Abstract
The management of thyroid cancer has been controversial and, as a result, the routine use of imaging in this disease, especially for pre-operative staging, has lagged behind other head and neck cancers. However, as more is known about the natural history of thyroid cancer, the role of imaging is becoming more established. This review focuses on how imaging now influences the staging and management of the primary cancer, nodal metastases and distant metastases. This is followed by a brief review of the role of imaging in planning post-operative radiotherapy and post-treatment surveillance.
Keywords: Thyroid cancer, imaging, MRI, ultrasound, CT, PET, staging
Introduction
Thyroid cancer has been increasing, partly as a result of the improved detection of subclinical cancer by imaging, and the incidence is now around 9 per 100,000[1],[2]. The four most common primary thyroid cancers, papillary (PC), follicular (FC), medullary (MC), and anaplastic (AC) carcinoma are discussed in this article with the emphasis on PC which accounts for up to 80% of primary thyroid cancers (for the background of each of these cancers, see Table 1). Papillary carcinoma is usually an indolent cancer but it can take an aggressive course in a small number of patients. As a result of this unpredictable behaviour, there has been controversy in the management of thyroid cancer and several different staging systems have developed. Currently the 6th edition of the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) is the most widely accepted. This classification is based on the TNM staging system and takes into account patient age (cut off 45 years) for PC/FC (Table 2). Irrespective of patient age, MC follows the PC/FC classification for patients 45 years and older, while all patients with AC are considered to have T4 primary tumours and hence advanced stage disease.
Table 1.
Features of thyroid cancer
| Carcinoma | Frequency (%) | Features |
|---|---|---|
| Papillary (PC) | 80 | Generally an indolent tumour with survival >90% at 10 years but a small number are aggressive. Propensity to spread to nodes |
| Follicular (FC) | 12–20 | Often grouped together with PC in studies of differentiated thyroid cancer. Propensity for haematogeneous spread. Hurtle cell carcinoma is a variant of FC which has a more aggressive course |
| Medullary (MC) | 5 | Higher mortality than the other differentiated cancers with a propensity for both lymphatic and haematogeneous spread. Familial or associated with the multiple endocrine neoplasia (MEN) syndromes in 25% of patients. Pre-operative biochemical screening for parathyroid and adrenal tumours (phaeochromocytomas are removed before thyroidectomy). Genetic screening is extended to family members in the inherited form |
| Anaplastic (AC) | 2 | Highly aggressive undifferentiated carcinoma with extrathyroidal extension, nodal and distant metastases. Mean survival only 4–12 months. Often arises in the elderly with multinodular goitre and is thought to develop from pre-existing PC/FC |
Table 2.
Sixth edition of the AJCC/UICC staging (papillary and follicular carcinoma)
| T | N | M | |
|---|---|---|---|
| Under 45 years | |||
| Stage 1 | Any T | Any N | M0 |
| Stage II | Any T | Any N | M1 |
| 45 years and older | |||
| Stage 1 | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1 | N1a | M0 | |
| T2 | N1a | M0 | |
| T3 | N1a | M0 | |
| Stage IVA | T4a | N0 | M0 |
| T4a | N1a | M0 | |
| T1 | N1b | M0 | |
| T2 | N1b | M0 | |
| T3 | N1b | M0 | |
| T4a | N1b | M0 | |
| Stage IVB | T4b | Any N | M0 |
| Stage IVC | Any T | Any N | M1 |
Primary thyroid cancer
Imaging primary thyroid cancer
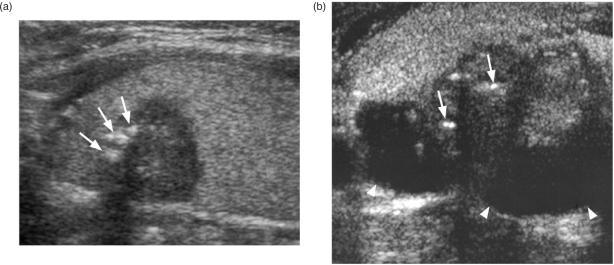
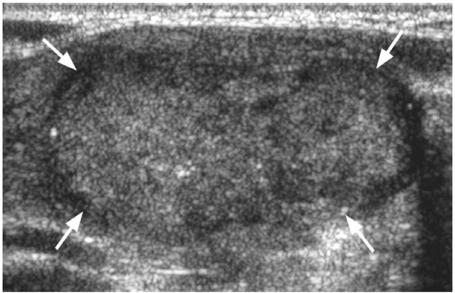
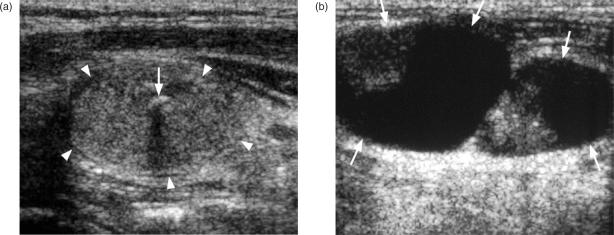
Ultrasound and ultrasound-guided fine needle aspiration cytology (FNAC) or core biopsy is the investigation of choice for diagnosing primary thyroid cancers. However, thyroid cancers can have a variable appearance on ultrasound and differentiation from benign nodules may sometimes be difficult, some nodules remaining indeterminate on both ultrasound and ultrasound-guided biopsy. A full description of the ultrasound features of thyroid nodules is beyond the scope of this article (for a full description of the ultrasound appearance of benign and malignant thyroid nodules readers are directed to the review of thyroid nodules by Wong et al.[3]), but in general benign nodules are frequently solid hyperechoic/isoechoic nodules or cystic, and may show ultrasound features such as comet tail artefact and rim calcification, while malignant nodules are frequently solid hypoechoic nodules with micro-calcification and blurred margins and a vascular pattern in which intranodular flow is more dominant than perinodular flow with a high resistive index[4],[5]. The most common thyroid cancer, PC, usually produces a solid hypoechoic nodule with a well or poorly defined margin, and up to 80% display small foci of punctate calcification[6] (Fig. 1a). This punctate calcification is responsible for much of the success of ultrasound in detecting small thyroid cancers[7], even in those patients with impalpable nodules or co-existing multinodular goitre. Papillary carcinoma may occasionally form cysts which can be the dominant feature (Fig. 1b)[8]. The other primary thyroid carcinomas most commonly present as solid hypoechoic nodules or masses (Fig. 2). Medullary carcinoma sometimes shows punctate calcification, calcification is rare in FC, and anaplastic carcinomas are usually large (average size 6 cm) necrotic tumours at diagnosis that extend outside the thyroid gland[9].
Figure 1.
Ultrasound of a papillary carcinoma (a) small hypoechoic solid nodule with punctate calcification (arrows), (b) predominantly cystic nodule (arrow heads) with smaller solid component with punctate calcification (arrows).
Figure 2.
Ultrasound of solid hypoechoic follicular thyroid cancer (arrows).
Computed tomography (CT) and magnetic resonance imaging (MRI) are inferior to ultrasound for characterizing thyroid nodules, and small carcinomas that are readily identified by ultrasound, may be undetectable[10],[11]. The main role of CT and MRI is to demonstrate extrathyroidal tumour extension (see staging below). When PC is demonstrated by computed tomography, punctate calcification or cystic components may be found[11] while on MRI the cystic components can have a high T1 signal intensity.
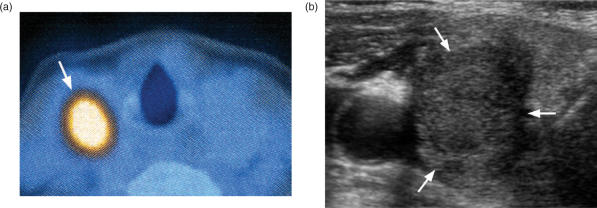
All four thyroid cancers have been shown to take up [18F]fluorodeoxyglucose (FDG), especially the less differentiated tumours. At present, the role of [18F]FDG positron emission tomography (PET) in primary thyroid cancer is unclear, but it appears to have a high negative predictive value, and so a role has been proposed to use a negative [18F]FDG PET in the inconclusive thyroid nodule to reduce the number of unnecessary thyroid resections for benign nodules[12],[13]. On the other hand, while [18F]FDG PET is a sensitive technique, it has a low specificity caused by uptake in non-malignant conditions such as benign thyroid nodules and thyroiditis. In daily clinical practice this causes problems because 1% of patients undergoing whole body staging for other cancers have ‘incidental’ uptake in the thyroid on [18F]FDG PET[14],[15]. About one-third of these patients turn out to have a malignant nodule, usually a primary thyroid cancer (Fig. 3a,b)[15]. Thyroid cancers tend to have a higher standardized uptake value (SUV) than benign disease but there is overlap, and ultrasound and biopsy are often required to make the diagnosis[12],[15].
Figure 3.
Thyroid nodule (arrows) (a) positive on [18F]FDG PET staging scan for a patient with a gastro-intestinal stromal tumour (GIST) and lung cancer; (b) ultrasound and FNAC confirmed a primary papillary carcinoma.
Staging thyroid cancer and impact of imaging on patient management (Table 3)
Table 3.
Sixth edition of the AJCC/UICC staging for primary tumour (T stage) (papillary, follicular, medullary and anaplastic carcinoma)
| TX | Primary tumour cannot be assessed |
| T0 | No evidence of primary tumour |
| T1 | Tumour 2 cm or less in greatest dimension limited to the thyroid |
| T2 | Tumour more than 2 cm but not more than 4 cm in greatest dimension limited to the thyroid |
| T3 | Tumour more than 4 cm in greatest dimension limited to the thyroid or any tumour with minimal extrathyroid extension (e.g., extension to sternothyroid muscle or perithyroid soft tissues) |
| T4a | Tumour of any size extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve |
| T4b | Tumour invades prevertebral fascia or encases carotid artery or mediastinal vessels |
| All anaplastic carcinomas are considered T4 tumours | |
| T4a | Intrathyroid anaplastic carcinoma – surgically resectable |
| T4b | Extrathyroid anaplastic carcinoma – surgically unresectable |
Stage T1–T2
When small thyroid cancers are confined to the gland, ultrasound is sufficient for staging and no further imaging of the primary is required, unless the tumour abuts ‘blind spots’ on ultrasound such as the midline posterior to the trachea. At present, imaging has little impact on the management of T1 and T2 stage cancer, but there are two controversial areas where ultrasound may become more important in the future. The first of these concerns small T1 tumours. Until recently T1 stage disease was defined as a cancer of ≤1 cm in size (the so-called micropapillary carcinoma), but in the current addition of the AJCC/UICC staging system this has been raised to 2 cm (Table 1). This change brings thyroid staging in line with other head and neck cancers, where 2 cm and 4 cm are the important thresholds for the size of primary tumours. However, this move has been controversial because there is evidence to show that patients with cancers of ≤1 cm have a better prognosis than those with cancers between 1 cm and 2 cm[16]. As a result, patients with ≤1 cm PC sometimes undergo lobectomy rather than total thyroidectomy[17]. This is especially the case when an unsuspected PC is found in the histology specimen of a patient who has undergone a lobectomy for multinodular goitre. In these patients completion thyroidectomy is often avoided. Ultrasound of the thyroid now provides a further method by which these subclinical ‘micropapillary’ cancers are detected, raising the question of whether these small ultrasound detected cancers should undergo lobectomy rather than total thyroidectomy.
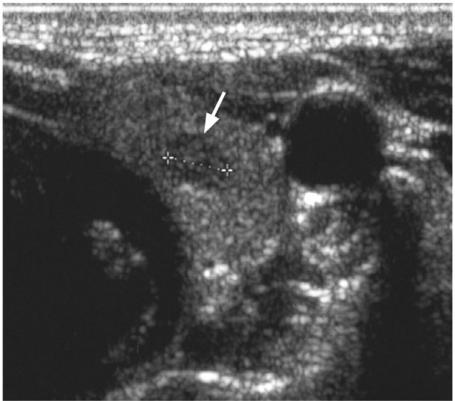
The second area of controversy is multifocal disease which is common in thyroid cancer, being found in PC (30% involving both lobes equally), MC (especially the familial form) and FC (8%). Multifocal disease can produce macroscopic deposits on ultrasound, the largest deposit being designated the primary cancer. Unfortunately many of the deposits are very small and frequently 2–3 mm hypoechoic nodules are identified by ultrasound which are too small to characterise even with ultrasound-guided FNAC (Fig. 4). The majority of patients with thyroid cancer undergo a total thyroidectomy anyway so pre-operative identification of possible multifocal disease on ultrasound will not change management. Where identification of these deposits could affect management is in those patients who are scheduled to undergo, or have already undergone, a lobectomy and are found to have small deposits in the opposite lobe on ultrasound. However, further management even in these cases is unclear because the clinical significance of multifocal disease is unknown.
Figure 4.
Ultrasound of a patient with multifocal papillary carcinoma with a small hypoechoic tumour deposit (arrow) in the opposite lobe to the primary thyroid cancer.
Stage T3–T4
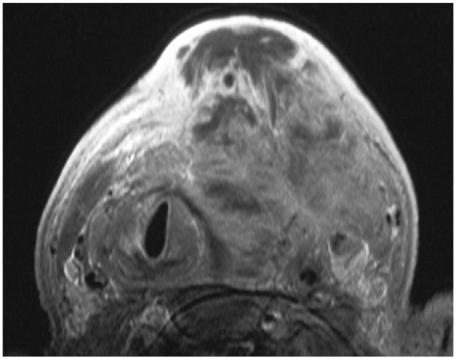
MRI or CT is required to image the full extent of large thyroid cancers or cancers with extrathyroidal extension, especially T4 tumours (Fig. 5). These modalities may also be required when the primary tumour abuts ‘blind spots’ on ultrasound such as the midline posterior to the trachea. MRI is preferred to CT for staging differentiated carcinomas because it avoids the use of iodinated contrast agents which interfere with the subsequent use of iodine-131/123 for whole body scintigraphy (WBS) and treatment of residual disease and distant metastases. Some patients are unable to tolerate an MR examination because of swallowing and breathing difficulties caused by extensive tumours. In these patients, as well as those with undifferentiated cancers who will not receive 131/123I, CT can be performed instead.
Figure 5.
Axial T1-weighted MRI post-contrast of a large anaplastic carcinoma with extensive extrathyroidal tumour extension.
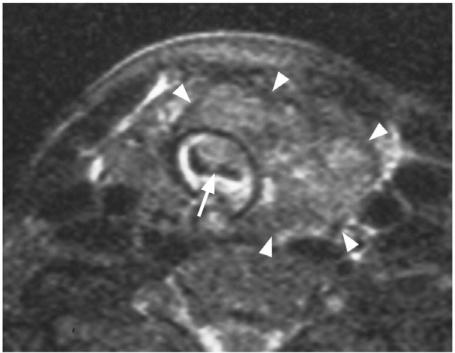
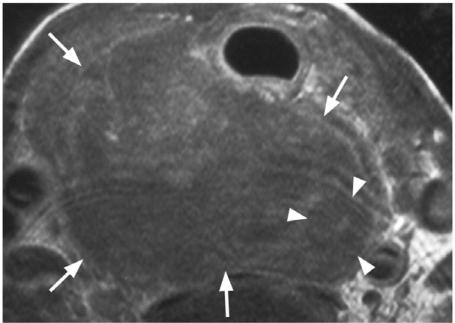
One of the main roles of imaging the primary cancer is to alert the surgeon to the presence of extrathyroidal spread into major structures of the neck. Invasion of these structures may require major reconstructive surgery or render the patient inoperable (Table 3). Tumour invasion into the trachea and oesophagus (T4a) are two sites where major reconstructive surgery may be required, but they are also two sites that are difficult to assess by imaging. Using MRI, tumour invasion into the trachea or oesophagus can be identified with 100% specificity when tumour is found in the inner wall/lumen (Fig. 6)[18],[19] and when tumour abuts 180° of the trachea[18] or 270° of the oesophagus[19] (Fig. 7). Unfortunately most patients with invasion of these structures do not show these advanced signs and other more sensitive imaging signs, such as a focal area of abnormality in the outer wall, result in false positive results[18–20]. The recurrent laryngeal nerve can be invaded by thyroid cancers leading to paralysis of the vocal cords. In these cases loss of the fatty tissue in the tracheo-oesphageal groove on one or more contiguous MRI sections has a sensitivity of 94% and specificity of 82% for tumour invasion of the nerve[21]. Cancers that are unresectable fall into the T4b category. Cancers surrounding >270° of the carotid artery or mediastinal vessels are unlikely to be resectable[22]. Unlike major artery invasion, invasion of the internal jugular vein, which is also well shown by ultrasound, does not influence staging or surgical resectability invasion of the prevertebral fascia tends to be diagnosed at the time of surgery rather than by imaging.
Figure 6.
Axial T2-weighted MRI with fat saturation showing a papillary carcinoma (arrow heads) invading into the tracheal lumen (arrow).
Figure 7.
Axial T1-weighted MRI post-contrast showing an anaplastic carcinoma (arrows) which is surrounding (>270°) and invading the oesophagus (arrow heads).
Nodal thyroid metastases
Imaging nodal metastases
Papillary carcinoma has a high propensity to spread to cervical nodes. The reported incidence varies from 30 to 90%, being higher from surgical series with systematic lymph node excision[23]. Medullary carcinoma (50%) and AC (40%) also have a high propensity to spread to neck nodes, while lymphatic spread is less common with FC (10%)[17]. Once again ultrasound is the method of choice for imaging nodal metastases. It is the best modality for assessing abnormalities in the internal architecture, it can be combined with ultrasound-guided FNAC, and the nodes and primary tumour can be evaluated in the same examination. Ultrasound features of metastatic nodes from thyroid cancer are similar to those from other head and neck cancers and include a round shape, loss of the nodal hilum, peripheral vascularity, necrosis and extranodal tumour spread, the latter two features being common in nodes from AC. In addition nodes from PC show calcification (50–69%), increased echogenicity (87%) and cysts (20%), the latter may be the dominant feature with some metastatic nodes being entirely cystic[24],[25] (Fig. 8a,b). Nodes of PC are frequently small and using a minimum diameter of 7 mm for level II and 6 mm for other levels on ultrasound, Rosario et al.[25] reported an accuracy of 89%. Ultrasound-guided biopsy improves diagnostic accuracy but patients with PC frequently have multiple very small nodes of less than 5 mm and unless punctate calcification is identified, it can be difficult to establish a pre-operative diagnosis. Diagnosis is further hampered by the fact that metastatic nodes from PC may show no change on serial imaging over several years.
Figure 8.
Ultrasound of metastatic nodes from papillary carcinoma which are (a) solid and hyperechoic (arrow heads) with punctate calcification (arrow), (b) predominantly cystic (arrows).
Although ultrasound is the primary investigation for PC metastatic nodes, both CT and MRI have imaging features that are helpful in diagnosis. Calcification can be seen on CT as small foci of nodal calcification (Fig. 9). Nodes may be cystic and on MRI can exhibit a high signal on T1-weighted MR images as a result of the thyroglobulin content[10],[26] (Fig. 10). Nodal calcification on CT and high T1 signal nodal cysts on MRI are both unusual features of nodes and when found in a patient being investigated for lymphadenopathy should lead to a hunt for an unknown primary thyroid cancer.
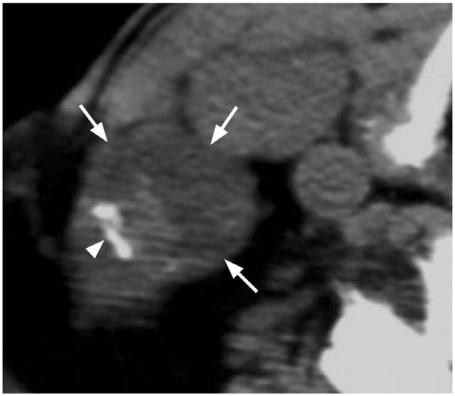
Figure 9.
Axial plain CT of a metastatic node from papillary carcinoma (arrows) with calcification (arrow head) which is relatively rare in other causes of lymphadenopathy.
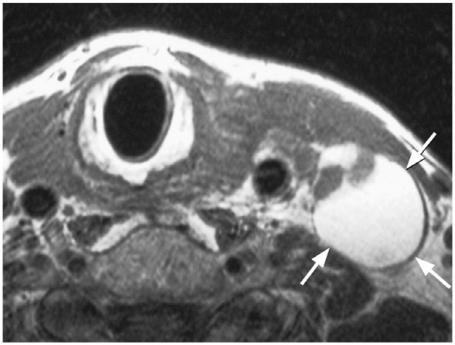
Figure 10.
Axial T1-weighted MRI of a metastatic node from papillary carcinoma which has a predominantly cystic component of high T1 signal intensity (arrows).
An 131/123I whole body scan is used following surgery to identify residual or recurrent nodal metastases in the neck and mediastinum. It can miss some iodine negative nodes which tend to be those that arise from less differentiated thyroid cancers. Nodal metastases in the neck and superior mediastinum show uptake on [18F]FDG PET/CT which can demonstrate nodes that are iodine negative. However, in common with all imaging modalities, both false positive and negative results are encountered and [18F]FDG PET/CT may not provide additional benefit over ultrasound and contrast enhanced CT[27].
Staging nodal metastases and impact of imaging on patient management (Table 4)
Table 4.
Sixth edition of the AJCC/UICC for staging regional lymph nodes (N stage) (papillary, follicular, medullary and anaplastic carcinoma)
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| N1a | Metastasis to Level VI (pretracheal, paratracheal and prelaryngeal/Delphian lymph nodes) |
| N1b | Metastasis to unilateral, bilateral, or contralateral cervical or superior mediastinal lymph nodes |
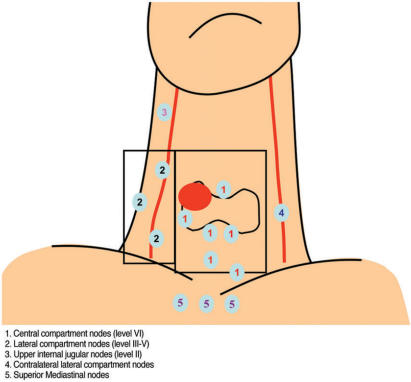
The clinical significance and management of cervical nodal metastases from PC is controversial. In the past, treatment has ranged from a modified radical neck dissection to a more conservative approach involving ‘cherry picking’ nodes at surgery and post-operative radioactive iodine for small nodes. However, with mounting evidence that nodal metastases are a significant risk factor for local recurrence and survival[28–30], selective neck dissection is becoming the standard treatment[31]. As a result imaging plays a more crucial role in planning surgery. A study by Stulak et al.[32] has shown that ultrasound has a sensitivity of 83% and specificity of 98% for detecting nodal metastases at presentation and alters the operative procedure even in those patients with palpable nodes. In order to understand how imaging influences management, one needs to be aware of the patterns of nodal spread from thyroid cancer (Fig. 11).
Figure 11.
Patterns of nodal metastases from differentiated thyroid cancer.
Central compartment nodes (stage N1a)
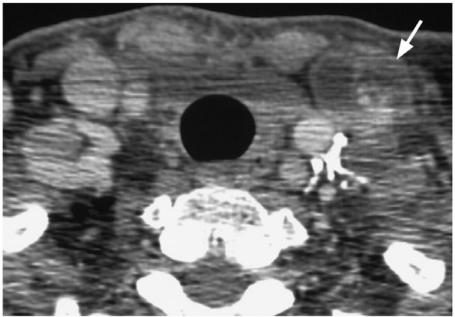
The first echelon of nodes to be involved from thyroid cancer are those in the central compartment (level VI) which includes the periglandular, paratracheal, paralaryngeal, and prelaryngeal nodes on the ipsilateral and sometimes the contralateral side. These nodes are difficult to identify by ultrasound, but for PC, a central compartment dissection is usually routinely performed in all patients undergoing a total thyroidectomy. The exception to this is removal of nodes in the paratracheal and paraoesphageal regions inferior to the thyroid around the level of the suprasternal notch. These nodes may not be removed in a standard central compartment resection and are believed to be responsible for some of the so-called ‘thyroid bed’ recurrences, which invade the trachea and oesphagus leading to a high mortality rate. These nodes may also provide an anatomical route by which nodes spread into the superior mediastinum[33]. Paratracheal nodes in the lower neck/cervicothoracic junction can be visualized by tilting the ultrasound probe down behind the sternum and can also be demonstrated by MRI or CT (Fig. 12a,b).
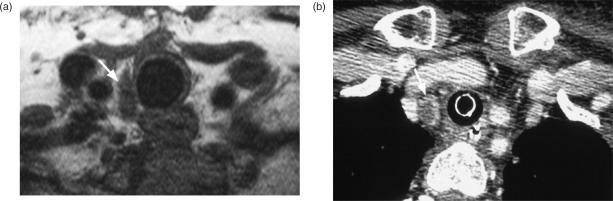
Figure 12.
Paratracheal nodes from papillary carcinoma (a) just inferior to the level of the thyroid demonstrated on axial T1-weighted MRI (arrow) and (b) more inferiorly at the suprasternal notch on axial CT post-contrast (arrow).
Lateral compartment nodes (stage N1b)
The lateral compartment is believed to be the second echelon of nodal spread from thyroid cancer, although not infrequently this may be the first site of lymphatic spread. A study by Machens et al.[34] has shown that this group of nodes are involved almost as frequently as the central group of nodes. Nodal metastases in the lateral compartment preferentially involve nodes in the mid and lower internal jugular chain (III and IV) but may spread into the posterior triangle (V) and supraclavicular fossa. This compartment is not dissected routinely and identification of metastatic nodes in the ipsilateral compartment of the neck by imaging will lead to a lateral compartment dissection involving nodal levels III, IV and V[35], dissection is not necessary in those patients with no nodes on ultrasound[36]. Less frequently nodes spread up to the upper internal jugular chain (II) which entails a more extensive surgical approach. Submandibular and submental nodal metastases (level 1) are rare at diagnosis unless there are other nodes present[37]. Nodal metastases to the contralateral neck are not uncommon from MC[34] but are believed to be rare with PC. However, with more widespread use of ultrasound for pre-operative staging, the identification of contralateral nodal metastases from PC is likely to prove more frequent than previously recognized.
Superior mediastinum compartment nodes (stage N1b)
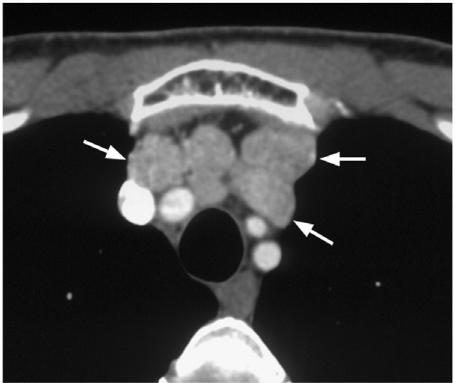
The true incidence of mediastinal nodes is largely unknown, although it is known that MC spreads more frequently to this region than PC (Fig. 13). These nodes can be resected via the transcervical route which has a low complication rate apart from hypoparathyroidism[33]. Pre-operative indications for scanning the mediastinum with MRI/CT are not well established but those patients known to be at greatest risk of mediastinal nodes include those with primary tumours invading the superior mediastinum, extensive central compartment nodes (possibly including lower paratracheal nodes identified by neck ultrasound), nodes in the lower lateral neck and contralateral neck nodes[33],[38] and, in the case of MC, re-operation for cervical nodes and T4 tumours[39]. At present, imaging of this region is usually only performed post-operatively using 131/123I WBS.
Figure 13.
Axial contrast-enhanced CT scan showing metastatic nodes from a medullary carcinoma in the superior mediastinum (arrows).
Distant thyroid metastases
Imaging and staging distant metastases (Table 5)
Table 5.
Sixth edition of the AJCC/UICC for staging distant metastases (M stage) (papillary, follicular, medullary and anaplastic carcinoma)
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Follicular carcinoma has a greater propensity for haematogeneous spread than PC, the incidence being 21–33% compared to 2–14%[17],[40]. Distant metastases from PC/FC have a more favourable prognosis than most other cancers of the body and patients with distant metastases <45 years are classified as only stage II disease. About 7% of patients with differentiated thyroid cancers have distant metastases at presentation, and in some patients the distant metastasis may be the presenting symptom (Fig. 14)[41]. Distant metastases may also arise many years after the initial diagnosis. Despite the long term survival of patients with well-differentiated cancers the distant metastases are still the major cause of mortality. The most common site for distant metastases from FC/PC are the lung (50%), bone (25%), lung and bone (20%), followed by other sites (5%)[41],[42]. Distant metastases from MC are present in 25% of patients and may also involve the liver where deposits over the liver surface are notoriously difficult to diagnose by imaging and may require laparoscopy and biopsy. Distant metastases from AC (stage IVc) are even more frequent, being found in 40% of patients[9]. Imaging distant metastases is usually performed post-operatively for the differentiated thyroid cancers and pre-operatively for AC. The following are the imaging modalities employed to identify distant metastases.
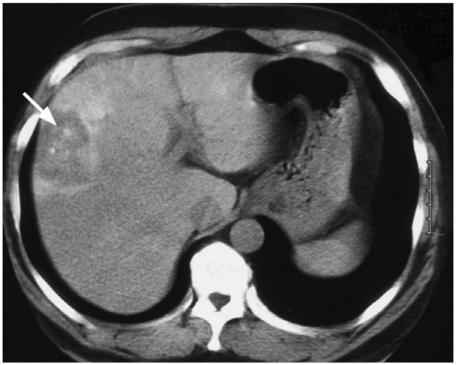
Figure 14.
Patient with a follicular carcinoma who presented with (a) a liver metastasis on CT (arrow) and over the next 10 years went on to develop multiple metastases in the lung and bone.
131/123I whole body scan
131/123I WBS can be used to detect residual locoregional disease in the neck and distant metastases from PC/FC and therefore M staging is performed after surgery (Fig. 15). While the indications for administrating 131/123I may vary among centres, it is commonly administered to a significant proportion of patients apart from those perceived to be at low risk (small localized primary PC or minimally invasive FC). However, such a diagnostic application is limited by the presence of normal residual thyroid remnants, which are usually present after total thyroidectomy, and which show up as uptake in the thyroid bed region on the post-thyroidectomy scan. In fact, it is increasingly popular to administer a high ‘ablative’ dose of 131I (such as 80 mCi), instead of a low diagnostic dose (such as 1–5 mCi), in the post-thyroidectomy patient, to serve the three-fold purpose of ablating thyroid remnants, screening for residual neck nodes and distant metastases, and eradicating any tumour foci which take up radioactive iodine. The high ablative dose has the advantage over a low diagnostic dose of a higher sensitivity of detection of residual tumour. The ablation of normal remnants serves to facilitate the interpretation of the tumour marker thyroglobulin, which could be ‘falsely’ elevated by the presence of residual normal thyroid tissue. 131/123I uptake is reduced by intravenous iodinated contrast agents used for CT and these can interfere with the uptake for at least 1–3 months. Not all thyroid cancers are identified by 131/123I with MC, AC and some less differentiated PC/FC (including some Hurtle cell carcinomas) being iodine-negative.
Figure 15.
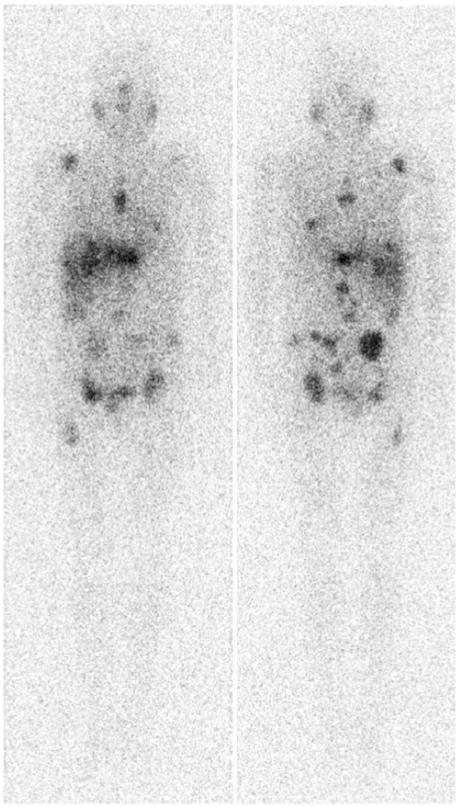
131I WBS of the neck and chest showing uptake in the remnant thyroid (arrow), residual metastatic cervical nodes (curved arrow) and lungs (arrow heads) in a patient with papillary carcinoma (courtesy of Dr Frankie Choi).
Chest X ray and CT thorax
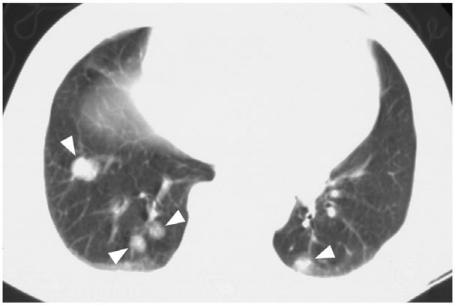
Pulmonary metastases produce a micronodular millary pattern or macronodular pattern with similar frequency (Figs. 16 and 17)[43],[44]. The chest X ray can be normal in one-third of patients with a positive 131I WBS[43], especially in those patients with diffuse military metastases. CT thorax improves the detection of pulmonary metastases but can be also negative in a small number of patients with a positive 131I WBS[45–47]. On the other hand, CT thorax may detect small pulmonary metastases that are not detectable by 131I WBS. In addition CT provides further evaluation of the mediastinal nodes, thoracic cage bony metastases and liver metastases (when the scan is extended down to cover the entire liver). The main role of CT thorax is to detect distant metastases in the post-operative patient with FC/PC who has a negative 131I WBS and chest X-ray but persistently raised thyroglobulin levels. CT thorax also has a role in the post-operative assessment of MC with raised calcitonin levels and the pre-operative staging of AC.
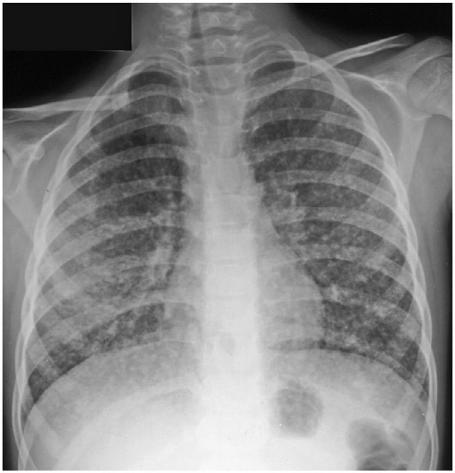
Figure 16.
Chest X-ray showing micronodular pulmonary metastases from a papillary carcinoma.
Figure 17.
Lung windows from an axial CT thorax showing macronodular pulmonary metastases from a patient with papillary carcinoma (arrow heads).
[18F]FDG PET and PET/CT
[18F]FDG PET/CT detects distant metastases from PC/FC, some of which are iodine-negative on 131I WBS[48],[49]. However, it can fail to detect small iodine-positive distant metastases such as miliary lung metastases[50] and it can produce false positive results in the chest from inflammation. The benefits of identifying iodine-negative deposits are limited to the small number of patients who have surgically resectable disease but this situation is likely to change in the future with the introduction of new therapeutic options. For now though, the indications for [18F]FDG PET in PC/FC are limited to identifying distant metastases in the post-operative patient with raised thyroglobulin and a negative 131I WBS. [18F]FDG PET has also been shown to identify distant metastases from MC and AC and so may have an even greater potential role in these patients with iodine-negative tumours.
Other imaging modalities
Other modalities such as bone radiographs, scintigraphy and MRI, liver ultrasound/CT, and brain CT can be positive when the 131I WBS and [18F]FDG PET are negative[51], but these modalities tend to be performed only when there are specific clinical indications. Laparoscopy and laparoscopic biopsy may be required for diagnosis of liver metastases from MC[52]. Scintigraphy is being used in some centres for post-operative assessment of MC using [111In]diethylenetriaminepentaacetic acid (DTPA)-octreotide, [99mTc(V)]dimercaptosuccinic acid (DMSA), and [131I]meta-iodobenzylguanidine (MIBG) which offer the prospect of targeted therapy in tumour positive cases[53] (Fig. 18). Radionuclide imaging using thallium-201 and technetium-99m labelled sestamibi are also used in some centres for thyroid cancer but discussion of these techniques is beyond the scope of this article.
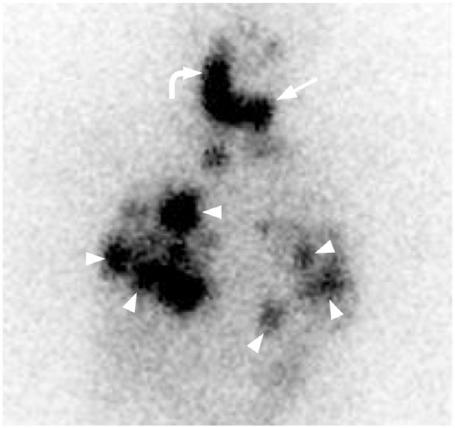
Figure 18.
131I-MIBG whole body scan in a patient with multiple bone and liver metastases from a medullary carcinoma (courtesy of Dr Frankie Choi).
Imaging for post-operative, pre-radiotherapy planning
Radiotherapy in the form of radioactive iodine (RAI) or external beam radiotherapy is used post-operatively to treat patients with microscopic or macroscopic residual disease. At present the indications for post-operative radiotherapy for thyroid cancer include positive microscopic resection margins, gross unresectable disease at the primary site, and the presence of extensive nodal metastases. Intensity modulated radiotherapy (IMRT) is being increasingly employed for the post-surgical treatment of thyroid cancer, in consideration of the improved distribution of radiation dose compared to conventional radiotherapy. IMRT delivers a high radiation dose to the tumour while shielding vital structures such as the spinal cord. The planning of radiotherapy is based on CT images acquired with the patient in the treatment position. With the use of IMRT, it is possible to assign different radiation dose levels to different regions perceived to be at different levels of risks. A higher dose is given to gross (i.e. imaging visible) tumour compared to a lower dose for sites at increased risk of occult nodal metastases and the site of a positive resection margin. For the purpose of focussing at the site of positive resection margin at the thyroid bed, a pre-operative CT scan or MRI is more helpful than ultrasound, as it is difficult to infer the site of extra-thyroidal extension seen on ultrasound in relation to the CT scan on which radiotherapy planning is executed. A post-operative ultrasound may be valuable to identify any residual nodal disease for radiotherapy planning purposes. In addition residual neck nodes greater than 1 cm detected by imaging may not respond to IMRT (this also applies to the administration of radioactive iodine)[17] and should be resected before further treatment (Fig. 19).
Figure 19.
Intensity modulated radiotherapy (IMRT) planning axial CT post-contrast showing a residual post-operative cystic nodal metastasis from papillary carcinoma (arrow). The patient underwent a further neck dissection to remove the node before IMRT was performed.
Imaging for post-treatment surveillance and recurrent cancer
After the first post-operative radioactive iodine scan performed with an ablative dose of radioactive iodine, further 131I scanning is not necessary if the thyroglobulin level is undetectable under thyroid stimulating hormone stimulation and the post-operative ultrasound is normal. Ultrasound of the neck has been shown to identify recurrent local tumours and neck node metastases in patients with a normal Tg level and normal 131I WBS[54],[55]. Therefore yearly ultrasound of the neck +/− FNA is advocated for surveillance of disease-free patients with PC/FC and normal thyroglobulin (Tg) and antithyroglobulin (TgAb), as well as for patients with MC and normal calcitonin levels. Patients with suspected recurrent cancer (rising biochemistry, ultrasound or clinical suspicion of recurrent tumour) need to undergo re-staging. The neck is imaged initially for local and nodal recurrence using ultrasound but this may need to be followed by MRI or CT. One difficulty for imaging is that metastatic nodes from PC can remain unchanged in size for many years. In addition, metastatic nodes treated with 131I may not resolve and can remain unchanged over many years. The 131/123I WBS is used for detection of distant metastases as well as assessing locoregional relapse for PC/FC. Limitations of 131/123I WBS for MC, AC and some less differentiated PC/FC may result in a greater role for CT thorax/liver and [18F]FDG PET. Other investigations depend on clinical indications and include bone radiographs, scintigraphy and MRI (spine, femur and pelvis), liver ultrasound/CT, brain CT and for MC, laparoscopy for liver metastases, [111In]DTPA-octreotide, [99mTc(V)]DMSA and [131I]MIBG scans.
Conclusions
Papillary carcinoma is by far the most frequently encountered thyroid cancer and the unpredictable behaviour of this cancer has led to controversies in the past regarding staging and management. However, as our understanding of this and other thyroid cancers improves, and as new treatments become available, it is clear that imaging will play a vital and ever-increasing role in the management of thyroid cancer.
Acknowledgements
I wish to thank Dr Frankie Choi, Dr Cheuk Lun Sham and Dr Sing Fai Leung for their advice on the nuclear medicine, surgical and radiotherapy aspects, respectively, of this article.
References
- [1].Davis L. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- [2].Colonna M, Guizard AV, Schvartz C, et al. A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983–2000) Eur J Cancer. 2007;43:891–900. doi: 10.1016/j.ejca.2006.11.024. [DOI] [PubMed] [Google Scholar]
- [3].KT Wong, AT Ahuja. Ultrasound of thyroid cancer. Cancer Imaging. 2005;5:157–66. doi: 10.1102/1470-7330.2005.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chammas MC, Gerhard R, de Oliveira IR, et al. Thyroid nodules: evaluation with power Doppler and duplex Doppler ultrasound. Otolaryngol Head Neck Surg. 2005;132:874–82. doi: 10.1016/j.otohns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [5].Cappelli C, Castellano M, Pirola I, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007;100:29–35. doi: 10.1093/qjmed/hcl121. [DOI] [PubMed] [Google Scholar]
- [6].Appetecchia M, Solivetti FM. The association of colour flow Doppler sonography and conventional ultrasonography improves the diagnosis of thyroid carcinoma. Hormone Res. 2006;66:249–56. doi: 10.1159/000096013. [DOI] [PubMed] [Google Scholar]
- [7].Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004;23:1455–64. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- [8].Yuan WH, Chiou HJ, Chou YH, et al. Gray-scale and color Doppler ultrasonographic manifestations of papillary thyroid carcinoma: analysis of 51 cases. Clinical Imaging. 2006;30:394–401. doi: 10.1016/j.clinimag.2006.09.024. [DOI] [PubMed] [Google Scholar]
- [9].Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103:1330–5. doi: 10.1002/cncr.20936. [DOI] [PubMed] [Google Scholar]
- [10].King AD, Ahuja AT, To EWH, Tse GMK, Metreweli C. Staging of papillary carcinoma of the thyroid: MR imaging vs ultrasound of the neck. Clin Radiol. 2000;55:222–6. doi: 10.1053/crad.1999.0373. [DOI] [PubMed] [Google Scholar]
- [11].Shetty SK, Maher MM, Hahn PF, Halpern EF, Aquino SL. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol. 2006;187:1349–56. doi: 10.2214/AJR.05.0468. (Erratum in AJR Am J Roentgenol 2007; 188: 8) [DOI] [PubMed] [Google Scholar]
- [12].De Geus-Oei LF, Pieters GFFM, Bonenkamp JJ, et al. 18F-FDG PET reduces unnecessary hemithyroidectomies for thyroid nodules with inconclusive cytologic results. J Nucl Med. 2006;47:770–5. [PubMed] [Google Scholar]
- [13].Mitchell JC, Grant F, Evenson AR, Parker JA, Hasselgren PO, Parangi S. Preoperative evaluation of thyroid nodules with 18FDG-PET/CT. Surgery. 2005;138:1166–75. doi: 10.1016/j.surg.2005.08.031. [DOI] [PubMed] [Google Scholar]
- [14].Chu QD, Connor MS, Lilien DL, Johnson LW, Turnage RH, Li BD. Positron emission tomography (PET) positive thyroid incidentaloma: the risk of malignancy observed in a tertiary referral center. Am Surg. 2006;72:272–5. [PubMed] [Google Scholar]
- [15].Bogsrud TV, Karantanis D, Nathan MA, et al. The value of quantifying 18F-FDG uptake in thyroid nodules found incidentally on whole-body PET-CT. Nucl Med Commun. 2007;28:373–81. doi: 10.1097/MNM.0b013e3280964eae. [DOI] [PubMed] [Google Scholar]
- [16].Passler C, Scheuba C, Asari R, Kaczirek K, Kaserer K, Niederle B. Importance of tumour size in papillary and follicular thyroid cancer. Br J Surg. 2005;92:184–9. doi: 10.1002/bjs.4795. [DOI] [PubMed] [Google Scholar]
- [17].Kebebew E, Clark OH. Differentiated thyroid cancer: ‘complete’ rational approach. World J Surg. 2000;24:942–51. doi: 10.1007/s002680010165. [DOI] [PubMed] [Google Scholar]
- [18].Wang JC, Takashima S, Takayama F, et al. Tracheal invasion by thyroid carcinoma. AJR. 2001;177:929–36. doi: 10.2214/ajr.177.4.1770929. [DOI] [PubMed] [Google Scholar]
- [19].Wang J, Takashima S, Matsushita T, Takayama F, Kobayashi T, Kadoya M. Esophageal invasion by thyroid carcinomas: prediction using magnetic resonance imaging. J Comput Assist Tomogr. 2003;27:18–25. doi: 10.1097/00004728-200301000-00004. [DOI] [PubMed] [Google Scholar]
- [20].Roychowdhury S, Loevner LA, Yousem DM, Chalian A, Montone KT. MR imaging for predicting neoplastic invasion of the cervical esophagus. AJNR Am J Neuroradiol. 2000;21:1681–7. [PMC free article] [PubMed] [Google Scholar]
- [21].Takashima S, Takayama F, Wang J, Kobayashi S, Kadoya M. Using MR imaging to predict invasion of the recurrent laryngeal nerve by thyroid carcinoma. AJR Am J Roentgenol. 2003;180:837–42. doi: 10.2214/ajr.180.3.1800837. [DOI] [PubMed] [Google Scholar]
- [22].Yousem DM, Hatabu H, Hurst RW, et al. Carotid artery invasion by head and neck masses: prediction with MR imaging. Radiology. 1995;195:715–20. doi: 10.1148/radiology.195.3.7754000. [DOI] [PubMed] [Google Scholar]
- [23].Noguchi S, Noguchi A, Murakami N. Papillary carcinoma of the thyroid I. developing pattern of metastasis. Cancer. 1970;26:1053–60. doi: 10.1002/1097-0142(197011)26:5<1053::aid-cncr2820260513>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [24].Ahuja AT, Chow L, Chik W, King W, Metreweli C. Metastatic cervical nodes in papillary carcinoma of the thyroid: ultrasound and histological correlation. Clin Radiol. 1995;50:229–31. doi: 10.1016/s0009-9260(05)83475-0. [DOI] [PubMed] [Google Scholar]
- [25].Rosario PW, de Faria S, Bicalho L, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005;24:1385–9. doi: 10.7863/jum.2005.24.10.1385. [DOI] [PubMed] [Google Scholar]
- [26].Som PM, Brandwein M, Lidov M, Biller HF. The varied presentations of papillary thyroid carcinoma cervical nodal disease: CT and MR findings. Am J Neuroradiol. 1994;15:1123–8. [PMC free article] [PubMed] [Google Scholar]
- [27].Jeong HS, Baek CH, Son YI, et al. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol. 2006;65:402–7. doi: 10.1111/j.1365-2265.2006.02612.x. [DOI] [PubMed] [Google Scholar]
- [28].Tubiana M, Schlumberger M, Rougier P, et al. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Caner. 1985;55:794–804. doi: 10.1002/1097-0142(19850215)55:4<794::aid-cncr2820550418>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [29].Noguchi S, Murakami N, Yamashita H, Toda M, Kawamoto H. Papillary thyroid carcinoma: modified radical neck dissection improves prognosis. Arch Surg. 1998;133:276–80. doi: 10.1001/archsurg.133.3.276. [DOI] [PubMed] [Google Scholar]
- [30].Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma. Cancer. 2006;106:524–31. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- [31].Palazzo FF, Gosnell J, Savio R, et al. Lymphadenectomy for papillary thyroid cancer: changes in practice over four decades. Eur J Surg Oncol. 2006;32:340–4. doi: 10.1016/j.ejso.2005.12.011. [DOI] [PubMed] [Google Scholar]
- [32].Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–96. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- [33].Khoo MLC, Freeman JL. Transcervical superior mediastinal lymphadenectomy in the management of papillary thyroid carcinoma. Head Neck. 2003;25:10–14. doi: 10.1002/hed.10173. [DOI] [PubMed] [Google Scholar]
- [34].Machens A, Hinze R, Thomusch O, Dralle H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg. 2002;26:22–8. doi: 10.1007/s00268-001-0176-3. [DOI] [PubMed] [Google Scholar]
- [35].Watkinson JC, Franklyn JA, Olliff JF. Detection and surgical treatment of cervical lymph nodes in differentiated thyroid cancer. Thyroid. 2006;16:187–94. doi: 10.1089/thy.2006.16.187. [DOI] [PubMed] [Google Scholar]
- [36].Ito Y, Tomoda C, Uruno T, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. Word J Surg. 2004;28:498–501. doi: 10.1007/s00268-004-7192-z. [DOI] [PubMed] [Google Scholar]
- [37].Amarasinghe IY, Perera NMA, Bahinathan N, Marzook HH, Peiris AKC. Review of distribution of nodal disease in differentiated thyroid cancers in an oncosurgical center in Sri Lanka. Ann Surg Oncol. 2007;14:1560–4. doi: 10.1245/s10434-006-9202-x. [DOI] [PubMed] [Google Scholar]
- [38].Sugenoya A, Asanuma K, Shingu K, et al. Clinical evaluation of upper mediastinal dissection for differentiated thyroid carcinoma. Surgery. 1993;113:541–5. [PubMed] [Google Scholar]
- [39].Machens A, Holzhausen HJ, Dralle H. Prediction of mediastinal lymph node metastasis in medullary thyroid carcinoma. Br J Surg. 2004;91:709–12. doi: 10.1002/bjs.4525. [DOI] [PubMed] [Google Scholar]
- [40].Benbassat CA, Mechlis-Frish S, Hirsch D. Clinicopathological characteristics and long-term outcome in patients with distant metastases from differentiated thyroid cancer. World J Surg. 2006;30:1088–95. doi: 10.1007/s00268-005-0472-4. [DOI] [PubMed] [Google Scholar]
- [41].Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8:148–56. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- [42].Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol. 2005;63:87–93. doi: 10.1111/j.1365-2265.2005.02304.x. [DOI] [PubMed] [Google Scholar]
- [43].Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging. 2004;48:12–9. [PubMed] [Google Scholar]
- [44].Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- [45].Kucuk ON, Gultekin SS, Aras G, Ibis E. Radioiodine whole-body scans, thyroglobulin levels, 99mTc-MIBI scans and computed tomography: results in patients with lung metastases from differentiated thyroid cancer. Nucl Med Commun. 2006;27:261–6. doi: 10.1097/00006231-200603000-00009. [DOI] [PubMed] [Google Scholar]
- [46].Bal CS, Kumar A, Chandra P, Dwivedi SN, Mukhopadhyaya S. Is chest x-ray or high-resolution computed tomography scan of the chest sufficient investigation to detect pulmonary metastasis in pediatric differentiated thyroid cancer? Thyroid. 2004;14:217–25. doi: 10.1089/105072504773297894. [DOI] [PubMed] [Google Scholar]
- [47].Ilgan S, Karacalioglu AO, Pabuscu Y, et al. Iodine-131 treatment and high-resolution CT: results in patients with lung metastases from differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:825–30. doi: 10.1007/s00259-004-1460-x. [DOI] [PubMed] [Google Scholar]
- [48].Chung JK, So Y, Lee JS, et al. Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med. 1999;40:986–92. [PubMed] [Google Scholar]
- [49].Wang W, Macapinlac H, Larson SM, et al. 18F-2-fluoro-2-deoxy-d-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic 131I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab. 1999;84:2291–302. doi: 10.1210/jcem.84.7.5827. [DOI] [PubMed] [Google Scholar]
- [50].Dietlein M, Scheidhauer K, Voth E Theissen P, Schicha H. Fluorine-18 fluorodeoxyglucose positron emission tomography and iodine-131 whole-body scintigraphy in the follow-up of differentiated thyroid cancer. Eur J Med. 1997;24:1342–8. doi: 10.1007/s002590050158. [DOI] [PubMed] [Google Scholar]
- [51].Shiga T, Tsukamoto E, Nakada K, et al. Comparison of 18F-FDG, 131I-Na, and 201T1 in diagnosis of recurrent or metastatic thyroid carcinoma. J Nucl Med. 2001;42:414–9. [PubMed] [Google Scholar]
- [52].Nanni C, Rubello D, Fanti S, et al. Role of 18F-FDG-PET and PET/CT imaging in thyroid cancer. Biomed Pharmacother. 2006;60:409–13. doi: 10.1016/j.biopha.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [53].Gao Z, Biersack HJ, Ezziddin S, Logvinski T, An R. The role of combined imaging in metastatic medullary thyroid carcinoma: 111In-DTPA octreotide and 131/123I-MIBG as predictors for radionuclide therapy. J Cancer Res Clin Oncol. 2004;130:649–56. doi: 10.1007/s00432-004-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Antonelli A, Miccoli P, Ferdeghini M, et al. Role of neck ultrasonography in the follow-up of patients operated on for thyroid cancer. Thyroid. 1995;5:25–8. doi: 10.1089/thy.1995.5.25. [DOI] [PubMed] [Google Scholar]
- [55].Frasoldati A, Pesenti M, Gallo M, Caroggio A, Salvo D, Valcavi R. Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer. 2003;97:90–6. doi: 10.1002/cncr.11031. [DOI] [PubMed] [Google Scholar]