Abstract
As imaging technologies advance, a paradigm shift is emerging in the assessment of tumor response to therapy. The traditional method of measuring tumor size may not reflect changes in tumor viability induced by chemotherapy and radiation therapy. Today's oncologists and radiologists seek objective methods for assessing tumor metabolism and blood flow, measures that provide earlier, more accurate information about treatment effects. Pediatric imaging presents unique challenges not encountered in adult imaging, including the need for sedation and consideration of the long-term effects of radiation exposure in a growing child. Therefore, the potential risks and benefits of new imaging approaches for monitoring anticancer treatment in children require careful consideration. Several new imaging techniques are currently under investigation for use in pediatric oncology. These include dynamic enhanced magnetic resonance imaging and quantitative contrast-enhanced ultrasonography for assessment of blood flow in solid tumors such as osteosarcoma and neuroblastoma, and nuclear imaging, including positron emission tomography–computed tomography, for assessment of pediatric musculoskeletal tumors and neuroblastoma. The potential value, relative advantages, and limitations of these new methods in monitoring anticancer therapy in children are discussed.
Keywords: Dynamic enhanced magnetic resonance imaging, contrast-enhanced ultrasonography, positron emission tomography–computed tomography
Introduction
In 2000, an international committee composed of members of the European Organization for Research and Treatment of Cancer, the National Cancer Institute of the United States, and the National Cancer Institute of Canada proposed the Response Evaluation Criteria in Solid Tumors (RECIST) as an alternative to the World Health Organization (WHO) criteria that had been in use since 1979. The RECIST incorporated advances in imaging technology and simplified the WHO methodology; however, both criteria rely on changes in tumor size to define tumor response or progression. Specifically, the primary tumor and metastatic sites are measured before the initiation of therapy and then at time points determined by tumor biology and the expected response to the therapeutic agent. This approach can result in patients receiving ineffective treatments for months before treatment failure is identified. Such patients are exposed to undue toxicity and most likely have a diminished probability of survival[1]. The ideal evaluation would use reliable, reproducible, non-invasive techniques to identify non-respondent tumors early in the course of therapy to reduce unnecessary toxicity and tailor case management to optimize patient survival.
Functional imaging offers methods for assessing tumor blood flow and metabolism, two parameters that reflect tumor viability and indicate the therapeutic response that occurs before the tumor size changes. Positron emission tomography–computed tomography (PET-CT), dynamic enhanced magnetic resonance imaging (DEMRI), perfusion-computed tomography, and contrast-enhanced ultrasonography (CEUS) all show promise as modalities for further development in pediatric oncology. When such imaging examinations are performed on children, the risk/benefit ratio must be carefully balanced. The potential detrimental effects of ionizing radiation on the growing child have been well described in recent literature[2],[3]. The need for sedation and anesthesia must be considered, especially when procedures are performed at adult facilities that may lack experienced personnel and equipment needed to treat pediatric patients. This review focuses on the current status of DEMRI, PET-CT, and CEUS as they apply to management of pediatric solid malignancies. The relative merits and limitations of each modality are addressed.
Dynamic enhanced magnetic resonance imaging in the assessment of bone tumors
The survival of children with osteosarcoma has improved dramatically with the use of preoperative, neoadjuvant therapy. The tumor's histologic response to neoadjuvant therapy, which is indicated by the percent necrosis of the resected specimen, predicts prognosis: patients with tumors that are 90% or more necrotic have a greater probability of survival than others[4]. Magnetic resonance imaging (MRI) is the conventional method used to monitor osteosarcoma response to therapy. However, this approach has limited value, because osseous tumors may respond well to therapy without substantially changing size. Furthermore, assessment of tumor necrosis by MRI is subjective and prone to interobserver variability. DEMRI provides a method of quantifying tumor blood flow, which better reflects tumor viability.
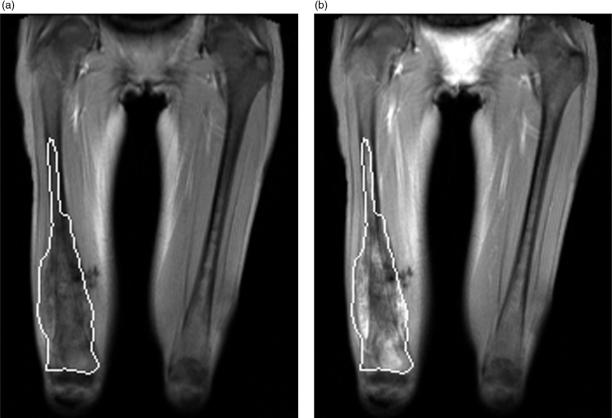
At St. Jude Children's Research Hospital (St. Jude), DEMRI is performed by acquiring sequential 10-mm-thick, coronal, T1-weighted images of the primary tumor in the plane with the largest tumor dimensions. One image is acquired every 12 s over approximately 6 min. A 2 ml/10 kg, intravenous, bolus injection of a low-molecular weight, paramagnetic contrast agent, followed by a 20-ml flush of normal saline, is given 1 min after the start of imaging[5]. The images are evaluated off-line using in-house software. A region of interest (ROI) is defined by the margins of the tumor, and several enhancement parameters are measured (Fig. 1). Because MRI contrast agents diffuse across the vascular membrane but cannot cross the cellular membrane, they are present only in the plasma and extracellular fluid space[5]. Therefore, a two-compartment pharmacokinetic model comprising the plasma volume and extracellular fluid space can be used to analyze contrast distribution within each voxel of an ROI.
Figure 1.
Dynamic enhanced MRI examination of a 14-year-old girl with right femoral osteosarcoma. (a) Coronal T1 weighted image showing the region of interest (ROI) encompassing the entire tumor before contrast administration and (b) at the end of the 6-min scanning period. This tumor was a non-responder (<90% tumor necrosis).
St. Jude investigators found that the modeled exchange rate of contrast agent between plasma and tumor extracellular fluid space, designated kep, and the dynamic vector magnitude (DVM), which incorporates the initial rate of contrast uptake and the maximum enhancement, are useful measures of osteosarcoma viability. Using a DVM threshold of 1.8 to distinguish responders (≥90% tumor necrosis) from non-responders (<90% necrosis), Reddick and colleagues found that tumor DVM measured at the completion of neoadjuvant therapy is 83% accurate, 81% sensitive, and 85% specific[5]. They also showed that the kep measured at the end of neoadjuvant therapy was significantly associated with disease-free survival, i.e., lower kep estimates predicted improved survival. Other investigators have applied this technique to Ewing sarcoma family of tumors (ESFTs) with similar results[6],[7].
Although DEMRI has proven to be a valuable tool for assessing pediatric bone tumors at St. Jude and other large academic centers, the reproducibility of the technique needs to be addressed in large multi-institutional trials. A typical DEMRI examination requires 15–20 min to perform. Because most pediatric patients with bone tumors are adolescents or young adults, DEMRI can usually be performed at the time of routine MRI examination without the need for sedation.
As anti-angiogenic agents are introduced into pediatric oncology clinical trials, DEMRI will inevitably play a valuable role. This technique is particularly attractive for use in children, because it involves no ionizing radiation. For young children with solid tumors, however, the time needed to perform DEMRI will be difficult to justify in cases where sedation is required. Nonetheless, DEMRI holds promise as a useful method for assessing vascular changes induced in tumors by therapeutic agents.
PET-CT in the assessment of pediatric musculoskeletal sarcomas and neuroblastoma
[18F]Fluorodeoxyglucose (FDG) PET-CT has emerged as a powerful metabolic-anatomic imaging tool for assessing various adult hematologic or solid malignancies. Although the value of PET and PET-CT in the assessment of adult cancers has been demonstrated, their value in the management of pediatric cancers (other than Hodgkin's lymphoma) is less well defined.
In primary bone malignancies, a reliable, non-invasive method such as PET for assessing tumor viability may permit the oncologist and surgeon to individualize management of tumors that are aggressive, respond poorly, or arise in surgically challenging sites. The FDG standardized uptake value (SUV) offers a semiquantitative measure of tumor metabolic activity. Because tumors are metabolically heterogeneous, the maximum SUV obtained from an ROI within the tumor is thought to provide the most reliable assessment of tumor metabolism[8]. Hawkins and colleagues recently investigated the value of the maximum SUV at diagnosis (SUV1) and after completion of neoadjuvant therapy (SUV2) in children and adults with the Ewings Sarcoma Family of Tumors (ESFTs). They found that patients whose SUV2 was less than 2 were more likely than others to have a favorable histologic response (≤10% viable tumor) and to have improved 4-year progression-free survival[9]. In an earlier study, Hawkins had reported a similar predictive value of SUV1 and SUV2 in patients with primary osteosarcoma[10].
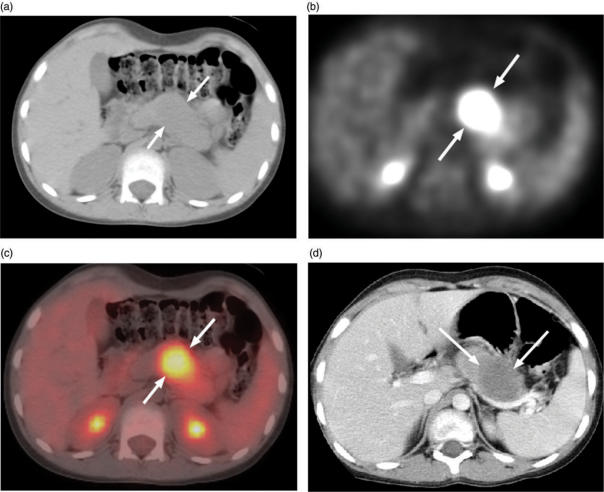
Upon review of our preliminary experience using PET-CT to evaluate 61 cases of various pediatric bone and soft tissue sarcomas[11], we found that PET-CT was especially valuable in assessing children with alveolar rhabdomyosarcoma, which has a propensity to metastasize to unusual soft tissue sites. In those patients, PET-CT identified metastatic disease in the breast, pancreas, peritoneum, and soft tissues of the extremities that had not been appreciated on physical examination or conventional diagnostic CT imaging (Fig. 2). In our experience, PET-CT has been a useful adjunct to the qualitative assessment of tumor response to chemotherapy, radiation therapy, or radiofrequency ablation and to the assessment of residual tumor in the surgical bed after resection[11].
Figure 2.
Axial abdominal PET-CT images of a 10-year-old girl with metastatic alveolar rhabdomyosarcoma. (a) Axial CT image at level of a pancreatic metastasis. The lesion (arrows) is not easily visible on this non-contrast enhanced image. (b) PET image showing intense FDG activity localized to (c) the body and tail of the pancreas on this fused PET-CT image. (d) This contrast enhanced CT image of the upper abdomen was obtained during a chest CT performed 1 week before the PET-CT. The pancreas mass (arrows) was overlooked.
PET-CT shows promise as a method of evaluating high-risk childhood neuroblastoma. Kushner and colleagues compared PET (but not PET-CT) with technetium-99m methylene diphosphonate (99mTc MDP) bone scan, [123I]meta-iodobenzylguanidine (MIBG) scan, CT or MRI, urinary catecholamines, and bone marrow biopsy in 51 children with high-risk disease at diagnosis and during follow-up. Their goal was to determine the minimum number of tests needed to detect disease. They found that PET revealed more osseous metastases than did 99mTc MDP bone scan. PET also either matched or surpassed MIBG for detecting skeletal abnormalities, with the notable exception of skull lesions that were obscured by intense, physiologic, brain FDG activity. Both PET and MIBG failed to detect minimal residual disease, which was detected by bone marrow biopsy. The investigators concluded that in the absence of or after the resolution of skull metastases and after resection of the primary tumor, PET and bone marrow biopsy are sufficient for the follow-up evaluation of this patient population[12]. Currently, studies are underway at St. Jude to prospectively evaluate the role of PET-CT in the management of high-risk neuroblastoma. Skull lesions overlooked by PET may be more obvious with the benefit of correlative CT imaging of the skull viewed in the bone window setting, which increases lesion conspicuity.
Significant strides have been made in demonstrating the value of PET and PET-CT in the evaluation of childhood solid malignancies. Both the subjective assessment of PET images and the measurement of the SUV hold promise as accurate methods for staging at baseline, assessing response to therapy, and detecting recurrences. The obvious limitation of PET-CT is the inherent risk of radiation exposure. Additionally, PET-CT examinations can be lengthy and may require sedation of young patients.
Contrast-enhanced ultrasonography for assessment of tumor vascularity
In 1971, Judah Folkman first hypothesized that tumor growth depends on the formation of new blood vessels[13]. Since then, considerable research has focused on validating this theory and applying it to preclinical models[14],[15]. In 2004, Hurwitz and colleagues demonstrated significantly improved survival of adults with metastatic colon cancer who received the anti-angiogenic agent bevacizumab in combination with conventional therapy, compared with that of patients who received conventional therapy alone[16]. That large randomized study sparked a surge of interest in anti-angiogenic agents. Multiple groups have shown that tumor shrinkage may not be an appropriate endpoint for evaluating anti-angiogenic agents, because restrictions in tumor microvasculature can occur without affecting tumor size[17],[18].
The successful introduction of anti-angiogenic therapies into cancer clinical trials will require reliable, non-invasive methods of assessing angiogenesis and its modification or inhibition in vivo[19]. Imaging modalities that show promise for assessing tumor blood flow include perfusion CT, DEMRI, and quantitative CEUS. Because perfusion CT exposes the patient to a substantial dose of ionizing radiation, it is not an attractive option for the assessment of pediatric patients. The merits and limitations of DEMRI in pediatric oncology have been addressed above.
Unlike MRI and CT contrast agents, ultrasound contrast agents are composed of microbubbles that approximate the size of red blood cells. Therefore, the agent remains within the vascular space. The microbubbles consist of an outer sphere that encapsulates a gas. These contrast agents are highly reflective on ultrasound imaging and offer a potential approach to enhance visualization of tumor neovasculature in small volumes of tissue and to monitor the early effects of anti-angiogenic therapy on small tumor neovessels, whose diameter is typically less than 50 µm[20].
We have shown that CEUS is a reliable method for assessing tumor blood flow in murine models treated with various anti-angiogenic agents[21–23]. Most recently, we investigated temporal vascular changes induced by bevacizumab in a murine neuroblastoma model by evaluating tumor perfusion at 24 h, 3 days, and 7 days after the administration of one dose of bevacizumab. We quantified tumor perfusion by using a clinical ultrasound machine (Acuson Sequoia, Siemens) and a 15L8 MHz transducer to measure the flow of the contrast agent Optison™ (Amersham Health, Inc.) into tumors. Mice were given a bolus injection of 0.1 ml Optison™ diluted in 0.1 ml of saline. Tumors were imaged in the transverse plane with a mechanical index of 0.4 or less to reduce microbubble destruction; images were recorded on cine-clips starting immediately before the injection and continuing for 30 s thereafter. Cine-clips were later analyzed on-line by using the Cadence contrast pulse sequence ACQ software (Siemens). An ROI was drawn to encompass the entire tumor, and the log-compressed signal intensity within the ROI was determined for each frame during the 30-s scan period.
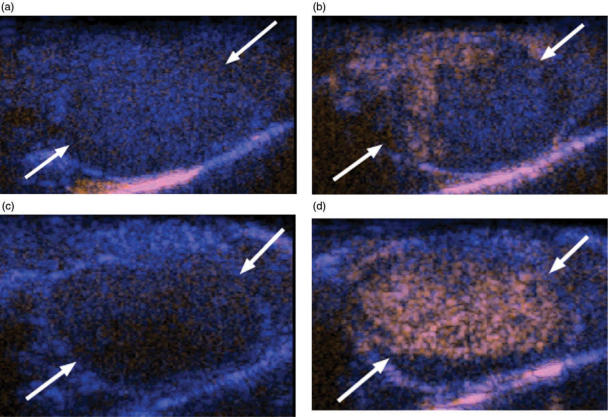
The change in signal intensity from baseline to peak (ΔSI) was significantly increased by 30.6% (17.38 ± 0.99 dB versus 13.30 ± 0.73 dB; P = 0.01) at 24 h and by 45.5% (14.25 ± 1.40 dB versus 9.29 ± 1.15 dB; P = 0.03) at day 3, in treated mice versus size-matched controls (Fig. 3). At day 7, the ΔSI was nearly equivalent for treated and control tumors (9.66 ± 0.24 dB versus 9.68 ± 0.69 dB; P = 0.92). These CEUS findings were confirmed by intravital microscopy, which showed normalization of tumor vessels at days 1 and 3 and vascular pruning at day 7. Immunohistochemical assessment of tumors with anti-CD34 and anti-smooth muscle actin antibodies showed increases in the vessel maturity index (functioning vessels) at days 1, 3, and 7. These findings suggest that bevacizumab transiently improved tumor vascularity early after its administration but then reduced tumor blood flow. Such information could be important in scheduling conventional chemotherapy given in combination with anti-angiogenic agents to optimize intratumoral drug penetration[22].
Figure 3.
Contrast enhanced ultrasound images of bevacizumab treated and control murine retroperitoneal neuroblastoma. (a) Before contrast administration and (b) at the peak of enhancement, this size matched control tumor (arrows) shows only minimal, peripheral perfusion (orange area). (c) Before contrast administration and at (d) peak enhancement, this tumor shows intense and homogenous perfusion 3 days after administration of one dose of bevacizumab.
Limitations of CEUS include interoperator variability, issues of reproducibility, and variations in body habitus and bowel gas that can impair image quality. However, CEUS offers several important advantages over DEMRI and perfusion CT. Perhaps most importantly, it does not involve potentially harmful exposure to ionizing radiation. Ultrasound is generally well tolerated and does not require sedation. Additionally, it can be performed at the bedside and is less expensive than other imaging modalities. These factors, coupled with encouraging preclinical findings, make CEUS especially appealing for future development in several ongoing clinical trials at St. Jude.
Conclusions
Ultimately, the value of an imaging modality in oncology depends on its ability to accurately predict the probability of patient survival. For pediatric applications, the ideal modality would minimize radiation exposure and the need for sedation. In the future, information obtained from functional imaging modalities should allow clinicians to intervene early and tailor the management of tumors to improve their response to therapy.
References
- [1].Fournier LS, Cuenod CA, Clement O, Siauve N, Frija G. Imaging of response to treatment in oncology. J Radiol. 2007;88:829–43. doi: 10.1016/s0221-0363(07)89885-4. [DOI] [PubMed] [Google Scholar]
- [2].Frush DP, Donnelly LF, Rosen NS. Computed tomography and radiation risks: what pediatric health care providers should know. Pediatrics. 2003;112:951–7. doi: 10.1542/peds.112.4.951. [DOI] [PubMed] [Google Scholar]
- [3].Brody AS, Frush DP, Huda W, Brent RL. Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–82. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- [4].Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- [5].Reddick WE, Wang S, Xiong X, et al. Dynamic magnetic resonance imaging of regional contrast access as an additional prognostic factor in pediatric osteosarcoma. Cancer. 2001;91:2230–7. [PubMed] [Google Scholar]
- [6].Fletcher BD, Hanna SL, Fairclough DL, Gronemeyer SA. Pediatric musculoskeletal tumors: use of dynamic, contrast-enhanced MR imaging to monitor response to chemotherapy. Radiology. 1992;184:243–8. doi: 10.1148/radiology.184.1.1319075. [DOI] [PubMed] [Google Scholar]
- [7].Dyke JP, Panicek DM, Healey JH, et al. Osteogenic and Ewing sarcomas: estimation of necrotic fraction during induction chemotherapy with dynamic contrast-enhanced MR imaging. Radiology. 2003;228:271–8. doi: 10.1148/radiol.2281011651. [DOI] [PubMed] [Google Scholar]
- [8].Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J Nucl Med. 2003;44:930–42. [PubMed] [Google Scholar]
- [9].Hawkins DS, Schuetze SM, Butrynski JE, et al. 18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–34. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- [10].Hawkins DS, Rajendran JG, Conrad EU, III, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–84. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- [11].McCarville MB, Christie R, Daw NC, Spunt SL, Kaste SC. PET/CT in the evaluation of childhood sarcomas. AJR Am J Roentgenol. 2005;184:1293–304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- [12].Kushner BH, Yeung HW, Larson SM, Kramer K, Cheung NK. Extending positron emission tomography scan utility to high-risk neuroblastoma: fluorine-18 fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J Clin Oncol. 2001;19:3397–405. doi: 10.1200/JCO.2001.19.14.3397. [DOI] [PubMed] [Google Scholar]
- [13].Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- [14].Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- [15].Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–24. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- [16].Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- [17].Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. 2002;94:883–93. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- [18].Padhani AR, Neeman M. Challenges for imaging angiogenesis. Br J Radiol. 2001;74:886–90. doi: 10.1259/bjr.74.886.740886. [DOI] [PubMed] [Google Scholar]
- [19].Anderson H, Price P, Blomley M, Leach MO, Workman P. Measuring changes in human tumour vasculature in response to therapy using functional imaging techniques. Br J Cancer. 2001;85:1085–93. doi: 10.1054/bjoc.2001.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferrara KW, Merritt CR, Burns PN, Foster FS, Mattrey RF, Wickline SA. Evaluation of tumor angiogenesis with US: imaging, Doppler, and contrast agents. Acad Radiol. 2000;7:824–39. doi: 10.1016/s1076-6332(00)80631-5. [DOI] [PubMed] [Google Scholar]
- [21].McCarville M, Streck C, Dickson PV, Li C-S, Nathwani A, Davidoff A. Quantitative, non-invasive assessment of intratumoral blood flow using contrast enhanced grayscale ultrasonography to evaluate the efficacy of angiogenesis inhibitors in a murine neuroblastoma model. Radiology. 2006;240:73–81. doi: 10.1148/radiol.2401050709. [DOI] [PubMed] [Google Scholar]
- [22].Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–50. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- [23].Dickson PV, Streck CJ, Ng CYC, et al. Continuous delivery of interferon-beta promotes sustained intratumoral vascular normalization and tumor growth restriction through upregulation of angiopoietin-1. Mol Cancer Res. 2007;5:531–42. doi: 10.1158/1541-7786.MCR-06-0259. [DOI] [PubMed] [Google Scholar]