; SECA, Hanover, MD). BMI was calculated as kilograms per meter squared and BMI percentiles and z-scores were determined using the Centers for Disease Control and Prevention updated growth charts (http://www.cdc.gov/growthcharts). Subjects were classified as being lean (L) if they were less than the 85th percentile of BMI by age and gender and overweight (O) if they were equal to or above the 85th percentile thereby including at risk of overweight and overweight children.
Laboratory
Venipuncture was performed after a minimum 10 hour fast. Plasma glucose was measured using a Hitachi model 704 glucose analyzer with intra-assay and inter-assay coefficients of variation (CV) of 1.2% and 1.6% respectively.[20] Plasma insulin was measured by radioimmunoassay using an anti-insulin serum raised in guinea pegs, 125I labeled insulin (Linco, St. Louis, MO) and a double antibody method to separate bound from free tracer. This assay has a sensitivity of 2 pmol and intra- and inter-assay CVs of 5% and 8%.[20] Hyperinsulinemia (H-I) was designated as a fasting insulin level that was >90th percentile for lean subjects in the study population. Subjects with insulin levels ≤ 90th percentile for lean subjects were classified as normal insulinemic (N-I).
Blood Pressure (BP) and Brachial Artery Distensibility (BrachD)
After 5 minutes of rest, trained personnel obtained three measures using a DynaPulse Pathway instrument (PulseMetric, Inc, San Diego, CA). Subject demographics were entered into a personal computer interfaced to the DynaPulse Pathway instrument. A BP cuff appropriate for the subject’s upper arm size was applied.[21] Three automatic BP recordings of systolic, diastolic, mean arterial BP, heart rate (HR) and brachial artery pressure curves were obtained. The curves were uploaded to the on-line automated system for calculation of BrachD via the technique of pulse wave form analysis.[5] The DynaPulse Pathway instrument derives brachial artery distensibility using the technique of pulse dynamic analysis of arterial pressure signals obtained from a standard cuff sphygmomanometer.[6] The pressure waveform is calibrated and incorporated into a physical model of the cardiovascular system, assuming a straight tube brachial artery and T-tube aortic system. Validation studies of this method have been previously published.[6, 7] Correlation between compliance measurements obtained during cardiac catheterization and brachial artery compliance derived with the noninvasive method was high (r = 0.83). Clinical reproducibility studies demonstrated intraclass correlation coefficient for arterial compliance of 0.72 and other analyses indicated that most of the variability in measurement was due to inter-individual variation.[5] Although body size is used to estimate baseline brachial artery diameter for calculation of compliance, distensibility is compliance normalized for baseline brachial artery diameter. Therefore, body size is in both the numerator and the denominator of the distensibility equation. Hence, a vascular measure that is independent of body size and baseline brachial artery diameter results.[5]
Statistical analyses
All analyses were performed with Statistical Analyses Software (SAS, version 9.1.3).[22] Average values for demographic, anthropometric, laboratory and hemodynamic variables were obtained for the entire group, by gender and by BMI-insulin group. ANOVA was performed to look for differences by BMI-insulin group with Bonferroni correction for multiple comparisons as appropriate. Pearson correlation coefficients were obtained between BrachD and continuous variables. BMI and insulin values were log transformed since they were not normally distributed. Correlations for categorical variables were assessed with Spearman correlation coefficients. Partial correlation analysis was employed to examine the correlation between BrachD and variables of interest after controlling for pulse pressure (PP). Controlling for PP is important in the evaluation of vascular function since the distending pressure of a vessel, namely the PP, may influence the absolute magnitude of the measurement.[5] Multiple regression modeling using backward elimination was then performed to determine significant contributions to BrachD. These were repeated with PP as a covariate. Due to significance of the BMI by Insulin group term, the regression was then repeated stratified by BMI by Insulin group. Since PP is such a strong determinant of distensibility, a final model was constructed excluding the BP variables to examine the importance of other determinants of BrachD.
Results
Table 1 lists average demographic, anthropometric, laboratory and hemodynamic variables stratified by BMI and fasting insulin. Mean values for age and height were similar in the L and O, N-I and H-I groups. By definition, L subjects weighed less than O and H-I subjects had higher insulin concentrations than N-I. The L group had lower SBP, DBP and PP. Subjects with H-I had higher HR and fasting plasma glucose concentrations. BrachD was normally distributed with an average of 6.73 ± 1.26mmHg-1, but average BrachD was lower in overweight subjects (6.01 ± 1.04 vs. 7.08 ± 1.21, p<0.0001). As seen previously, females had greater distensibility than males (7.01 ± 1.29 vs. 6.37 ± 1.12, p<0.0001)[5], and Caucasians had greater distensibility than non-Caucasians (6.80 ± 1.24 vs. 6.64 ± 1.28, p=0.0215). There was no difference in BrachD by age. Correlation analyses (Table 2) also showed decreased BrachD in males, non-Caucasians and subjects with larger body size, and higher BP, PP, fasting plasma glucose and log of fasting insulin (p<0.0001). After adjusting for PP, significant correlations remained between BrachD and gender, adiposity, BP, HR and metabolic variables (glucose and log of fasting insulin) (all p<0.05).
Table 1.
Average values by BMI-Insulin group. Mean ± SD.
| Variable* | Lean / N-I† (N=582) |
Lean / H-I‡ (N=65) |
Overweight / N-I
(N=166) |
Overweight / H-I
(N=156) |
|---|---|---|---|---|
| Age (years) | 17.7 ± 1.8 | 17.6 ± 1.8 | 17.8 ± 1.9 | 17.8 ± 1.9 |
| Height (cm) | 169.0 ± 9.1 | 166.8 ± 8.6 | 169.8 ± 8.8 | 169.2 ± 9.4 |
| Weight (kg)a,b,d,e,f,g,h | 61.9 ± 9.9 | 62.2 ± 10.2 | 85.3 ± 15.4 | 98.3 ± 21.5 |
| BMI (kg/m2)a,b,d,e,f,g,h | 21.6 ± 2.1 | 22.3 ± 2.5 | 29.6 ± 4.5 | 34.4 ± 6.6 |
| BMI Z-score BMIa,b,d,e,f,g,h | -0.01 ± 0.71 | 0.20 ± 0.78 | 1.57 ± 0.39 | 1.99 ± 0.49 |
| K1 SBP (mmHg)a,b,d,e,f,g | 112.5 ± 10.1 | 114.2 ± 10.4 | 119.4 ± 11.4 | 122.0 ± 10.3 |
| K4 DBP (mmHg)a,b,d,e,g,h | 68.4 ± 7.3 | 68.7 ± 7.1 | 71.0 ± 7.6 | 73.4 ± 7.1 |
| Pulse Pressure (mmHg)a,b,d,e,g | 44.2 ± 7.7 | 45.5 ± 9.0 | 48.4 ± 8.5 | 48.6 ± 7.3 |
| HR (beats/min)b,c,e,m,h | 72.2 ± 11.6 | 78.2 ± 10.1 | 71.6 ± 10.1 | 76.9 ± 9.7 |
| Glucose (mmol/L)a,b,c,e,m,h | 4.10 ± 0.48 | 4.38 ± 0.59 | 4.14 ± 0.46 | 4.35 ± 0.53 |
| Insulin (μmol/l)a,b,c,e,m,h | 65.2 ± 24.8 | 200.5 ± 124.3 | 78.8 ± 24.8 | 222.9 ± 125.4 |
| Brachial Distensibility (%/mmHg)i,j,k,l,m,n | 7.1 ± 1.2 | 6.8 ± 1.3 | 6.2 ± 1.0 | 5.8 ± 1.1 |
P values < 0.05: A = L<O; B = N-I<H-I; C = L/N-I<L/H-I; D = L/N-I<O/N-I; E = L/N-I<O/H-I; F = L/H-I<O/N-I; G = L/H-I<O/H-I; H = O/N-I<O/H-I; I = L>O; J = N-I>H-I; K = L/N-I>O/N-I; L = L/N-I>O/H-I; M = L/H-I>O/N-I; N = L/H-I>O/H-I
N-I = Normo-Insulinemic
H-I = Hyper-Insulinemic
Table 2.
Correlation Coefficients between BrachD and variables of interest with Partial Correlation coefficients after adjusting for the covariate of PP.
| Variable | Pearson | Point Bi-Serial
(Spearman) |
Partial Correlation
Coefficient after adjusting for PP |
|---|---|---|---|
| Age | 0.01 | -0.003 | |
| Gender | -0.26* | 0.16* | |
| Ethnicity | -0.07* | -0.05 | |
| Height | -0.25* | 0.06 | |
| Weight | -0.50* | -0.33* | |
| Log BMI | -0.48* | -0.39* | |
| K1 | -0.56* | -0.09* | |
| K4 | -0.06 | -0.09* | |
| PP | -0.71* | N/A | |
| HR | -0.03 | -0.26* | |
| Glucose | -0.19* | -0.12* | |
| Log Insulin | -0.26* | -0.28* |
P<0.05
Multiple regression modeling revealed that PP, HR, gender, glucose, BMI and a BMI x Insulin interaction term were significant determinates of BrachD, and explained 59% of the variance in BrachD. This modeling was then repeated with stratification by the four BMI x Insulin groups. Table 3 demonstrates that PP and HR were major determinates for all groups with age remaining in the model for only the O/H-I group. Glucose was significant for subjects with a normal insulin level regardless of adiposity (both L and O). In the model excluding BP variables, glucose remained important in subjects with normal insulin levels but gender was now significant for all. HR retained significance only in overweight subjects whether normal- or hyper-insulinemic.
Table 3.
Results for Backward Elimination Regression Model for dependent variable BrachD.
| Group | Variables Remaining
(full model) |
r2* | Variables Remaining
(BP variables excluded) |
r2* |
|---|---|---|---|---|
| Lean / NL-I† | PP, HR, Glucose | 0.52 | Gender, Glucose | 0.12 |
| Lean / H-I‡ | PP, HR | 0.65 | Gender | 0.12 |
| Overweight / NL-I | PP, HR, Glucose | 0.51 | Gender, HR, Glucose | 0.15 |
| Overweight / H-I | PP, Age, HR | 0.45 | Gender, HR | 0.06 |
All testing performed at alpha level = 0.10.
N-I = Normo-Insulinemic
H-I = Hyper-Insulinemic
BrachD was also examined within BMI by insulin groups using ANOVA (Table 1 and Figures 1 and 2). BrachD was highest in L/N-I group, with a progressive decrease seen in L/H-I, O/N-I, and O/H-I regardless of gender (Table 1 & Figures 1 and 2). There were significant differences in BrachD between the L and O groups (p<0.05) but no difference within BMI group by insulin status for the entire study population or for females when stratified by gender. For males, significant difference was found between L/N-I and all O subjects (p<0.05).
Figure 1.
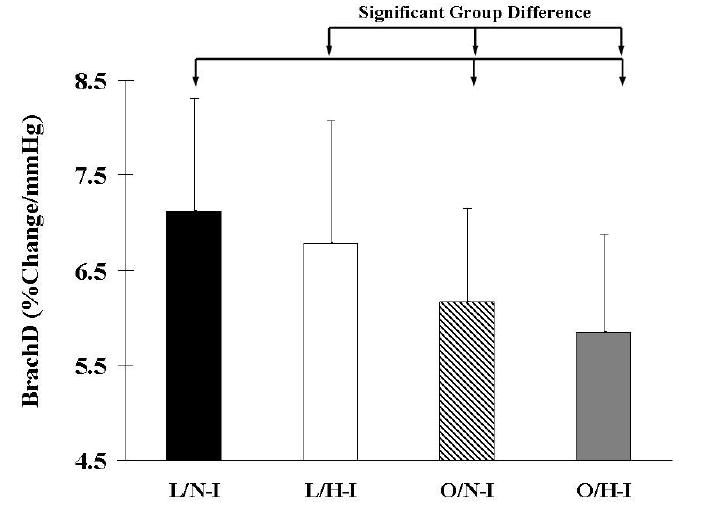
Brachial Artery Distensibility (mean with SD bars) by BMI and Insulin groups. N = 969. Significant (P<0.05) group differences indicated by arrow bars. L = Lean; O = Overweight; N-I = Normo-Insulinemic; H-I = Hyper-Insulinemic.
Figure 2.
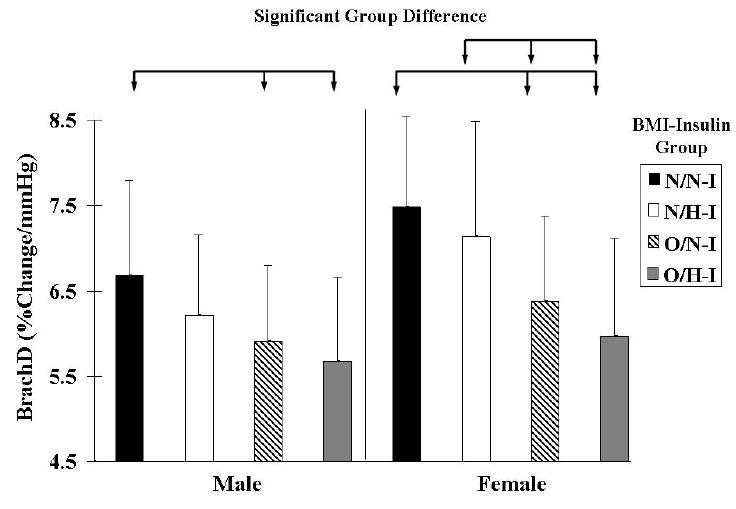
Brachial Artery Distensibility (mean with SD bars) by BMI and Insulin groups stratified by gender. N = 969. Significant (P<0.05) group differences indicated by arrow bars. L = Lean; O = Overweight; N-I = Normo-Insulinemic; H-I = Hyper-Insulinemic.
Discussion
The current study demonstrates lower levels of BrachD in subjects who are male, overweight, have a higher BP, HR and increased fasting glucose or insulin concentrations even after controlling for PP. Analysis by BMI-Insulin group revealed that BrachD was lower in obese subjects. A trend was seen for decrease in BrachD with the addition of hyper-insulinemia to either BMI category. The magnitude of decrease in BrachD with adiposity was greater than the difference between normal and high insulin levels within the same BMI category. These data suggest that overweight may have a greater effect on vascular function than hyperinsulinemia and that the combination of excess adiposity and hyperinsulinemia will produce the greatest decline. In addition, regression models with PP as a covariate demonstrated that gender was also an important determinate of BrachD with fasting glucose retaining significance for subjects with a normal insulin level. HR was important for the overweight group. Thus, this cross-sectional study demonstrates that gender, obesity, BP, glucose level and hyperinsulinemia may provide individual contributions towards lower levels of vascular function in healthy adolescents and young adults.
Recently, Whincup et al reported a strong, graded, inverse relationship between brachial distensibility and DBP, adiposity, and fasting insulin in adolescents in the United Kingdom.[18] Similar to the present study, an inverse relationship between BrachD and DBP was found in both genders. In contrast to the present study, Whincup did not find a relationship between BrachD and adiposity or fasting insulin when the group was stratified by gender. In addition, no relationship was seen between BrachD and SBP or fasting plasma glucose. There are a number of differences between the present study and Whincup’s report aside from the different methods employed, that may account for these discrepancies. We present data from a broader sampling of a healthy population versus contrasts between cohorts at low or high risk for adult CV disease. Our higher number of participants (969 vs. 383) offers greater power and use of only morning studies to control for circadian variation also support the validity of our observations while adding to the previously published results [18].
Many investigations in adults have documented differences in vascular stiffness by gender. Male gender was found to be an independent determinant of pulse wave velocity, a measure of central arterial compliance[23] and Young’s elastic pressure modulus, a measure of carotid stiffness.[24] Studies of the muscular brachial artery in adults whether using the wall-tracker method[25] or own technique,[5] also demonstrate reduced distensibility of this artery in men. Estrogen may play a role in these gender differences since hormone replacement therapy in post-menopausal women improves arterial stiffness (reduced pulse wave velocity) independently of change in BP.[26] Furthermore, in the limited data relating gender to arterial stiffness in pediatric subjects, girls were found to have greater distensibility of large arteries than boys but only after puberty had occurred.[27] Our data extend these observations on gender differences in brachial artery properties by providing the largest number of measurements in adolescents to date. Furthermore, our method accounts for baseline brachial diameter in the calculation of distensibility thus decreasing the likelihood that the decline in BrachD seen in males is due to gender difference in arterial size.
Large studies in adults demonstrate increased carotid and aortic stiffness related to obesity even after adjustment for mean arterial pressure.[11] Although few data are available in children, adiposity has been related to decreased brachial distensibility [18, 28] and lower carotid compliance in small studies of adolescents.[15] Our findings on a larger population of youth confirm the adverse effects of obesity while emphasizing the greater effect of overweight as compared to hyperinsulinemia on the vascular properties of the brachial artery in the young.
The relationship between blood pressure and vascular stiffness is complex. Although decreased arterial compliance is associated with hypertension,[29] whether the increased stiffness is the cause or the effect of the arterial pressure elevation is debated.[30] Data supporting the role of arterial stiffness in the pathophysiology of hypertension include the observation that normotensive adults at genetic risk for hypertension have reduced brachial artery distensibility when measured via the same technique as employed in the current study.[31] Furthermore, decreased arterial compliance at baseline was associated with increased SBP later in adulthood in a study that employed radial artery tonometry.[32] Few data are available in children concerning the effect of blood pressure on vascular function. Brachio-radial pulse wave velocity was independently correlated with mean arterial pressure healthy children[33] while carotid artery elasticity was reduced in children with hypertension.[34] Our data provide additional observations demonstrating that reduction in brachial artery distensibility, a vascular territory less well studied in children, is associated with higher blood pressure levels at a young age independent of baseline PP and may indicate an increased risk for future development of clinical hypertension.
Insulin is known to affect autonomic tone.[35] Therefore, it is not surprising that higher HR was found in our hyperinsulinemic group. However, an earlier study in adults using the same measurement device as used in the current study did not find HR to be a significant determinate of BrachD in multivariate modeling.[5] Although studies using other techniques have demonstrated a relationship between HR and arterial stiffness in central and leg arteries,[36], no relationship was found between sympathetic tone measured with heart rate variability and brachial distensibility in hypertensive adults[37] or subjects with type 1 diabetes.[38] One investigator suggested that sympathetic tone only impacted the brachial artery in the distal muscular rather than proximal elastic portion.[39] Another explanation for this discrepancy may involve the wider distribution of normal HR values found in children. It is possible the effect of insulin on the more narrow range of adult HR is insufficient to change BrachD as our results suggest that insulin-mediated sympathetic stimulation of HR as found in obesity may indeed affect brachial artery stiffness in younger individuals.
Adults with glucose-intolerance demonstrate increased arterial stiffness with abnormalities in vascular function predicting adverse CV outcomes.[40] Even “high normal” levels of fasting glucose are independently related to carotid stiffness[41] and brachial distensibility.[5] Furthermore, decreased aortic compliance has been found in non-diabetic adults with a family history of diabetes.[42] Children with type 1 diabetes also demonstrate increased aortic augmentation index, a measure of large artery stiffness.[43] However, the relationship between glucose levels and distensibility of the brachial artery in healthy youth and those with Type 2 diabetes or hyperinsulinemia has not been studied systematically. Similar to our findings, Whincup’s study of adolescents[18] found decreased brachial distensibility with increasing insulin resistance, but no relationship with fasting glucose. However, our analyses went on to stratify subjects by insulin status. With this approach, we found our normo-insulinemic subjects had reduced brachial distensibility associated with higher fasting glucose levels even though they remained within the normal range. This suggests that glucose metabolism exerts a greater influence on distensibility in non-hyperinsulinemic, healthy youth.
In conclusion, gender, BMI, BP, HR, fasting plasma glucose concentration, and hyperinsulinemia have individual adverse effects on brachial artery distensibility in healthy adolescents and young adults. Defining the individual contributions and mechanisms by which each of these factors contributes to vascular dysfunction is required in order to develop targeted, effective strategies to prevent, reverse, or limit the development and progression of cardiovascular disease.
Acknowledgments
This work was supported by NIH grants DK59183, 0M01 RR 08084, and a Trustee Grant from Cincinnati Children’s Hospital. The authors gratefully acknowledge the work of the PSD research team and the administration, staff, teachers, students, and parents of the Princeton School District.
Supported in part by National Institutes of Health Landmarks in the Progression to type 2 diabetes grant (R01 DK59183).
Footnotes
The authors have no conflicts of interests to report.
Conflict of Interest Disclosures None of the authors or persons mentioned in the acknowledgment section have potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. http://www.cdc.gov/
- 2.Berenson G, Srinivasan S, Nicklas T. Atherosclerosis: a nutritional disease of childhood. American Journal of Cardiology. 1998;82:22T–29T. doi: 10.1016/s0002-9149(98)00719-x. [DOI] [PubMed] [Google Scholar]
- 3.McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–8. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 4.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 5.Urbina EM, Brinton TJ, Elkasabany A, Berenson GS. Brachial artery distensibility and relation to cardiovascular risk factors in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;89:946–51. doi: 10.1016/s0002-9149(02)02244-0. [DOI] [PubMed] [Google Scholar]
- 6.Brinton TJ, Cotter B, Kailasam MT, Brown DL, Chio S-S, O’Conor DT, DeMaria AN. Development and validation of a noninvasive method to determine arterial pressure and vascular compliance. Am J Cardiol. 1997;80:323–330. doi: 10.1016/s0002-9149(97)00353-6. [DOI] [PubMed] [Google Scholar]
- 7.Brinton TJ, Walls ED, Chio SS. Validation of Pulse Dynamic Blood pressure Measurement by Auscultation. Blood Pressure Monitoring. 1998;3:121–124. [PubMed] [Google Scholar]
- 8.Budoff MJ, Flores F, Tsai J, Frandsen T, Yamamoto H, Takasu J. Measures of brachial artery distensibility in relation to coronary calcification. Am J Hypertens. 2003;16:350–5. doi: 10.1016/s0895-7061(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M, Sugawara S, Arakawa N, Nagano M, Shizuka T, Shimoda Y, et al. Reduced vascular compliance is associated with impaired endothelium-dependent dilatation in the brachial artery of patients with congestive heart failure. Journal of Cardiac Failure. 2004;10:36–42. doi: 10.1016/s1071-9164(03)00585-2. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira I, Henry R, Twisk J, et al. The metabolic syndrome, cardiopulmonary fitness, and subctuaneous trunk fat as independent determinants of arterial stiffness: the Amsterdam Growth and Health Longitudinal Study. Archives of Internal Medicine. 2005;165:875–882. doi: 10.1001/archinte.165.8.875. [DOI] [PubMed] [Google Scholar]
- 11.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–46. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 12.Urbina EM, Kieltkya L, Tsai J, Srinivasan SR, Berenson GS. Impact of multiple cardiovascular risk factors on brachial artery distensibility in young adults: the Bogalusa Heart Study. Am J Hypertens. 2005;18:767–71. doi: 10.1016/j.amjhyper.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Kosch M, Barenbrock M, Kisters K, Rahn KH, Hausberg M. Relationship between muscle sympathetic nerve activity and large artery mechanical vessel wall properties in renal transplant patients. Journal of Hypertension. 2002;20:501–8. doi: 10.1097/00004872-200203000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Mietus-Snyder M, Malloy MJ. Endothelial dysfunction occurs in children with two genetic hyperlipidemias: improvement with antioxidant vitamin therapy. Journal of Pediatrics. 1998;133:35–40. doi: 10.1016/s0022-3476(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 15.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 16.Woo KS, Chook P, Yu CW, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. International Journal of Obesity & Related Metabolic Disorders: Journals of the International Association for the Study of Obesity. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 17.Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–5. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 18.Whincup PH, Gilg JA, Donald AE, Katterhorn M, Oliver C, Cook DG, Deanfield JE. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112:1789–97. doi: 10.1161/CIRCULATIONAHA.104.532663. [DOI] [PubMed] [Google Scholar]
- 19.Goodman E, Adler NE, Daniels SR, Morrison JA, Slap GB, Dolan LM. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obesity Research. 2003;11:1018–26. doi: 10.1038/oby.2003.140. [DOI] [PubMed] [Google Scholar]
- 20.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–51. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 21.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 22.SAS Institute. SAS OnlineDoc, Version 8 [Google Scholar]
- 23.Oren A, Vos L, Uiterwaal C, et al. Change in body mass index from adolescence to young adulthood and increased carotid intima-media thickness at 28 years of age: the Atherosclerosis Risk in Young Adults study. International Journal of Obesity & Related Metabolic Disorders: Journals of the International Association for the Study of Obesity. 2003;27:1383–1390. doi: 10.1038/sj.ijo.0802404. [DOI] [PubMed] [Google Scholar]
- 24.Urbina EM, Srinivasan SR, Kieltyka RL, Tang R, Bond MG, Chen W, Berenson GS. Correlates of carotid artery stiffness in young adults: The Bogalusa Heart Study. Atherosclerosis. 2004;176:157–64. doi: 10.1016/j.atherosclerosis.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 25.van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension. 2000;35:637–42. doi: 10.1161/01.hyp.35.2.637. [DOI] [PubMed] [Google Scholar]
- 26.Kawecka-Jaszcz K, Czarnecka D, Olszanecka A, Rajzer M, Jankowski P. The effect of hormone replacement therapy on arterial blood pressure and vascular compliance in postmenopausal women with arterial hypertension. J Hum Hypertens. 2002;16:509–16. doi: 10.1038/sj.jhh.1001431. [DOI] [PubMed] [Google Scholar]
- 27.Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. Journal of Clinical Endocrinology & Metabolism. 2003;88:5375–80. doi: 10.1210/jc.2003-030722. [DOI] [PubMed] [Google Scholar]
- 28.Singhal A, Farooqi IS, Cole TJ, O’Rahilly S, Fewtrell M, Kattenhorn M, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–24. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 29.Bouthier JD, De Luca N, Safar ME, Simon AC. Cardiac hypertrophy and arterial distensibility in essential hypertension. American Heart Journal. 1985;109:1345–52. doi: 10.1016/0002-8703(85)90364-3. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL, Jr, Neutel J, Kerwin LJ, et al. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108:1592–8. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 31.Brinton TJ, Kailasam MT, Wu RA, Cervenka JH, Chio SS, Parmer RJ, et al. Arterial compliance by cuff sphygmomanometer. Application to hypertension and early changes in subjects at genetic risk. Hypertension. 1996;28:599–603. doi: 10.1161/01.hyp.28.4.599. [DOI] [PubMed] [Google Scholar]
- 32.Arnett DK, Glasser SP, McVeigh G, Prineas R, Finklestein S, Donahue R, et al. Blood pressure and arterial compliance in young adults: the Minnesota Children’s Blood Pressure Study. Am J Hypertens. 2001;14:200–5. doi: 10.1016/s0895-7061(00)01262-0. [DOI] [PubMed] [Google Scholar]
- 33.Cheung YF, Wong KY, Lam BC, Tsoi NS. Relation of arterial stiffness with gestational age and birth weight. Archives of Disease in Childhood. 2004;89:217–21. doi: 10.1136/adc.2003.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litwin M, Trelewicz J, Wawer Z, Antoniewicz J, Wierzbicka A, Rajszys P, Grenda R. Intima-media thickness and arterial elasticity in hypertensive children: controlled study. Pediatr Nephrol. 2004;19:767–74. doi: 10.1007/s00467-004-1480-6. [DOI] [PubMed] [Google Scholar]
- 35.Liao D, Cai J, Brancati FL, Folsom A, Barnes RW, Tyroler HA, Heiss G. Association of vagal tone with serum insulin, glucose, and diabetes mellitus--The ARIC Study. Diabetes Res Clin Pract. 1995;30:211–21. doi: 10.1016/0168-8227(95)01190-0. [DOI] [PubMed] [Google Scholar]
- 36.Sa Cunha R, Pannier B, Benetos A, Siche JP, London GM, Mallion JM, Safar ME. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. Journal of Hypertension. 1997;15:1423–30. doi: 10.1097/00004872-199715120-00009. [DOI] [PubMed] [Google Scholar]
- 37.Kosch M, Hausberg M, Barenbrock M, Kisters K, Rahn KH. Studies on cardiac sympathovagal balance and large artery distensibility in patients with untreated essential hypertension. J Hum Hypertens. 1999;13:315–9. doi: 10.1038/sj.jhh.1000806. [DOI] [PubMed] [Google Scholar]
- 38.van Ittersum FJ, Schram MT, van der Heijden-Spek JJ, Van Bortel LM, Elte JW, Biemond P, et al. Autonomic nervous function, arterial stiffness and blood pressure in patients with Type I diabetes mellitus and normal urinary albumin excretion. Journal of Human Hypertension. 2004;18:761–8. doi: 10.1038/sj.jhh.1001751. [DOI] [PubMed] [Google Scholar]
- 39.Bjarnegard N, Ryden Ahlgren A, Sonesson B, Lanne T. The effect of sympathetic stimulation on proximal brachial artery mechanics in humans--differential behaviour within the length of the brachial artery? Acta Physiologica Scandinavica. 2004;182:21–7. doi: 10.1111/j.1365-201X.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 40.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 41.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–43. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins KD, Lehmann ED, Jones RL, Turay RC, Gosling RG. A family history of NIDDM is associated with decreased aortic distensibility in normal healthy young adult subjects. Diabetes Care. 1996;19:501–3. doi: 10.2337/diacare.19.5.501. [DOI] [PubMed] [Google Scholar]
- 43.Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF, et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004;27:2911–7. doi: 10.2337/diacare.27.12.2911. [DOI] [PubMed] [Google Scholar]


