Abstract
In the 1930s, Otto Warburg reported that anaerobic metabolism of glucose is a fundamental property of all tumours, even in the presence of an adequate oxygen supply. He also demonstrated a relationship between the degree of anaerobic metabolism and tumour growth rate. Today, this phenomenon forms the basis of tumour imaging with fluorodeoxyglucose positron emission tomography (FDG-PET). More recently, Folkman has demonstrated that malignant growth and survival are also dependent on tumour vascularity which is increasingly evaluated in vivo using techniques such as contrast enhanced computed tomography or magnetic resonance imaging (MRI). Although it is reasonable to hypothesise that the metabolic requirements of tumours are mirrored by alterations in tumour haemodynamics, the relationship between tumour blood flow and metabolism is in fact complex. A well-developed tumour vascular supply is required to ensure a sufficient delivery of glucose and oxygen to support the metabolism essential for tumour growth. However, an inadequate vascularisation of tumour will result in hypoxia, a factor that is known to stimulate anaerobic metabolism of glucose. Thus, the balance between tumour blood flow and metabolism will be an important indicator of the biological status of a tumour and hence the tumour's likely progression and response to treatment. This article reviews the molecular biology of tumour vascularisation and metabolism, relating these processes to currently available imaging techniques while summarising the imaging studies that have compared tumour blood flow and metabolism. The potential for vascular metabolic imaging to assess tumour aggression and sub-classify treatment response is highlighted.
Keywords: Tumour perfusion, tumour metabolism, angiogenesis, tumour characterisation, response evaluation
Introduction
The phenomena known as the ‘Warburg Effect’ was described by Otto Warburg (1883–1970) during his lifetime of work into cellular metabolism and respiration, for which he was awarded the Nobel Prize in 1931[1],[2]. He recognised that glucose can be metabolised either by combination with oxygen, i.e. respiration, or by glycolysis to produce lactate. He also observed that a change from oxidative phosphorylation to the less energy efficient glycolysis, even in the presence of an adequate supply of oxygen, is a fundamental property of the metabolism of cancer cells and that the rate of glycolysis correlated with tumour growth. Today, Warburg's findings underpin the principles of tumour imaging with fluorodeoxyglucose positron emission tomography (FDG-PET).
The Warburg Effect and oxygen delivery
The later identification of hypoxia inducible factor 1 (HIF-1) by Gregg Semenza in 1991 has provided further understanding of the mechanism by which cancer cells exhibit the increased aerobic glycolysis described by Warburg[1]. HIF-1 is a transcription factor which up-regulates a large number of cellular processes that confer a survival advantage to cancer cells. In particularly, HIF-1 increases expression of Glut-1 glucose transporters and hexokinase which are the major determinates of glucose uptake and metabolism. Another important effect of increased HIF-1 activity is increased production of vascular growth factors that stimulate new vessel formation and increased blood flow. Folkman has demonstrated that such new vessel formation (angiogenesis) also promotes tumour growth and survival[3].
HIF-1 is frequently expressed constitutively by tumours as a consequence of oncogene mutations including the p53 and Von Hippel Lindau (VHL) genes. Mutations in p53 are commonly found in a variety of tumour types; VHL mutations are particularly associated with renal cancer. The linkage between oncogene mutation, increased expression of HIF and accumulation of FDG has been demonstrated recently by microPET studies showing a two-fold increase in glucose metabolism in VHL knockdown tumour xenografts[4]. Clinical PET studies show that approximately 50–70% of renal cancers demonstrate FDG uptake, consistent with the expected frequency of VHL oncogene mutations in this tumour type[4].
On the basis of constitutive expression of HIF-1 by tumours, it would be reasonable to expect tumour blood flow and metabolism to increase in parallel. Indeed, high levels of angiogenesis and elevated glucose metabolism are both associated with increased metastatic potential and poor patient survival for a range of cancers[5–12]. However, tumour HIF-1 activity can be increased further by tissue hypoxia, which occurs when a tumour outgrows its blood supply. This additional HIF-1 activity ensures adaptation of the tumour to the hypoxic environment by producing an even greater increase in glucose metabolism beyond that secondary to oncogene effects alone, along with other metabolic changes which further increase tumour aggression and resistance to treatment[13].
Therefore, the balance between tumour vascularity and metabolic status offers important information concerning the tumour microenvironment. High glucose metabolism with increased vascularity represents a different biological status within the tumour than high metabolism with poor vascularity, the latter indicating adaptation to hypoxia. Low glucose metabolism with poor vascularity suggests a failure of the adaptive response to hypoxia and/or reduced oncogene effects.
Techniques for imaging tumour blood flow and metabolism
FDG-PET has become an established technique for imaging tumour metabolism in clinical practice and research. Although tumour perfusion imaging is used less frequently in clinical practice, a range of techniques is available including positron emission tomography (PET) with 15O-labelled water, dynamic contrast enhanced magnetic resonance imaging (MRI), contrast-enhanced perfusion computed tomography (CT) and ultrasound. However, there is a growing interest in the use of intravenous contrast media during PET-CT[14]. Extending these applications for contrast media to include perfusion CT would enable anatomical information about tumours to be co-registered with not only metabolic information but also perfusion data in a single examination, without the need for an on-site cyclotron (Fig. 1). The use of CT to assess perfusion as opposed to administration of a second PET tracer such as 15O-labelled water circumvents the need for an on-site cyclotron. Furthermore, because CT depicts perfusion data with higher spatial resolution, some of the limitations of PET perfusion studies can be avoided, including the underestimation of perfusion values in small tumours due to the partial volume effect and the spillover of counts from adjacent structures with high blood flow (e.g. heart, aorta, liver)[15]. CT measurements of perfusion in tumours have also been shown to correlate with polarographic probe measurements of tumour oxygenation[16].
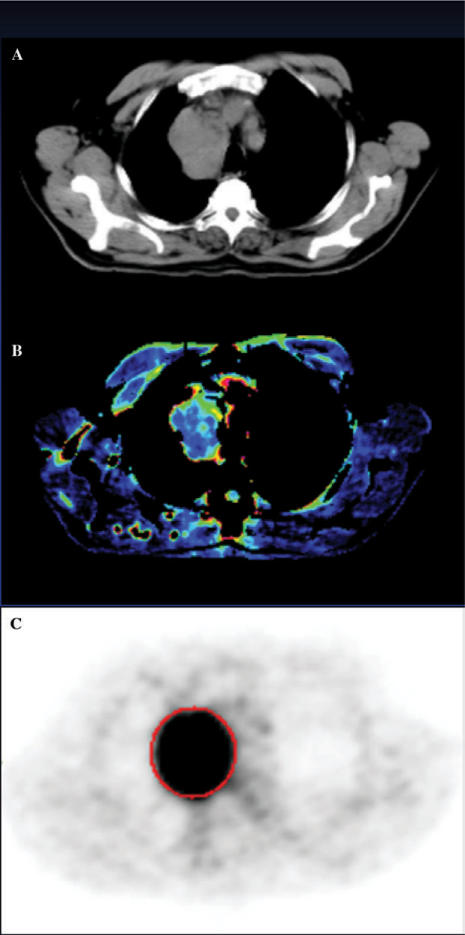
Figure 1.
Conventional CT (A) and images of tumour blood flow (B) and glucose metabolism (C) acquired using perfusion CT and FDG-PET, respectively, from a patient with non-small cell lung cancer.
Imaging studies of tumour blood flow and metabolism
Imaging studies using a range of techniques have shown the relationship between tumour blood flow and glucose metabolism to be highly variable[17–28] (Table 1). Factors influencing this relationship include tumour type, grade and size. Moderate correlations between tumour vascularity and metabolism have been observed in cerebral glioma and breast cancer[17–19]. In non-small cell lung cancer (NSCLC) and cancers of the head and neck, the relationship between tumour circulation and metabolism appears to be dependent on tumour size. Blood flow and metabolism correlate in NSCLCs smaller than 2.5–3.0 cm in diameter, whereas larger tumours exhibit glucose metabolism in excess of blood flow[20–23]. In head and neck cancer, Hirasawa et al.[28] observed no correlation between perfusion and metabolism for tumours smaller than 8 cm2, whereas an inverse correlation was found for larger tumours. Uncoupling of flow and metabolism has also been observed in pulmonary metastases[24]. Studies of liver tumours have shown a negative correlation between blood flow and metabolism, with the ratio of metabolism to blood flow increasing as tumours grow larger[25–27].
Table 1.
Summary of imaging studies comparing tumour vascularity and metabolism
| Study | Tumour type | Techniques | Findings |
|---|---|---|---|
| Aronen et al.[17] | Glioma | DC-MRI, FDG-PET | Maximum CBV correlates with maximum FDG (r = 0.573, p = 0.023) |
| Mankoff et al.[18] | Breast | H215O-PET, FDG-PET | Perfusion and metabolism weekly correlated. High metabolism-flow ratio predicts poor treatment response |
| Semple et al.[19] | Breast | DC-MRI, FDG-PET | Moderate correlation between vascularity and metabolism |
| Hunter et al.[20] | NSCLC | DC-MRI, FDG-PET | Correlation between vascular physiology and glucose metabolism in Stage IIIA (r = 0.76, p < 0.01) |
| Tateishi et al.[21] | NSCLC | Perf CT, FDG-PET | Vascularity and metabolism correlate in surgically resectable tumours |
| Hoekstra et al.[22] | NSCLC | H215O-PET, FDG-PET | No correlation between perfusion and metabolism in stage IIIA-N2 |
| Miles et al.[23] | NSCLC | Perf CT, FDG-PET | Correlation between vascularity and metabolism in small tumours only (r = 0.85, p = 0.03) |
| Veronesi et al.[24] | Lung metastases | Perf CT, FDG-PET | FDG uptake and angiogenesis independent |
| Fukuda et al.[25] | HCC, CCC and colorectal liver metastases | H215O-PET, FDG-PET | Negative correlation (r = −0.713, p = 0.006) |
| Stewart et al.[26] | Liver tumours (animal model) | Perf CT, FDG-PET | Glucose metabolism increases and blood flow decreases as tumours grow |
| Williams et al.[27] | Colorectal liver metastases | Perf CT, FDG-PET | Ratio of metabolism to blood flow increases with tumour size |
| Hirasawa et al.[28] | Head and neck cancer | Perf CT, FDG-PET | Negative correlation between perfusion and metabolism for tumours >8 cm2 |
DC-MRI, dynamic contrast-enhanced magnetic resonance imaging; CBV, cerebral blood volume; H215O, oxygen-15 labelled water; HCC, hepatocellular carcinoma; CCC, cholangiocarcinoma; Perf CT, perfusion CT.
An association between mismatched tumour blood flow and metabolism and adverse tumour biology has been illustrated by many of these studies. Aronen et al.[17] found that uncoupling of vascularity and metabolism was a feature of high-grade gliomas; Mankoff et al.[18] showed that breast cancers with a high ratio of glucose metabolism to perfusion were less likely to respond favourably to treatment. Miles et al.[23] found a high metabolic flow difference to be more likely in advanced NSCLC. This adverse impact of high glucose metabolism with low vascularity is also illustrated by separate imaging studies of head and neck cancer that show low perfusion and high FDG uptake to be independent predictors for poor local control following treatment[29],[30].
Imaging can also demonstrate regional areas of uncoupling of vascularity and metabolism within tumours (Fig. 2). In their study of locally advanced breast cancer, Mankoff et al.[18] illustrated a case in which high metabolism but low blood flow were observed at the centre of the tumour. Following chemotherapy, there was a substantial reduction in tumour size but the patient was left with a core of residual viable tumour, suggesting that regional areas of mismatch may have prognostic implications. Imaging may also depict areas of flow–metabolic mismatch adjacent to a region of frank necrosis (Fig. 2). This finding is in accordance with the results of autoradiographic studies of tumour allografts in which the greatest FDG uptake was found adjacent to areas of necrosis, correlating with local increases in the expression of Glut-1 and hexokinase[31]. Galie et al.[32] demonstrated that the epithelial and mesenchymal compartments of syngeneic tumour models exhibit reciprocal patterns of vascularity and metabolism. High vascularity relative to metabolism was found in the stromal capsule and intra-tumoural connectival septa, whereas tumour parenchyma exhibited lower vascularity but greater metabolism.
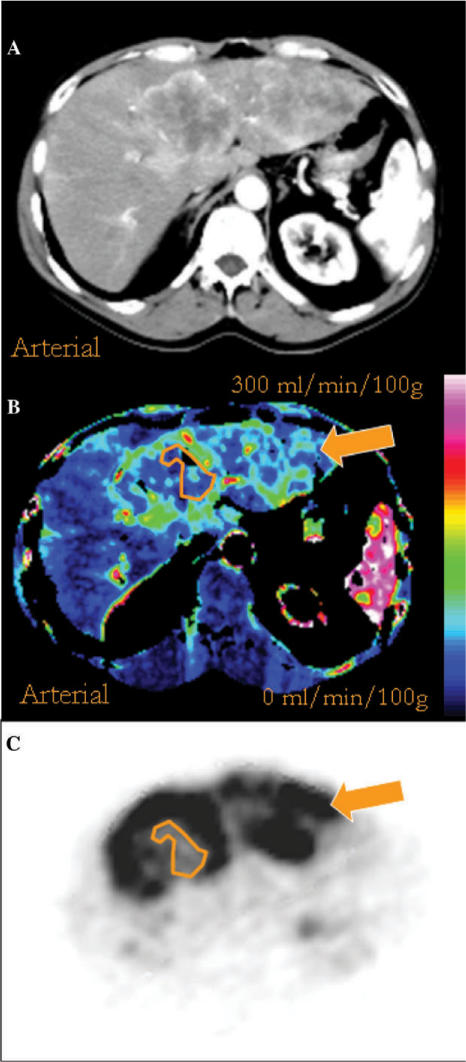
Figure 2.
Conventional contrast-enhanced CT (A), perfusion CT (B) and FDG-PET (C) images of a large hepatic metastasis from colorectal cancer demonstrating regional areas of mismatch between vascularity and metabolism. The orange polygon outlines an area of tumour necrosis with markedly reduced vascularity and metabolism. Regions of reduced vascularity but increased FDG uptake can be seen adjacent to the necrotic zone and in the left lobe of the liver (arrow).
Changes in tumour blood flow and metabolism following therapy
The application of FDG-PET and tumour perfusion imaging as markers of tumour response is increasing in research and clinical settings as the limitations of current structural imaging approaches are realised. Generally, these functional imaging techniques have been used in isolation. However, there have been a few studies in which both perfusion and FDG uptake have been measured before and after treatment. The findings show that perfusion and glucose metabolism may not change in parallel in response to therapy[33–36] (Fig. 3). Tumour type, drug type and dose, and time since therapy are all factors that may affect the relative magnitude of change in each parameter.
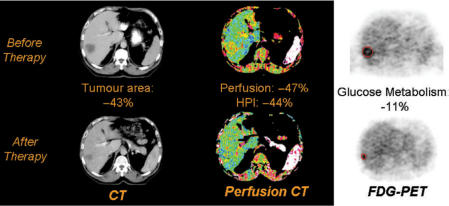
Figure 3.
Changes in tumour size (left), perfusion (centre) and metabolism (right) of a colorectal liver metastases following chemotherapy. There has been a partial morphological response with a predominantly vascular functional response. This combination may indicate adaptation of the tumour to the development of hypoxia during therapy. This response pattern could potentially indicate a need to adapt therapy in order to achieve a full response.
A study of rectal cancer by Willett et al.[33] using perfusion CT and FDG-PET reported significant falls in perfusion but no change in glucose metabolism when the vascular endothelial growth factor (VEGF) antibody, bevacizumab, was given alone. A reduction in glucose metabolism was only seen when bevacizumab was given in combination with radiotherapy. A study of locally advanced breast cancer by Mankoff et al.[34] found that following neoadjuvant chemotherapy, glucose metabolism tended to fall irrespective of the final pathological response, whereas perfusion increased in tumours failing to respond to treatment but decreased in tumours that subsequently proceeded to partial or complete response. On the other hand, a study of patients with androgen independent prostate cancer treated using thalidomide found that prostate-specific antigen (PSA) response correlated positively with change in glucose metabolism but negatively with change in perfusion[35]. The effect of drug dose is seen in a study by Herbst et al.[36] in which the anti-vascular agent endostatin, when given in high doses, resulted in decreased tumour perfusion but increased glucose metabolism. These studies suggest that uncoupling of flow and metabolism appears to be particularly likely following anti-angiogenic therapy, probably reflecting drug-induced hypoxia and secondary stimulation of glucose metabolism.
Based on the results of these studies, it is possible to propose a sub-classification of therapeutic responses into those that are (a) balanced (i.e. a significant reduction in both glucose metabolism and tumour vascularity), (b) predominantly vascular, and (c) predominantly metabolic (Table 2)[37]. A balanced response seems most likely to be associated with a good outcome. It is likely that the predominantly vascular and predominantly metabolic responses will carry different clinical significance. The possibility of modulating tumour responses by adapting therapy for individual patients on the basis of their imaging findings can be envisaged. For example, it may be appropriate to add an anti-vascular drug to a treatment regime producing a predominantly metabolic response. On the other hand, addition of a hypoxia agent may be appropriate if the response is predominantly vascular. The ultimate goal would be to tailor an individual patient's therapy to the vascular–metabolic response exhibited by their tumour.
Table 2.
Sub-classification of functional tumour response based upon perfusion and metabolic imaging
| Unchanged or increased perfusion | Reduced perfusion | |
|---|---|---|
| Unchanged or increased metabolism | No response. Likely poor outcome | Predominantly vascular partial response. ?adapt therapy to target hypoxia |
| Reduced metabolism | Predominantly metabolic partial response. ?adapt therapy to target neovasculature | Balanced response. Likely good outcome |
The putative clinical significance of each response class, given in italics, requires confirmation by further clinical trials. Adapted from Miles[37].
Summary
Knowledge of tumour biochemistry and molecular biology dating back to Warburg is fundamental to understanding the application of the techniques currently available for imaging tumour blood flow and metabolism. Tumours exhibit anaerobic metabolism of glucose even in the presence of adequate oxygen. However, glucose metabolism can be further stimulated in the presence of hypoxia associated with poor blood flow. Uncoupling of blood flow and metabolism implying hypoxic stimulation of glucose metabolism is frequently encountered in cancer, particularly in large aggressive tumours and following therapy. Imaging tumour blood flow and metabolism has potential applications for non-invasive characterisation of tumour aggression and may allow novel sub-classification of response with opportunities for personalised cancer care.
References
- [1].Garber K. Energy boost: the Warburg Effect returns in a new theory of cancer. J Natl Cancer Inst. 2004;96:1805–6. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- [2].Nobel Prize in Medicine 1931 Otto Warburg. [(accessed 25 April, 2006).];The official website of the Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1931/warburg-bio.html.
- [3].Folkman J. Tumour angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- [4].Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–7. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- [5].Ahuja V, Coleman RE, Herndon J, Patz EF., Jr The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer. 1998;83:918–24. [PubMed] [Google Scholar]
- [6].Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on 18F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999;17:3201–6. doi: 10.1200/JCO.1999.17.10.3201. [DOI] [PubMed] [Google Scholar]
- [7].Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- [8].Jeong HJ, Min JJ, Park JM, et al. Determination of the prognostic value of [18F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun. 2002;23:865–70. doi: 10.1097/00006231-200209000-00010. [DOI] [PubMed] [Google Scholar]
- [9].Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–60. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- [10].Fontanini G, Bigini D, Vignati S, et al. Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol. 1995;177:57–63. doi: 10.1002/path.1711770110. [DOI] [PubMed] [Google Scholar]
- [11].Meert AP, Paesmans M, Martin B, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694–701. doi: 10.1038/sj.bjc.6600551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tanaka F, Yanagihara K, Otake Y, et al. Prognostic factors in resected pathologic (p-) stage IIIA-N2, non-small-cell lung cancer. Ann Surg Oncol. 2004;11:612–8. doi: 10.1245/ASO.2004.07.013. [DOI] [PubMed] [Google Scholar]
- [13].Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–7. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- [14].Antoch G, Freudenberg LS, Beyer T, Bockisch A, Debatin JF. To enhance or not to enhance? 18F-FDG and CT contrast agents in dual-modality 18F-FDG PET/CT. J Nucl Med. 2004;45(Suppl 1):S56–65. [PubMed] [Google Scholar]
- [15].Bacharach SL, Libutti SK, Carrasquillo JA. Measuring tumor blood flow with H215O: practical considerations. Nucl Med Biol. 2000;27:671–767. doi: 10.1016/s0969-8051(00)00136-0. [DOI] [PubMed] [Google Scholar]
- [16].Haider MA, Milosevic M, Fyles A, et al. Assessment of the tumor microenvironment in cervix cancer using dynamic contrast enhanced CT, interstitial fluid pressure and oxygen measurements. Int J Radiat Oncol Biol Phys. 2005;62:1100–7. doi: 10.1016/j.ijrobp.2004.12.064. [DOI] [PubMed] [Google Scholar]
- [17].Aronen HJ, Pardo FS, Kennedy DN, et al. High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin Cancer Res. 2000;6:2189–200. [PubMed] [Google Scholar]
- [18].Mankoff DA, Dunnwald LK, Gralow JR, et al. Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med. 2002;43:500–9. [PubMed] [Google Scholar]
- [19].Semple SI, Gilbert FJ, Redpath TW, et al. The relationship between vascular and metabolic characteristics of primary breast tumours. Eur Radiol. 2004;14:2038–45. doi: 10.1007/s00330-004-2454-6. [DOI] [PubMed] [Google Scholar]
- [20].Hunter GJ, Hamberg LM, Choi N, Jain RK, McCloud T, Fischman AJ. Dynamic T1-weighted magnetic resonance imaging and positron emission tomography in patients with lung cancer: correlating vascular physiology with glucose metabolism. Clin Cancer Res. 1998;4:949–55. [PubMed] [Google Scholar]
- [21].Tateishi U, Nishihara H, Tsukamoto E, Morikawa T, Tamaki N, Miyasaka K. Lung tumors evaluated with FDG-PET and dynamic CT: the relationship between vascular density and glucose metabolism. J Comput Assist Tomogr. 2002;26:185–90. doi: 10.1097/00004728-200203000-00004. [DOI] [PubMed] [Google Scholar]
- [22].Hoekstra CJ, Stroobants SG, Hoekstra OS, Smit EF, Vansteenkiste JF, Lammertsma AA. Measurement of perfusion in stage IIIA-N2 non-small cell lung cancer using H215O and positron emission tomography. Clin Cancer Res. 2002;8:2109–15. [PubMed] [Google Scholar]
- [23].Miles KA, Griffiths MR, Keith CJ. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:22–8. doi: 10.1007/s00259-005-1932-7. [DOI] [PubMed] [Google Scholar]
- [24].Veronesi G, Landoni C, Pelosi G, et al. Fluoro-deoxy-glucose uptake and angiogenesis are independent biological features in lung metastases. Br J Cancer. 2002;86:1391–5. doi: 10.1038/sj.bjc.6600262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fukuda K, Taniguchi H, Koh T, Kunishima S, Yamagishi H. Relationships between oxygen and glucose metabolism in human liver tumours: positron emission tomography using 15O and 18F-deoxyglucose. Nucl Med Commun. 2004;25:577–83. doi: 10.1097/01.mnm.0000126627.01919.1d. [DOI] [PubMed] [Google Scholar]
- [26].Stewart EE, Chen X, Hadway J, Lee TY. Correlation between hepatic tumor blood flow and glucose utilization in a rabbit liver tumor model. Radiology. 2006;239:740–50. doi: 10.1148/radiol.2393041382. [DOI] [PubMed] [Google Scholar]
- [27].Williams RE, Miles KA. British Nuclear Medicine Society, Autumn Meeting. 2007. Quantitative studies into perfusion and metabolism in colorectal liver metastases using perfusion CT and FDG-PET. p. 7. [Google Scholar]
- [28].Hirasawa S, Tsushima Y, Takei H, et al. Inverse correlation between tumor perfusion and glucose uptake in human head and neck tumors. Acad Radiol. 2007;14:312–8. doi: 10.1016/j.acra.2006.12.017. [DOI] [PubMed] [Google Scholar]
- [29].Hermans R, Meijerink M, Van den Bogaert W, Rijnders A, Weltens C, Lambin P. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:1351–6. doi: 10.1016/s0360-3016(03)00764-8. [DOI] [PubMed] [Google Scholar]
- [30].Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;5:1295–300. doi: 10.1016/j.ijrobp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- [31].Zhao S, Kuge Y, Mochizuki T, et al. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46:675–82. [PubMed] [Google Scholar]
- [32].Galiè M, Farace P, Nanni C, et al. Epithelial and mesenchymal tumor compartments exhibit in vivo complementary patterns of vascular perfusion and glucose metabolism. Neoplasia. 2007;9:900–8. doi: 10.1593/neo.07541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mankoff DA, Dunnwald LK, Gralow JR, et al. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nucl Med. 2003;44:1806–14. [PubMed] [Google Scholar]
- [35].Kurdziel KA, Figg WD, Carrasquillo JA, et al. Using positron emission tomography 2-deoxy-2-[18F]fluoro-D-glucose, 11CO, and 15O-water for monitoring androgen independent prostate cancer. Mol Imaging Biol. 2003;5:86–93. doi: 10.1016/s1536-1632(03)00039-8. [DOI] [PubMed] [Google Scholar]
- [36].Herbst RS, Mullani NA, Davis DW, et al. Development of biologic markers of response and assessment of antiangiogenic activity in a clinical trial of human recombinant endostatin. J Clin Oncol. 2002;20:3804–14. doi: 10.1200/JCO.2002.05.102. [DOI] [PubMed] [Google Scholar]
- [37].Miles KA. Perfusion CT-PET: opportunities for combined assessment of tumor vascularity and metabolism. In: Miles KA, Cuenod C-A, editors. Multidetector computed tomography in oncology: CT perfusion Imaging. London: Informa Healthcare; 2007. pp. 215–29. [Google Scholar]