Abstract
Malignant lymph nodes in the neck include metastases and lymphoma. Cervical nodal metastases are common in patients with head and neck cancers, and their assessment is important as it affects treatment planning and prognosis. Neck nodes are also a common site of lymphomatous involvement and an accurate diagnosis is essential as its treatment differs from other causes of neck lymphadenopathy. On ultrasound, grey scale sonography helps to evaluate nodal morphology, whilst power Doppler sonography is used to assess the vascular pattern. Grey scale sonographic features that help to identify metastatic and lymphomatous lymph nodes include size, shape and internal architecture (loss of hilar architecture, presence of intranodal necrosis and calcification). Soft tissue oedema and nodal matting are additional grey scale features seen in tuberculous nodes or in nodes that have been previously irradiated. Power Doppler sonography evaluates the vascular pattern of nodes and helps to identify the malignant nodes. In addition, serial monitoring of nodal size and vascularity are useful features in the assessment of treatment response.
Keywords: Cervical lymph nodes, metastases, lymphoma, ultrasound
Introduction
Assessment of nodal status is essential in patients with head and neck carcinomas as it predicts prognosis and helps in the selection of treatment options[1],[2]. In patients with proven head and neck carcinomas, the presence of a unilateral metastatic node reduces the 5-year survival rate by 50%, whereas the presence of bilateral metastatic nodes reduces the 5-year survival rate to 25%[3]. Metastatic cervical lymph nodes from head and neck carcinomas are usually site specific with respect to the location of the primary tumour. Therefore, assessment of the distribution of metastatic nodes in patients with unknown primary may provide a clue to the site of the primary tumour. Moreover, metastatic nodes in an unexpected site indicates that the primary tumour is biologically more aggressive[4].
Besides metastases, lymphoma is also a common malignant disease and head and neck involvement is relatively common[5]. Clinically, lymphomatous cervical lymph nodes are difficult to differentiate from other causes of lymphadenopathy including metastatic nodes. As the treatment options differ, accurate identification of the nature of the diseases is essential.
The role of ultrasound in the assessment of cervical lymphadenopathy is well established. It is particularly sensitive compared to clinical examination (96.8% and 73.3% respectively) in patients with previous head and neck cancer with post-radiation neck fibrosis[6]. When combined with guided fine needle aspiration cytology (FNAC), the specificity of ultrasound is as high as 93%[7]. Although computed tomography (CT) and magnetic resonance imaging (MRI) are also used to evaluate cervical lymph nodes, the nature and internal architecture of small lymph nodes (<5 mm) may not be readily assessed. In addition, MRI may not identify intranodal calcification which is a useful feature in predicting metastatic nodes from papillary carcinoma of the thyroid[1],[3],[8],[9]. On contrast-enhanced CT, the reported sensitivity and specificity in the evaluation of metastatic cervical lymph nodes are 90.2% and 93.9% respectively[10]. On high resolution MRI, the sensitivity and specificity in assessing metastatic nodes are 86% and 94% respectively, whereas those in evaluating lymphomas are 85% and 95% respectively[11]. Positron emission tomography (PET) has a relatively lower sensitivity (80.3%) and specificity (92.8%) in the evaluation of metastatic nodes, but the sensitivity (91.8%) and specificity (98.9%) are higher when PET/CT is used[10]. Among different imaging modalities, ultrasound has the highest sensitivity in the assessment of malignant cervical nodes, whereas PET/CT has the highest specificity in the diagnosis.
This article reviews the grey scale and Doppler sonographic features in the assessment of metastatic and lymphomatous cervical lymph nodes. In the sonographic assessment of cervical lymph nodes, grey scale ultrasound assesses the nodal site, size, shape, border, internal architecture (echogenicity, echogenic hilus, calcification and necrosis), matting and adjacent soft tissue oedema. The vascular pattern of lymph nodes is evaluated with colour or power Doppler ultrasound, whilst the blood flow velocity and vascular resistance are measured using spectral Doppler ultrasound.
Metastases
Metastatic cervical nodes from head and neck primaries are site-specific[4],[12]. Common nodal metastatic sites for head and neck primaries are[1],[12–29]:
pharynx, larynx, oesophagus, papillary carcinoma of thyroid metastasize along internal jugular chain
tumours in the oral cavity metastasize to the submandibular and upper cervical regions, although carcinoma of the tongue may give rise to skip metastases in the lower neck.
infraclavicular primaries from breast and lung metastasize to supraclavicular fossa and posterior triangle.
nasopharyngeal carcinoma commonly spreads to upper cervical and posterior triangle nodes.
Grey scale evaluation of metastatic nodes
Size
Nodal size is one of the criteria used to differentiate reactive from metastatic nodes[15],[30]. Although larger nodes tend to have a higher incidence of malignancy, reactive nodes can be as large as metastatic nodes. Therefore, different cut-offs of the nodal size to differentiate reactive and metastatic nodes have been reported (5 mm, 8 mm and 10 mm)[30–32]. However, when a lower cut-off of nodal size is used, the diagnostic sensitivity increases while the specificity decreases and vice versa[33]. Therefore, nodal size alone cannot be used to distinguish reactive from metastatic lymph nodes. However, the size of lymph nodes is useful in two clinical situations: (1) increase in nodal size on serial examinations in a patient with a known carcinoma is highly suspicious for metastatic involvement; (2) serial reduction in nodal size is a useful indicator in monitoring patient's response to treatment[34].
Shape
Metastatic nodes tend to be round with a short to long axes ratio (S/L ratio) greater than 0.5, while reactive or benign lymph nodes are elliptical in shape (S/L ratio <0.5)18,[32],[35–37]. Although the round shape helps to identify a metastatic lymph node, it should not be used as the sole criterion of nodal assessment as normal submandibular and parotid nodes are also round[36]. Irrespective of size, eccentric cortical hypertrophy, which is due to focal tumour infiltration within the lymph node, is a useful sign to identify metastatic nodes[37].
Border
Contrary to common belief, metastatic lymph nodes tend to have sharp borders (Fig. 1), whilst benign lymph nodes usually show unsharp borders[31]. This sharp border in metastatic nodes is due to intranodal tumour infiltration which causes an increase in the acoustic impedance difference between intranodal and surrounding tissues[31]. However, metastatic nodes in advanced stages may demonstrate ill-defined borders, indicating extracapsular spread[38]. Nodal border alone is therefore not a reliable criterion in distinguishing normal from abnormal nodes in routine clinical practice. However, the presence of ill-defined borders in a proven metastatic node indicates extracapsular spread and is useful in predicting patient prognosis.
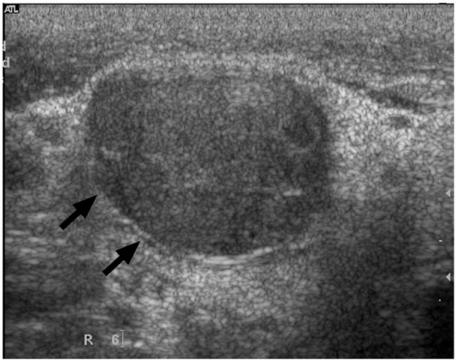
Figure 1.
Grey scale sonogram showing a metastatic lymph node which is enlarged, hypoechoic, well-defined and without an echogenic hilus (arrows).
Echogenicity
Metastatic lymph nodes are predominantly hypoechoic relative to the adjacent musculature[18],[19],[24],[39]. However, metastatic nodes from papillary carcinoma of the thyroid are usually hyperechoic (Fig. 2), and this is believed to be related to the intranodal deposition of thyroglobulin originating from the primary tumour[8],[26].
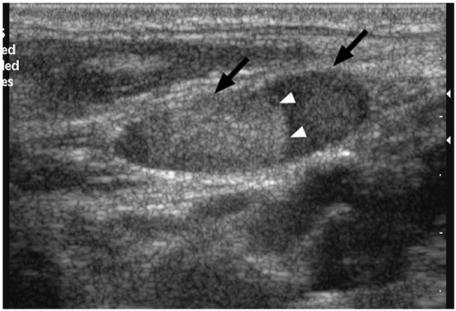
Figure 2.
Grey scale sonogram showing a metastatic lymph node from papillary carcinoma of the thyroid (arrows). Note the hyperechoic component within the node which may be related to intranodal deposition of thyroglobulin (arrowheads).
Echogenic hilus
On ultrasound, the echogenic hilus appears as an echogenic intranodal linear structure which is continuous with the adjacent perinodal fat[40–42]. The echogenic hilus is mainly the result of multiple medullary sinuses, which act as acoustic interfaces and partially reflect the ultrasound waves to produce an echogenic structure[2],[40],[42]. In the normal neck, about 90% of nodes with a maximum transverse diameter greater than 5 mm will demonstrate an echogenic hilus on high resolution ultrasound[43]. Metastatic lymph nodes usually do not show an echogenic hilus (Fig. 1), and the presence of an echogenic hilus within lymph nodes was previously considered a sign of benignity[44]. However, studies have shown that echogenic hilus may also be found in malignant nodes2,[18],[19],[40]. Therefore, the presence/absence of echogenic hilus cannot be used as the sole criterion in the evaluation of cervical lymph nodes.
Intranodal necrosis
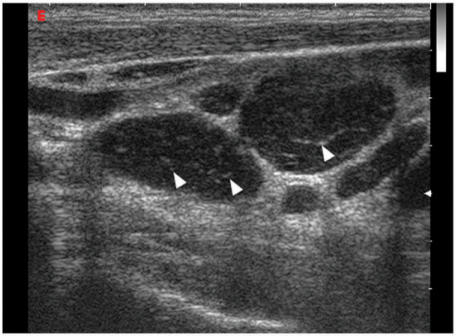
Intranodal necrosis may be seen as a cystic (cystic or liquefaction necrosis) or echogenic (coagulation necrosis) area within the node. Cystic necrosis is the more common form of intranodal necrosis which appears as an echolucent area within the nodes (Fig. 3). Coagulation necrosis is a less common sign, and appears as an echogenic focus within lymph nodes but is not continuous with the surrounding fat and does not produce acoustic shadowing[41],[42]. Intranodal necrosis may be found in metastatic and tuberculosis nodes4,[19],[24],[26], and regardless of nodal size, the presence of intranodal necrosis should be considered pathologic[4].
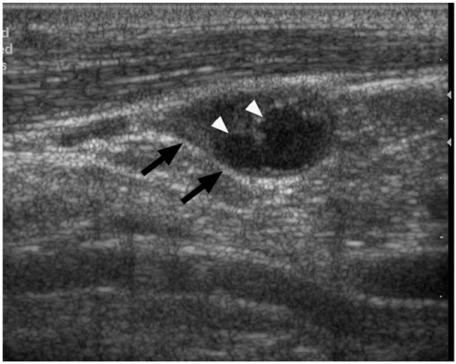
Figure 3.
Longitudinal grey scale sonogram showing a metastatic cervical node (arrows) with intranodal cystic necrosis which appears ill-defined and echolucent (arrowheads).
Calcification
Calcification within lymph nodes is uncommon, however, metastatic cervical nodes from papillary carcinoma of the thyroid tend to show calcification (Fig. 4)[4],[8],[26]. The calcification in these lymph nodes is usually punctate, peripherally located with acoustic shadowing using a high resolution transducer[26]. The relatively higher incidence of calcification in metastatic nodes from papillary carcinoma of the thyroid makes this a useful feature in predicting the nature of the adenopathy and directing a search for the primary tumour in the thyroid gland. Although metastatic lymph nodes from medullary carcinoma of the thyroid may also show calcification, the incidence is substantially lower than metastatic nodes from papillary carcinoma of the thyroid.
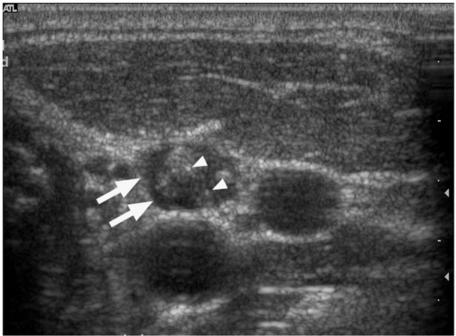
Figure 4.
Transverse grey scale sonogram of a metastatic lymph node from papillary carcinoma of the thyroid (arrows) with echogenic, punctate calcification (arrowheads).
Ancillary features
On grey scale ultrasound, the presence/absence of ancillary features such as matting of lymph nodes and adjacent soft tissue oedema should also be evaluated. Although matting and adjacent soft tissue oedema are common in tuberculous nodes, metastatic nodes with extracapsular spread can invade adjacent soft tissues and cause oedema, and patients with previous radiation therapy of the neck may also show post-radiation soft tissue oedema and nodal matting[24],[25],[45].
Doppler evaluation of metastatic nodes
Vascular distribution
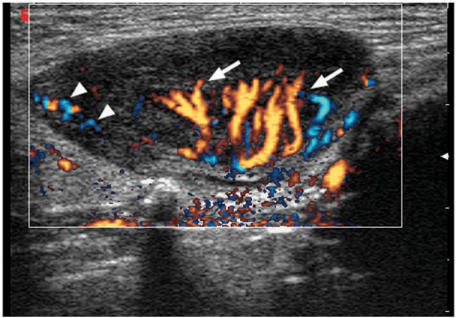
Evaluation of the vascular pattern of cervical lymph nodes has been reported to be highly reliable, with a repeatability of 85%[46]. On power Doppler ultrasound, approximately 90% of normal lymph nodes with a maximum transverse diameter greater than 5 mm will show hilar vascularity[43]. Normal and reactive nodes usually show hilar vascularity, or appear apparently avascular[47–50]. However, peripheral or mixed vascularity (the presence of both hilar and peripheral vascularity) are common in metastatic nodes[47–49],[51],[52]. Therefore, the presence of peripheral vessels in lymph nodes is a useful indicator of malignancy (Fig. 5). The peripheral vascularity in metastatic nodes is believed to be related to tumour infiltration of the lymph nodes in which the tumour cells produce tumour angiogenetic factor (TAF), which causes angiogenesis and recruitment of peripheral vessels[47–49],[51]. Mixed vascularity is seen in malignant nodes because angiogenesis occurs and peripheral vessels are induced, but the pre-existing hilar vessels are preserved until they are destroyed by the tumour cells at a later stage[48].
Figure 5.
Power Doppler sonogram of a metastatic lymph node with peripheral vascularity (arrowheads).
Vascular resistance
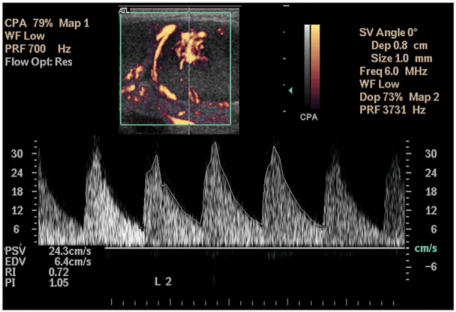
With the use of spectral Doppler ultrasound, the vascular resistance in terms of resistive index (RI) and pulsatility index (PI) can be evaluated (Fig. 6). However, the value of vascular resistance in differentiating malignant from benign lymph nodes remains unclear. Some reports have shown that the vascular resistance of metastatic nodes is higher than that of reactive nodes48,[49],[51],[53],[54], whereas others have suggested that metastatic nodes have a lower or similar vascular resistance compared to benign nodes[55],[56]. Different cut-off values of RI (0.6, 0.7 and 0.8) and PI (1.1, 1.5 and 1.6) with different sensitivities (RI, 47–81%; PI, 55–94%) and specificities (RI, 81–100%; PI, 97–100%) in differentiating metastatic and reactive lymph nodes have been reported48,[49],[51],[55]. In our experience the optimum cut-off values for RI and PI are 0.7 and 1.4, with a sensitivity of 86% and 80%, and a specificity of 70% and 86%, respectively[52]. In view of the inconsistency between various reports and the technical difficulties involved in obtaining suitable/repeatable values, the role of intranodal vascular resistance in routine clinical practice is limited.
Figure 6.
Spectral Doppler sonogram showing measurement of the resistive index (RI) and pulsatility index (PI) of a metastatic lymph node. Measurement of the peak systolic velocity (PSV) and end diastolic velocity (EDV) is also demonstrated. Note the measurements are obtained from three consecutive waveforms.
Lymphoma
Lymphoma in the head and neck region can be classified into Hodgkin's, and the more common non-Hodgkin's type. Involved lymph nodes are usually found in the submandibular, upper cervical chain and posterior triangle regions[5],[57],[58].
Grey scale evaluation of lymphomatous nodes
Size
The size of lymphomatous lymph nodes varies significantly[58]. Although lymphomatous nodes tend to be enlarged with a minimum transverse diameter of 10 mm or larger[5],[6],[59], nodal size alone is not an accurate criterion for differentiating lymphomatous nodes from normal or other pathologic lymph nodes. Nevertheless, similar to metastatic lymph nodes, progressive and substantial reduction in nodal size is a useful parameter to indicate good treatment response[60].
Shape, border, echogenicity, echogenic hilus
On grey scale ultrasound, lymphomatous nodes tend to be round in shape, well-defined, appear hypoechoic and are usually without an echogenic hilus29,[57],[59],[61], features which are similar to most metastatic lymph nodes. Therefore, nodal shape, border sharpness, echogenicity and the presence/absence of an echogenic hilus may not be useful sonographic criteria to differentiate lymphoma from metastases.
Intranodal reticulation
Previous studies have suggested that pseudo-cystic appearance and posterior acoustic enhancement are characteristic features of lymphomatous nodes, especially in non-Hodgkin's lymphoma29,[57],[59],[61],[62]. It was believed that the pseudo-cystic appearance was related to the homogeneous and diffuse histologic pattern of non-Hodgkin's lymphoma, which allows easy propagation of ultrasound resulting in a hypoechoic echopattern and posterior enhancement (67–90%)6,[18],[25],[29],[57],[63]. However, with the use of newer high-resolution transducers, the pseudocystic appearance in non-Hodgkin's lymphoma is not often seen, whilst intranodal reticulation (micronodular echopattern) is commonly found in lymphomatous nodes (Fig. 7)[64].
Figure 7.
Grey scale sonogram showing multiple hypoechoic lymphomatous nodes. Arrowheads indicate the intranodal reticulation, commonly seen in lymphomatous nodes using high-resolution transducers.
Intranodal necrosis and calcification
Lymphomatous nodes seldom show cystic necrosis unless the patient has received previous radiation therapy or chemotherapy, or has advanced disease[5],[25]. Similarly, intranodal calcification is uncommon in lymphomatous lymph nodes. However, calcification may be found in lymphomatous nodes after treatment, and the calcification in these nodes is usually dense with posterior acoustic shadowing.
Doppler evaluation of lymphomatous nodes
Vascular distribution
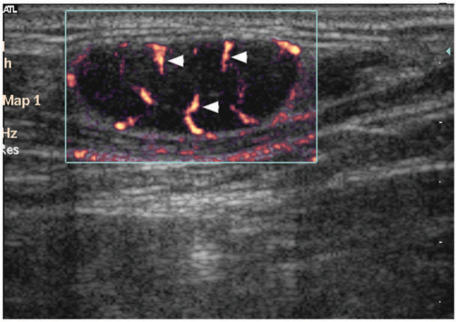
On power Doppler ultrasound, lymphomatous lymph nodes tend to have both hilar and peripheral vessels (62–90%, Fig. 8)48,[51],[54],[65],[66]. Unlike metastatic nodes, the presence of peripheral vascularity alone is not common in lymphomatous nodes (5%)[66]. The high incidence of hilar vascularity in lymphomatous nodes is thought to be related to the fact that intranodal necrosis or keratinisation is not common in lymphoma, and therefore the hilar vessels of the nodes are preserved[65],[66].
Figure 8.
Directional power Doppler sonogram showing a lymphomatous lymph node with both hilar (arrows) and peripheral (arrowheads) vascularity, which are commonly seen in lymphoma.
Vascular resistance
Similar to metastatic lymph nodes, the role of vascular resistance in the assessment of lymphomatous nodes is not clear because of insufficient information in the literature and inconsistent findings[9],[48],[51]. The reported RI and PI of lymphomatous nodes varies from 0.64 to 0.84 and from 1.2 to 2.2, respectively48,[51],[54],[66]. Nevertheless, it is generally believed that the RI and PI of lymphomatous nodes are higher than those of reactive, tuberculous and normal nodes, and are lower than those of metastatic nodes[48],[51],[66].
Doppler sonographic assessment is useful in monitoring the treatment response of lymphomatous lymph nodes. On colour/power Doppler sonography, rapid reduction of nodal vascularity is a sensitive sign of positive treatment response, and is useful in predicting patient prognosis. Patients with lymph nodes of rapidly diminishing vascularity tend to remain in remission, whereas patients with lymph nodes with prolonged high vascularity following chemotherapy tend to have subsequent relapse after chemotherapy[60]. Since the RI and PI do not significantly correlate with the response to chemotherapy, evaluation of the vascular resistance in post-chemotherapy lymphomatous nodes has limited prognostic value[60].
Contrast enhanced ultrasound of lymph nodes
Contrast enhancement in the evaluation of superficial nodes appears it to be more sensitive in characterizing lymph node pathology[67],[68]. Contrast-enhancement demonstrates more lymph node vessels, which allows more accurate characterization of nodal vascularity. Real-time sonography during contrast administration (dynamic contrast enhancement) adds a new, time-dependent dimension to the evaluation of lymph node vascularity, and has been shown to provide information on lymph node parenchymal perfusion[68]. Dynamic contrast scanning using ultrasound is advantageous over similar techniques using CT or MRI in that it is radiation-free, has a high spatial resolution yet maintains a high frame rate, and can be performed repeatedly during the same examination.
Our preliminary experience with dynamic sonographic contrast enhancement in Hodgkin's and non-Hodgkin's lymphoma[69] showed a delay in the time to peak enhancement after treatment (Figs. 9–11). On the other hand, the change in the magnitude of peak enhancement was variable after treatment (nodes in some patients had more enhancement and some had less enhancement after treatment). This delay to peak enhancement may be due to arteriolar constriction, an increase in capillary resistance, of a decrease in capillary density after treatment.
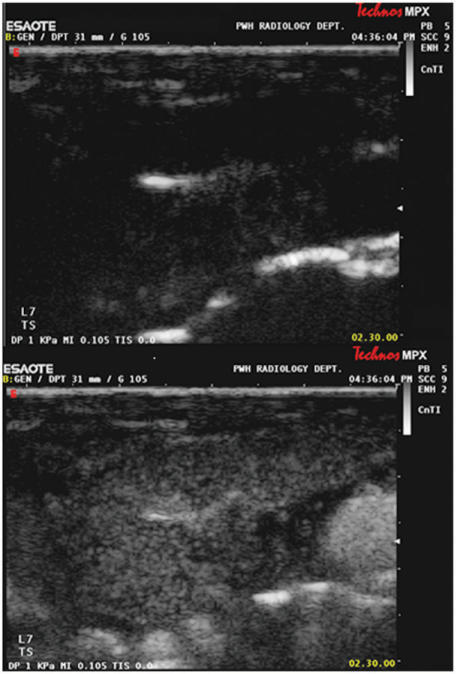
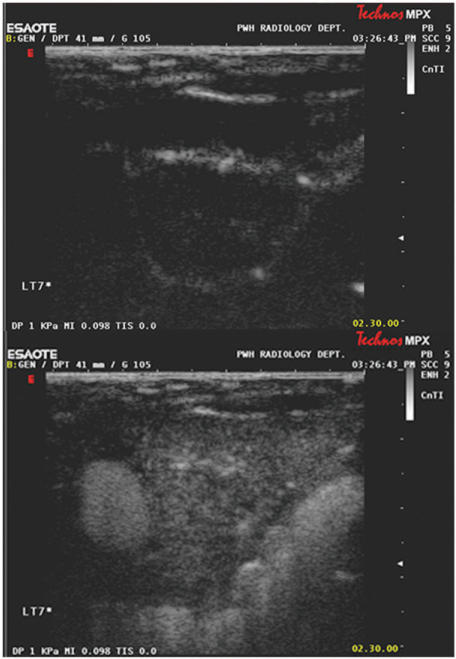
Figure 9.
Grey scale ultrasound images of a lymphomatous cervical lymph node at the start (top) and at peak enhancement (bottom) of contrast administration. The lymph node parenchyma enhances uniformly with contrast. A region of interest is drawn to include the lymph node to calculate a time-enhancement curve.
Figure 10.
Grey scale ultrasound images of the same lymph node (as in Fig. 9) after chemotherapy, at the start (top) and at peak enhancement (bottom) of contrast administration. The lymph node is smaller in size, the parenchyma enhances less (lower peak enhancement) and enhancement is more heterogeneous.
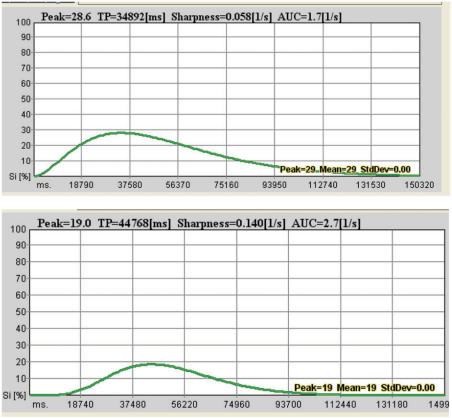
Figure 11.
Dynamic time-enhancement curves before (top) and after chemotherapy (bottom) for the same affected cervical lymph node as in Figs. 9 and 10. The time to peak contrast enhancement has lengthened from 34.9 s to 44.8 s. The peak and total (area under curve) contrast enhancement are both lower after treatment.
Dynamic contrast enhancement appears to provide a new, time-dependent dimension in the assessment of lymph node pathology and supplements the morphological information provided by grey scale and Doppler sonographic interrogation.
Conclusion
Ultrasound is a useful examination in the evaluation of malignant nodes in the neck. It helps in identifying the abnormal nodes, confirms the nature (with guided FNAC) and objectively assesses the response to treatment.
References
- [1].Ishii JI, Amagasa T, Tachibana T, Shinozuka K, Shioda S. US and CT evaluation of cervical lymph node metastasis from oral cancer. J Cranio-Max-Fac Surg. 1991;19:123. doi: 10.1016/s1010-5182(05)80575-x. [DOI] [PubMed] [Google Scholar]
- [2].Vassallo P, Edel G, Roos N, Naguib A, Peters PE. In-vitro high-resolution ultrasonography of benign and malignant lymph nodes. A sonographic-pathologic correlation. Invest Radiol. 1993;28:698. doi: 10.1097/00004424-199308000-00009. [DOI] [PubMed] [Google Scholar]
- [3].Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. Am J Roentgenol. 1992;158:961. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- [4].Som PM. Lymph nodes of the neck. Radiology. 1987;165:593. doi: 10.1148/radiology.165.3.3317494. [DOI] [PubMed] [Google Scholar]
- [5].DePena CA, Van Tassel P, Lee YY. Lymphoma of the head and neck. Radiol Clin North Am. 1990;28:723. [PubMed] [Google Scholar]
- [6].Bruneton JN, Normand F. Cervical lymph nodes. In: Bruneton JN, editor. Ultrasonography of the neck. Berlin: Springer-Verlag. 1987;p. 81 [Google Scholar]
- [7].Baatenburg de Jong RJ, Rongen RJ, Lameris JS, Harthoorn M, Verwoerd CD, Knegt P. Metastatic neck disease. Palpation vs ultrasound examination. Arch Otolaryngol Head Neck Surg. 1989;115:689. doi: 10.1001/archotol.1989.01860300043013. [DOI] [PubMed] [Google Scholar]
- [8].Som PM, Brandwein M, Lidov M, Lawson W, Biller HF. The varied presentations of papillary thyroid carcinoma cervical nodal disease: CT and MR findings. Am J Neuroradiol. 1994;15:1123. [PMC free article] [PubMed] [Google Scholar]
- [9].Ahuja A, Ying M. An overview of neck node sonography. Invest Radiol. 2002;37:333. doi: 10.1097/00004424-200206000-00005. [DOI] [PubMed] [Google Scholar]
- [10].Jeong HS, Baek CH, Son YI, et al. Use of integrated 18F-FDG PET/CT to improve the accuracy of initial cervical nodal evaluation in patients with head and neck squamous cell carcinoma. Head Neck. 2007;29:203. doi: 10.1002/hed.20504. [DOI] [PubMed] [Google Scholar]
- [11].Sumi M, Van Cauteren M, Nakamura T. MR microimaging of benign and malignant nodes in the neck. AJR Am J Roentgenol. 2006;186:749. doi: 10.2214/AJR.04.1832. [DOI] [PubMed] [Google Scholar]
- [12].Ahuja A, Ying M. Grey-scale sonography in assessment of cervical lymphadenopathy: review of sonographic appearances and features that may help a beginner. Br J Oral Maxillofac Surg. 2000;38:451. doi: 10.1054/bjom.2000.0446. [DOI] [PubMed] [Google Scholar]
- [13].Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- [14].Komisar A. Treatment of the node negative neck. In: Vogl SE, editor. Head and neck cancer. New York: Churchill Livingstone; 1988. p. 19. [Google Scholar]
- [15].van Overhagen H, Lameris JS, Berger MY, et al. Supraclavicular lymph node metastases in carcinoma of the esophagus and gastroesophageal junction: assessment with CT, US, and US-guided fine- needle aspiration biopsy. Radiology. 1991;179:155. doi: 10.1148/radiology.179.1.2006268. [DOI] [PubMed] [Google Scholar]
- [16].van Overhagen H, Lameris JS, Zonderland HM, Tilanus HW, van Pel R, Schutte HE. Ultrasound and ultrasound-guided fine needle aspiration biopsy of supraclavicular lymph nodes in patients with esophageal carcinoma. Cancer. 1991;67:585. doi: 10.1002/1097-0142(19910201)67:3<585::aid-cncr2820670310>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [17].Van Overhagen H, Lameris JS, Berger MY, et al. Improved assessment of supraclavicular and abdominal metastases in oesophageal and gastro-oesophageal junction carcinoma with the combination of ultrasound and computed tomography. Br J Radiol. 1993;66:203. doi: 10.1259/0007-1285-66-783-203. [DOI] [PubMed] [Google Scholar]
- [18].Ahuja A, Ying M, King W, Metreweli C. A practical approach to ultrasound of cervical lymph nodes. J Laryngol Otol. 1997;111:245. doi: 10.1017/s0022215100137004. [DOI] [PubMed] [Google Scholar]
- [19].Ying M, Ahuja AT, Evans R, King W, Metreweli C. Cervical lymphadenopathy: sonographic differentiation between tuberculous nodes and nodal metastases from non-head and neck carcinomas. J Clin Ultrasound. 1998;26:383. doi: 10.1002/(sici)1097-0096(199810)26:8<383::aid-jcu2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [20].Sugama Y, Kitamura S. Ultrasonographic evaluation of neck and supraclavicular lymph nodes metastasized from lung cancer. Intern Med. 1992;31:160. doi: 10.2169/internalmedicine.31.160. [DOI] [PubMed] [Google Scholar]
- [21].Yao ZH, Wu AR. Supraclavicular lymph node metastasis from carcinoma of the uterine cervix after radiotherapy – analysis of 219 patients. Chung Hua Chung Liu Tsa Chih. 1988;10:230. [PubMed] [Google Scholar]
- [22].Kiricuta IC, Willner J, Kolbl O, Bohndorf W. The prognostic significance of the supraclavicular lymph node metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 1994;28:387. doi: 10.1016/0360-3016(94)90062-0. [DOI] [PubMed] [Google Scholar]
- [23].Cervin JR, Silverman JF, Loggie BW, Geisinger KR. Virchow's node revisited. Analysis with clinicopathologic correlation of 152 fine-needle aspiration biopsies of supraclavicular lymph nodes. Arch Pathol Lab Med. 1995;119:727. [PubMed] [Google Scholar]
- [24].Ahuja A, Ying M, Evans R, King W, Metreweli C. The application of ultrasound criteria for malignancy in differentiating tuberculous cervical adenitis from metastatic nasopharyngeal carcinoma. Clin Radiol. 1995;50:391. doi: 10.1016/s0009-9260(05)83136-8. [DOI] [PubMed] [Google Scholar]
- [25].Swartz JD, Yussen PS, Popky GL. Imaging of the neck: nodal disease. Crit Rev Diagn Imaging. 1991;31:413. [PubMed] [Google Scholar]
- [26].Ahuja AT, Chow L, Chick W, King W, Metreweli C. Metastatic cervical nodes in papillary carcinoma of the thyroid: ultrasound and histological correlation. Clin Radiol. 1995;50:229. doi: 10.1016/s0009-9260(05)83475-0. [DOI] [PubMed] [Google Scholar]
- [27].Attie JN, Setzin M, Klein I. Thyroid carcinoma presenting as an enlarged cervical lymph node. Am J Surg. 1993;166:428. doi: 10.1016/s0002-9610(05)80348-4. [DOI] [PubMed] [Google Scholar]
- [28].De Jong SA, Demeter JG, Jarosz H, Lawrence AM, Paloyan E. Primary papillary thyroid carcinoma presenting as cervical lymphadenopathy: the operative approach to the “lateral aberrant thyroid”. Am Surg. 1993;59:172. [PubMed] [Google Scholar]
- [29].Ahuja A, Ying M, Yang WT, Evans R, King W, Metreweli C. The use of sonography in differentiating cervical lymphomatous lymph nodes from cervical metastatic lymph nodes. Clin Radiol. 1996;51:186. doi: 10.1016/s0009-9260(96)80321-7. [DOI] [PubMed] [Google Scholar]
- [30].Hajek PC, Salomonowitz E, Turk R, Tscholakoff D, Kumpan W, Czembirek H. Lymph nodes of the neck: evaluation with US. Radiology. 1986;158:739. doi: 10.1148/radiology.158.3.3511503. [DOI] [PubMed] [Google Scholar]
- [31].Shozushima M, Suzuki M, Nakasima T, Yanagisawa Y, Sakamaki K, Takeda Y. Ultrasound diagnosis of lymph node metastasis in head and neck cancer. Dentomaxillofac Radiol. 1990;19:165. doi: 10.1259/dmfr.19.4.2097226. [DOI] [PubMed] [Google Scholar]
- [32].Solbiati L, Rizzatto G, Bellotti E, Montali G, Cioffi V, Croce F. High-resolution sonography of cervical lymph nodes in head and neck cancer: criteria for differentiation of reactive versus malignant nodes. Radiology. 1988;169(P):113. [Google Scholar]
- [33].Ying M, Ahuja A, Metreweli C. Diagnostic accuracy of sonographic criteria for evaluation of cervical lymphadenopathy. J Ultrasound Med. 1998;17:437. doi: 10.7863/jum.1998.17.7.437. [DOI] [PubMed] [Google Scholar]
- [34].Ahuja A, Leung SF, Ying M, Metreweli C. Echography of metastatic nodes treated by radiotherapy. J Laryngol Otol. 1999;113:993. doi: 10.1017/s0022215100145797. [DOI] [PubMed] [Google Scholar]
- [35].Tohnosu N, Onoda S, Isono K. Ultrasonographic evaluation of cervical lymph node metastases in esophageal cancer with special reference to the relationship between the short to long axis ratio (S/L) and the cancer content. J Clin Ultrasound. 1989;17:101. doi: 10.1002/jcu.1870170206. [DOI] [PubMed] [Google Scholar]
- [36].Ying M, Ahuja A, Brook F, Brown B, Metreweli C. Sonographic appearance and distribution of normal cervical lymph nodes in a Chinese population. J Ultrasound Med. 1996;15:431. doi: 10.7863/jum.1996.15.6.431. [DOI] [PubMed] [Google Scholar]
- [37].Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992;183:215. doi: 10.1148/radiology.183.1.1549675. [DOI] [PubMed] [Google Scholar]
- [38].Johnson JT. A surgeon looks at cervical lymph nodes. Radiology. 1990;175:607. doi: 10.1148/radiology.175.3.2188292. [DOI] [PubMed] [Google Scholar]
- [39].van den Brekel MW, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177:379. doi: 10.1148/radiology.177.2.2217772. [DOI] [PubMed] [Google Scholar]
- [40].Evans RM, Ahuja A, Metreweli C. The linear echogenic hilus in cervical lymphadenopathy – a sign of benignity or malignancy? Clin Radiol. 1993;47:262. doi: 10.1016/s0009-9260(05)81135-3. [DOI] [PubMed] [Google Scholar]
- [41].Sakai F, Kiyono K, Sone S, et al. Ultrasonic evaluation of cervical metastatic lymphadenopathy. J Ultrasound Med. 1988;7:305. doi: 10.7863/jum.1988.7.6.305. [DOI] [PubMed] [Google Scholar]
- [42].Rubaltelli L, Proto E, Salmaso R, Bortoletto P, Candiani F, Cagol P. Sonography of abnormal lymph nodes in vitro: correlation of sonographic and histologic findings. Am J Roentgenol. 1990;155:1241. doi: 10.2214/ajr.155.6.2122673. [DOI] [PubMed] [Google Scholar]
- [43].Ying M, Ahuja A, Brook F, Metreweli C. Vascularity and grey-scale sonographic features of normal cervical lymph nodes: variations with nodal size. Clin Radiol. 2001;56:416. doi: 10.1053/crad.2000.0680. [DOI] [PubMed] [Google Scholar]
- [44].Solbiati L, Cioffi V, Ballarati E. Ultrasonography of the neck. Radiol Clin North Am. 1992;30:941. [PubMed] [Google Scholar]
- [45].Ahuja A, Ying M, Leung SF, Metreweli C. The sonographic appearance and significance of cervical metastatic nodes following radiotherapy for nasopharyngeal carcinoma. Clin Radiol. 1996;51:698. doi: 10.1016/s0009-9260(96)80241-8. [DOI] [PubMed] [Google Scholar]
- [46].Ying M, Ahuja A, Brook F. Repeatability of power Doppler sonography of cervical lymph nodes. Ultrasound Med Biol. 2002;28:737. doi: 10.1016/s0301-5629(02)00523-9. [DOI] [PubMed] [Google Scholar]
- [47].Ariji Y, Kimura Y, Hayashi N, et al. Power Doppler sonography of cervical lymph nodes in patients with head and neck cancer. Am J Neuroradiol. 1998;19:303. [PMC free article] [PubMed] [Google Scholar]
- [48].Na DG, Lim HK, Byun HS, Kim HD, Ko YH, Baek JH. Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. Am J Roentgenol. 1997;168:1311. doi: 10.2214/ajr.168.5.9129432. [DOI] [PubMed] [Google Scholar]
- [49].Wu CH, Chang YL, Hsu WC, Ko JY, Sheen TS, Hsieh FJ. Usefulness of Doppler spectral analysis and power Doppler sonography in the differentiation of cervical lymphadenopathies. Am J Roentgenol. 1998;171:503. doi: 10.2214/ajr.171.2.9694484. [DOI] [PubMed] [Google Scholar]
- [50].Ying M, Ahuja A, Brook F, Metreweli C. Power Doppler sonography of normal cervical lymph nodes. J Ultrasound Med. 2000;19:511. doi: 10.7863/jum.2000.19.8.511. [DOI] [PubMed] [Google Scholar]
- [51].Steinkamp HJ, Maurer J, Cornehl M, Knobber D, Hettwer H, Felix R. Recurrent cervical lymphadenopathy: differential diagnosis with color- duplex sonography. Eur Arch Otorhinolaryngol. 1994;251:404. doi: 10.1007/BF00181966. [DOI] [PubMed] [Google Scholar]
- [52].Ahuja AT, Ying M, Ho SS, Metreweli C. Distribution of intranodal vessels in differentiating benign from metastatic neck nodes. Clin Radiol. 2001;56:197. doi: 10.1053/crad.2000.0574. [DOI] [PubMed] [Google Scholar]
- [53].Maurer J, Willam C, Schroeder R, et al. Evaluation of metastases and reactive lymph nodes in Doppler sonography using an ultrasound contrast enhancer. Invest Radiol. 1997;32:441. doi: 10.1097/00004424-199708000-00002. [DOI] [PubMed] [Google Scholar]
- [54].Dragoni F, Cartoni C, Pescarmona E, et al. The role of high resolution pulsed and color Doppler ultrasound in the differential diagnosis of benign and malignant lymphadenopathy: results of multivariate analysis. Cancer. 1999;85:2485. doi: 10.1002/(sici)1097-0142(19990601)85:11<2485::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- [55].Chang DB, Yuan A, Yu CJ, Luh KT, Kuo SH, Yang PC. Differentiation of benign and malignant cervical lymph nodes with color Doppler sonography. Am J Roentgenol. 1994;162:965. doi: 10.2214/ajr.162.4.8141027. [DOI] [PubMed] [Google Scholar]
- [56].Adibelli ZH, Unal G, Gul E, Uslu F, Kocak U, Abali Y. Differentiation of benign and malignant cervical lymph nodes: value of B-mode and color Doppler sonography. Eur J Radiol. 1998;28:230. doi: 10.1016/s0720-048x(97)00174-5. [DOI] [PubMed] [Google Scholar]
- [57].Ishii J, Fujii E, Suzuki H, Shinozuka K, Kawase N, Amagasa T. Ultrasonic diagnosis of oral and neck malignant lymphoma. Bull Tokyo Med Dent Univ. 1992;39:63. [PubMed] [Google Scholar]
- [58].Lee YY, Van Tassel P, Nauert C, North LB, Jing BS. Lymphomas of the head and neck: CT findings at initial presentation. Am J Roentgenol. 1987;149:575. doi: 10.2214/ajr.149.3.575. [DOI] [PubMed] [Google Scholar]
- [59].Bruneton JN, Normand F, Balu-Maestro C, et al. Lymphomatous superficial lymph nodes: US detection. Radiology. 1987;165:233. doi: 10.1148/radiology.165.1.3306785. [DOI] [PubMed] [Google Scholar]
- [60].Ho SS, Ahuja AT, Yeo W, Chan TC, Kew J, Metreweli C. Longitudinal colour Doppler study of superficial lymph nodes in non- Hodgkin's lymphoma patients on chemotherapy. Clin Radiol. 2000;55:110. doi: 10.1053/crad.1999.0229. [DOI] [PubMed] [Google Scholar]
- [61].Ying MTC. Department of Optometry and Radiography, The Hong Kong Polytechnic University, Hong Kong; 1996. Ultrasound evaluation of cervical lymph nodes in a Chinese population. p. 235. MPhil thesis. [Google Scholar]
- [62].Callen PW, Marks WM. Lymphomatous masses simulating cysts by ultrasonography. J Can Assoc Radiol. 1979;30:244. [PubMed] [Google Scholar]
- [63].Bruneton JN, Roux P, Caramella E, Demard F, Vallicioni J, Chauvel P. Ear, nose, and throat cancer: ultrasound diagnosis of metastasis to cervical lymph nodes. Radiology. 1984;152:771. doi: 10.1148/radiology.152.3.6463260. [DOI] [PubMed] [Google Scholar]
- [64].Ahuja AT, Ying M, Yuen HY, Metreweli C. ‘Pseudocystic’ appearance of non-Hodgkin's lymphomatous nodes: an infrequent finding with high-resolution transducers. Clin Radiol. 2001;56:111. doi: 10.1053/crad.2000.0642. [DOI] [PubMed] [Google Scholar]
- [65].Steinkamp HJ, Mueffelmann M, Bock JC, Thiel T, Kenzel P, Felix R. Differential diagnosis of lymph node lesions: a semiquantitative approach with colour Doppler ultrasound. Br J Radiol. 1998;71:828. doi: 10.1259/bjr.71.848.9828794. [DOI] [PubMed] [Google Scholar]
- [66].Ying MTC. Department of Optometry and Radiography, The Hong Kong Polytechnic University, Hong Kong; 2002. Power Doppler sonography of normal and abnormal cervical lymph nodes; p. 236. in PhD thesis. [Google Scholar]
- [67].Moritz JD, Ludwig A, Oestmann JW. Contrast-enhanced color Doppler sonography for evaluation of enlarged cervical lymph nodes in head and neck tumors. Am J Roentgenol. 2000;174:1279. doi: 10.2214/ajr.174.5.1741279. [DOI] [PubMed] [Google Scholar]
- [68].Rubaltelli L, Khadivi Y, Tregnaghi A, et al. Evaluation of lymph node perfusion using continuous mode harmonic ultrasonography with a second-generation contrast agent. J Ultrasound Med. 2004;23:829. doi: 10.7863/jum.2004.23.6.829. [DOI] [PubMed] [Google Scholar]
- [69].Lee YLP, Antonio GE, Ho SSY, et al. Serial dynamic sonographic contrast enhancement changes in cervical lymph nodes: before and after treatment for lymphoma.. International & 9th National Head and Neck Cancer Conference; 7–11 September 2007; Urumqi, China. 2007. [Google Scholar]